Abstract
Background
Pazopanib is an angiogenesis inhibitor approved for the treatment of renal cell carcinoma and soft tissue sarcoma. Post hoc analysis of a clinical trial demonstrated a relationship between pazopanib trough concentrations (Cmin) and treatment efficacy. The aim of this study was to explore the pharmacokinetics and exposure-survival relationships of pazopanib in a real-world patient cohort.
Patients and methods
Renal cell cancer and soft tissue sarcoma patients who had at least one pazopanib plasma concentration available were included. Using calculated Cmin values and a threshold of > 20 mg/L, univariate and multivariate exposure-survival analyses were performed.
Results
Sixty-one patients were included, of which 16.4% were underexposed (mean Cmin < 20 mg/L) using the 800 mg fixed-dosed schedule. In univariate analysis Cmin > 20 mg/L was related to longer progression free survival in renal cell cancer patients (34.1 vs. 12.5 weeks, n = 35, p = 0.027) and the overall population (25.0 vs. 8.8 weeks, n = 61, p = 0.012), but not in the sarcoma subgroup (18.7 vs. 8.8 weeks, n = 26, p = 0.142). In multivariate analysis Cmin > 20 mg/L was associated with hazard ratios of 0.25 (p = 0.021) in renal cancer, 0.12 (p = 0.011) in sarcoma and 0.38 (p = 0.017) in a pooled analysis.
Conclusion
This study confirms that pazopanib Cmin > 20 mg/L relates to better progression free survival in renal cancer and points towards a similar trend in sarcoma patients. Cmin monitoring of pazopanib can help identify patients with low Cmin for whom individualized treatment at a higher dose may be appropriate.
Keywords: Pazopanib, Renal cell carcinoma, Soft tissue sarcoma, Pharmacokinetics, Dose optimization, Personalized medicine
Introduction
Pazopanib is an angiogenesis inhibitor, targeting the vascular endothelial growth factor receptor (VEGFR)-1,2,3, platelet derived growth factor receptor (PDGFR) α/β, fibroblast growth factor receptor and the stem cell receptor/ c-Kit [1, 2].
Pazopanib increased progression free survival in renal cell carcinoma and in soft tissue sarcoma compared to placebo, resulting in market approval for both tumor types by the Food and Drug Administration and European Medicine Agency [3, 4]. A retrospective analysis from clinical trial data showed an increased median progression free survival in patients with pazopanib plasma trough concentrations (Cmin) ≥ 20.5 mg/L compared to patients with lower concentrations (52.0 vs. 19.6 weeks, n = 177, p = 0.004) [5].
Pazopanib has a complex pharmacokinetic profile, described by low, non-linear and time-dependent bioavailability and large inter-individual variability [6–9]. This results in a subset of patients receiving less than optimal exposure [5]. It has been estimated in clinical trials that on the approved 800 mg dose, approximately 20% of patients may not reach the > 20 mg/L threshold [5].
In routine clinical care pharmacokinetic variability may be even greater, as patients are likely to have more comorbidities and concomitant medication, may be older, have impaired renal or hepatic function and have suboptimal therapy adherence [10]. In particular the elderly are known to be underrepresented in clinical trials [11]. Moreover, it has been reported that only 39.0% of renal cancer patients treated with targeted therapies in routine clinical practice would be eligible for enrolment in the pivotal phase III trials of their respective therapy [12].
The above underscores the need for exploration of the proportion of patients that risk suboptimal efficacy due to low exposure in real-word patient cohorts. Especially, since it has been shown that increasing the pazopanib dose based on a low Cmin is a feasible and safe option that could lead to improved treatment outcomes [13].
We now report an observational unselected cohort study in renal cell carcinoma and soft tissue sarcoma patients to identify the number of patients at risk of suboptimal treatment due to low exposure. Additionally, we perform exposure–response and exposure-toxicity analyses and explore if patient characteristics could predict the occurrence of low pazopanib Cmin.
Materials and methods
Patient inclusion and data collection
An observational study was performed in the outpatient clinic of the Netherlands Cancer Institute, Amsterdam, The Netherlands. Plasma sampling for concentration monitoring was performed as part of routine care in all patients treated with pazopanib at this hospital (however, no dose increments above 800 mg based on low Cmin were performed during the study period). In the current study, data from routine clinical care including pazopanib plasma concentrations were used retrospectively, which has been authorized in the institute.
Renal cell carcinoma and soft tissue sarcoma patients who received pazopanib treatment as part of standard of care and who had at least one pazopanib plasma concentration measured were included.
Visits were planned according to standard of care in accordance with respective treatment guidelines. Clinical characteristics including demographic data, medical history, pazopanib dose, treatment duration, reason for discontinuation and progression free survival were collected retrospectively from medical records.
Pharmacokinetics
Blood samples were drawn at routinely scheduled visits to the outpatient clinic. Date and time of last intake of pazopanib dose and the time of blood sampling were recorded. Plasma pazopanib levels were determined using a validated liquid chromatography tandem mass spectrometry assay [14]. Cmin values were calculated based on the measured concentration and interval between last ingested dose and sample time using the algorithm developed previously for imatinib [15]. Samples drawn before Tmax (2 h) [8] or more than 24 h after the last dose were excluded from the analysis.
Relationships between Cmin and available patient characteristics were explored, including tumor type, age, weight, gender, (lowest) pazopanib dose and World Health Origination (WHO) performance status. Binary variables were tested using two-sided t tests, categorical variables using analysis of variance, numerical variables using linear regression. p values < 0.05 were considered significant. All statistical analyses were performed in R 3.3.1 [16].
Exposure-survival analysis
For the purpose of exposure-survival analyses the mean of all available Cmin levels per patient was used as parameter for exposure during the entire treatment period, as described previously [13]. Progression free survival of patients with a mean Cmin above or below the pharmacokinetic threshold of > 20 mg/L was analyzed in univariate (Kaplan–Meier analysis plus log-rank test) and multivariate analyses using Cox regression. In multivariate analysis performances status, (lowest) pazopanib dose, number of prior lines of therapy, age and sex were included as covariates. For the exposure-survival analyses in sarcoma, the tumor subtype (leiomyosarcoma, synovial sarcoma or other) was included as an additional covariate. Results are reported as hazard ratios plus 95% confidence intervals (95% CI). A pooled analysis of all patients was also performed. Here, tumor type (renal cancer versus sarcoma) was also included in the multivariate Cox regression.
Exposure-toxicity analysis
Pharmacokinetic exposure was compared between patients who discontinued pazopanib therapy due to toxicity and those who did not. Both the average Cmin per patient and the last Cmin closest to the discontinuation event (due to toxicity or progressive disease) were analyzed.
Results
Evaluable patients
From April 2013 to November 2016, 61 patients were included in the analysis, of whom 35 had renal cell carcinoma and 26 soft tissue sarcoma. A full overview of patient characteristics, including WHO performance status, pazopanib dose, previous lines of therapy, age, weight, sex and number of samples is given in Table 1.
Table 1.
Characteristics of included patients
| Renal cell carcinoma | Soft tissue sarcoma | Overall | |
|---|---|---|---|
| Patients (n) | 35 | 26 | 61 |
| Gender (n (%)) | |||
| Male | 22 (62.9) | 14 (53.8) | 36 (59.0) |
| Female | 13 (37.1) | 12 (46.2) | 25 (41.0) |
| Age (mean (range)) | 62 (45–77) | 61 (32–91) | 61 (32–91) |
| Weight (mean (CV%)) | 84 (23.1) | 77 (17.5) | 81 (21.6) |
| Performance status (n (%)) | |||
| 0 | 13 (37.1) | 11(42.3) | 24 (39.3) |
| 1 | 16 (45.7) | 14 (53.8) | 30 (49.2) |
| 2 | 6 (17.1) | 1 (3.8) | 7 (11.5) |
| Pazopanib dose (n (%))* | |||
| 200 mg | 3 (8.6) | 1 (3.8) | 4 (6.6) |
| 400 mg | 5 (14.3) | 2 (7.7) | 7 (11.5) |
| 600 mg | 6 (17.1) | 2 (7.7) | 8 (13.1) |
| 800 mg | 21 (60.0) | 21 (80.8) | 42 (68.9) |
| Previous lines of systemic therapy (median (range)) | 1 (1–4) | 1 (0–2) | 1 (0–4) |
| Number of samples (n) | 151 | 76 | 227 |
| Samples per patients (mean (range)) | 4 (1–17) | 3 (1–9) | 4 (1–17) |
| Mean (CV%) Cmin per patient (mg/L) | 26.9 (36.4) | 31.9 (36.3) | 29.0 (37.1) |
| Patients with mean Cmin < 20 mg/L (n (%)) | 6 (17.1) | 4 (15.4) | 10 (16.4) |
C min Pazopanib trough level/minimum concentration, CV% coefficient of variation
*Lowest dose per patient
The subtypes of the sarcoma patients included leiomyosarcoma (n = 12), synovial sarcoma (n = 6), pleomorphic sarcoma (n = 2), epithelioid sarcoma, malignant peripheral nerve sheath tumor, angiosarcoma, solitary fibrous tumor, myxofibrosarcoma and undifferentiated spindle cell sarcoma (all n = 1).
Pharmacokinetics
In total, 227 plasma samples were included. Overall, a mean (range) of 4 (1–17) samples were available per patient, 3 (1–9) for the sarcoma and 4 (1–17) for the renal cancer patients. In aggregate, mean (coefficient of variation (CV%)) pazopanib Cmin was 28.1 (39.7) mg/L, ranging from 6.90 to 77.8 mg/L. Median [range] sampling time was 6 [1–44] months since start of therapy. With 5% of samples taken < 4 weeks after start. Median interpatient variability (quantified as CV% of the multiple Cmin values per patient on the same dose) was 24.8%.
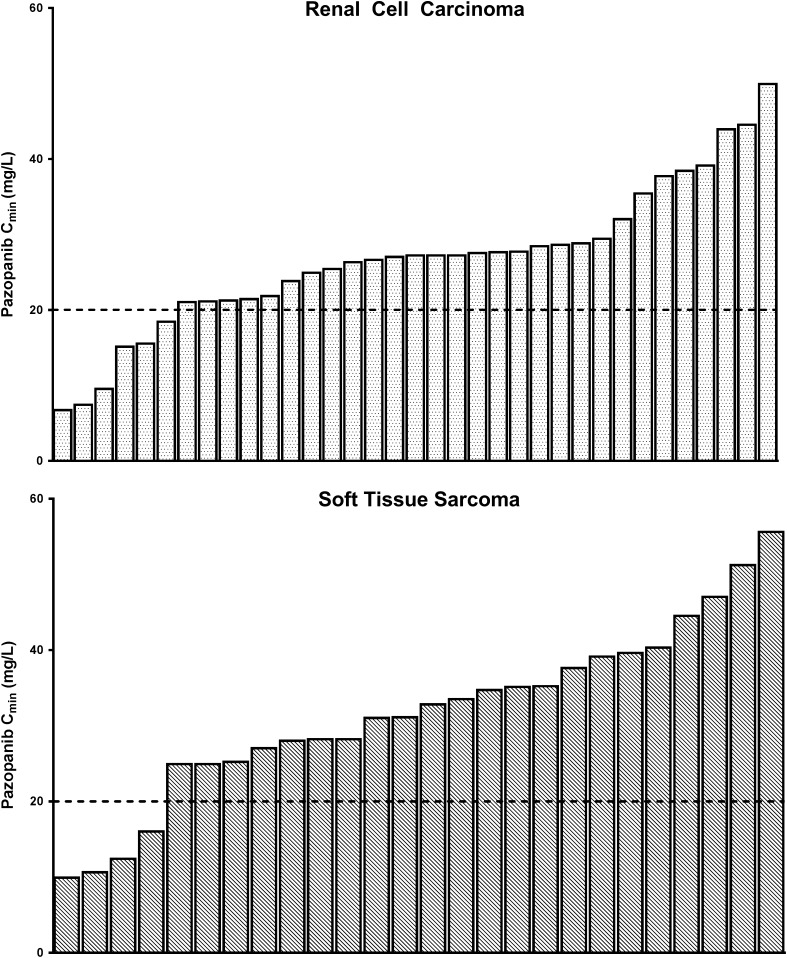
An overview of the distribution of average Cmin per patients per tumor type is provided in Fig. 1 and Table 1. In renal cancer patients mean (CV%) Cmin per patient was 26.9 (36.4) mg/L compared to 31.9 (36.3) mg/L in the sarcoma patients. The overall average Cmin per patient was 29.0 (37.1) mg/L.
Fig. 1.
Distribution of the mean calculated pazopanib Cmin per patient for renal cell carcinoma n = 35 (upper panel) and soft tissue sarcoma patients n = 26 (lower panel). The dotted line indicates the threshold of 20 mg/L. In renal cell carcinoma 6 (17.1%) of patients and in soft tissue sarcoma 4 (15.4%) of patients seem underexposed using the 800 mg fixed-dosed schedule
Although Cmin was higher in sarcoma compared to renal cancer patients (Table 1), this difference was not statistically significant (p = 0.081). In renal cell carcinoma 6 (17.1%) and in soft tissue sarcoma 4 (15.4%) patients were underexposed (mean Cmin < 20 mg/L) using the 800 mg fixed-dose schedule.
Of all explored clinical parameters, none were found to be significantly predictive of low pazopanib Cmin except gender in renal cell cancer patients and age in sarcoma patients. Female sex was associated with a higher Cmin (mean (CV%) of 33.1 (32.5) mg/L versus 23.2 (30.1) p = 0.005). Of the six renal cancer patients with low Cmin only one was female.
In sarcoma patients, linear regression indicated that patients with higher age had lower Cmin and was associated with a slope of − 0.454 and Pearson’s r of − 0.414 (p = 0.035).
Exposure-survival analysis
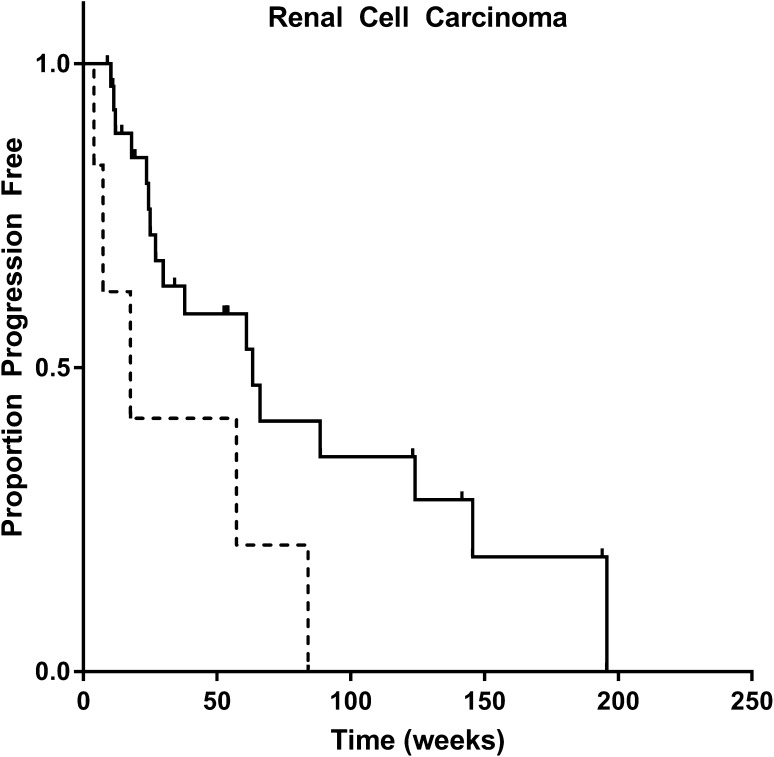
In renal cell carcinoma, Cmin > 20 mg/L was significantly related to improved progression free survival in univariate analysis (p = 0.027, see Fig. 2 and Table 2). Median progression free survival was 34.1 weeks for patients with high and 12.5 weeks for patients with low exposure.
Fig. 2.
Kaplan–Meier plot of progression free survival (weeks) for renal cell carcinoma patients with an average Cmin above (n = 29, solid line) or below (n = 6, dashed line) the exposure target of > 20 mg/L. Median progression free survival was 34.1 weeks for patients with high and 12.5 weeks for patients with low exposure, p = 0.027 (log-rank test)
Table 2.
Overview of exposure-survival analysis outcomes
| Renal cell carcinoma | Soft tissue sarcoma | Overall | |
|---|---|---|---|
| Patients (n) | 35 | 26 | 61 |
| Median PFS (weeks) | 29.9 | 18.3 | 24.4 |
| Median PFS Cmin > 20 mg/L (weeks) | 34.1 | 18.7 | 25.0 |
| Median PFS Cmin < 20 mg/L (weeks) | 12.5 | 8.8 | 8.8 |
| Univariate p value (log-rank test) | 0.027 | 0.142 | 0.012 |
| Hazard ratio (95% CI)* | 0.25 (0.076–0.81) | 0.12 (0.024–0.61) | 0.38 (0.17–0.92) |
| Multivariate p value (Cox regression) | 0.021 | 0.011 | 0.017 |
C min pazopanib trough level/minimum plasma concentration
PFS progression free survival
95% CI 95% confidence interval
*Hazard ratios are based on the multivariate Cox regression analysis
In multivariate analysis, Cmin above or below 20 mg/L resulted in a hazard ratio of 0.25 (95% CI 0.076–0.81, p = 0.021). Female gender was also significantly related to increased progression free survival (p = 0.008).
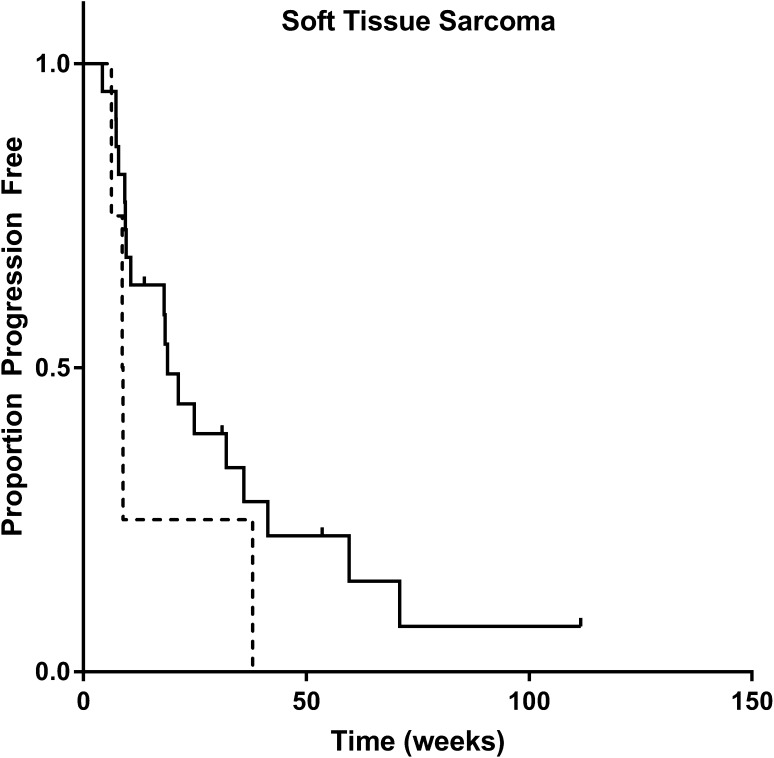
In soft tissue sarcoma, median progression free survival was 18.7 weeks for patients with high and 8.8 weeks for patients with low Cmin (p = 0.142, log-rank test, see Fig. 3; Table 2). In Cox regression, Cmin > 20 mg/L was significantly related to progression free survival and associated with an hazard ratio of 0.12 (95% CI 0.024–0.61, p = 0.011). In the sarcoma subgroup, worse performance status (p = 0.035) and lower age (p = 0.017) were also associated with shorter progression free survival.
Fig. 3.
Kaplan–Meier plot of progression free survival (weeks) for soft tissue sarcoma patients with an average Cmin above (n = 22, solid line) or below (n = 4, dashed line) the exposure target of > 20 mg/L. Median progression free survival was 18.7 weeks for patients with high and 8.80 weeks for patients with low exposure, p = 0.142 (log-rank test)
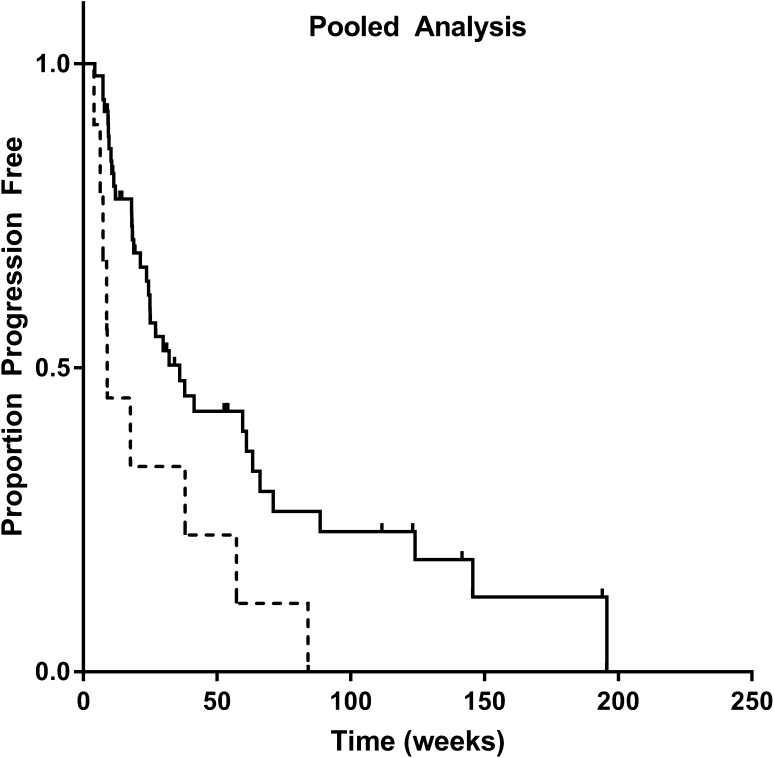
In the pooled analysis, Cmin > 20 mg/L was related to improved survival in univariate analysis (25.0 vs. 8.8 weeks, p = 0.012, see Fig. 4 and Table 2). Here, multivariate analysis resulted in a hazard ratio of 0.38 (95% CI 0.17–0.92, p = 0.017) for Cmin > 20 mg/L. Worse WHO performance status and sarcoma as tumor type were also both associated with worse treatment outcome in the Cox model, p = 0.004 and p < 0.001, respectively.
Fig. 4.
Kaplan–Meier plot of progression free survival (weeks) in a pooled analysis of both renal cell carcinoma and soft tissue sarcoma patients with an average Cmin above (n = 51, solid line) or below (n = 10, dashed line) the exposure target of > 20 mg/L. Median progression free survival was 25.0 weeks for patients with high and 8.80 weeks for patients with low exposure, p = 0.012 (log-rank test)
An overview of the main univariate and multivariate exposure-survival outcomes is provided in Table 2.
Exposure-toxicity analysis
Of the 61 included patients, 44 discontinued treatment due to progressive disease and 5 due to toxicity. Reasons for discontinuation included, hepatotoxicity, hypertension, pancreatitis, dyspnea and multiple grade 2 toxicities (all n = 1). Mean Cmin was 37.3 mg/L in those who discontinued due to toxicity, compared to 27.5 mg/L in those had experienced progressive disease. However, this difference was not statistically significant (p = 0.176). Mean (CV%) last Cmin (the last available sample) was 37.7 (36.4) mg/L compared to 26.4 (46.9) mg/L (p = 0.177).
Discussion
Pazopanib is administered at a fixed 800 mg dose, which is only adjusted in case of severe toxicity. Yet, based on the available data this may lead to suboptimal treatment outcomes in a subset of patients [5]. We now show in an unselected cohort that approximately 16.4% of patients is underexposed using the pazopanib fixed-dosing schedule applying the predefined Cmin target of > 20 mg/L (Fig. 1, Table 1).
No clinical characteristics, except for gender and age were found to be significantly related to pazopanib Cmin. In general, the ability of clinical characteristics to predict which patients experienced low Cmin was limited. This underscores the relevance of routine pazopanib Cmin monitoring, as the subgroup at risk of lower efficacy cannot be identified employing clinical and demographic characteristics. Furthermore, the use of potential interacting medication is carefully monitored during routine care and, therefore, no effects of concomitantly used medication on PK exposure could be identified.
We demonstrate that in renal cancer patients Cmin > 20 mg/L was significantly related to longer progression free survival (34.1 vs. 12.5 weeks, Table 2 and Fig. 2). Our data, therefore, confirm the findings of Suttle et al. in an independent patient cohort [5].
As no pharmacokinetic sampling was performed in the pivotal phase II trial in soft tissue sarcoma,[4] no pazopanib exposure threshold has been proposed yet in sarcoma. This is the first study to investigate a relationship between exposure and survival in sarcoma. However, possibly due to the limited size of the sarcoma subgroup in our cohort and the relatively lower effect size of pazopanib in sarcoma, our result did not reach statistical significance in univariate analysis (progression free survival of 18.7 vs. 8.8 weeks, p = 0.142, Fig. 3). Another possible explanation for the lack of significance in the univariate exposure-survival analysis could be the diversity of sarcoma subtypes. This heterogeneity may, therefore, explain differences in response rates and response duration between disease subtypes. However, in the multivariate analysis in sarcoma this difference in progression free survival for patients with Cmin > 20 mg/L was statistically significant (p = 0.011).
In a pooled exposure-survival analysis (Fig. 4) higher pazopanib Cmin was significantly related to improved treatment outcomes. Furthermore, the existence of a similar exposure–response relationship is theoretically supported by the fact that efficacy of pazopanib is mediated by inhibition of the same target proteins (mainly VEGFR) in both tumor types. However, this exploratory pooled analysis should be interpreted with caution given the variability in sensitivity of the different tumor types.
Previous exposure-toxicity relationships have been reported for pazopanib related adverse events such as hepatotoxicity and hypertension and dose-limiting toxicity in pediatric patients [5, 17, 18]. Although not statistically significant, in this cohort we did find a numerically higher exposure in patients discontinuing due to toxicity (n = 5), this result was not statistically significant (37.3 vs. 27.5 mg/L, p = 0.176).
Drawbacks of this study are its retrospective nature, relatively limited number of patients in each tumor type and the heterogeneity in the availability and timing of plasma samples. Furthermore, not actual but calculated Cmin values (using an therapeutic drug monitoring algorithm as validated for imatinib) were used. However, this algorithm describes a general exponential decline in exposure with a specified plasma half-life and would, therefore, also be suitable for pazopanib.
Yet despite these limitations, it is the first pharmacokinetic study that reports exposure-survival and exposure-toxicity relationships for pazopanib in a real-world cohort of renal cell carcinoma and soft tissue sarcoma patients and identifies a subgroup of approximately 16.4% of patients which may benefit from individualized Cmin-guided pazopanib dosing.
Given the currently presented results and the previous work by Suttle et al [5], one could argue that a fixed dosing strategy for pazopanib is becoming increasingly inappropriate in the era of personalized medicine [19, 20]. Recommendations for individualized dosing of pazopanib and other tyrosine kinase inhibitors have been made previously [21, 22]. Moreover, specifically for pazopanib the safety and feasibility of individualized dosing has been established in a prospective clinical trial [13].
Collectively, the current study and data available in the literature [5, 13] point towards the need to validate the strategy of individualized pazopanib dosing in a prospective randomized clinical trial.
Conclusion
In conclusion, at the currently approved fixed dose regimen a relevant subgroup of 16.4% of patients treated with pazopanib is underexposed in routine care and may be at risk of suboptimal treatment efficacy.
Our study further confirms that the previously established threshold of Cmin > 20 mg/L is related to longer progression free survival in renal cell carcinoma patients (34.1 vs. 12.5 weeks, n = 35, p = 0.027). Moreover, exploratory analyses point towards a similar association of increased progression free survival with higher exposure in soft tissue sarcoma patients (18.7 vs. 8.8 weeks, n = 26, p = 0.142).
Plasma Cmin monitoring of pazopanib can help identify patients with low Cmin for whom treatment at a higher dose may be appropriate.
Acknowledgements
N. Steeghs received an unrestricted grant (funding and study medication) from GlaxoSmithKline as Principal Investigator for an investigator initiated study with Pazopanib. Pazopanib is an asset of Novartis as of June 2015.
Funding
No funding was received for this research.
Compliance with ethical standards
Conflict of interest
R. B. Verheijen, L. E. Swart, J. H. Beijnen, J. H. M. Schellens and A. D. R. Huitema declare they have no conflicts of interest to disclose.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of retrospective study formal consent was not required.
References
- 1.Kumar R, Knick VB, Rudolph SK, et al. Pharmacokinetic-pharmacodynamic correlation from mouse to human with pazopanib, a multikinase angiogenesis inhibitor with potent antitumor and antiangiogenic activity. Mol Cancer Ther. 2007;6:2012–2021. doi: 10.1158/1535-7163.MCT-07-0193. [DOI] [PubMed] [Google Scholar]
- 2.Hamberg P, Verweij J, Sleijfer S. Pre-)clinical pharmacology and activity of pazopanib, a novel multikinase angiogenesis inhibitor. Oncologist. 2010;15:539–547. doi: 10.1634/theoncologist.2009-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 4.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 5.Suttle AB, Ball H, Molimard M, et al. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br J Cancer. 2014;111:1–8. doi: 10.1038/bjc.2014.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu H, van Erp N, Bins S, et al. Development of a pharmacokinetic model to describe the complex pharmacokinetics of pazopanib in cancer patients. Clin Pharmacokinet. 2016 doi: 10.1007/s40262-016-0443-y. [DOI] [PubMed] [Google Scholar]
- 7.Deng Y, Sychterz C, Suttle AB, et al. Bioavailability, metabolism and disposition of oral pazopanib in patients with advanced cancer. Xenobiotica. 2013;43:443–453. doi: 10.3109/00498254.2012.734642. [DOI] [PubMed] [Google Scholar]
- 8.Hurwitz HI, Dowlati A, Saini S, et al. Phase I trial of pazopanib in patients with advanced cancer. Clin Cancer Res. 2009;15:4220–4227. doi: 10.1158/1078-0432.CCR-08-2740. [DOI] [PubMed] [Google Scholar]
- 9.Verheijen RB, Beijnen JH, Schellens JHM, et al. Clinical pharmacokinetics and pharmacodynamics of pazopanib: towards optimized dosing. Clin Pharmacokinet. 2017 doi: 10.1007/s40262-017-0510-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell AP, Harrison MR, Walker MS, et al. Clinical trial participants with metastatic renal cell carcinoma differ from patients treated in real-world practice. J Oncol Pract. 2015;11:491–497. doi: 10.1200/JOP.2015.004929. [DOI] [PubMed] [Google Scholar]
- 13.Verheijen RB, Bins S, Mathijssen RHJ, et al. Individualized pazopanib dosing: a prospective feasibility study in cancer patients. Clin Cancer Res. 2016;22:5738–5746. doi: 10.1158/1078-0432.CCR-16-1255. [DOI] [PubMed] [Google Scholar]
- 14.Herbrink M, de Vries N, Rosing H, et al. Quantification of 11 therapeutic kinase inhibitors in human plasma for therapeutic drug monitoring using liquid chromatography coupled with tandem mass spectrometry. Ther Drug Monit. 2016;38:649–656. doi: 10.1097/FTD.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Chia YL, Nedelman J, Schran H. A therapeutic drug monitoring algorithm for refining the imatinib trough level obtained at different sampling times. Ther Drug Monit. 2009;31:579–584. doi: 10.1097/FTD.0b013e3181b2c8cf. [DOI] [PubMed] [Google Scholar]
- 16.R Core Development Team (2016) A language and environment for statistical computing. R Found Statistical Computing, Vienna. https://www.r-project.org/
- 17.Food and Drug Administration. Center for Drug Evaluation and Research (2008) Pazopanib clinical pharmacology and biopharmaceutics review. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022465s000_ClinPharmR.pdf. Accessed 17 Oct 2017
- 18.Glade Bender JL, Lee A, Reid JM, et al. Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children’s oncology group phase I consortium report. J Clin Oncol. 2013;31:3034–3043. doi: 10.1200/JCO.2012.47.0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ornstein MC, Rini BI. Pharmacokinetically-guided dosing of oral drugs: true precision oncology? Clin Cancer Res. 2016;22:5626–5628. doi: 10.1158/1078-0432.CCR-16-1833. [DOI] [PubMed] [Google Scholar]
- 20.Beumer JH. Without therapeutic drug monitoring, there is no personalized cancer care. Clin Pharmacol Ther. 2013;93:228–230. doi: 10.1038/clpt.2012.243. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Steeghs N, Nijenhuis CM, et al. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. Clin Pharmacokinet. 2014;53:305–325. doi: 10.1007/s40262-014-0137-2. [DOI] [PubMed] [Google Scholar]
- 22.de Wit D, Guchelaar H-J, den Hartigh J, et al. Individualized dosing of tyrosine kinase inhibitors: are we there yet? Drug Discov Today. 2015;20:18–36. doi: 10.1016/j.drudis.2014.09.007. [DOI] [PubMed] [Google Scholar]