Abstract
Background:
There is some evidence that shows the symptoms of anemia are fewere in overweight and obese people, so, the purpose of this research was to study the relationship between anemia and iron deficiency anemia (IDA) with lipid profile status of the elderly.
Methods:
This cross-sectional study came from the Amirkola Health and Ageing Project (AHAP). A demographic questionnaire was given to the older people and a blood sample was obtained to assay their lipid indexes (triglyceride, cholesterol, HDL and LDL) and the parameters related to anemia after 12 hours fasting. The data were analyzed by chi-square test, t-test and Pearson correlation using SPSS. A p<0.05 was considered as the significance level of the tests.
Results:
The average age of the people was 68.95±7.43 years old. In this study, the prevalence of anemia and IDA was 31% and 9%, respectively. The mean concentration of serum triglyceride, cholesterol and LDL in the anemia group and the IDA group was less than the control groups. The amount of HDL in different groups was almost the same, although the difference was statistically significant with respect to variables like age and obesity (P=0.001).
Conclusion:
The study showed that the amount of lipid profile (triglyceride and cholesterol) in the elderly with anemia and IDA was less compared to other people. This result was achieved in some other research studies too, but further research is suggested to find possible mechanisms.
Key Words: Elderly, Anemia, Hemoglobin, Iron Deficiency Anemia, Lipid Profile, Cholesterol, Triglyceride
Anemia is a common blood disorder as 26% of adults in the developing countries are anemic (1). In Iran, 30% of people aged 3-65 are suffering from anemia (2). Symptoms of moderate anemia include fatigue, loss of energy, shortness of breath, and palpitations (especially during physical activity) (1). One of the most important types of anemia is iron deficiency anemia (IDA) (3). Iron is an essential element in the physiology of the body, such as the transport of oxygen and enzymatic reactions. However, excess iron, due to production of free radicals, is harmful and causes tissue damage (4, 5). Iron deficiency reduces the serum level of iron and ferritin and thereby causes impaired erythropoiesis (6). About 50% of anemia cases in the world can be attributed to iron deficiency which is responsible for about 841,000 deaths in the world every year (1). A study conducted in Iran in 2001 showed that anemia and iron deficiency occursin a high percentage of the population in different age groups. Accordingly, about 23% of children under 2, 26% of children aged 6, 23% of male and female adolescents, and 43% of pregnant women suffer from iron deficiency (7). In a study conducted in the US, it was reported that nearly 4.7 million Americans suffer from anemia and its prevalence increases with age, as 44.4% of people aged over 85 are anemic (8).
Elevation of serum lipid levels increases the risk of atherosclerosis and coronary heart disease (4). Because of the role of abnormal lipid levels in atherogenesis, its effects on health increase with age, thus great attention is paid to abnormal levels of lipids and its associated factors (9, 10). The results of some studies show that the symptoms of anemia are fewer in overweight or obese people than those with normal weight (11, 12). On the other hand, the findings of another study indicate that anemia is associated with increased risk of long-term complications of cardiovascular events and death, especially in obese patients (13).
In a number of epidemiological studies, high iron reserves in the body have been reported to be associated with increased risk of coronary heart disease, the most important risk factor is hyperlipidemia (14, 15). In this regard, although the relationship between iron intake and serum lipid level has been found in animal models, this relationship has not been studied extensively in humans (16-18). Since few studies have been conducted on the effect of anemia on serum lipid profile, the present research aimed to study the relationship between lipid profile and IDA.
Methods
The present cross-sectional research was part of a cohort study conducted on 1616 elderly people aged 60 or above in Amirkola, Babol, Mazandaran Province (19). Project protocol was approved by the Babol University of Medical Sciences Research Ethics Committee, and a written consent was obtained from all subjects after briefing them on the research objectives. Patients with chronic renal failure along with anemia and blood cancer or the elderly whose information on their serum lipid level and anemia indices was incomplete or unavailable were excluded from the study. Then, the subjects were asked to fill out a questionnaire containing the demographic information. Fasting blood samples were collected and biochemical markers were measured according to the standard method in the laboratory of Cellular and Molecular Biology Research Center, Babol University of Medical Sciences. Lipid indices tests (triglyceride, cholesterol, HDL, and LDL) were conducted based on ELISA method using the kits manufactured by Pars Azmoon Company.
Parameters related to blood cell count and anemia were studied using Sysmex XS-1000i. TIBC and serum iron levels were measured based on ELISA kits manufactured by Pars Azmoon Company. In addition, ELISA kits of DiaMetra Company were used for the measurement of ferritin. Hemoglobin level less than 14 gr/dl for men and less than 12 gr/dl for adult women, diagnosed anemia cases by the physician and cases under treatment were defined as anemia (1). Patients with a ferritin level of less than 20 µg/dl, transferrin saturation less than or equal to 10%, and serum iron of less than 30 µg/dl and the patients for whom iron supplement was prescribed by the physician were regarded as the cases of IDA (1).
It is noteworthy that the patients receiving anti-lipid drugs were excluded from the comparison of serum lipid level in anemia and IDA groups. The obtained data and information were statistically analyzed using chi-square, t-test, and correlation coefficient test in SPSS software. The level of significance in this study was determined to be p<0.05.
Results
Among the 1616 elderly people studied, 22 (1.4%) individuals people were excluded from the study according to the predetermined criteria. Finally, 868 men and 726 women with the mean age of 68.95±7.43 were selected as the subjects. Table 1 shows the demographic information, status of anemia indices, and lipid profile of subjects.
Table 1.
Demographic information, status of anemia indices, and lipid profile of subjects
| Women | Men | Variable |
|---|---|---|
| 68.24±7.18 | 69.38±7.82 | Age (year) |
| 28.23±4.16 | 26.03±4.65 | BMI (Kg/m2) |
| 12.02±1.11 | 14.17±1.08 | Hemoglobin (mg/dL) |
| 83.11±10.12 | 84.31±9.12 | MCV |
| 78.23±32.63 | 87.15±37.09 | Serum iron (µg/dL) |
| 154.21±117.54 | 167.13±123.61 | Ferritin (µg/L) |
| 285.16±36.12 | 280.12±38.24 | TIBC (µg/dL) |
| 173.81±91.19 | 147.21±77.20 | Triglyceride (mg/dL) |
| 211.34±42.08 | 191.16±37.5 | Cholesterol (mg/dL) |
| 39.38±4.12 | 38.06±4.39 | HDL (mg/dL) |
| 144.95±46.32 | 126.81±39.28 | LDL (mg/dL) |
Among the subjects, 300 people were taking anti-lipid drugs and 58 people were receiving iron supplement, which was taken into account in data analysis. Among the subjects, 497 (31%) elderly were anemic, with a frequency of 23.8% and 37.3% among among women and men, respectively (P<0.001). Moreover, 157 (9%) subjects had IDA, with a prevalence of 11.9% among females, and 8.2% among males (P<0.001). Table 2 presents the mean serum level of triglyceride, cholesterol, HDL, and LDL in two groups of anemic and non-anemic. After excluding the subjects who were taking anti-lipid drugs, the mean level of triglyceride in anemic and non-anemic groups was 141.98 mg/dl and 166.11 mg/dl, respectively.
Table 2.
Mean serum lipid profile in anemic (404) and non-anemic (894) elderly groups
| Pvalue |
Non anemic
(Mean±SD) |
Anemic
(Mean±SD) |
Variable |
|---|---|---|---|
| 0.001 | 166.11±87.23 | 141.94±76.85 | Triglyceride(mg/dL) |
| 0.001 | 206.02±40.38 | 187.43±39.25 | Cholesterol (mg/dL) |
| 0.23 | 38.93±4.29 | 38.60±4.55 | HDL (mg/dL) |
| 0.001 | 139.43±44.95 | 121.89±36.54 | LDL (mg/dL) |
The difference between these two groups was statistically significant in this regard (p=0.001). Moreover, serum cholesterol level in anemic group (187.43 mg/dl) was significantly lower than the control (206.02 mg/dl). The mean HDL was almost the same in anemic and non-anemic groups (P=0.23), while the mean LDL in the anemic group was significantly lower than the non-anemic group (P=0.001) (table 3).
Table 3.
Mean lipid profile in elderly with iron deficiency anemia (117) and the control (without iron deficiency anemia) (1176)
| Pvalue |
Control
(mean±SD) |
Iron deficiency anemia
(mean±SD) |
Variable |
|---|---|---|---|
| 0.001 | 158.98±82.15 | 152.18±108.23 | Triglyceride(mg/dL) |
| 0.001 | 200.54±40.03 | 193.21±42.18 | Cholesterol (mg/dL) |
| 0.036 | 38.73±4.6 | 37.03±3.8 | HDL (mg/dL) |
| 0.001 | 134.65±43.21 | 129.19±44.28 | LDL (mg/dL) |
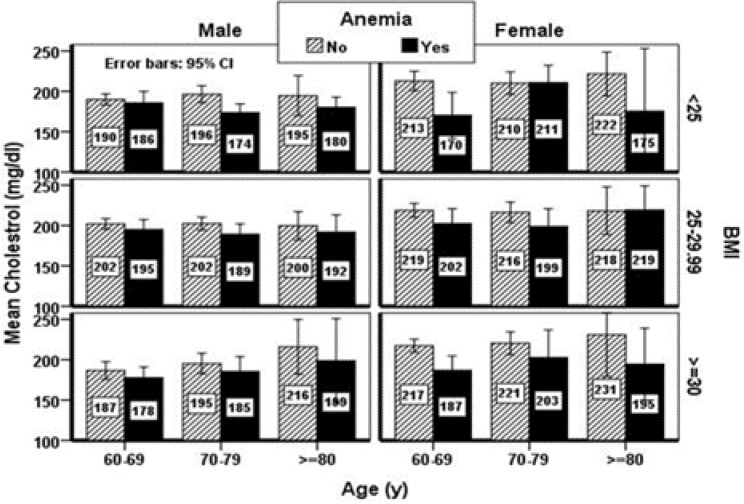
The mean serum cholesterol level in anemic and non-anemic elderly of both genders in different groups of body mass index (BMI) is shown in figure 1. Based on this figure, the prevalence of anemia and the mean level of cholesterol are higher among females. Furthermore, the prevalence of anemia slightly increases with the reduction in BMI in both genders. Hemoglobin level showed a significant relationship with triglyceride, cholesterol, and LDL (p=0.001). In addition, TIBC was found to have a significant relationship with cholesterol, HDL, and LDL, while ferritin was only significantly associated with triglyceride (p=0.002). On the other hand, MVC showed a significant relationship with cholesterol and LDL (p=0.002, 0.004). Age was found to be significantly related to cholesterol and triglyceride (p=0.03, 0.001) (table 4).
Figure 1.
Mean serum level of cholesterol in studied elderly by age, gender, and BMI
Table 4.
Relationship of blood parameters and age with lipid profile
| HDL | Triglyceride | Cholesterol | Hemoglobin | Variable | |
|---|---|---|---|---|---|
| -0.004 0.87 |
0.081 0.001 |
0.103 0.001 |
1 | Correlation covariance P value |
Hemoglobin |
| 0.329 0.001 |
0.370 0.001 |
1 | 0.103 0.001 |
Correlation covariance P value |
Cholesterol |
| 0.111 0.001 |
1 | 0.370 0.001 |
0.081 0.001 |
Correlation covariance P value |
Triglyceride |
| 1 | 0.111 0.001 |
0.329 0.001 |
-0.004 0.87 |
Correlation covariance P value |
HDL |
| 0.198 0.001 |
0.348 0.001 |
0.802 0.011 |
0.091 0.001 |
Correlation covariance P value |
LDL |
| 0.008 0.75 |
0.019 0.44 |
0.108 0.001 |
0.256 0.001 |
Correlation covariance P value |
Serum iron |
| -0.012 0.63 |
0.076 0.002 |
-0.024 0.33 |
0.015 0.54 |
Correlation covariance P value |
Ferritine |
| 0.058 0.02 |
-0.029 0.34 |
-0.093 0.001 |
-0.203 0.001 |
Correlation covariance P value |
TIBC |
| -0.028 0.27 |
-0.022 0.38 |
0.073 0.004 |
0.366 0.001 |
Correlation covariance P value |
MCV |
| -0.018 0.46 |
-0.124 0.001 |
-0.052 0.03 |
-0.160 0.001 |
Correlation covariance P value |
Age |
Discussion
In the present research, the association between triglyceride, cholesterol, HDL, and LDL was studied in the elderly people with anemia and iron deficiency. The results showed that the values of lipid profile including triglyceride and cholesterol in subjects with anemia and IDA were lower than the control. This was also true even after taking into account the effects of age, gender, and BMI.
In the present study, the prevalence of anemia among the ageing individuals was 31%. This figure has been different in various studies. In a study conducted by the Ministry of Health and Medical Education of Iran in December 2004, anemia was observed in 10-11% of the older people (7). In a study conducted in the US, it was reported that nearly 4.7 million Americans suffer from anemia and its prevalence increases with age, as 44.4% of people aged over 85 suffer from it (8). In this study, the prevalence of IDA in the elderly was 9%. About 50% of anemia cases in the world can be attributed to iron deficiency which is responsible for about 841,000 deaths worldwide annually (1).
A study conducted in Iran in 2001 showed that anemia and iron deficiency occurred in the high percentage of the population in different age groups. Accordingly, about 23% of children under 2, 26% of children aged 6, 23% of male and female adolescents, and 43% of pregnant women suffer from iron deficiency (7). The results of the present study confirmed the findings of Au (20) who reported some degree of association of anemia with lower serum levels of cholesterol. El-Hazmiet al. (21) reported that serum level of cholesterol in patients with sickle cell anemia is significantly lower than the control with normal hemoglobin. In the study conducted by Mitrache et al. (22), anemic patients showed a significantly lower level of serum cholesterol. In the present study, serum level of cholesterol and triglyceride in anemic group was significantly lower than non-anemic group. Ohira et al. (23) found that the serum level of cholesterol increases following the increased hemoglobin through blood transfusion. They argued that the amount of red blood cells probably affects cholesterol synthesis or its displacement from tissue to plasma.
The relationship between hypercholesterolemia and anemia and also the effect of anemia on atherosclerosis were reported in some studies (24-26). In a study conducted by Ohiraet al. (28), no significant relationship existed between triglyceride and hemoglobin. By contrast, Choi et al. (27) observed a significant relationship between triglyceride and hemoglobin, which is consistent with the findings of the present study. In a study carried out by Bunyaratvej et al. (28), there was no relationship between MCV and triglyceride, but a significant inverse relationship was noted between MCV and cholesterol. In the present study, MCV showed a significant relationship between cholesterol and LDL in all subjects, but there was no significant relationship between MCV and triglyceride.
Choi et al. (29) reported no significant relationship between MVC and cholesterol among the elderly. Changes in serum level of cholesterol during iron reduction have been different in many studies conducted on animals. The results of the present study were consistent with the findings of Bristow-Craig et al. (18) who showed that consumption of high-iron diet in mice is followed by the elevation of serum cholesterol level. These observations suggest that changes in serum cholesterol level is associated with age, gender, diet, and animal model studies.
By contrast, Stangle and Kirchgessner (30) reported that hypertriglyceridemia in mice is associated with low-iron diets. Guthrie et al. (31) and Amine et al. (32) mentioned that anemia is caused by low-iron diets, it leads to hyperlipidemia, which is inconsistent with the findings of the present study.
On the other hand, Ece et al. (33) noted that iron deficiency has no effect on lipid profile. Since low-iron diets cause loss of energy and protein and thereby lead to a hypocaloric diet, it causes hyperlipidemia. Dabbagh et al. (25) showed that high iron increases serum level of HDL in mice. Nevertheless, in another study, they did not confirm the hypothesis that increased iron reserves increase the risk of coronary artery disease. In the present study, there was significant difference between iron deficiency anemia group and the control in terms of HDL level. One of the main strengths of the present study was the considerable number of subjects and its major weakness was its cross-sectional method which lowers the power of assessment of causal relationship. One of the constraints of this research was the non-delivery or not mentioning the name of drugs related to anemia and hyperlipidemia by the subjects.
In conclusion, the results of the present study showed that the values of lipid profile including triglyceride and cholesterol in subjects with anemia and iron deficiency anemia were lower than the control, which is consistent with the findings of some other studies. Nonetheless, to identify the possible mechanisms, further studies are recommended to be carried out.
Acknowledgments
The authors would like to thank the elderly participants and our associates for their kind cooperation.
Funding:
This research was financially supported by the Department for Research and Technology, Babol University of Medical Sciences.
Conflict of Interest:
None
References
- 1.Longo DL, Fauci AS, Kasper DL, et al. Harrison’ principles of internal medicine (hematology disorders) 18th ed. New York: McGraw Hill ; 2011. pp. 212–15. [Google Scholar]
- 2.Samadpour K, Sheikholeslam R, Abodollahi Z, Salehi F, Mazandarani F. The effect of weekly dose of iron supplementation for 16 and 20 week on the iron status of adolescent girls in Iran. Asia Pac J Clin Nutr. 2004;13:S135. [Google Scholar]
- 3.Bijani A, Ghadimi R, Mikaniki E, et al. Cohort Profile Update: The Amirkola Health and Ageing Project (AHAP) Caspian J Intern Med. 2017;8:205–12. doi: 10.22088/cjim.8.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S, Chen XY, Xu XP. The relationship between lipoprotein-associated phospholipase A (2), cholesterylester transfer protein and lipid profile and risk of atherosclerosis in women with iron deficiency anaemia. Clin Lab. 2015;61:1463–9. doi: 10.7754/clin.lab.2015.150202. [DOI] [PubMed] [Google Scholar]
- 5.Zaribaf F, Entezari MH, Hassanzadeh A, Mirzaian S. Association between dietary iron, iron stores, and serum lipid profile in reproductive age women. J Educ Health Promot. 2014;3:15. doi: 10.4103/2277-9531.127586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emokpae A, Kuliya-Gwarzo A. The influence of decreased levels of high density lipoprotein cholesterol on hematological indices in sickle cell disease patients. Ann Med Health Sci Res. 2014;4:157–61. doi: 10.4103/2141-9248.129020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iran’s Ministry of health and medical education. Islamic Republic of Iran’s fourth economic, social and cultural development plan. 2004. Available at: http://global-disease-burden.healthgrove.com/l/23481/Iron-Deficiency-Anemia-in-Iran.
- 8.Brill JR, Baumgardner DJ. Normocytic anemia. Am Fam Physician. 2000;62:2255–64. [PubMed] [Google Scholar]
- 9.Ashar S, Sultan S, Irfan SM, Sheeraz A. Serum fasting lipid profile in children and adolescents with β-thalassaemia majorin southern Pakistan. Malays J Pathol. 2015;37:233–8. [PubMed] [Google Scholar]
- 10.Sotuneh N, Hosseini SR, Shokri-Shirvani J, Bijani A, Ghadimi R. Helicobacter pylori infection and metabolic parameters: is there an association in elderly population? Int J Prev Med. 2014;5:1537–42. [PMC free article] [PubMed] [Google Scholar]
- 11.Qin Y, Melse-Boonstra A, Pan X, et al. Anemia in relation to body mass index and waist circumference among Chinese women. Nutr J. 2013;12:10. doi: 10.1186/1475-2891-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ausk KJ, Ioannou GN. Is obesity associated with anemia of chronic disease? Obesity (Silver Spring) 2008;16:2356–61. doi: 10.1038/oby.2008.353. [DOI] [PubMed] [Google Scholar]
- 13.Winther SA, Finer N, Sharma A, Trop-Pedersen C, Anderson C. Association of anemia with the risk factor of cardiovascular adverse events in overweight/obese patients. Int J Obes. 2014;38:432–7. doi: 10.1038/ijo.2013.111. [DOI] [PubMed] [Google Scholar]
- 14.Avila F, Echeverría G, Pérez D, et al. Serum ferritin is associated with metabolic syndrome and red meat consumption. Oxid Med Cell Longev. 2015;2015:769739. doi: 10.1155/2015/769739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guglielmi V, D'Adamo M, Bellia A, et al. Iron status in obesity: An independent association with metabolic parameters and effect of weight loss. Nutr Metab Cardiovasc Dis. 2015;25:541–7. doi: 10.1016/j.numecd.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Bristow-Craig HE, Strain JJ, Welch RW. Iron status, blood lipids and endogenous antioxidants in response to dietary iron level in male and female rats. Int J Vit Nutr Res. 1994;64:324–9. [PubMed] [Google Scholar]
- 17.Verma U, Shankar N, Madhu SV, et al. Relationship between iron deficiency anaemia and serum lipid levels in Indian adults. J Indian Med Assoc. 2010;108:555–8. [PubMed] [Google Scholar]
- 18.Ahmed U, Latham PS, Oates PS. Interactions between hepatic iron and lipid metabolism with possible relevance to steatohepatitis. World J Gastroenterol. 2012;18:4651–8. doi: 10.3748/wjg.v18.i34.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosseini SR, Cumming RG, Kheirkhah F, et al. Cohort profile: The Amirkola health and aging project. Int J Epidemiol. 2014;43:1393–400. doi: 10.1093/ije/dyt089. [DOI] [PubMed] [Google Scholar]
- 20.Kaluza J, Madej D. Effect of iron and zinc supplementation and its discontinuation on lipid profile in rats. J Trace Elem Med Biol. 2014;28:298–302. doi: 10.1016/j.jtemb.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 21.El-Hazmi MAF, Jabbar FA, Warsy AS. Cholesterol and triglyceride level in patients with sickle cell anaemia. Scan J Clin Lab Invest. 1987;47:351–4. [PubMed] [Google Scholar]
- 22.Mitrache C, Passweg JR, Libura J, et al. Anemia: an indicator for malnutrition in the elderly. Ann Hematol J. 2001;80:295–8. doi: 10.1007/s002770100287. [DOI] [PubMed] [Google Scholar]
- 23.Ohira Y, Edgerton VR, Gardner GW, Senewiratne B. Serum lipid levels in iron deficiency anemia and effects of various treatments. J Nutr Sci Vitaminol. 1980;26:375–9. doi: 10.3177/jnsv.26.375. [DOI] [PubMed] [Google Scholar]
- 24.Bari MA, Rahman MA. Effect of iron deficiency anemia on the development of atherosclerosis in chicks. Atherosclerosis. 1975;21:401–8. doi: 10.1016/0021-9150(75)90052-0. [DOI] [PubMed] [Google Scholar]
- 25.Dabbagh AJ, Mannion T, Lynch SM, Frei B. The effect of iron overload on rat plasma and liver oxidant status in vivo. Biochem J. 1994;300:799–803. doi: 10.1042/bj3000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dabbagh AJ, Shwaery GT, Keaney JF Jr, Frei B. Effect of iron overload and iron deficiency on atherosclerosis in the hypercholesterolemic rabbit. Arterioscler Thromb Vasc Biol. 1997;17:2638–45. doi: 10.1161/01.atv.17.11.2638. [DOI] [PubMed] [Google Scholar]
- 27.Choi J, Kim S, Pai S. Changes in serum lipid concentrations during iron depletion and after iron supplementation. Ann Clin Lab Sci J. 2001;31:151–6. [PubMed] [Google Scholar]
- 28.Bunyaratvej P, Komindr S, Wisedpanichkij R. Different reticulocyte volume in diabetes mellitus patients with and without hypercholesterolemia and/or hypertriglyceridemia. J Med Assoc Thail. 2000;83:790–6. [PubMed] [Google Scholar]
- 29.Choi JW, Pai SH. Influences of hypercholesterolemia on red cell indices and erythrocyte sedimentation rate in elderly persons. Clin Chim Acta. 2004;341:117–21. doi: 10.1016/j.cccn.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Stangl GI, Kirchgessner M. Different degrees of moderate iron deficiency modulate lipid metabolism of rats. Lipids. 1998;33:889–95. doi: 10.1007/s11745-998-0285-8. [DOI] [PubMed] [Google Scholar]
- 31.Guthrie HA, Froozani M, Sherman AR, Barron GP. Hyperlipidemia in offspring of iron-deficient rats. J Nutr. 1974;104:1273–8. doi: 10.1093/jn/104.10.1273. [DOI] [PubMed] [Google Scholar]
- 32.Amine EK, Desilets EJ, Hegsted DM. Effect of dietary fats on lipogenesis in iron deficiency anemic chicks and rats. J Nutr. 1976;106:405–11. [Google Scholar]
- 33.Ece A, Yigitoglu MR, Vurgun N, Guven H, Iscan A. Serum lipid and lipoprotein profile in children with iron deficiency anemia. Pediatr Int. 1999;41:168–73. doi: 10.1046/j.1442-200x.1999.4121036.x. [DOI] [PubMed] [Google Scholar]