Abstract
Rationale: Although epidemiological studies consistently show that chronic obstructive pulmonary disease is associated with an increased risk of lung cancer, debate exists as to whether there is a linear relationship between the severity of airflow limitation and lung cancer risk.
Objectives: We examined this in a large, prospective study of older heavy smokers from the American College of Radiology Imaging Network subcohort of the National Lung Screening Trial (ACRIN). Airflow limitation was defined by prebronchodilator spirometry subgrouped according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) grades 1–4.
Methods: In the National Lung Screening Trial–ACRIN cohort of 18,473 screening participants, 6,436 had airflow limitation (35%) and 12,037 (65%) had no airflow limitation. From these groups, 758 lung cancer cases were prospectively identified. Participants with airflow limitation were stratified according to GOLD groups 1 (n = 1,607), 2 (n = 3,528), 3 (n = 1,083), and 4 (n = 211). Lung cancer incidence at study end (mean follow-up, 6.4 yr) was compared between the GOLD groups and those with no airflow limitation (referent group).
Measurements and Main Results: Compared with those with no airflow limitation, where lung cancer incidence was 3.78/1,000 person years, incidence rates increased in a simple linear relationship: GOLD 1 (6.27/1,000 person yr); GOLD 2 (7.86/1,000 person yr); GOLD 3 (10.71/1,000 person yr); and GOLD 4 (13.25/1,000 person yr). All relationships were significant versus the reference group at a P value of 0.0001 or less.
Conclusions: In a large prospective study of high-risk cigarette smokers, we report a strong linear relationship between increasing severity of airflow limitation and risk of lung cancer.
Keywords: National Lung Screening Trial, chronic obstructive pulmonary disease, airflow limitation, lung cancer, risk
Over the past 30 years, both cross-sectional and prospective studies have consistently shown that the presence of chronic obstructive pulmonary disease (COPD), characterized by irreversible airflow limitation and reduced expiratory flow rates, confers a greater risk of lung cancer (1–10). This association is independent of smoking history and, strongest when COPD is defined by spirometry with the control group confirmed to have normal lung function (1, 4, 5).
In the population-based National Health and Nutrition Examination Survey (4), increasing severity of spirometry-defined COPD was associated with greater risk of lung cancer. The risk associated with mild COPD was triple that of smokers with normal baseline lung function and sixfold greater in those with moderate to severe COPD (4).
A similar magnitude of risk (four- to sixfold) was found in a cross-sectional study of moderate to heavy smokers with lung cancer when compared with a randomly selected group matched for age, sex, and smoking histories (5). When the lung cancer risk conferred by FEV1 was examined, an inverse dose–response relationship was found where even a small decrease in percent predicted FEV1 (<90%) was associated with an increased risk of lung cancer (8).
This increased risk extends to those with “restrictive” lung disease (4). However, in a recent study using a clinic-based COPD cohort, subjects with Global Initiative for Chronic Obstructive Lung Disease (GOLD) grades 1–2 were found to have a greater risk of lung cancer than those of GOLD grade 4 (10). This unexpected observation argues against a linear relationship between airflow limitation and lung cancer risk, leading to some debate (11, 12).
Underlying this debate is the observation that reduced expiratory flow rates have also been associated with increased all-cause, lung cancer and cardiovascular mortality (3), implicating a differential survival effect (11). This debate is highly relevant to the current era of screening for lung cancer by chest computed tomographic (CT) imaging, because risk stratifying eligible smokers is considered a key element of screening, where the risk of lung cancer must be balanced against the harm-to-benefit ratio of screening relative to dying of other causes (9).
Why smokers with underlying airflow obstruction are at greater risk of lung cancer than smokers with normal lung function remains unknown (13–16). Early hypotheses suggested that patients with COPD have a greater accumulation of carcinogens in the airway (1, 2). Other hypotheses link lung cancer with underlying emphysema and lung remodeling (13, 14). The innate immune response has been implicated in both COPD and lung cancer in large prospective studies (15–18). Systemic inflammation, reflecting innate immune hyperresponsiveness, has been linked to both progression of lung remodeling leading to COPD and DNA damage leading to lung cancer (18).
In 2009, it was suggested that the microclimate of remodeling underlying COPD, characterized by excess metalloproteinases and growth factors, might initiate premalignant transformation, termed epithelial–mesenchymal transition (EMT) (15). Since 2010, when EMT was first identified in patients with COPD (19), it has been associated with worsening airflow limitation, and has subsequently been reported by three further research groups using surgical samples from patients with or without COPD with lung cancer (20). EMT is considered a precursor to many other epithelial-based cancers, and has been shown to be potentially reversible with immune-modulatory treatment (15).
As spirometry is not routinely performed in the workup of all unscreened cases of lung cancer, unbiased data on airflow limitation in lung cancer are limited. With the recent interest in CT screening for lung cancer, the relationship between airflow limitation and lung cancer can be more robustly examined (6–8). Using data from the American College of Radiology, Imaging Network (ACRIN) cohort of the National Lung Screening Trial (NLST), a large, prospective study in 18,714 subjects, we re-examined the relationship between GOLD-based airflow limitation and the development of lung cancer. Preliminary results of this study have been reported in abstract form (21).
Methods
Subjects
The recruitment and study design of the full NLST, involving 53,452 screening participants, yielding 2,058 histology-confirmed lung cancers, has been described elsewhere (22). In the ACRIN cohort of the NLST, participants from 23 centers agreed to undergo baseline prebronchodilator spirometry (n = 18,714). From this cohort, 768 histology-confirmed lung cancer cases were diagnosed over the study period of 7.5 years (22).
Pulmonary Function Testing
In the NLST-ACRIN cohort, prebronchodilatory spirometry was measured at baseline screening (T0) in the majority of participants meeting the following criteria: no chest infection in the preceding 3 weeks and no use of a short-acting bronchodilator inhaler in the preceding 6 hours, or long-acting bronchodilator in the preceding 24 hours. Those not meeting these criteria were rescheduled for spirometry testing at a later visit. The spirometry was measured by trained staff using a Spiropro spirometer (eResearchTechnology, GmbH, Estenfeld, Germany). The severity of airflow limitation was defined according to the GOLD criteria grades 1–4 (www.GOLD.org, accessed February 24, 2016).
Lung Cancer Case Rates
Lung cancer cases included all those diagnosed during the trial, whether screen or nonscreen detected, or prevalent (diagnosed during the first year or T0) or incident lung cancers (diagnosed during subsequent years [T1–T6]; lung cancer prevalence is the number of lung cancer cases diagnosed as a percentage of the total number of screening participants including both prevalent lung cancers [diagnosed in the first year of screening (T0)] and incident cancers [diagnosed during subsequent years T1–T6], described as a percentage; lung cancer incidence rate [IR] is the number of lung cancer cases diagnosed over a defined period as a function of the total number of person years calculated according to the total number of screening participants and years of screening, described as rate/1,000 person years; lung cancer IR ratio (IRR) is the ratio of one lung cancer IR relative to a reference lung cancer IR, and provides a crude estimate of relative risk, but in the context of comparing two IRs rather than two prevalence rates). All lung cancers cases were confirmed on histological sampling according to accepted international classification criteria (21). We report the old bronchioloalveolar cancer (BAC) subgroup separately from adenocarcinomas, because they have been previously associated with “histology shift” and overdiagnosis (22).
Lung function results and lung cancer histology results were available for 758 of the 768 lung cancer cases (99% of total). In a sensitivity analysis, in which only spirometry meeting strict American Thoracic Society criteria (grade A) were included in the analysis (n = 13,530, 72% of the total), the relationships identified in the larger cohort (n = 18,714) were reanalyzed.
For comparative purposes, the lung cancer IRs (per 1,000 person yr) and incident rate ratios (IRR) in this NLST-ACRIN cohort were compared with those from two other prospective lung cancer studies, a non-screening study (4) and screening study (7), where airflow limitation was assessed using spirometry at study baseline. In a further analysis we sub-grouped those with no airflow obstruction and compared lung cancer IRs in those with restrictive lung disease (4) and GOLD unclassified (GOLD U) (23), relative to “healthy” smokers.
Statistical Analysis
Differences in lung cancer IRs, stratified by severity of airflow limitation based on GOLD grades 1–4 were compared. Differences in lung cancer IRs were compared using IR per 1,000 person years and IRR. Differences in lung cancer histology were compared according to GOLD status using Fisher’s exact test and mid-P exact test. Confidence intervals for IRs and IRRs were estimated using the exact method. Significance was defined as a two-tailed P less than 0.05. All statistical analyses were performed using SAS (v9.4, SAS Institute, Cary, NC) or STATA statistical software (StataCorp, 2015. Stata Statistical Software; Release 14; College Station, TX).
Results
Table 1 shows a comparison of the demographic characteristics of the full NLST trial cohort (n = 53,452) and lung cancer cases (n = 2,058) compared with the NLST-ACRIN cohort (n = 18,714) and lung cancer cases (n = 768). The NLST-ACRIN cohort participants are very similar to the full NLST study participants (22). Based on the prebronchodilator pulmonary function testing in the NLST-ACRIN cohort, 64.3% had no airflow limitation, a further 27.4% had airflow limitation approximating GOLD 1–2 COPD, 5.8% had GOLD 3, and 1.1% had GOLD 4 severity COPD (Table 1). There were missing lung function data for 239 participants and missing FEV1 % predicted in 7 participants with airflow limitation.
Table 1.
Comparison of baseline demographics of the Full National Lung Screening Trial Study subjects and National Lung Screening Trial–American College of Radiology Imaging Network cohort where spirometry defines severity of airflow limitation using global initiative for chronic obstructive lung disease grading
| Screening Trial | NLST–Main Study (n = 53,452) |
NLST–ACRIN Cohort (n = 18,714) |
||
|---|---|---|---|---|
| Screening Participants | Lung Cancer Cases | Total Cohort | Lung Cancer Cases | |
| Subject demographics | ||||
| Number | 53,452 | 2,058 | 18,714 | 768 |
| Mean age (SD), yr | 61.4 (5.0) | 63.7 (5.3) | 61.6 (5.0) | 63.6 (5.2) |
| Male, % | 59 | 60 | 55 | 56 |
| Mean pack years (SD) | 56.0 (23.9) | 64.9 (27.1) | 55.9 (23.5) | 63.9 (27.0) |
| Current smokers, % | 48 | 60 | 50 | 60 |
| Family history of lung cancer, % | 22 | 26 | 23 | 26 |
| Self-reported COPD, %* | 17 | 27 | 20 | 32 |
| Body Mass Index (SD) | 27.9 (5.0) | 26.8 (4.7) | 27.8 (5.1) | 26.9 (4.9) |
| Pulmonary function tests | ||||
| Total† | ND | ND | 18,714 | 768 |
| GOLD 1 | ND | ND | 1,607 (8.6%) | 78 (10.2%) |
| GOLD 2 | ND | ND | 3,528 (18.9%) | 213 (27.7%) |
| GOLD 3–4‡ | ND | ND | 1,294 (6.9%) | 109 (14.2%) |
| GOLD U (due to missing height data)§ | 7 (<1%) | 1 (<1%) | ||
| Airflow limitation | ND | ND | 6,436 (34.4%) | 401 (52.2%) |
| No airflow limitation | ND | ND | 12,037 (64.3%) | 357 (46.5%) |
| Missing spirometry data | ND | ND | 241 (1.3%) | 10 (1.3%) |
Definition of abbreviations: ACRIN = American College of Radiology Imaging Network; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; GOLD 1 = FEV1/FVC < 0.70, FEV1 % predicted ≥ 80%; GOLD 2 = FEV1/FVC < 0.70, FEV1 % predicted = 50–79%; GOLD 3 = FEV1/FVC < 0.70, FEV1 % predicted = 30–49%; GOLD 4 = FEV1/FVC < 0.70, FEV1 % predicted < 30%; GOLD U = GOLD unclassified; ND = not done; NLST = National Lung Screening Trial.
Self-reported COPD in the NLST was based on questionnaire responses referring to the past diagnosis of COPD, emphysema, chronic bronchitis, or a combination of these.
Pulmonary function results were available for 99% of screening participants and lung cancer cases.
Stage 4 COPD—1.1% in total cohort and 2.7% in lung cancer cases.
Airflow limitation based on FEV1/FVC < 0.70, but FEV1 % predicted not known.
A comparison of the demographic variables, lung cancer rates, and histology, according to the presence or absence of airflow limitation, is shown in Table 2. Regardless of the screening interval, airflow limitation was associated with a twofold greater lung cancer IR than those with no airflow limitation (P < 0.0001 for screening and follow-up intervals). Airflow limitation was also associated with significantly less BAC (now redefined as adenocarcinoma in situ or minimally invasive adenocarcinoma) and significantly more non–small cell lung cancer histology (Table 2). Airflow limitation was also associated with marginally more squamous cell cancers and marginally less adenocarcinoma. These differences are compared further in Table 3, where demographic variables, lung function, and cancer histology are compared across severity of airflow limitation based on GOLD grade.
Table 2.
Comparison of the demographics, lung cancer prevalence, lung cancer incidence rate, and histology according to airflow limitation in the National Lung Screening Trial–American College of Radiology Imaging Network cohort (n = 18,473)
| Characteristics | No Airflow Limitation | Airflow Limitation | Total | P Value |
|---|---|---|---|---|
| n (%) | 12,037 (65.2) | 6,436 (34.8) | 18,473 | — |
| Mean age (SD), yr | 61.0 (4.9) | 62.5 (5.2) | 61.6 (5.1) | <0.0001 |
| Male, % | 53 | 60 | 55 | <0.0001 |
| Mean pack-years (SD) | 53.8 (22.3) | 59.7 (24.9) | 55.8 (23.4) | <0.0001 |
| Current smokers, % | 47 | 56 | 50 | <0.0001 |
| Family Hx of lung cancer, % | 23 | 24 | 23 | 0.55 |
| Self-reported COPD, % | 14 | 31 | 20 | <0.0001 |
| Mean BMI (SD) | 28.5 (5.2) | 26.7 (4.8) | 27.8 (5.1) | <0.0001 |
| Lung cancers by screening arm, n (%) | 357 (47) | 401 (53) | 758 | <0.0001 |
| CXR | 164 (45) | 201 (55) | 365 | 0.27 |
| CT | 193 (49) | 200 (51) | 393 | <0.0001 |
| Lung cancer prevalence (%)* | 2.97 | 6.23 | 4.10 | |
| Lung cancer incidence rate per 1,000 person years | ||||
| T0–T6 (total study) | 3.78 | 8.12 | 5.27 | <0.0001† |
| T0–T2 (screening interval) | 6.01 | 12.73 | 8.33 | <0.0001‡ |
| T3–T6 (follow-up interval) | 2.36 | 5.14 | 3.31 | <0.0001§ |
| CXR | 4.28 | 9.66 | <0.0001|| | |
| CT | 4.99 | 9.82 | <0.0001¶ | |
| Histology, n (%) | 0.0035 | |||
| Small cell | 51 (14) | 60 (15) | 111 (15) | |
| Squamous cell | 73 (20) | 95 (24) | 168 (22) | |
| Adenocarcinoma | 129 (36) | 127 (32) | 256 (34) | |
| BAC** | 40 (11) | 19 (5) | 59 (8) | |
| Large Cell | 14 (4) | 16 (4) | 30 (4) | |
| Non–small cell | 50 (14) | 81 (20) | 131 (17) | |
| Other | 357 | 3 (<1) | 3 (<1) | |
| Total | 401 | 758 |
Definition of abbreviations: BAC = bronchioloalveolar cancer; BMI = body mass index; COPD = chronic obstructive pulmonary disease; CT = computed tomography; CXR = chest X-ray; Hx = history; T0 = baseline; T6 = combination of follow-up years 6 and 7.
Combines prevalent lung cancers (T0) and incident lung cancers (T1–T6).
Incidence rate ratio (IRR) = 2.15 (95% confidence interval [CI] = 1.86–2.48).
IRR = 2.12 (95% CI = 1.76–2.55).
IRR = 2.18 (95% CI = 1.72–2.76).
IRR = 2.26 (95% CI = 1.84–2.77).
IRR = 1.97 (95% CI = 1.62–2.40).
BAC has been redefined as adenocarcinoma: in situ and minimally invasive adenocarcinoma and defined separately above from adenocarcinoma as they are representative of lung cancers identified by CT screening and associated with “histology shift” and “overdiagnosis” relative to CXR screening (21).
Table 3.
Demographics and lung function at baseline in the Full National Lung Screening Trial–American College of Radiology Imaging Network cohort and those with lung cancer, according to severity of airflow limitation by global initiative for chronic obstructive lung disease
| Cohorts, Demographic Variables | Presence of Airflow Limitation and Its Severity |
|||||
|---|---|---|---|---|---|---|
| No Airflow Limitation | GOLD 1 | GOLD 2 | GOLD 3–4 | P Trend | P Trend GOLD* | |
| Full cohort at baseline (n = 18,466) | 12,037 | 1,607 | 3,528 | 1,294 | ||
| Mean age (SD) | 61.0 (4.9) | 62.2 (5.3) | 62.5 (5.2) | 63.2 (5.2) | <0.0001 | <0.0001 |
| Male, % | 53 | 64 | 58 | 58 | <0.0001 | 0.0006 |
| Mean pack years (SD) | 53.8 (22.3) | 56.6 (23.0) | 59.8 (24.7) | 63.4 (27.2) | <0.0001 | <0.0001 |
| Current smokers, % | 47 | 57 | 58 | 53 | <0.0001 | 0.0159 |
| Family history of lung cancer, % | 23 | 24 | 23 | 25 | 0.63 | 0.51 |
| Self-reported COPD, %† | 14 | 17 | 29 | 55 | <0.0001 | <0.0001 |
| Mean body mass index (SD) | 28.5 (5.2) | 25.9 (4.0) | 26.9 (4.8) | 26.9 (5.5) | <0.0001 | <0.0001 |
| Mean observed FEV1 (SD) | 2.7 (0.8) | 2.9 (0.7) | 2.1 (0.5) | 1.2 (0.3) | <0.0001‡ | <0.0001‡ |
| Mean FEV1 % predicted (SD) | 89.2 (16.8) | 91.1 (12.2) | 65.9 (8.3) | 38.8 (8.4) | <0.0001‡ | <0.0001‡ |
| Mean FEV1/FVC (SD) | 78.7 (54.7) | 65.4 (4.9) | 61.5 (6.8) | 48.3 (10.4) | <0.0001‡ | <0.0001‡ |
| Lung cancer cases (n = 757) | 357 | 78 | 213 | 109 | ||
| Mean age (SD) | 62.8 (5.2) | 65.2 (5.4) | 64.0 (5.0) | 64.3 (5.3) | 0.0003 | <0.0001 |
| Male, % | 52 | 69 | 58 | 57 | 0.0324 | 0.16 |
| Mean pack-years (SD) | 60.9 (24.6) | 64.5 (28.6) | 63.4 (26.4) | 69.6 (33.4) | 0.0288 | <0.0001 |
| Current smokers, % | 56 | 68 | 67 | 53 | 0.0151 | 0.0413 |
| Family history of lung cancer, % | 27 | 19 | 25 | 28 | 0.52 | 0.42 |
| Self-reported COPD, %† | 22 | 14 | 39 | 58 | <0.0001 | <0.0001 |
| Mean body mass index (SD) | 27.8 (5.1) | 25.3 (3.7) | 26.1 (4.8) | 26.8 (4.7) | <0.0001 | <0.0001 |
| Mean observed FEV1 (SD) | 2.5 (0.8) | 2.9 (0.9) | 2.0 (0.5) | 1.2 (0.3) | <0.0001‡ | <0.0001‡ |
| Mean FEV1 % predicted (SD) | 85.1 (17.9) | 93.8 (19.1) | 64.4 (8.2) | 38.5 (8.2) | <0.0001‡ | <0.0001‡ |
| Mean FEV1/FVC (SD) | 76.9 (7.4) | 64.9 (5.5) | 60.8 (6.9) | 49.2 (8.2) | <0.0001‡ | <0.0001‡ |
| Small cell, % | 14 | 12 | 17 | 14 | 0.70§ | 0.55§ |
| Squamous cell, % | 20 | 18 | 23 | 28 | 0.27 | 0.26 |
| Adenocarcinoma, % | 36 | 36 | 32 | 28 | 0.43 | 0.56 |
| Bronchioloalveolar, %|| | 11 | 9 | 4 | 4 | 0.003 | 0.19 |
| Large cell, % | 4 | 6 | 5 | 1 | 0.20 | 0.10 |
| Non–small cell, % | 14 | 19 | 19 | 24 | 0.08 | 0.55 |
| Other, % | 0 | 0 | 1 | 1 | ||
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; GOLD 1 = FEV1/FVC < 0.70, FEV1 % predicted ≥ 80%; GOLD 2 = FEV1/FVC < 0.70, FEV1 % predicted = 50–79%; GOLD 3 = FEV1/FVC < 0.70, FEV1 % predicted = 30–49%; GOLD 4 = FEV1/FVC < 0.70, FEV1 % predicted < 30%.
Testing difference between GOLD categories only.
Self-reported COPD in the National Lung Screening Trial was based on questionnaire.
Please note that, as GOLD was defined by spirometry parameters, differences are highly significant
P values from the exact test of the association between each histology (yes/no) and COPD groups. The significance level was adjusted via Bonferroni correction to be 0.05/6 = 0.008 to control for multiple comparisons.
Bronchioloalveolar cancer has been redefined as adenocarcinoma: in situ and minimally invasive adenocarcinoma.
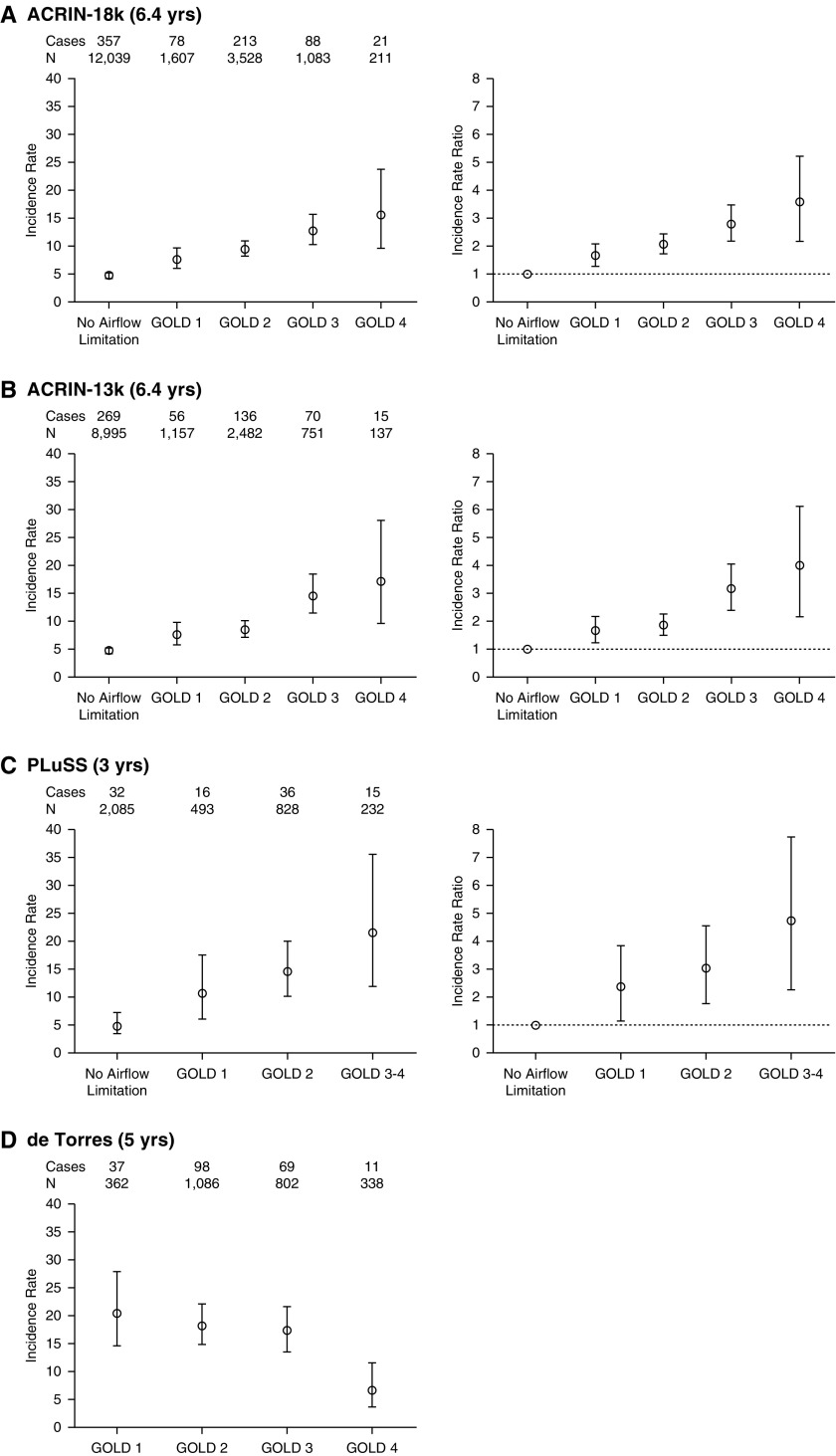
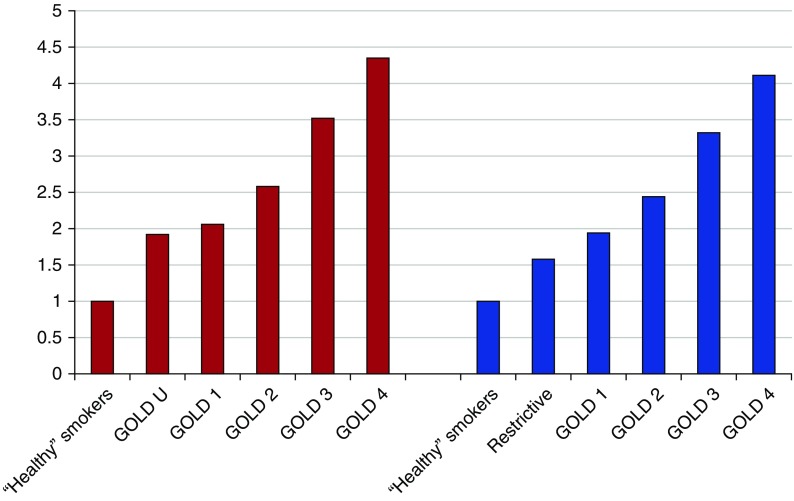
The lung cancer incidence (per 1,000 person yr) and IRRs (referenced against those with no airflow limitation or GOLD 1 airflow limitation), are shown in Table 4 and Figure 1. In the whole cohort (n = 18,714), the incidence in each of the GOLD groups 1–4 were significantly greater than for the referent group with “no airflow limitation,” and showed a linear relationship indicating that increasing severity of airflow limitation is directly associated with an increased risk of lung cancer (P < 0.001 for trend). This linear relationship extended to include GOLD U (see Table 5 and Figure 2).
Table 4.
Lung cancer incidence rate per 1,000 person years and incidence rate ratio, according to severity of airflow limitation in screening and nonscreening prospective studies (4, 7, 10)
| Study (Follow-Up) | Total Cohort n | Lung Cancer Cases n | Absolute Incidence |
Rate Versus No Airflow Limitation |
Rate versus Gold 1 Airflow Limitation |
|||
|---|---|---|---|---|---|---|---|---|
| IR | IR 95% CI | IRR* | IRR 95% CI | IRR† | IRR 95% CI | |||
| ACRIN 18K (6.4 yr) | 18,466 | 758 | ||||||
| No Airflow limitation | 12,037 | 357 | 3.78 | 3.40–4.19 | Ref | — | — | — |
| GOLD 1 | 1,607 | 78 | 6.27 | 4.96–7.82 | 1.66‡ | 1.28–2.12 | Ref | — |
| GOLD 2 | 3,528 | 213 | 7.86 | 6.85–8.99 | 2.08§ | 1.75–2.47 | 1.25 | 0.96–1.65 |
| GOLD 3 | 1,083 | 88 | 10.71 | 8.60–13.18 | 2.83§ | 2.22–3.89 | 1.71‡ | 1.25–2.35 |
| GOLD 4 | 211 | 21 | 13.25 | 8.22–20.19 | 3.51§ | 2.14–5.44 | 2.11|| | 1.24–3.46 |
| Airflow limitation | 6,429 | 401 | 8.11 | 7.34–8.94 | 2.14§ | 1.85–2.48 | — | — |
| ACRIN 13K (6.4 yr) | 13,522 | 546 | ||||||
| No Airflow limitation | 8,995 | 269 | 3.81 | 3.37–4.30 | Ref | — | — | — |
| GOLD 1 | 1,157 | 56 | 6.25 | 4.73–8.11 | 1.64|| | 1.21–2.19 | Ref | — |
| GOLD 2 | 2,482 | 136 | 7.10 | 5.96–8.39 | 1.86§ | 1.50–2.30 | 1.14 | 0.83–1.58 |
| GOLD 3 | 751 | 70 | 12.39 | 9.67–15.62 | 3.25§ | 2.46–4.24 | 1.98‡ | 1.37–2.87 |
| GOLD 4 | 137 | 15 | 14.68 | 8.24–24.09 | 3.85§ | 2.12–6.46 | 2.35|| | 1.23–4.21 |
| Airflow limitation | 4,527 | 277 | 7.96 | 7.06–8.96 | 2.09§ | 1.76–2.48 | — | — |
| PLuSS (3 yr)7 | 3,638 | 99 | ||||||
| No Airflow limitation | 2,085 | 32 | 5.12 | 3.50–7.22 | Ref | — | — | — |
| GOLD 1 | 493 | 16 | 10.82 | 6.18–17.57 | 2.12 | 1.16–3.85 | Ref | — |
| GOLD 2 | 828 | 36 | 14.49 | 10.15–20.06 | 2.83§ | 1.76–4.56 | 1.34 | 0.74–2.41 |
| GOLD 3–4 | 232 | 15 | 21.55 | 12.05–35.55 | 4.21§ | 2.28–7.78 | 1.99 | 0.99–4.03 |
| Airflow Limitation | 1,553 | 67 | 9.07 | 7.37–11.04 | 1.77|| | 1.19–2.64 | — | — |
| NHANES (17.9 yr)4 | 5,402 | 291 | ||||||
| Normal lung function | 2,359 | 165 | 0.92 | 0.66–1.26 | Ref | — | — | — |
| Mild COPD | 296 | 14 | 2.64 | 1.44–4.43 | 2.86|| | 1.55–5.27 | Ref | 1.55–5.27 |
| Mod/Severe | 393 | 38 | 5.40 | 3.82–7.41 | 5.85§ | 3.74–9.14 | 2.86‡ | — |
| All COPD | 689 | 52 | 4.22 | 3.15–5.53 | 4.57§ | 3.01–6.91 | — | — |
| Restrictive | 300 | 12 | 2.24 | 1.13–3.90 | 2.42|| | 1.27–4.62 | — | — |
| Nonsmokers | 2,054 | 10 | 0.27 | 0.13–0.50 | 0.29‡ | 0.15–0.59 | — | — |
| de Torres (5 yr)10 | 2,588 | 215 | ||||||
| GOLD 1 | 362 | 37 | 20.40 | 14.60–27.88 | — | — | Ref | — |
| GOLD 2 | 1,086 | 98 | 18.10 | 14.73–21.90 | — | — | 0.88 | 0.60–1.29 |
| GOLD 3 | 802 | 69 | 17.21 | 13.49–21.65 | — | — | 0.84 | 0.56–1.26 |
| GOLD 4 | 338 | 11 | 6.51 | 3.42–11.31 | — | — | 0.31‡ | 0.16–0.62 |
| All COPD (100%) | 2,588 | 215 | 16.62 | 14.50–18.95 | — | — | — | — |
Definition of abbreviations: ACRIN = American College of Radiology Imaging Network; CI = confidence interval; COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; GOLD 1 = FEV1/FVC < 0.70, FEV1 % predicted ≥ 80%; GOLD 2 = FEV1/FVC < 0.70, FEV1 % predicted = 50–79%; GOLD 3 = FEV1/FVC < 0.70, FEV1 % predicted = 30–49%; GOLD 4 = FEV1/FVC < 0.70, FEV1 % predicted < 30%; IR = incidence rate; IRR = IR ratio; NHANES = National Health and Nutrition Examination Survey; PLuSS = Pittsburgh Lung Cancer Screening Study; Ref = referent group.
IRR compares GOLD groups with no airflow limitation (referent).
IRR compares GOLD groups with GOLD 1 or mild COPD (referent).
P < 0.001.
P < 0.0001.
P < 0.01.
Figure 1.
Comparison of incidence rate (IR), IR ratios, and 95% confidence interval in the lung cancer studies according to severity of airflow limitation in the National Lung Screening Trial–American College of Radiology Imaging Network (ACRIN) cohort (A and B), Pittsburgh Lung Screening Study (PLuSS) (7) screening cohort (C), and chronic obstructive pulmonary disease clinic population (10) (D). GOLD=Global Initiative for Chronic Obstructive Lung Disease.
Table 5.
Lung cancer incidence rate per 1,000 person years and incident rate ratio, according to GOLD grade severity with subphenotyping of those with no airflow limitation into those who are “healthy” smokers, those with “restrictive” spirometry (4), or those with GOLD unclassified criteria (23)
| Subphenotype | Total Cohort n | Lung Cancer Cases n | Absolute Incidence |
Rate versus Healthy Smokers |
Rate versus GOLD U or Restrictive |
|||
|---|---|---|---|---|---|---|---|---|
| IR | IR 95% CI | IRR* | IRR 95% CI | IRR† | IRR 95% CI | |||
| ACRIN 18K (6.4 yr) | 18,466 | 757 | ||||||
| Healthy smokers | 8,845 | 212 | 3.04 | 2.65–3.48 | Ref | — | — | — |
| GOLD U‡ | 3,192 | 145 | 5.85 | 4.94–6.88 | 1.92§ | 1.55–2.39 | Ref | — |
| GOLD 1 | 1,607 | 78 | 6.27 | 4.96–7.82 | 2.06§ | 1.57–2.68 | 1.07 | 0.80–1.42 |
| GOLD 2 | 3,528 | 213 | 7.86 | 6.85–8.99 | 2.58§ | 2.13–3.14 | 1.34|| | 1.08–1.67 |
| GOLD 3 | 1,083 | 88 | 10.71 | 8.60–13.18 | 3.52§ | 2.71–4.53 | 1.83§ | 1.39–2.40 |
| GOLD 4 | 211 | 21 | 13.25 | 8.22–20.19 | 4.35§ | 2.64–6.83 | 2.26|| | 1.36–3.59 |
| Healthy smokers | 8,436 | 214 | 3.23 | 2.81–3.69 | Ref | — | — | — |
| Restrictive¶ | 3,601 | 143 | 5.10 | 4.30–6.00 | 1.58§ | 1.27–1.96 | Ref | — |
| GOLD 1 | 1,607 | 78 | 6.27 | 4.96–7.82 | 1.94§ | 1.48–2.53 | 1.23 | 0.92–1.63 |
| GOLD 2 | 3,528 | 213 | 7.86 | 6.85–8.99 | 2.44§ | 2.01–2.96 | 1.54** | 1.24–1.92 |
| GOLD 3 | 1,083 | 88 | 10.71 | 8.60–13.18 | 3.32§ | 2.56–4.27 | 2.10§ | 1.59–2.76 |
| GOLD 4 | 211 | 21 | 13.25 | 8.22–20.19 | 4.11§ | 2.49–6.44 | 2.60** | 1.56–4.13 |
Definition of abbreviations: ACRIN = American College of Radiology Imaging Network; CI = confidence interval; GOLD = Global Initiative for Chronic Obstructive Lung Disease; GOLD 1 = FEV1/FVC < 0.70, FEV1 % predicted ≥ 80%; GOLD 2 = FEV1/FVC < 0.70, FEV1 % predicted = 50–79%; GOLD 3 = FEV1/FVC < 0.70, FEV1 % predicted = 30–49%; GOLD 4 = FEV1/FVC < 0.70, FEV1 % predicted < 30%; GOLD U = GOLD unclassified; IR = incidence rate; IRR = IR ratio; Ref = referent group.
IRR (per 1,000 person years) referenced against “healthy smokers.”
IRR (per 1,000 person years) referenced against GOLD U or restrictive.
GOLD U = FEV1/FVC ≥ 0.70 and FEV1 % predicted < 80%.
P < 0.0001.
P < 0.01.
Restrictive = FEV1/FVC ≥ 0.70 and FVC % predicted < 80%.
P < 0.001.
Figure 2.
Incidence rate ratio (IRR) of lung cancer according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) severity with subphenotyping of those with no airflow limitation into those who are “healthy” smokers (referent), those with “restrictive” spirometry (4) or those with GOLD unclassified (GOLD U) criteria (23).
In a sensitivity analysis, when only those whose spirometry met strict American Thoracic Society criteria were included (73% of the total, n = 13,530), we found almost identical lung cancer IRs across the GOLD groups (see Table 4; see also Figure E1 in the online supplement). The relationship between severity of airflow limitation and risk of lung cancer was again linear (P < 0.001 for trend).
In Table 4, lung cancer incidence stratified by GOLD grade according to baseline spirometry, is compared with two other prospective studies (4, 7), one a nonscreening study (4) and the other a screening study (7). Despite representing different risk populations and having different study designs, the lung cancer incidence (and thus risk) consistently shows a linear relationship, with GOLD 3–4 subjects having a three- to sixfold greater risk for lung cancer than those with no airflow limitation and two- to threefold greater risk for those with GOLD 2 COPD. We found the same relationship across GOLD groups regardless of whether lung cancer was diagnosed in the CT or chest X-ray arms, where the latter better simulates unscreened lung cancers (Table E1). We also found that FEV1 % predicted less than 90% was inversely related to lung cancer incidence (Figure E2). In addition, lung cancer rate ratios were increased for those with restrictive spirometry (Figure 2).
Importantly, although lung cancer prevalence was linearly related to the severity of airflow obstruction after stratification for smoking status, lung cancer detection status, and radiological presence of emphysema at baseline (Figure E3), this association was much reduced for lung cancer mortality. Lastly, we assessed the relative contribution of age, pack-years, and FEV1 % predicted to lung cancer risk in a multivariate analysis comparable to that reported by Tockman and colleagues (1). This showed that FEV1 % predicted was the single most important predictor of lung cancer relative to age or pack-years (Table E3).
Discussion
In an analysis of the NLST-ACRIN cohort, we found a direct linear relationship between increasing severity of airflow limitation, according to GOLD 1–4 spirometry grade, and increasing risk of lung cancer. To our knowledge, this is the largest prospective study to date that has looked at this relationship. In a second analysis (Figure 2), we found that this linear relationship extended to smokers meeting GOLD U and restrictive spirometry (4, 23) with a magnitude comparable to those with airflow limitation of GOLD 1–2 severity. This study also shows that, not only is a reduced expiratory flow rate an important risk variable for developing lung cancer among heavy smokers, it is also one of the most important risk variables relative to those of age and pack-years (Tables 1 and 2, Table E3) (1, 2, 5, 8, 24). This suggests that airflow limitation represents a global barometer of susceptibility to the key smoking-related lung diseases, including lung cancer (25).
The results reported here, showing that the severity of airflow limitation according to GOLD grade correlates in a linear relationship with lung cancer risk, are consistent with several other published studies (4, 7, 8). This finding extends to even mild to moderate reductions in airflow limitation (Figure E1) according to FEV1 % predicted (8), a finding confirmed in a meta-analysis (26).
These findings contrast with those reported by de Torres and colleagues (10), who found that lung cancer risk is greatest in those with mild to moderate COPD. Relative strengths of the NLST are the prospective design, large sample size, and focus on older, asymptomatic smokers, otherwise eligible for CT screening. This contrasts with the participants studied by de Torres and colleagues, who were symptomatic patients with COPD of greater severity. In the de Torres study, the risk of lung cancer was greatest in those who had higher FEV1, but lower diffusing capacity of carbon monoxide (DlCO) and lower body mass index (10). In their study, variables, such as age and pack-years, were no longer a risk factor for getting lung cancer.
These findings are hard to reconcile with other larger prospective studies that report age, pack-years, and worsening COPD as the strongest risk variables for lung cancer (1, 4, 27–29). One possible explanation for these conflicting results is the very high proportion of patients with COPD with GOLD 3–4 severity present in the clinic population reported in the de Torres study (44%) compared with participants in NLST (20%) and Pittsburgh Lung Screening Study (PLuSS) (15%). This suggests that the clinic population, while being representative of symptomatic severe COPD, and very likely worse emphysema, are not representative of the larger group of relatively unselected smokers at risk of lung cancer.
This is important for several reasons. First, although cancer is one of the most important causes of death among patients with COPD with mild disease (40–50%), respiratory disease, cardiovascular disease, and non–cancer-related causes are more common in moderate to severe COPD (where cancer accounts for only 20% of deaths) (30). It has been argued that, in a clinic COPD population, the frequency of lung cancer was higher in those with mild COPD secondary to a survivor effect, where those with GOLD 3–4 severity are dying before cancer develops (11, 12). Unlike the strong linear association between severity of airflow limitation and risk of lung cancer (Figures 1 and 2), the association with lung cancer mortality is substantially weakened (Figure E3). This contrasts with the strong linear association we found between severity of airflow limitation and non–lung cancer–related mortality in this cohort (unpublished data), which is the subject of further publications.
Second, in PLuSS, the odds ratio for lung cancer shows a linear relationship for GOLD 1–4 (Table 4), but a curvilinear (nonlinear) relationship for CT-based emphysema (CTE) severity (semiquantitative), where the highest risk for lung cancer was conferred by mild emphysema (Table E2) (7). It is therefore possible that the GOLD 1–2 group, identified by de Torres to be at greatest risk of lung cancer, had a greater prevalence of mild emphysema than our screening participants and more severe emphysema overall. This is consistent with their findings that low DlCO and low body mass index also confer increased lung cancer risk (10). Lastly, in the de Torres study (10), over 90% of subjects were men and, given potential sex differences in susceptibility, aeropollutant exposures, COPD prevalence, and histological subtypes, their results may consequently be specific to the cohort they studied. In the current study, where 55% of screening participants were male, we found no difference in lung cancer incidence between genders.
The results of the current study are notable in showing that the prevalence of airflow limitation in the NLST-ACRIN screening cohort of high-risk smokers (35%) was substantially higher in those who went on to develop lung cancer (53%). This means that past lung cancer epidemiological studies, which have almost exclusively failed to account for this difference in prevalence of airflow limitation (or COPD), may spuriously report associations with lung cancer that result from confounding effects with COPD (31–34).
Our results extend those of other studies showing worsening airflow limitation is also associated with more aggressive lung cancer histological subtypes (i.e., less BAC/adenocarcinomas and more squamous/non–small cell cancers) (21, 35–37), most notable in those with severe disease (Table 3). In a secondary analysis, we also show that smokers meeting GOLD U criteria (also labeled as spirometry grade-undefined [SGU] or preserved ratio impaired spirometry [PRISm]) have an increased risk of lung cancer relative to healthy smoker (nearly twofold) and comparable to those with GOLD 1–2 airflow limitation. In the latter smokers, with only mild reductions in expiratory flow rates, more than 70–80% do not know they have airflow limitation (COPD), conferring a greater risk of lung cancer.
With the recent interest in CT screening for lung cancer, there is growing interest in better defining those smokers at greatest risk to maximize the benefit and minimize the harms from screening (9). Our results confirm that smokers with underlying airflow limitation are at greater risk of lung cancer, and this is associated with a two- to fourfold greater lung cancer incidence in screening studies (9, 38). In a simulation study, Lowry and colleagues (39) examined the effect of competing causes of death in CT screening participants, and concluded that those with COPD may disproportionately benefit from lung cancer screening. However, some argue that screening patients with COPD may not necessarily translate into mortality benefit due to factors such as competing causes of premature death (40).
In a preliminary analysis of the NLST-ACRIN cohort, we have found that lung cancer-specific mortality reduction was attenuated in those with airflow limitation (15%, P > 0.05) and enhanced in those with no airflow limitation (28%, P < 0.05) (41). We suggest that, in those with airflow limitation, more aggressive lung cancers (see previous descriptions here) or competing cause of premature death from causes other than lung cancer may indeed be an issue (11, 41). This also suggests that those at greatest risk of lung cancer may not necessarily achieve the greatest benefit from screening (40, 42). This is important, because risk models for lung cancer share the same variables associated with COPD (24), often including a past diagnosis of COPD (27, 28), or the presence of airflow limitation based on spirometry (29).
Personalized risk prediction is a recognized feature of CT screening for lung cancer and helps assess the relative benefits and harms of screening (43). Our results in the NLST-ACRIN cohort not only suggest that the severity of airflow limitation confers differential effects on lung cancer risk and histology (22), but that it may also affect outcomes according to lung cancer–specific and all-cause mortality (41). The latter is termed the “competing cause of death” effect, and is beyond the scope of this study. However, this effect has relevance to optimizing the selection of “healthy” smokers for lung cancer screening (the “sweet spot” [44]) and is the subject of further investigations in this cohort.
Limitations
There are several limitations to this study. First, although comparable with other CT screening studies (7, 45–47), spirometry in the NLST was performed as a prebronchodilator measurement rather than postbronchodilator, as recommended for clinical purposes. This means that a proportion of subjects we have classified as having airflow limitation may have had asthma or COPD–asthma overlap rather than COPD (i.e., full, partial, or minimal reversible airflow limitation, respectively), although this is likely to be only a small proportion of this elderly cohort of chronic smokers (mean age, 64 yr, and minimum 30 pack-years of smoking).
A second limitation of the study is that people with a life expectancy of less than 5 years were excluded from the NLST (22). This might explain the relatively low prevalence of subjects with GOLD grade 3 and 4 in this study. A third limitation is that we do not yet have any measure of emphysema severity in the NLST-ACRIN screening participants so that the relationship between emphysema score (severity) and lung cancer incidence could not be examined across the screening participants. Moreover, other CT-based phenotypes, such as airway size, gas trapping, and interstitial lung disease, were not routinely recorded in the NLST study. There remains much debate as to which of the COPD phenotypes, airflow limitation or CTE, is more closely associated with lung cancer risk (6, 7).
A fourth limitation of our study is that lung cancers identified during CT screening are not identical to those diagnosed in an unscreened cohort (22, 48, 49). Results from the full NLST bear this out with an excess of both adenocarcinomas and BAC (now subclassified under adenocarcinomas) reflecting an “histology shift” (22). Collectively, these results suggest that the lung cancers identified during screening include more indolent lung cancers than occurs without screening (47). Results from the Dutch–Belgian Lung Cancer Screening Trial (NELSON) trial using volumetric assessment will help answer this question. If the Danish Lung Cancer Screening Trial is representative of the histological differences observed between screened and unscreened lung cancer (46), then 38 cancers of the 45 excess cancers diagnosed in the CT arm (65% excess compared with no screening) were BAC and adenocarcinoma subtypes. A similar excess was reported by the Detection and Screening of Early Lung Cancer with Novel imaging Technology (DANTE) trial, suggesting that CT screening identifies a subgroup of cancers that would not otherwise come to clinical attention if no screening was done (48, 49). A comparison of the lung cancer histology, after stratification by COPD, from the European trials will help confirm this observation.
Lastly, although we have been able to show that the GOLD severity relationship with lung cancer risk is maintained after stratification by screening arm (Table E1), smoking status, presence of CTE, lung cancer detection (Figure E3), and sex (unpublished findings), we are unable to comment on ethnicity, due to insufficient numbers in minority groups.
Conclusions
The presence of airflow limitation identifies smokers at greatest risk for developing lung cancer, with increasing severity being associated with an increased lung cancer IR (37, 38). Airflow limitation is also associated with marginally more aggressive cancers and significantly less (or minimal) overdiagnosis (22). Another finding of this study is that the risk of lung cancer is directly related to a reduced FEV1 % predicted in a simple linear relationship, where even only minor reductions in expiratory flow rate (<90% in FEV1 % predicted, GOLD U, or restrictive subgroups) confer an increased risk. Our results do not support the view that smokers with mild to moderate COPD are at more risk than those with GOLD 3–4 (10–12). The results of this study confirm previous studies, showing the severity of airflow limitation contributes substantially to differentiating smokers at low or high risk of lung cancer (7, 9, 28, 29, 38).
We believe that our findings support the routine use of spirometry in asymptomatic adult smokers (50), especially those otherwise eligible for CT screening, where a “sweet spot” may help define those who will achieve the greatest benefit.
Supplementary Material
Footnotes
Supported by grants U01-CA-80098 and U01-CA-79778 to the American College of Radiology Imaging Network.
Author Contributions: all authors contributed to the conception and design of the study, acquisition, analysis, and interpretation of the data, and drafting and review for important intellectual content and final approval of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med. 1987;106:512–518. doi: 10.7326/0003-4819-106-4-512. [DOI] [PubMed] [Google Scholar]
- 2.Burrows B, Knudson RJ, Cline MG, Lebowitz MD. Quantitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis. 1977;115:195–205. doi: 10.1164/arrd.1977.115.2.195. [DOI] [PubMed] [Google Scholar]
- 3.Hole DJ, Watt GCM, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313:711–715. doi: 10.1136/bmj.313.7059.711. discussion 715–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: data from the first National Health and Nutrition Examination Survey follow-up. Arch Intern Med. 2003;163:1475–1480. doi: 10.1001/archinte.163.12.1475. [DOI] [PubMed] [Google Scholar]
- 5.Young RP, Hopkins RJ, Christmas T, Black PN, Metcalf P, Gamble GD. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34:380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 6.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, Pueyo JC, Villanueva A, Lozano MD, Montes U, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132:1932–1938. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 7.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calabrò E, Randi G, La Vecchia C, Sverzellati N, Marchianò A, Villani M, Zompatori M, Cassandro R, Harari S, Pastorino U. Lung function predicts lung cancer risk in smokers: a tool for targeting screening programmes. Eur Respir J. 2010;35:146–151. doi: 10.1183/09031936.00049909. [DOI] [PubMed] [Google Scholar]
- 9.Young RP, Hopkins RJ. Diagnosing COPD and targeted lung cancer screening. Eur Respir J. 2012;40:1063–1064. doi: 10.1183/09031936.00070012. [DOI] [PubMed] [Google Scholar]
- 10.de Torres JP, Marín JM, Casanova C, Cote C, Carrizo S, Cordoba-Lanus E, Baz-Dávila R, Zulueta JJ, Aguirre-Jaime A, Saetta M, et al. Lung cancer in patients with chronic obstructive pulmonary disease—incidence and predicting factors. Am J Respir Crit Care Med. 2011;184:913–919. doi: 10.1164/rccm.201103-0430OC. [DOI] [PubMed] [Google Scholar]
- 11.Chang KC, Leung CC. Lung cancer is more common in early GOLD stages of COPD: a spurious association? Am J Respir Crit Care Med. 2012;185:1128. doi: 10.1164/ajrccm.185.10.1128. [DOI] [PubMed] [Google Scholar]
- 12.de Torres JP, Zulueta JJ, Casanova C, Aguirre-Jaime A, Celli BR. Lung cancer is more common in early GOLD stages of COPD: a spurious association? Response. Am J Respir Crit Care Med. 2012;185:1128–1129. doi: 10.1164/ajrccm.185.10.1128. [DOI] [PubMed] [Google Scholar]
- 13.Houghton AM, Mouded M, Shapiro SD. Common origins of lung cancer and COPD. Nat Med. 2008;14:1023–1024. doi: 10.1038/nm1008-1023. [DOI] [PubMed] [Google Scholar]
- 14.Caramori G, Adcock IM, Casolari P, Ito K, Jazrawi E, Tsaprouni L, Villetti G, Civelli M, Carnini C, Chung KF, et al. Unbalanced oxidant-induced DNA damage and repair in COPD: a link towards lung cancer. Thorax. 2011;66:521–527. doi: 10.1136/thx.2010.156448. [DOI] [PubMed] [Google Scholar]
- 15.Young RP, Hopkins R, Eaton TE. Pharmacological actions of statins: potential utility in COPD. Eur Respir Rev. 2009;18:222–232. doi: 10.1183/09059180.00005309. [DOI] [PubMed] [Google Scholar]
- 16.Crystal RG. Airway basal cells: the “smoking gun” of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:1355–1362. doi: 10.1164/rccm.201408-1492PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Man SFP, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61:849–853. doi: 10.1136/thx.2006.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is associated with incident cancer and survival in patients with cancer. J Clin Oncol. 2009;27:2217–2224. doi: 10.1200/JCO.2008.19.8440. [DOI] [PubMed] [Google Scholar]
- 19.Sohal SS, Reid D, Soltani A, Ward C, Weston S, Muller HK, Wood-Baker R, Walters EH. Reticular basement membrane fragmentation and potential epithelial mesenchymal transition is exaggerated in the airways of smokers with chronic obstructive pulmonary disease. Respirology. 2010;15:930–938. doi: 10.1111/j.1440-1843.2010.01808.x. [DOI] [PubMed] [Google Scholar]
- 20.Gohy ST, Hupin C, Fregimilicka C, Detry BR, Bouzin C, Gaide Chevronay H, Lecocq M, Weynand B, Ladjemi MZ, Pierreux CE, et al. Imprinting of the COPD airway epithelium for dedifferentiation and mesenchymal transition. Eur Respir J. 2015;45:1258–1272. doi: 10.1183/09031936.00135814. [DOI] [PubMed] [Google Scholar]
- 21.Hopkins RJ, Young RP, Duan F, Chiles C, Greco E, Gamble GD, Aberle D. Airflow limitation and lung cancer risk in a large prospective screening study (NLST-ACRIN cohort analysis, n = 18,714) [abstract] Am J Respir Crit Care Med. 2016;193:A6178. [Google Scholar]
- 22.Young RP, Duan F, Chiles C, Hopkins RJ, Gamble GD, Greco EM, Gatsonis C, Aberle D. Airflow limitation and histology shift in the National Lung Screening Trial: the NLST-ACRIN cohort study (n = 18,714) Am J Respir Crit Care Med. 2015;192:1060–1067. doi: 10.1164/rccm.201505-0894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan ES, Hokanson JE, Murphy JR, Regan EA, Make BJ, Lynch DA, Crapo JD, Silverman EK COPDGene Investigators. Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med. 2011;184:57–63. doi: 10.1164/rccm.201101-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young RP, Duan F, Hopkins RJ, et al. COPD-related risk factors in screen detected lung cancer: preliminary results of a sub analysis of the NLST [abstract] Am J Respir Crit Care Med. 2013;187:A2345. [Google Scholar]
- 25.Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J. 2007;30:616–622. doi: 10.1183/09031936.00021707. [DOI] [PubMed] [Google Scholar]
- 26.Fry JS, Hamling JS, Lee PN. Systematic review with meta-analysis of the epidemiological evidence relating FEV1 decline to lung cancer risk. BMC Cancer. 2012;12:498. doi: 10.1186/1471-2407-12-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tammemagi CM, Pinsky PF, Caporaso NE, Kvale PA, Hocking WG, Church TR, Riley TL, Commins J, Oken MM, Berg CD, et al. Lung cancer risk prediction: Prostate, Lung, Colorectal And Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst. 2011;103:1058–1068. doi: 10.1093/jnci/djr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, Chaturvedi AK, Silvestri GA, Riley TL, Commins J, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tammemagi MC, Lam SC, McWilliams AM, Sin DD. Incremental value of pulmonary function and sputum DNA image cytometry in lung cancer risk prediction. Cancer Prev Res (Phila) 2011;4:552–561. doi: 10.1158/1940-6207.CAPR-10-0183. [DOI] [PubMed] [Google Scholar]
- 30.Berry CE, Wise RA. Mortality in COPD: causes, risk factors, and prevention. COPD. 2010;7:375–382. doi: 10.3109/15412555.2010.510160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- 32.El-Zein RA, Young RP, Hopkins RJ, Etzel CJ. Genetic predisposition to chronic obstructive pulmonary disease and/or lung cancer: important considerations when evaluating risk. Cancer Prev Res (Phila) 2012;5:522–527. doi: 10.1158/1940-6207.CAPR-12-0042. [DOI] [PubMed] [Google Scholar]
- 33.Carlsten C, Sagoo GS, Frodsham AJ, Burke W, Higgins JP. Glutathione S-transferase M1 (GSTM1) polymorphisms and lung cancer: a literature-based systematic HuGE review and meta-analysis. Am J Epidemiol. 2008;167:759–774. doi: 10.1093/aje/kwm383. [DOI] [PubMed] [Google Scholar]
- 34.Young RP, Hopkins RJ, Hay BA, Gamble GD. GSTM1 null genotype in COPD and lung cancer: evidence of a modifier or confounding effect? Appl Clin Genet. 2011;4:137–144. doi: 10.2147/TACG.S21517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young RP, Hopkins RJ. Estimating overdiagnosis of lung cancer. Ann Intern Med. 2013;158:635–636. doi: 10.7326/0003-4819-158-8-201304160-00013. [DOI] [PubMed] [Google Scholar]
- 36.de-Torres JP, Casanova C, Marín JM, Zagaceta J, Alcaide AB, Seijo LM, Campo A, Carrizo S, Montes U, Cordoba-Lanus E, et al. Exploring the impact of screening with low-dose CT on lung cancer mortality in mild to moderate COPD patients: a pilot study. Respir Med. 2013;107:702–707. doi: 10.1016/j.rmed.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Young RP, Hopkins RJ. CT screening in COPD: the impact on lung cancer mortality. Respir Med. 2014;108:813–814. doi: 10.1016/j.rmed.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 38.Young RP, Hopkins RJ. Targeted CT image screening and its effect on lung cancer detection rate. Chest. 2013;144:1419–1420. doi: 10.1378/chest.13-1321. [DOI] [PubMed] [Google Scholar]
- 39.Lowry KP, Gazelle GS, Gilmore ME, Johanson C, Munshi V, Choi SE, Tramontano AC, Kong CY, McMahon PM. Personalizing annual lung cancer screening for patients with chronic obstructive pulmonary disease: a decision analysis. Cancer. 2015;121:1556–1562. doi: 10.1002/cncr.29225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young RP, Hopkins RJ, Midthun DE.Computed tomographic screening for lung cancer JAMA 20123081320–1321.author reply 1320–1321. [DOI] [PubMed] [Google Scholar]
- 41.Young RP, Duan F, Greco E, Hopkins RJ, Chiles C, Gamble GD, Aberle D. Lung cancer–specific mortality reduction with CT screening: outcomes according to airflow limitation in the ACRIN-NLST study (n = 18,475) (abstract) Am J Respir Crit Care Med. 2016;193:A6166. [Google Scholar]
- 42.Ruano-Ravina A, Heleno B, Fernandez-Villar A. Lung cancer screening with low-dose CT (LDCT), or when a public health intervention is beyond the patient’s benefit. J Epidemiol Community Health. 2015;69:99–100. doi: 10.1136/jech-2014-204293. [DOI] [PubMed] [Google Scholar]
- 43.Mazzone P, Powell CA, Arenberg D, Bach P, Detterbeck F, Gould MK, Jaklitsch MT, Jett J, Naidich D, Vachani A, et al. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society policy statement. Chest. 2015;147:295–303. doi: 10.1378/chest.14-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gould MK. Lung cancer screening in individuals with chronic obstructive pulmonary disease: finding the sweet spot. Am J Respir Crit Care Med. 2015;192:1027–1028. doi: 10.1164/rccm.201508-1594ED. [DOI] [PubMed] [Google Scholar]
- 45.Veronesi G, Maisonneuve P, Bellomi M, Rampinelli C, Durli I, Bertolotti R, Spaggiari L. Estimating overdiagnosis in low-dose computed tomography screening for lung cancer: a cohort study. Ann Intern Med. 2012;157:776–784. doi: 10.7326/0003-4819-157-11-201212040-00005. [DOI] [PubMed] [Google Scholar]
- 46.Saghir Z, Dirksen A, Ashraf H, Bach KS, Brodersen J, Clementsen PF, Døssing M, Hansen H, Kofoed KF, Larsen KR, et al. CT screening for lung cancer brings forward early disease: the randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012;67:296–301. doi: 10.1136/thoraxjnl-2011-200736. [DOI] [PubMed] [Google Scholar]
- 47.Horeweg N, van der Aalst CM, Thunnissen E, Nackaerts K, Weenink C, Groen HJ, Lammers JW, Aerts JG, Scholten ET, van Rosmalen J, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med. 2013;187:848–854. doi: 10.1164/rccm.201209-1651OC. [DOI] [PubMed] [Google Scholar]
- 48.Infante M, Cavuto S, Lutman FR, Passera E, Chiarenza M, Chiesa G, Brambilla G, Angeli E, Aranzulla G, Chiti A, et al. DANTE Study Group. Long-term follow-up results of the DANTE trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med. 2015;191:1166–1175. doi: 10.1164/rccm.201408-1475OC. [DOI] [PubMed] [Google Scholar]
- 49.Young RP, Hopkins RJ. Mortality reduction, overdiagnosis, and the benefit-to-harm ratio of computed tomography screening. Am J Respir Crit Care Med. 2015;192:398–399. doi: 10.1164/rccm.201504-0801LE. [DOI] [PubMed] [Google Scholar]
- 50.Young RP, Hopkins RJ.A clinical practice guideline update on the diagnosis and management of stable chronic obstructive pulmonary disease Ann Intern Med 201215668–69.author reply 69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.