Abstract
Animals anticipate the timing of food availability via the food-entrainable oscillator (FEO). The anatomical location and timekeeping mechanism of the FEO are unknown. Several studies showed the circadian gene, Period 2, is critical for FEO timekeeping. However, other studies concluded that canonical circadian genes are not essential for FEO timekeeping. In this study, we re-examined the effects of the Per2 Brdm1 mutation on food entrainment using methods that have revealed robust food anticipatory activity in other mutant lines. We examined food anticipatory activity, which is the output of the FEO, in single Period mutant mice. Single Per1, Per2, and Per3 mutant mice had robust food anticipatory activity during restricted feeding. In addition, we found that two different lines of Per2 mutant mice (ldc and Brdm1) anticipated restricted food availability. To determine if FEO timekeeping persisted in the absence of the food cue, we assessed activity during fasting. Food anticipatory (wheel-running) activity in all Period mutant mice was also robust during food deprivation. Together, our studies demonstrate that the Period genes are not necessary for the expression of food anticipatory activity.
Introduction
The food-entrainable oscillator (FEO) is an enigmatic circadian pacemaker that is entrained by temporally restricted food availability1. During daytime restricted feeding, mice display anticipatory activity (the output of the FEO) prior to food availability. The self-sustained nature of the FEO is evidenced by the persistence of anticipatory activity during fasting subsequent to restricted feeding. Numerous attempts to identify the locus of the FEO have been unsuccessful2. However, the FEO does not reside in the master circadian pacemaker in the suprachiastmatic nucleus (SCN) since food anticipatory activity persists in SCN-lesioned animals3–5.
Recent studies have shown that the molecular timekeeping mechanism of the FEO operates differently compared to canonical circadian oscillators (e.g. SCN, liver, lung). This was first demonstrated in homozygous Clock Δ19 mutant mice that have arrhythmic SCN-controlled nocturnal activity, but normal FEO-controlled food anticipatory activity6. Similarly, mice lacking both functional Cryptochrome (Cry)1 and Cry2, or both Period (Per)1 and Per2, or Per1, Per2, and Per3 exhibit food anticipatory activity (albeit sometimes abnormal or with a non-24h period) when nocturnal activity is arrhythmic7–9.
However, several studies suggested that some canonical circadian genes are necessary for FEO timekeeping (Table 1). Three studies showed that food anticipatory activity was absent in Per2 mutant mice (the Brdm1 strain)10–12. Another study used mice with a conditional Per2 allele and showed that total-body and liver-specific Per2 mutant mice did not express FAA13. In contrast, Storch and Weitz showed that a different line of Per2 mutant mice (the ldc strain) had robust food anticipatory activity8. In this study, we sought to re-examine the roles of the Period genes in food entrainment.
Table 1.
Summary of previous studies of food anticipatory activity in Period mutant mice.
| Genotype | Genetic background | Age (weeks) | Length of food availability | RF phase | Results | Reference |
|---|---|---|---|---|---|---|
| Per1 −/− | C57BL/6 × 129SvEvBrd | 12 to 28 | 8 h | ZT4–12 | FAA present | 10 |
| C57BL/6 × 129SvEvBrd | Not reported | 6 h | ZT6–12 | FAA present | 11 | |
| C57BL/6 J (>N12) | 8 | 4 h | ZT4–8 | FAA present | 12 | |
| mPer2 Brdm1−/− | C57BL/6 × 129SvEvBrd | 12 to 28 | 8 h | ZT4–12 | FAA absent | 10 |
| C57BL/6 × 129SvEvBrd | Not reported | 6 h | ZT6–12 | FAA absent | 11 | |
| C57BL/6 J (>N12) | 8 | 4 h | ZT4–8 | FAA very weak or absent | 12 | |
| mPer2 ldc−/− | 129/C57BL/6 | 7 to 9 | 3 h | ZT6–9 | FAA present | 8 |
| mPer1 ldc−/− / mPer2 ldc−/− | 129 | 7 to 9 | 3 h | ZT6–9 | FAA present | 8 |
| mPer1 ldc−/− / mPer2 ldc−/− / mPer3 −/− | C57BL/6 J | 9 to 20 | 6 h | ZT8–14 | FAA present | 9 |
*All studies were performed in 12 L:12D, except the triple Per1/2/3 mutant mice study that was performed in 18L:6D. Wheel-running food anticipatory activity (FAA) was measured during restricted feeding (RF) in mice with intact (not lesioned) SCN. Results from studies where caloric restriction was combined with restricted feeding are not reported.
Results
Period mutant (ldc strain) mice have robust food anticipatory activity during restricted feeding and fasting
We first determined if C57BL/6 J Period1 (mPer1 ldc−/−), Period2 (mPer2 ldc−/−), and Period3 (mPer3 −/−) mutant mice14 expressed food anticipatory activity during daytime restricted feeding (ZT6-10) and subsequent fasting. During ad libitum feeding, all mice had minimal daytime wheel-running activity (Fig. 1: AL1; actograms of all mice shown in Figs. S1–S4). In contrast, during 4-h restricted feeding, wheel-running activity began 2 to 4 hours before feeding time and continued until food was provided at ZT6 in wild-type and mPer1 ldc−/−, mPer2 ldc−/−, and mPer3 −/− mice (RF in Fig. 1, Fig. 2a). When mice were returned to ad libitum feeding, food anticipatory activity disappeared (Fig. 1: ALII). However, when we food deprived mice after 1 week of ad libitum feeding, food anticipatory activity reappeared at a similar phase in Per mutant and wild-type mice (Fig. 1: FD). As we previously reported, food anticipatory activity was weak or absent on the first day of fasting (Fig. 2b), but was robust in all genotypes on the second day of food deprivation (Fig. 2c).
Figure 1.
PERIOD-deficient (ldc) mice have robust wheel-running food anticipatory activity during restricted feeding and food deprivation. Representative double-plotted actograms (a–d) and group average activity profiles (e–l) of wild-type (a,e,i), mPer1 ldc−/− (b,f,j), mPer2 ldc−/− (c,g,k), and mPer3 −/− (d,h,l) mice. The time when food was available is indicated by gray shading on the left half of each actogram and in the activity profiles. The light-dark cycle is indicated by the white and black bars, respectively. The black traces in the group activity profiles represent the mean number of wheel revolutions (in counts/10-minute bin) relative to the light-dark cycle where 0 is lights on and 12 is lights off. The SEM is shown in dark gray shading in each activity profile. ALI, RF, ALII, and FD in a-d indicate the days used to generate the activity profiles ad libitum I, restricted feeding, ad libitum II (e–h) and food deprivation (i–l), respectively. Forty-eight hours of continuous food deprivation is shown (i–l) and the dotted lines indicate when food was available during the preceding restricted feeding.
Figure 2.

Food anticipatory activity from individual wild-type and Period mutant (ldc strain) mice. (a) Food anticipatory activity (FAA) during 9 days of restricted feeding (RF) of wild-type (n = 5), mPer1 ldc−/− (n = 4), mPer2 ldc−/− (n = 7), and mPer3 −/− (n = 5) mice was determined by totaling the number of wheel revolutions per minute from 4 hours before feeding time to the end of feeding time (total of 8 hours). FAA during fasting was defined as the total number of wheel revolutions per minute from 4 hours before feeding time to the end of previous feeding time (total of 8 hours). Wheel-running FAA for each mouse was determined separately for the first (Day 1; b) or second (Day 2; c) day of fasting. Each black circle is data from one mouse. The mean of each group is a horizontal line.
To determine if genotype-specific differences in total daily activity affected the expression of food anticipatory activity, we expressed food anticipatory as a ratio of total daily activity (Fig. S5). We found that wild-type and mPer1 ldc−/−, mPer2 ldc−/−, and mPer3 −/− mice had similar food anticipatory activity ratios during RF (Fig. S5a), day 1 fasting (Fig. S5b), and day 2 fasting (Fig. S5c). We also found that the ages of the mice were not correlated with their food anticipatory activity ratios (Fig. S6).
Period2 mutant (Brdm1 strain) mice have robust food anticipatory activity during restricted feeding and fasting
Three previous studies found that food anticipatory activity was absent or very weak in mPer2 Brdm1−/− mice10–12. The mPer2 Brdm1−/− mice are a distinct strain from the mPer2 ldc−/− mice we used in our first experiment14,15. These are 2 distinct lines of Per2 mutants produced by different laboratories. mPer2 Brdm1−/− mice express a mutant transcript that lacks most of the PAS domain, while mPer2 ldc−/− mice are null mutants that do not express PERIOD2 protein14,15. Moreover, in 2 studies of food anticipatory activity in the mPer2 Brdm1−/− strain, the mice were on a hybrid C57BL/6 × 129S5/SvEvBrd genetic background10,11. Thus, we next performed daytime restricted feeding in mPer2 Brdm1−/− mice on a hybrid genetic background (Fig. 3; actograms of all mice shown in Figs S7–S13). During ad libitum feeding, wild-type and mPer2 Brdm1−/− mice had minimal daytime activity (Fig. 3: ALI). During 4-h restricted feeding (ZT6-10; ZT5-9 in Figs S7–S9), the wheel-running activity of wild-type and mPer2 Brdm1−/− mice increased prior to feeding (Fig. 3: RF; Fig. 4a). Food anticipatory activity disappeared during ad libitum feeding after restricted feeding (Fig. 3: ALII). Robust daytime activity reappeared at the predicted phase in some mPer2 Brdm1−/− mice during the second, but not first, day of fasting (Fig. 3: FD; Fig. 4b, c). Later we released the mice into constant darkness and confirmed that mPer2 Brdm1−/− mice had short free-running periods of activity compared to wild-type mice as previously reported (Figs S7–S13)15.
Figure 3.
Food anticipatory activity in Period2 mutant (Brdm) mice. Representative double-plotted actograms (a,b) and group average activity profiles (c–j) of wild-type (a,c,e,g,i) and mPer2 Brdm1−/− (b,d,f,h,j) mice. The time when food was available is indicated by gray shading on the left half of each actogram and in the activity profiles. The light-dark cycle is indicated by the white and black bars, respectively. The black traces in the group activity profiles represent the mean number of wheel revolutions (in counts/10-minute bin) plotted relative to the light-dark cycle where 0 is lights on and 12 is lights off. The SEM is shown in dark gray shading in each activity profile. ALI, RF, ALII, and FD in a-d indicate the days used to generate the activity profiles ad libitum I (c,d), restricted feeding (e,f), ad libitum II (g,h) and food deprivation (I,j), respectively. Forty-eight hours of continuous food deprivation is shown (I,j) and the dotted lines indicate when food was available during the preceding restricted feeding.
Figure 4.
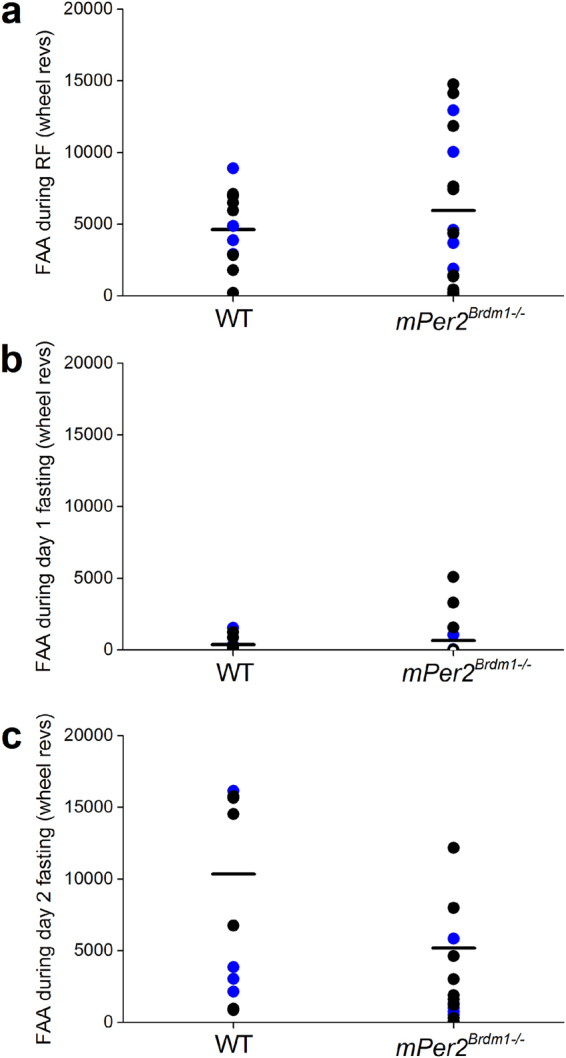
Food anticipatory activity from individual wild-type and Period mutant (Brdm strain) mice. (a) Food anticipatory activity (FAA) during 9 days of restricted feeding (RF) of wild-type (n = 12) and mPer2 Brdm1−/− (n = 17) mice was determined by totaling the number of wheel revolutions per minute from 4 hours before feeding time to the end of feeding time (total of 8 hours). FAA during fasting was defined as the total number of wheel revolutions per minute from 4 hours before feeding time to the end of previous feeding time (total of 8 hours). Wheel-running FAA for each mouse was determined separately for the first (Day 1; b) or second (Day 2; c) day of fasting. Black circles are individual mice fed from ZT6-10. Blue circles are individual mice fed from ZT5-9. The mean of each group is a horizontal line.
The food anticipatory activity ratio, which normalizes anticipatory activity to daily activity levels, was robust in wild-type and mPer2 Brdm1−/− mice during restricted feeding (Fig. S14a) and day 2 of fasting (Fig. S14c). Moreover, food anticipatory activity occurred on day 2 of fasting only in mice that had been previously exposed to restricted feeding. Naïve (never exposed to restricted feeding) mPer Brdm1−/− mice did not have elevated daytime activity during 48-h fasting (Fig. S15). The ages of the mice were also not correlated with their food anticipatory activity ratios (Fig. S16).
Discussion
The Period genes are critical for timekeeping in canonical circadian clocks. For example, mice with non-functional Per1 and Per2 have arrhythmic SCN and locomotor activity, while mice lacking functional Per3 have altered circadian rhythms in peripheral tissue clocks14,16. The molecular timekeeping mechanism of the FEO, however, is still unknown. More than a decade ago, mice without functional Per2 were reported to lack food anticipatory activity during restricted feeding10. This study (and 2 other studies that confirmed this finding in mPer2 Brdm1−/− mice) strongly suggested that the FEO uses a canonical molecular timekeeping similar to the SCN and peripheral clocks10–12. However, other studies have shown that canonical circadian genes are not required for FEO timekeeping. For example, both double Per1/2 and triple Per1/2/3 mutant mice have robust anticipatory activity during restricted feeding. In this study, we re-examined these discrepant results and showed that single Per1, Per2, and Per3 mutant mice have food anticipatory activity during restricted feeding. Moreover, we found that 2 different lines of Per2 mutant mice anticipated restricted food availability.
Three previous studies did not observe food anticipatory activity during restricted feeding in mPer2 Brdm1−/− mice. These results are in contrast with our study where we observed clear food anticipatory during restricted feeding in this same line of Per2 mutant mice. We hypothesize that subtle differences in experimental conditions account for this discrepancy. First, we provided food for only 4-h per day, while previous studies performed 6-h or 8-h restricted feeding [but see Li et al. (2015) for 4-h restricted feeding]. Second, we fed the mice beginning at ZT5 or ZT6, while previous studies sometimes began restricted feeding at ZT410,12. We previously showed that the phase of restricted feeding regulated the robustness of food anticipatory activity so incremental changes in the phase of restricted feeding could permit or conceal the expression of anticipatory activity17. Finally, we measured wheel-running food anticipatory activity, which enhances food anticipatory activity compared to mice without running wheels17. Thus, it appears that the combination of an aggressive restricted feeding protocol (4-h/day) at mid-day phases (ZT6-10 and ZT5-9) with running wheels permitted the detection of food anticipatory activity in mPer2 Brdm1−/− mice in the current study.
Notably, we did observe individual differences in the robustness of food anticipatory activity during restricted feeding in both mPer2 Brdm1−/− mice and their wild-type littermates. This could be due to the mixed genetic background of these mice. We have also previously shown that food anticipatory is more robust in long photoperiods (18 L:6D) compared to 12 L:12D. If we had performed our experiments in 18L:6D, we may have reduced the individual variability in the robustness of food anticipatory activity in both wild-type and mPer2 Brdm1−/− mice17,18.
We also examined food anticipatory activity during fasting to determine if the output of the FEO was sustained in the absence of the temporal food cue. We used an optimized 48-h fasting protocol that we previously showed maximizes the expression of food anticipatory activity on the second day of food deprivation (after ~40 h of fasting)18. We hypothesize that mice must be sufficiently hungry to express food anticipatory activity, which is why anticipatory activity is weak on day 1 of fasting and robust on day 2 of fasting. Food anticipatory wheel-running activity in wild-type, mPer1 ldc−/−, mPer2 ldc−/−, and mPer3 −/− mice was robust on the second day of fasting. Our results in mPer1 ldc−/− mice are consistent with a previous study that showed a different strain of Per1 −/− mice also had food anticipatory activity during fasting10.
Likewise, some mPer2 Brdm1−/− mice and their wild-type littermates had food anticipatory activity on the second day of fasting. However, there was individual variability among mice. In addition, a greater proportion of wild-type mice expressed food anticipatory activity on the second day of fasting compared to mPer2 Brdm1−/− mice. We propose that an optimized fasting protocol may be required for the expression of food anticipatory activity in mutant mice. A previous study did not detect food anticipatory activity during 36-h fasting in mPer2 Brdm1−/− mice10. This could be due to the length of fasting (36-h fasting in the previous study vs. 48-h fasting in our study) and phase when food was removed (24 h before predicted food anticipatory activity in the previous study vs. 42 h before predicted food anticipatory activity in our study). Also, fasting was performed in constant darkness in the previous study, so it was impossible to distinguish the SCN-controlled free-running activity from FEO-controlled anticipatory activity. To avoid this complication, fasting should be performed in the light-dark cycle or in SCN-lesioned mice. In sum, using an optimized fasting protocol, our study showed that the FEO in mPer2 Brdm1−/− mice is functional and keeps time in the absence of the food cue.
Together our data show that food anticipatory is present in single Period mutant mice, including mPer2 Brdm1−/− mice, during restricted feeding and subsequent food deprivation. These data demonstrate that the FEO is functional in Period mutant mice. Moreover, our data further support the hypothesis that the FEO uses a non-canonical timekeeping mechanism and that Period2 is not critical for expression of food anticipatory activity.
Methods
Animals
Period1 (mPer1 ldc−/−), Period2 (mPer2 ldc−/−), and Period3 (mPer3 −/−) mutant mice14 were obtained from Dr. David Weaver on a 129/sv background and backcrossed to Jackson Laboratory C57Bl/6 J mice for 10 to 11 generations. Experimental mice and wild-type controls were generated from heterozygote breeding pairs for each genotype. Mice were born and raised in 12 L:12D at Vanderbilt University and fed chow (LabDiet 5001) ad libitum. Genotype was determined by PCR amplification of tail DNA as previously described14,19. Male and female mice, aged 6 to 13 weeks at the beginning of the experiment, were used. All procedures at Vanderbilt University were approved by the Institutional Animal Care and Use Committee at Vanderbilt University.
mPer2 Brdm1−/− mice were purchased from Jackson Laboratory (stock number 003819; genetic background: 129S7/SvEvBrd-Hprt backcrossed to C57BL/6Brd-Tyr c-Brd strain for at least 5 generations) and crossed with C57BL/6 J (Jackson Laboratory) for 1 generation to generate mPer2 Brdm1+/− heterozygous mice. These heterozygotes were intercrossed to generate mPer2 Brdm1−/− and wild-type control mice for experiments. These mice were born and raised in 12 L:12D at the University of Kentucky and fed chow (Teklad 2918) ad libitum. m Per2 Brdm1−/− mice were genotyped according to the Jackson Laboratory protocol. Male and female mice, aged 6 to 18 weeks at the beginning of the experiment, were used. All procedures at University of Kentucky were approved by the Institutional Animal Care and Use Committee at University of Kentucky.
All procedures were conducted in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Activity recording
Mice were single-housed in cages (33 × 17 × 14 cm) with running wheels (diameter: 11 cm) in light-tight boxes in 12 L:12D and fed ad libitum chow (LabDiet 5001 at Vanderbilt and Teklad 2918 at University of Kentucky). At Vanderbilt University (mPer1 ldc−/−, mPer2 ldc−/−, and mPer3 −/− mice), light sources were white fluorescent bulbs and light intensity was 250-350 lux at the bottom of the cages. At the University of Kentucky (mPer2 Brdm1+/+ and mPer2 Brdm1−/− mice), light sources were white LEDs and light intensity was 200-300 lux at the bottom of the cages. Wheel-running revolutions were collected every minute using the ClockLab acquisition system (Actimetrics Inc, Wilmette, IL).
Restricted Feeding
After several days of ad libitum chow, food was removed for 24 h beginning at ZT4. Then food availability was gradually reduced. Food was available from ZT4-12 for 2 days, then from ZT4-10 for 2 days, and then from ZT6-10 for 9-10 days. Mice were then fed ad libitum for 6 days. To determine if food anticipatory activity persisted in the absence of the food cue, we fasted the mice for 48 h, beginning at ZT12. The timing of fasting is critical as food anticipatory activity becomes more robust as the length of fasting increases (note that food anticipatory activity is more robust on day 2 of fasting compared to day 1 in all genotypes)18.
Analysis
ClockLab Analysis software was used to make double-plotted actograms (10-min bins, normalized format). Mean activity profiles were generated in ClockLab using the following procedure. For each mouse, an activity profile was generated for 3 days of ad libitum feeding (ALI), 9 days of 4-h restricted feeding (RF), and 6 days of subsequent ad libitum feeding (ALII). Since food anticipatory activity becomes more robust with length of fasting, the entire 48-h of fasting was plotted. Then, the mean activity profiles of all mice of each genotype were plotted. The SEM shown in the activity profiles represents the variability among the mice in the group.
Food anticipatory activity during restricted feeding was defined as the 4 h before and 4 h during food availability (e.g. wheel revolutions between ZT2-10 will be summed for restricted feeding beginning at ZT6). Similarly, food anticipatory activity during fasting was defined as the 4 h before and 4 h during the time when food was previously available. Food anticipatory activity during fasting was quantified separately for the first and second days of fasting. The food anticipatory activity ratio was calculated by dividing the number of wheel revolutions 4 h before and 4 h during food availability by the total number of daily wheel revolutions.
Data Availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Electronic supplementary material
Acknowledgements
We thank David Weaver for Period mutant mice and Wataru Nakamura for discussions of his unpublished data in mPer2 Brdm1−/− mice. This research was supported by National Institutes of Health grants R21NS099809 (to S.Y.), P20GM103527 (to J.S.P.), K01DK098321 (to J.S.P.), R03DK098321 (to J.S.P.), National Science Foundation grant IOS-1146908 (to S.Y.), an American Physiological Society Undergraduate Research Fellowship (to R.H.W.), and the University of Kentucky.
Author Contributions
J.S.P. and S.Y. conceived the experiments. J.S.P., R.H.W., R.C.S. and C.D.K. conducted the experiments. J.S.P. and R.H.W. analyzed the results. J.S.P. and S.Y. wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15332-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 2.Davidson AJ. Lesion studies targeting food-anticipatory activity. The European journal of neuroscience. 2009;30:1658–1664. doi: 10.1111/j.1460-9568.2009.06961.x. [DOI] [PubMed] [Google Scholar]
- 3.Boulos Z, Rosenwasser AM, Terman M. Feeding schedules and the circadian organization of behavior in the rat. Behav Brain Res. 1980;1:39–65. doi: 10.1016/0166-4328(80)90045-5. [DOI] [PubMed] [Google Scholar]
- 4.Marchant EG, Mistlberger RE. Anticipation and entrainment to feeding time in intact and SCN-ablated C57BL/6j mice. Brain research. 1997;765:273–282. doi: 10.1016/S0006-8993(97)00571-4. [DOI] [PubMed] [Google Scholar]
- 5.Stephan FK, Swann JM, Sisk CL. Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol. 1979;25:346–363. doi: 10.1016/S0163-1047(79)90415-1. [DOI] [PubMed] [Google Scholar]
- 6.Pitts S, Perone E, Silver R. Food-entrained circadian rhythms are sustained in arrhythmic Clk/Clk mutant mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R57–67. doi: 10.1152/ajpregu.00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iijima M, et al. Altered food-anticipatory activity rhythm in Cryptochrome-deficient mice. Neurosci Res. 2005;52:166–173. doi: 10.1016/j.neures.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6808–6813. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pendergast JS, Oda GA, Niswender KD, Yamazaki S. Period determination in the food-entrainable and methamphetamine-sensitive circadian oscillator(s) Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14218–14223. doi: 10.1073/pnas.1206213109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feillet CA, et al. Lack of food anticipation in Per2 mutant mice. Current biology: CB. 2006;16:2016–2022. doi: 10.1016/j.cub.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 11.Mendoza J, Albrecht U, Challet E. Behavioural food anticipation in clock genes deficient mice: confirming old phenotypes, describing new phenotypes. Genes Brain Behav. 2010;9:467–477. doi: 10.1111/j.1601-183X.2010.00576.x. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, et al. Sex-related difference in food-anticipatory activity of mice. Horm Behav. 2015;70:38–46. doi: 10.1016/j.yhbeh.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Chavan R, et al. Liver-derived ketone bodies are necessary for food anticipation. Nat Commun. 2016;7:10580. doi: 10.1038/ncomms10580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae K, et al. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/S0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 15.Zheng B, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22659. [DOI] [PubMed] [Google Scholar]
- 16.Pendergast JS, Niswender KD, Yamazaki S. Tissue-specific function of Period3 in circadian rhythmicity. PloS one. 2012;7:e30254. doi: 10.1371/journal.pone.0030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flores DE, Bettilyon CN, Jia L, Yamazaki S. The Running Wheel Enhances Food Anticipatory Activity: An Exploratory Study. Front Behav Neurosci. 2016;10:143. doi: 10.3389/fnbeh.2016.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pendergast JS, et al. Robust food anticipatory activity in BMAL1-deficient mice. PloS one. 2009;4:e4860. doi: 10.1371/journal.pone.0004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shearman LP, Jin X, Lee C, Reppert SM, Weaver DR. Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Molecular and cellular biology. 2000;20:6269–6275. doi: 10.1128/MCB.20.17.6269-6275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bunger MK, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103:1009–1017. doi: 10.1016/S0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).




