Abstract
Background:
Hearing disorders are reported in thalassemia patients treated with deferoxamine. This study aimed to assess hearing loss in Iranian thalassemia major patients treated with deferoxamine.
Methods:
This review article was designed based on PRISMA guidelines. To review the literature, two researchers studied national and international databases including Iranmedex, Magiran, Medlib, SID, Scopus, PubMed, Science Direct, Web of Science and Google Scholar without time limit until May 2017. Cochran's Q test and I2 index were used to assess the heterogeneity of the studies. The data were analyzed using Comprehensive Meta-Analysis software version 2 and p<0.05 was considered significant.
Results:
A total of 17 articles involving 1,835 Iranian thalassemia major patients treated with deferoxamine were included in the meta-analysis. The overall prevalence of hearing loss was estimated 27.3% (95% confidence intervals (CI): 19-37.6). The prevalence of sensorineural, conductive and mixed hearing loss was estimated 10.6% (95% CI: 5.7-18.8), 14.6% (95% CI: 10.5-20.6) and 9.1% (95% CI: 5.6-14.6), respectively. No significant differences were noted regarding the relationship hearing loss and mean serum ferritin (P=0.29) and average daily deferoxamine (P=0.30). Meta-regression model showed an increased significance in the prevalence of hearing loss based on the year of studies (p<0.0001).
Conclusions:
There is a high prevalence of hearing loss in Iranian thalassemia major patients treated with deferoxamine. Therefore, periodic hearing assessments and regular check-ups after the initiation of chelation therapy are necessary.
Key Words: Hearing loss, Thalassemia major, Deferoxamine, Systematic review, Meta-analysis, Iran
Thalassemia is a common disorder in Southeast Asia caused by genetic defects in the synthesis of hemoglobin chain leading to chronic hypochromic microcytic anemia (1). These patients can be treated by receiving regular monthly blood transfusions that decreases the acute symptoms of the disease. On the other hand, frequent blood transfusions can lead to hemosiderosis and tissue dysfunction including hepatic, cardiac and endocrine complications (2-5). These patients die in the second decade of life due to the mentioned complications. But today, the survival rate has greatly improved because of the advances in therapies, especially after the chelation therapy. A relatively long lifetime, is expected for the patients with appropriate treatment (6). Despite the benefits of chelation therapy in these patients, complications are unavoidable. Hearing disorders are reported in thalassemia patients treated with deferoxamine (DFO) (7-8).
A simple review of the documents indicated that the prevalence of hearing loss in patients with thalassemia major treated with DFO varied in Iran (9-13) and now a meta-analysis is necessary to summarize the results and provide an overview of the problem. Meta-analysis is a method of collecting and analyzing the results of several studies with the same goal and providing a reliable estimation of the effect of some medical interventions or observations (14-15). Based on this method, as the number of samples increased, the range of changes and possibilities decreased and the significance of the statistical results increased. This method is more reliable due to the certain conditions of meta-analysis (16-17). This study aimed to assess hearing loss in patients with thalassemia major treated with DFO in Iran using the meta-analysis method.
Methods
Study protocol : This review study was designed based on the recommendations of PRISMA (18). To avoid bias in this study, all procedures were done by two researchers independently. Group discussion was used in case of divergence in the results.
Inclusion and exclusion criteria: The main inclusion criteria were all the epidemiological studies that assessed hearing loss in patients with thalassemia major. Exclusion criteria included: (1) non-random sample size for prevalence estimate; (2) non-Iranian patients; (3) patients not treated with DFO; (4) being irrelevant to the topic; and (5) review studies, letters to the editor, case reports.
Search strategy: To review the literature, researchers studied the national and international databases, including Iranmedex, Magiran, Medlib, SID (Scientific Information Database), Scopus, PubMed, Science Direct, Web of Science and Google Scholar without time limit until May 2017. To maximize the comprehensiveness of the research in the databases, we used MeSH keywords including Prevalence, Epidemiology, Hearing Loss, Sensorineural, Drug-Related Side Effects, Deferoxamine, Ferritin, Iron Overload, Chelation Therapy, Thalassemia and Iran and then combined our search with operators AND/OR in English databases. PubMed combination search is shown in appendix 1. References were also evaluated to find further studies.
Quality assessment: Researchers examined the selected articles using STROBE checklist (19), which is a standard and an international checklist for the quality assessment of the studies. The authors have adopted a simple method for scoring. Each question was scored between 0-2 points. The maximum point was 44 and the minimum acceptable point for inclusion in the meta-analysis was 16. The researchers focused on a group discussion in case of discrepancies.
Data Extraction: To reduce the errors in data collection, data extraction was done using a data extraction form including authors’ name, data publication, year of study, number of participants, the overall prevalence of hearing loss, prevalence of conductive hearing loss, prevalence of sensorineural hearing loss, dB HL (decibels hearing level), the association between hearing loss and ferritin (mean serum ferritin in case and control groups and number of patients with hearing loss; equal or above 3000 vs. [versus] lower than 3000 ng/ml), the association between hearing loss and age (number of patients with hearing loss; equal or above 10 years-old vs. below 10) and for the association between hearing loss and average daily DFO use (mean daily DFO use in case and control groups). we asked specific questions or ambiguities from the authors of the articles via email.
Statistical analysis: The binomial distribution was used to calculate the variance of each study for the prevalence of hearing loss in patients with thalassemia major. To study the relationship between hearing loss and ferritin, average daily DFO use and age, odds ratio (OR) and the standardized mean difference (SMD) were used. Cochran's Q test and I2 index were used to assess heterogeneity of the studies. In this study, the heterogeneity was found to be 92.5% for the prevalence of hearing loss, which was a high heterogeneity (I2 index less than 25% is considered as low heterogeneity, between 25-75% is moderate heterogeneity and more than 75% is high heterogeneity). So, the random effects model was used in the meta-analysis.
To investigate the effect of ferritin (≥3000 vs. <3000) on hearing loss, random effects model was used due to high heterogeneity and for other variables, fixed effects model was used due to low heterogeneity (20-21). To find the source of high heterogeneity for the prevalence of hearing loss, subgroup analysis was conducted based on province and thresholds of sound intensity in audiometry. To investigate the relationship between hearing loss and the year of studies, meta-regression model was used. The data were analyzed using Comprehensive Meta-Analysis software Version 2 and p<0.05 was considered significant.
Results
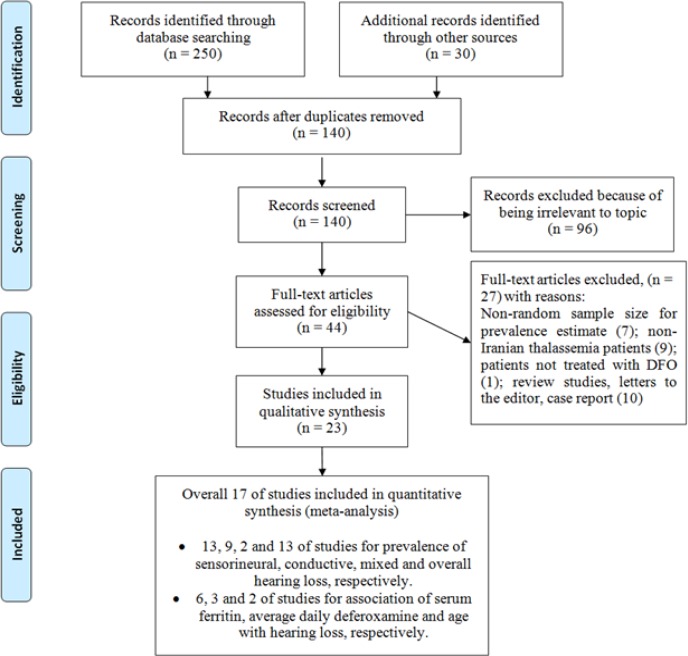
Search results: 280 relevant studies were found in the initial systematic review by two authors and 140 studies were excluded due to duplication. After screening the titles and abstracts, 96 studies were excluded because of their irrelevance to the topic. Finally, 17 studies with appropriate quality entered the meta-analysis (figure 1).
Figure1.
A flowchart of the literature searches for the systematic review of studies
Characteristics of the included studies: The participants of this study were 1,835 Iranian thalassemia major patients treated with DFO.
The mean age of the participants was 12.5 yr (95% confidence intervals (CI): 10.0-14.9). Details of the studies that entered the meta-analysis are shown in table 1.
Table 1.
Summary of the included studies
|
Prevalence of hearing loss (%)
|
Age Mean±SD | Sample size | Year | Place | The first author, publication date | Ref | |||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Mixed | Conductive | Sensorineural | ||||||
| 19.8 | 9.4 | 9.4 | 9.3±1.4 | 32 | 2007 | Birjand | Chahkandi T, 2011 | 9 | |
| 43.1 | 16 | 4.6 | 195 | 2002 | Tehran | Nili S, 2002 | 10 | ||
| 48.4 | 95 | 2001 | Gorgan | Taziki MH, 2004 | 11 | ||||
| 12 | 12 | 100 | 2006 | Babol | Kiakojouri K, 2008 | 12 | |||
| 1.8 | 178 | 2003 | Kerman | Mozafarinia K, 2005 | 13 | ||||
| 47.5 | 8.75 | 17.5 | 21.2 | 14.2±2.3 | 80 | 2009 | Tehran | Ashrafi M, 2011 | 22 |
| 30.9 | 9.5 | 9.5 | 11.9 | 12.8±5.7 | 84 | 2007 | Sanandaj | Company F, 2009 | 23 |
| 63 | 8.2 | 54.8 | 73 | 2010 | Yazd | Doosti A, 2013 | 24 | ||
| 10.3 | 10.3 | 0 | 78 | 2001 | Shahrkord | Raesi N, 2004 | 25 | ||
| 14 | 14 | 10.0±2.5 | 50 | 1998 | Arak | Hashemieh M, 1999 | 26 | ||
| 3.5 | 293 | 2006 | Shiraz | Faramarzi A, 2010 | 27 | ||||
| 48.7 | 156 | 2003 | Isfahan | Sonboestan M, 2005 | 28 | ||||
| 56 | 32.3 | 11.7 | 14.4±5.0 | 128 | 2000 | Shiraz | Karimi M, 2002 | 29 | |
| 7.4 | 14.3±5.1 | 67 | 2006 | Tehran | Shamsian B.B, 2008 | 8 | |||
| 5.7 | 53 | 2006 | Tehran | Azizi G, 2008 | 30 | ||||
| 25 | 20 | 5 | 100 | 1991 | Shiraz | Kaviani M, 1992 | 31 | ||
| 11.8 | 73 | 1994 | Babol | Nowruzi F, 1995 | 32 | ||||
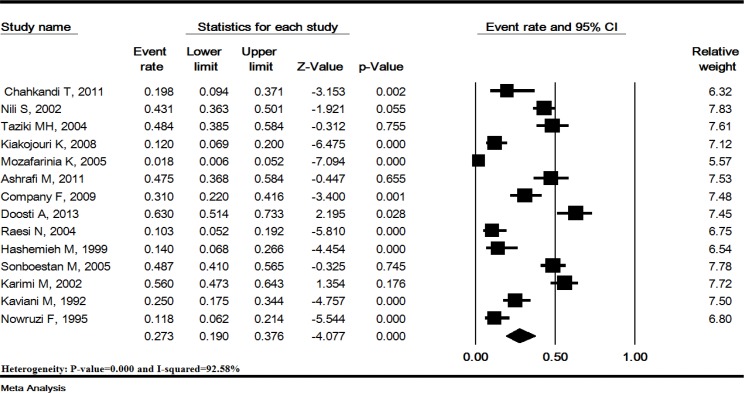
Overall prevalence of hearing loss: The rate heterogeneity in this study was 92.5% (p<0.0001). In 14 studies including1,422 Iranian thalassemia major patients treated with DFO, the overall prevalence of hearing loss was estimated 27.3% (95% CI: 19-37.6). The lowest prevalence of hearing loss was reported in a study conducted in Kerman (1.8%) while the highest prevalence was reported in a study conducted in Yazd (63%) (figure 2).
Figure 2.
Prevalence of hearing loss in Iranian thalassemia major patients treated with deferoxamine. Random effects model.
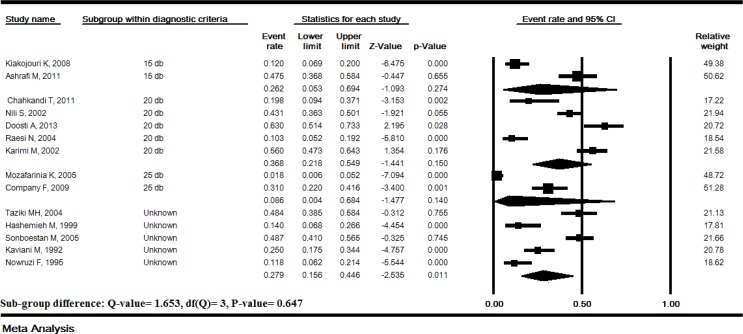
Subgroup analysis of hearing loss prevalence based on threshold s: The prevalence of hearing loss based on the thresholds 15, 20 and 25 dB HL of sound intensity in audiometry was 26.2% (95% CI: 5.3-69.4), 36.8% (95% CI: 21.8-54.6) and 8.6% (95% CI: 0.4-68.4), respectively and the subgroup difference was not significant (P=0.64) (figure 3).
Figure 3.
Subgroup analysis of hearing loss prevalence based on thresholds of sound intensity in Iranian thalassemia major patients treated with deferoxamine. Random effects model
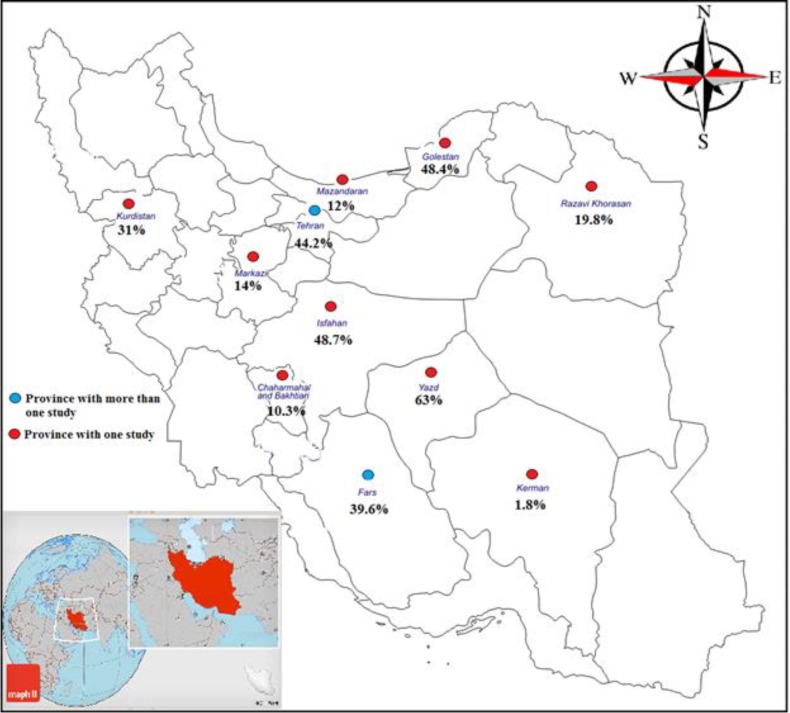
Subgroup analysis of hearing loss prevalence based on provinces: The prevalence of hearing loss based on provinces was shown in figure 4 and the subgroup difference was significant (p<0.0001).
Figure 4.
Geographical distribution of prevalence of hearing loss in Iranian thalassemia major patients treated with deferoxamine
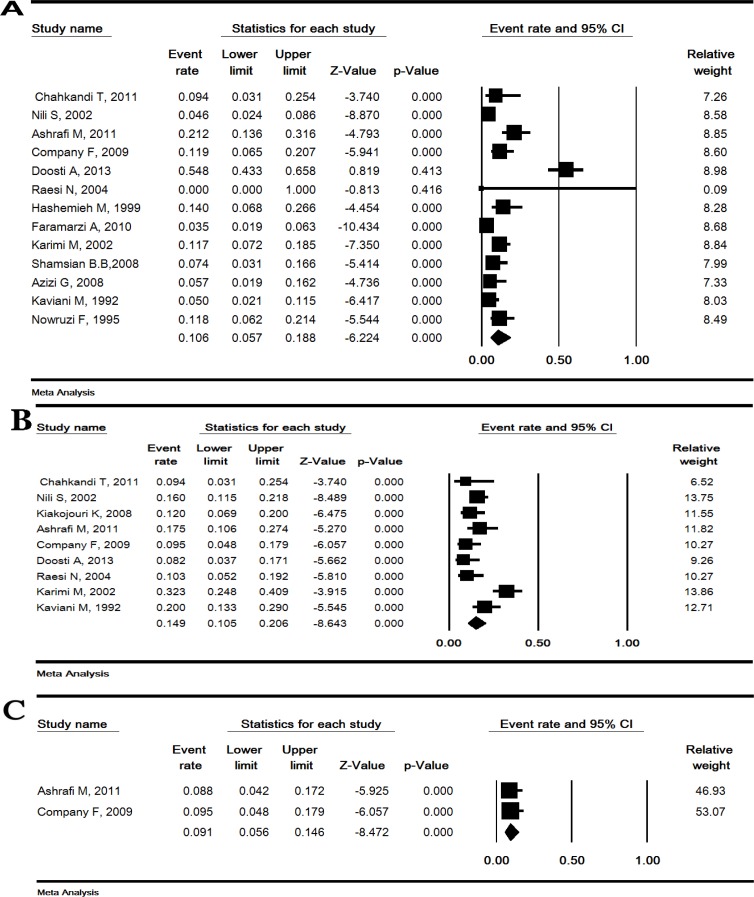
Prevalence of sensorineural, conductive and mixed hearing loss: The prevalence of sensorineural, conductive and mixed hearing loss was estimated 10.6% (95% CI: 5.7-18.8), 14.6% (95% CI: 10.5-20.6) and 9.1% (95% CI: 5.6-14.6), respectively (figure 5).
Figure 5.
Prevalence of sensorineural (A), conductive (B) and mixed (C) hearing loss in Iranian thalassemia major patients treated with deferoxamine. Random effects model
The relationship between hearing loss and age, serum ferritin, and average daily dose of deferoxamine:
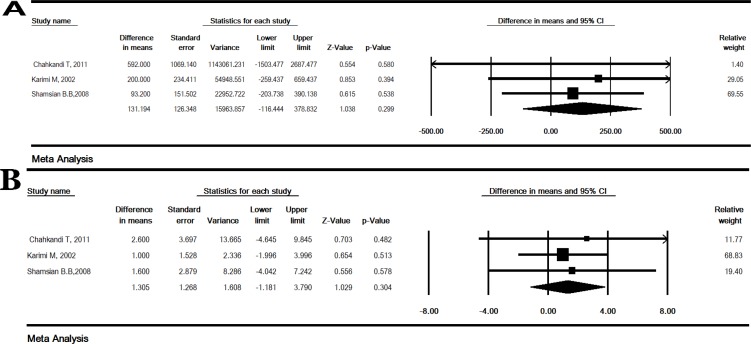
The OR for the relationship between age (≥10 vs. <10 years) and hearing loss was 1.93 (95% CI: 1.24-3.02) and was statistically significant (p=0.004) (table 2). The relationship between serum ferritin (≥3000 vs. <3000 ng/ml) and hearing loss was estimated and OR was 3.34 (95% CI: 1.53-7.27), which was significant (p=0.002) (table 2). But this relationship between mean serum ferritin in patients with hearing loss versus without hearing loss was not significant (SMD: 131.19 [95% CI: -116.44 to 378.83], P=0.29) (figure 6 A). The SMD for the relationship of average daily DFO and hearing loss was 1.30 (95% CI: -1.18 to 3.79) and was not significant (P=0.30) (figure 6 B).
Table 2.
The association between age and serum ferritin with hearing loss in Iranian thalassemia major patients treated with deferoxamine
| P-Value | 95% CI c | OR b |
Heterogeneity
|
Without hearing loss
(Na) |
With hearing loss
(Na) |
Studies (N a ) | Variable | |||
|---|---|---|---|---|---|---|---|---|---|---|
| P -Value | I 2 | All | Event | All | Event | |||||
| 0.004 | 1.24-3.02 | 1.93 | 0.81 | 0 | 80 | 29 | 82 | 58 | 2 | Age ≥10 vs. <10 years |
| 0.002 | 1.53-7.27 | 3.34 | 0.19 | 38.16 | 181 | 26 | 97 | 46 | 3 | Ferritin ≥3000 vs. <3000 ng/ml |
Number;
odds ratio;
confidence interval
Figure 6.
The the standardized mean difference for the mean serum ferritin (A) and average daily deferoxamine use (B) in Iranian thalassemia major patients treated with deferoxamine. Fixed effects model
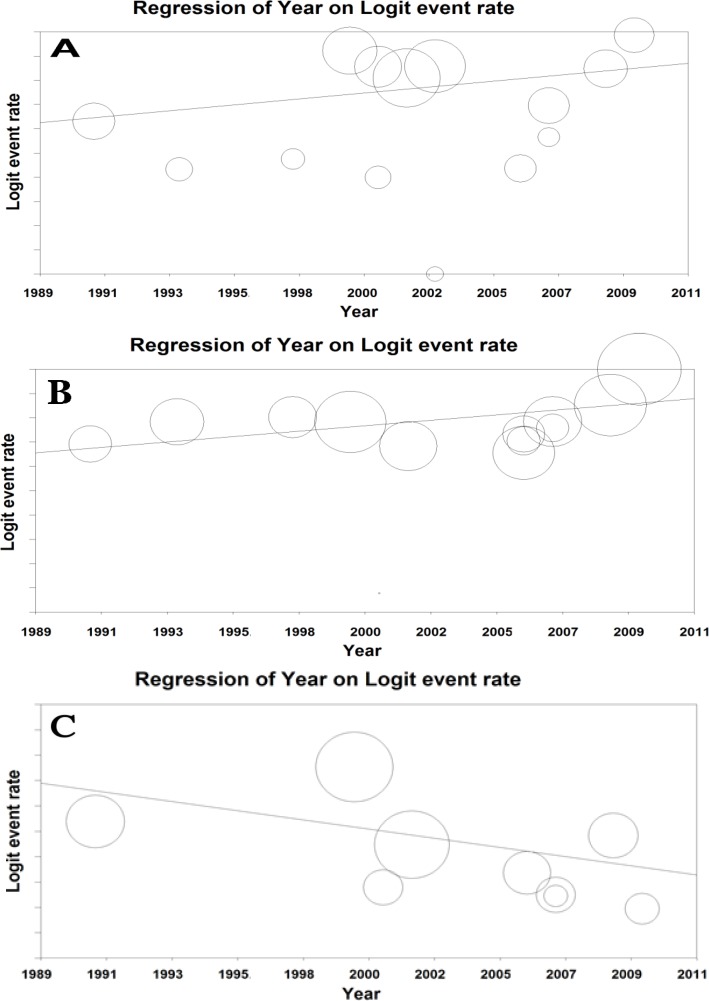
Meta-regression model: Meta-regression model was used to assess the relationship between sensorineural, conductive and overall prevalence of hearing loss and year of studies and the p-value was <0.0001, which was statistically significant (figure 7).
Figure 7.
Meta-regression between the prevalence of overall (A), sensorineural (B) and conductive (C) hearing loss in Iranian patients with thalassemia major and year of studies. Larger circles indicate the larger sample size
Discussion
The present study is the first systematic review and meta-analysis about the prevalence of hearing loss in patients with thalassemia major treated with DFO in Iran. The overall prevalence of hearing loss was estimated 27.3% (19%-37.6%). The prevalence of sensorineural, conductive and mixed hearing loss was estimated 10.6%, 14.6% and 9.1%, respectively. The subgroup analysis of hearing loss prevalence based on provinces and thresholds of sound intensity was conducted and the difference was statistically significant (p<0.01). In other countries, the prevalence of sensorineural and conductive hearing loss in patients with thalassemia major treated with DFO were reported to be 3.8 - 57% (33-38) and 4.5 - 59.2% (34, 39-40), respectively. The variety in the prevalence of hearing loss can be attributed to genetic factors, individual characteristics, DFO administration, the length of DFO administration and different follow-up periods in different countries.
According to thalassemia prevention program (Ministry of Health, 2000), there are 20,000 patients with thalassemia receiving blood transfusions and iron chelation in Iran. DFO and deferiprone are considered as iron chelators in Iran, while DFO is more common and deferiprone is less common due to the complications such as agranulocytosis and the need for the routine evaluation of white blood count (WBC) (8). In a systematic review among patients with thalassemia major in Iran, almost all patients were treated with iron chelation drugs, but only 55% of the patients were treated with regular (41), which can be attributed to glandular and cardiac complications in patients with thalassemia major in Iran (42-44). In this study, the difference is mean serum ferritin was not significant in patients with hearing loss and without hearing loss, but the relationship between serum ferritin and hearing impairment was significant (≥ 3000 vs. 3000 ng/ml). The reason for this significant relationship can be attributed to the invasive treatment of deferoxamine to reduce iron overload in patients with ferritin levels above 3000 ng/ml. However, the small number of studies involved in the meta-analysis process can be another reason, which needs to be considered. Moreover, the relationship between age (≥10 vs. <10 years) and hearing loss was demonstrated. Therefore, a thalassemia major patient treated with DFO older than 10 years was more exposed to hearing complications. The mechanism of neurotoxicity of DFO chelation is not completely specified yet (45). A high dosage of DFO may cause a high level of unbound drug and this may directly result in neurotoxicity or interaction with other very rare elements (46-47). Young age of starting treatment, the dose of drug administration, and serum ferritin may increase the risk for ototoxicity (45, 47). Sensorineural hearing loss is not directly associated with serum ferritin or dose of DFO. However, other factors including genetic and constitutional characteristics may be associated with sensorineural hearing loss (8). Porter et al. found that the high dose of DFO is associated with low serum ferritin level (<2000 ng/ml) and is a major risk factor for desferal ototoxicity (45). Ambrosetti et al. demonstrated that there is no significant relationship between hearing loss and age, serum ferritin level and therapeutic index (39). Considering the changes in the prevalence of hearing loss based on the year in the meta-regression, the value is increasing based on the years of study (1998-2010), which is significant?
The limitations of this study include: 1. Low sensitivity of national databases for combined search; 2. Failure to provide statistics on the prevalence of hearing loss according to gender; 3. The limitation of the number of studies regarding the relationship between hearing impairment and age, and the mean ferritin that may affect the results.
In conclusion, the prevalence of hearing loss in patients with thalassemia major treated with DFO in Iran is high. Potential lesions causing hearing impairment may be present in any part of the auditory pathway. Therefore, periodical hearing assessments and regular check-ups after onset of chelation therapy are necessary.
Conflict of interest:
No conflict of interest in this article.
References
- 1.Albera R, Pia F, Morra B, et al. Hearing loss and desferrioxamine in homozygous beta-thalassemia. Int J Audiol. 1988;27:207–14. doi: 10.3109/00206098809081591. [DOI] [PubMed] [Google Scholar]
- 2.Azami M, Gheisoori A, Sayehmiri F, Sayehmiri K. Prevalence of hypothyroidism in patients with Beta thalassemia major in Iran: A systematic review and meta-analysis. J Kurdistan Univ Med Sci. 2016;21:104–16. [Google Scholar]
- 3.Azami M, Tardeh Z, Abangah GH, Sayemiri K. The prevalence of impaired glucose tolerance in patients with thalassemia major in Iran: a systematic review and meta-analysis. J Shahid Sadoughi Uinv Med Sci. 2016;23:912–22. [Google Scholar]
- 4.Azami M, Sayehmiri K. Prevalence of diabetes mellitus in Iranian patients with thalassemia major: a systematic review and meta-analysis. J Mazandaran Univ Med Sci. 2016;26:192–204. [Google Scholar]
- 5.Sayehmiri K, Tardeh Z, Mansouri A, Borji M, Azami M. The prevalence of hypogonadism in patients with thalassemia major in Iran–a systematic review and meta-analysis study. J Shahrekord Univ Med Sci. 2016;18:140–51. [Google Scholar]
- 6.De Sanctis V, Tangerini A, Testa MR, et al. Final height and endocrine function in thalassaemia intermedia. J Pediatr Endocrinol Metab. 1998;11:965–71. [PubMed] [Google Scholar]
- 7.Sonbolestan M, Mokhtarinejad F, Omrani M. An Evaluation of sensory neural hearing loss in thalassaemic patients treated with desferrioxamine and its risk factors. J Res Med Sci. 2005;10:210–6. [Google Scholar]
- 8.Shamsian BS, Aminasnafi A, Moghadassian H, et al. Sensory neural hearing loss in beta-thalassemia major patients treated with deferoxamine. Pediatr Hematol Oncol. 2008;25:502–8. doi: 10.1080/08880010802234911. [DOI] [PubMed] [Google Scholar]
- 9.Chahkandi T, Mofatteh MR, Sharifzadeh Gh, Azarkar Z. Hearing impairment in patients with major thalassemia in Southern Khorasan Province, 2007. J Birjand Univ Med Sci. 2011;18:102–8. [Google Scholar]
- 10.Nili S, Karimi Yazdi AR, Sharifian RA, Jalaei S. Hearing evaluation of thalassemic patients with Desferrioxamine therapy in Qazvin thalassemia center and Tehran pediatrics’ Medical Center. J Audiology. 2002;11:31–5. [Google Scholar]
- 11.Taziki MH, Golalipour MJ, Behnampour N. The determination of hearing loss in thalassemia major. J Gorgan Univ Med Sci. 2005;6:35–9. [Google Scholar]
- 12.Kiakojouri K, Tamaddoni A, Mahmoodi Nesheli H, Jahanian Bahnemiri Z, Gholipour S. Correlation of hearing impairment with desferal and serum ferritin level in β thalassemia major patients. J Babol Univ Med Sci. 2008;10:48–53. [Google Scholar]
- 13.Mozafari Nia K, Farahmandi Nia Z, Ghazvini M. Evaluation of hearing threshold in patients with beta thalassemic major receiving regular chelation therapy with Desferrioxamine (DFO) Kerman Univ Med Sci. 2005;12:93–8. [Google Scholar]
- 14.Azami M, Hafezi Ahmadi MR, Sayehmiri K. Hepatitis B vaccination efficacy in Iranian healthcare workers: a meta-analysis study. Hepat Mon. 2017;17:e37781. [Google Scholar]
- 15.Spector TD, Thompson SG. The potential and limitations of meta-analysis. J Epidemiol Community Health. 1991;45:89–92. doi: 10.1136/jech.45.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azami M, Khataee M, Bigam Bigdeli-Shamlo M, et al. Prevalence and risk factors of hepatitis b infection in pregnant women of Iran: a systematic review and meta-analysis. IJOGI. 2016;19:17–30. [Google Scholar]
- 17.Azami M, Nasirkandy MP, Mansouri A, et al. Global prevalence of helicobacter pylori infection in pregnant women: a systematic review and meta-analysis study. Int J Women's Health Reprod Sci. 2017;5:30–6. [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 19.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 20.Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. 2005;25:646–54. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- 21.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Syn Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 22.Ashrafi M, Mohammadzadeh A. Hearing status of thalassemic patients treated with dessfroxamine. Behbood J. 2011;15:365–71. [Google Scholar]
- 23.Company F, Rezaei N, Yosefi G. Evaluation of hearing loss and otolaryngeal disorders in beta thalassemic patients treated with deferoxamine. Sci J Kurdistan Univ Med Sci. 2009;14:47–55. [Google Scholar]
- 24.Dousti A, Akhondi R, Atighechi S. Auditory evaluation of patients with major beta thalassemia under treatment with desferrioxamine. J Res Hearing Speech Language. 2014;1:57–62. [Google Scholar]
- 25.Reissi N, Kargoshaie A. Prevalence of hearing loss in patients with Beta thalassemia major, Hajar hospital of Shahrekord 2002. J Shahrekord Univ Med Sci. 2004;6:31–6. [Google Scholar]
- 26.Hashemieh M, Kobar Fard F. The incidence of hearing loss among thalassemic patients in Arak city. Arak Univ Med Sci (Rahavard Danesh)1999. 2:38–42. [Google Scholar]
- 27.Faramarzi A, Karimi M, Heydari ST, Shishegar M, Kaviani M. Frequency of sensory neural hearing loss in major beta-thalassemias in Southern Iran. Iran J Pediatr. 2010;20:308–12. [PMC free article] [PubMed] [Google Scholar]
- 28.Sonbolestan M, Mokhtarinejad F, Omrani M. An evaluation of sensory neural hearing loss in thalassaemic patients treated with desferrioxamine and its risk factors. J Res Med Sci. 2005;10:210–6. [Google Scholar]
- 29.Karimi M, Asadi-Pooya AA, Khademi B, Asadi-Pooya K. Evaluation of the incidence of sensorineural hearing loss in beta thalassemia major patients under regular chelation therapy with desferrioxamine. Acta Haematol. 2002;108:79–83. doi: 10.1159/000064748. [DOI] [PubMed] [Google Scholar]
- 30.Azizi G, Habibian N. The relationship between sensorineural hearing loss with the use of chelation therapy in patients with B-thalassemia major (Special Medical Center, Tehran, 2006). Thesis. Tehran: Azad University of Medical Sciences; 2008. [in Persian] [Google Scholar]
- 31.Kaviani M, Rekiabi Bana H, Haghshenas M. ENT Complications in Beta thalassemia major. Iran J Med Sci. 1992;17:55–8. Available at: http://elib.sums.ac.ir/cgi-bin/koha/opac-detail.pl?bib=133000309. [Google Scholar]
- 32.Nowruzi F. Hearing disorders caused by chelation therapy in patients with thalassemia major. Thesis. Babol: Babol University of Medical Sciences ; 1995. [in Persian] [Google Scholar]
- 33.Prasansuk S. Incidence/prevalence of sensorineural hearing impairment in Thailand and Southeast Asia. Audiology. 2000;39:207–11. [PubMed] [Google Scholar]
- 34.Tanphaichitr A, Kusuwan T, Limviriyakul S, et al. Incidence of ototoxicity in pediatric patients with transfusion-dependent thalassemia who are less well-chelated by mono- and combined therapy of iron chelating agents. Hemoglobin. 2014;38:345–50. doi: 10.3109/03630269.2014.940462. [DOI] [PubMed] [Google Scholar]
- 35.Kong MH, Goh BS, Hamidah A, Zarina AL. The Prevalence of Sensorineural Hearing Loss in β-thalassaemia patient treated with Desferrioxamine. Med J Malaysia. 2014;69:9–12. [PubMed] [Google Scholar]
- 36.Kontzoylou G, Koussi A, Economou M, et al. Long term audiological evaluation of beta-thalassemia patients. Acta Otorhinolaryngol Belg. 2004;58:113–7. [PubMed] [Google Scholar]
- 37.Osma U, Kurtoglu E, Eyigor H, Yilmaz MD, Aygener N. Sensorineural hearing loss in β-thalassemia patients treated with iron chelation. Ear Nose Throat J. 2015;94:481–5. [PubMed] [Google Scholar]
- 38.Chen SH, Liang DC, Lin HC, et al. Auditory and visual toxicity during deferoxamine therapy in transfusion-dependent patients. J Pediatr Hematol Oncol. 2005;27:651–3. doi: 10.1097/01.mph.0000194019.95096.b6. [DOI] [PubMed] [Google Scholar]
- 39.Ambrosetti U, Dondè E, Piatti G, Cappellini MD. Audiological evaluation in adult beta-thalassemia major patients under regular chelation treatment. Pharmacol Res. 2000;42:485–7. doi: 10.1006/phrs.2000.0722. [DOI] [PubMed] [Google Scholar]
- 40.Onerci M, Aslan S, Gumruk F, et al. Audiologic and impedancemetric findings within thalassaemic patients. Int J Pediatr Otorhinolaryngol. 1994;28:167–72. doi: 10.1016/0165-5876(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 41.Azami M, Nikpay S, Abangah G, Sayehmiri K. Evaluation of the incidence of splenectomy and frequency of regular iron chelation therapy in patients with thalassemia Major in Iran: a meta-analysis. Sci J Iran Blood Transfus Organ. 2016;13:146–55. [Google Scholar]
- 42.Azami M, Rahmati S, Sayehmiri K. Prevalence of Hyperparathyroidism in Patients with Thalassemia Major in Iran. J Babol Univ Med Sci. 2016;18:39–48. [Google Scholar]
- 43.Sayehmiri K, Sharifi Sh, Noruzi S, Mansouri A, Azami M. Prevalence of diabetes, impaired fasting glucose and impaired glucose tolerance in patients with thalassemia major in Iran-a meta-analysis. Caspian J Intern Med. 2017;8:1–15. [PMC free article] [PubMed] [Google Scholar]
- 44.Azami M, Parizad N, Kourosh Sayehmiri K. Prevalence of hypothyroidism, hypoparathyroidism and the frequency of regular chelation therapy in patients with thalassemia major in Iran: a systematic review and meta-analysis study. Iran J Ped Hematol Oncol. 2016;6:260–75. [Google Scholar]
- 45.Porter JB, Jaswon MS, Huehns ER, East CA, Hazell JW. Desferrioxamine ototoxicity: Evaluation of risk factors in thalassaemic patients and guidelines for safe dosage. Br J Haematol. 1989;73:403–9. doi: 10.1111/j.1365-2141.1989.tb07761.x. [DOI] [PubMed] [Google Scholar]
- 46.De Virgiliis S, Congia M, Turco MP, et al. Depletion of trace elements and acute ocular toxicity induced by desferrioxamine in patients with thalassaemia. Arch Dis Child. 1988;63:250–5. doi: 10.1136/adc.63.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kontzoglou G, Koussi A, Tsatra J, et al. Sensorineural hearing loss in children with thalassemia major in Northern Greece. Int J Pediatr Otorhinolaryngol. 1996;35:223–30. doi: 10.1016/0165-5876(95)01308-3. [DOI] [PubMed] [Google Scholar]