Abstract
Despite the benefits associated with the use of food waste (FW), there are mixed consumer perceptions regarding pork quality harvested from pigs fed FW. Twenty crossbred pigs were selected for the present study. Ten pigs were fed a conventional diet (control group), and the other 10 pigs were given a conventional diet and FW (FW group) during different growth stages. Meat quality in the FW group showed deteriorative qualities with higher lightness and yellowness synonymous to pale soft exudative meat. Drip loss in the experimental group was significantly higher than that in the control group (p<0.01). The contents of polyunsaturated fatty acids in the FW group were higher and those of saturated and monounsaturated fatty acids were lower than those in the control group. The contents of thiobarbituric acid were significantly different between the control and FW groups (p<0.05). There was also a significant difference between the control and FW groups in terms of off-flavor (p<0.05) after sensory evaluation. To conclude, the off-flavor noted, including other inferior pork quality traits, in the FW group implies that FW should not be used as swine feed.
Keywords: food waste, meat quality, polyunsaturated fatty acids, thiobarbituric acid
Introduction
Feed costs constitute approximately 60-70% of the total cost of pig production (AHDB Market Intelligence, 2014). Increasing feed costs have a negative impact on profit margins. Conventional monogastric animal diets are composed of corn and soybean as sources of energy and protein, respectively. Corn is the most preferred energy source for swine feed owing to its good feeding value (Stein et al., 2009). In recent years, corn and soybean prices have been gradually increasing due to the increased demand by pig producers as they attempt to satisfy their growing market base exacerbated by population growth (McGlone, 2013). Some countries use corn as their staple food, resulting in competition between animals and people, thus further increasing demand (Robinson and Pozzi, 2011).
Food waste (FW) is defined as any edible material or byproduct generated in the production, processing, transportation, distribution, or consumption of food (Westendorf et al., 1996). The main sources of FWs are plate and kitchen wastes from restaurants, institutions, schools, and military camps (Westendorf et al., 1998). FW is a common issue in many countries, particularly in areas adjacent to major metropolitan areas (Aschemann-Witzel et al., 2015). The successful use of FWs as feed can be beneficial to the environment. Feeding FWs to pigs to increase their energy content is a usual practice in most countries rather than a novel innovation (Rohella et al., 2015). However, FWs show considerable variations in chemical composition and nutritional value. FWs generally contain more fat and salt contents than conventional feeds (Murto et al., 2004). Pigs exposed to high fat and salt rations are likely to yield pork that has a soft texture and that is quick to get rancid (Matlock et al., 1984).
Although issues have been raised regarding the advantages and disadvantages of using FWs as an alternative source of animal feed, further studies are required on this subject because food resources continue to diminish due to the growing human population. Therefore, the present study was designed to investigate the influence of FWs as swine feed on carcass traits, meat quality, and fat characteristics including rancidity during storage.
Materials and Methods
Animals and diets
Totally, 20 crossbred pigs (Landrace × Yorkshire × Duroc) were used in the experiment for 18 wk (6 wk for the growing period and 12 wk for the finishing period). The pigs in the control group (n=10) were fed a standard commercial diet (CON) that met the nutrient requirements of growing-finishing pigs provided by the National Research Council (National Research Concil, 2012) during the growing-finishing period. The experimental pigs (n=10) were fed FW and CON (FW group); FW was given during the growing-finishing period and then CON for 4 wk before slaughtering. FW used in the present study was collected from different restaurants located in Gyeonggi-do. Inedible materials (e.g., toothpicks, napkins, or vinyl bags) were removed from FW prior to boiling it once for preventing food-borne pathogens and as a precaution against other sanitation issues before providing it to the pigs. FW comprised only FW without any other diet. FW used during the experiment was analyzed for moisture, ash, and crude protein contents through proximate analysis (Gonçalves et al., 2010).
After chilling at 2°C for 24 h, the right carcass side was weighed and midline back fat thickness was measured at the first rib, 10th rib, last rib, and last lumbar vertebra. Moisture, ash, crude protein, and total lipid contents were determined using proximate analysis (Gonçalves et al., 2010).
Proximate analysis of feed and pork
Measurement of moisture
Moisture content was determined according to AOAC (2000). Pork strips and feed samples were dried in an oven at 102°C for 24 h, and the total moisture content of individual pork strips and feed samples was determined by dry weights expressed as a percentage of pre-dry weights.
Measurement of ash
Ash content was determined by the gravimetric method as described by AOAC (2000) using a muffle furnace. Fresh minced meat sample (5 g) and feed (5 g) were transferred in pre-weighed crucibles into a muffle furnace at 550°C for 4-5 h. Sample ashes were transferred to a desiccator containing silica gel as a desiccant. After 1 h, the dishes were weighed, and the ash content was expressed as a percentage of the sample weight.
Measurement of crude protein
Muscle and feed samples to be analyzed were weighed into individual digestion flasks and then digested by heating in the presence of sulfuric acid, anhydrous sodium sulfate, and a copper catalyst to speed up the reaction. Nitrogen in the samples was converted to ammonia during the process. After digestion was complete, the digestion flask was connected to a receiving flask by a tube. The solution in the digestion flask was then made alkaline by the addition of sodium hydroxide, which converted ammonium sulfate to ammonia gas. The ammonia gas formed was liberated from the solution into the receiving flask containing excess boric acid. The low pH of the solution in the receiving flask converted ammonia gas to ammonium ions. The nitrogen content was then estimated by titrating ammonium borate formed with standard sulfuric or hydrochloric acid, using a suitable indicator to determine the endpoint of the reaction. Once the nitrogen content had been determined, it was converted to protein content using the appropriate conversion factor (6.25).
Measurement of total lipids in feed
Three test tubes for each diet, FW and CON, were dried in an oven at 60°C for 3 h. One gram of the respective diets was added to each tube, and 2 mL of Folch solution was added for lipid extraction (Folch et al., 1957). The tubes were then gently rotated for 30 min at room temperature, followed by filtering the mixture into new preweighed tubes. A solution of 0.74% KCl was added to each of the six tubes; these tubes were later vortexed and centrifuged at 1200 rpm. The resulting supernatant after centrifugation was aspirated, and tubes containing the lower phase were placed in a nitrogen evaporator for 10 min. The weight of the lipids per gram of each diet was determined as the average of the tube weights with dried lipids.
Measurement of Ca and P
One gram of each diet, FW and CON, was incubated to ashes in an oven at 550°C overnight, digested with 2 mL of 16 M HNO3 for 30 min, and transferred to 1000 mL volumetric flasks pre-cleaned with 0.2 N HNO3 overnight. Distilled water (H2O) was used to fill up the volumetric flasks with the digested ash. Ca and P contents were determined using inductively coupled plasma optical emission spectrometry (Varian Vista MPX; Varian, USA) with a 1000-ppm standard solution.
Meat quality measurements
pH
Muscle pH at 45 min and 24 h postmortem was directly measured at the seventh and eighth thoracic vertebrae of each cold carcass using a portable meat pH meter (Testo 206-pH2 meter; Testo AG, Germany).
Salinity
Salinity was measured using a digital salinity meter (TM-30D; Takemura Electric Works Ltd., Japan).
Meat color
Meat color was measured using a Minolta chroma meter (CR-400; Minolta Co., Japan), following a slightly modified version of the method by Honikel (1998). The samples were cut from the pork loin at 24 h postmortem and were placed on a table for 30 min, exposing their surfaces to air without any packaging before measuring meat color. The average of triplicate measurements was recorded, and the results were expressed as Commission Internationale de l'Eclairage (CIE) lightness (L*), redness (a*), and yellowness (b*).
Water holding capacity
To evaluate water holding capacity, drip loss, cooking loss, and filter-paper fluid uptake (FFU) were measured using the method by Honikel (1998). To determine drip loss, meat samples were cut from the pork loin at 24 h postmortem and immediately weighed (initial weight for drip loss). The samples were placed in a netting and suspended in an inflated bag, ensuring that the samples were not in contact with the bag. After a 48-h storage period at 4°C, the samples were extracted from the bag, gently blotted dry, and re-weighed. Drip loss was expressed as a percentage of the final sample weight after dripping over the initial sample weight.
To measure cooking loss, different samples were freshly cut from the pork loin at 24 h postmortem and weighed (initial weight for cooking loss). The samples were put in thin-walled polyethylene bags and then placed in a continuously boiling water bath. Then, the samples were cooked at 75°C internal temperature. When the endpoint temperature was reached, the bags were removed from the water bath. Thereafter, the samples were cooled in ice slurry and kept under chilled conditions (1-5°C) until equilibration. The samples were then removed from the bag, blotted dry, and re-weighed. Cooking loss was expressed as a percentage of the final sample weight after cooking over the initial sample weight.
To determine FFU, different samples were cut from the pork loin at 24 h postmortem. A filter paper (Whatman #2, 42.5 mm in diameter) was pre-weighed, placed on the surface of the sample for less than 2 s to absorb fluids, and re-weighed. FFU was expressed as milligrams of exudate absorbed onto the filter paper.
Shear force
To measure shear force, the pork loin was cut into 20-mm thick chops. Pork chops from each sample were cooked to a final core temperature of 75°C in a continuously boiling water bath. After cooking, six cores (1.27 cm diameter) parallel to the longitudinal orientation of the muscle fibers were extracted from each pork chop for Warner-Bratzler shear (WBs) force measurements. WBs force was determined using an Instron Universal Testing Machine (Model Series IX; Instron Corp., USA) equipped with a Warner-Bratzler shearing device. The samples were sheared perpendicular to the long axis of the core, and the WBs force was the peak force of the curve (Honikel, 1998).
Fatty acid composition and lipid peroxidation
Lipids were extracted, and the content was determined using the modified Folch method (Folch et al., 1957). Extracted lipids were used for determining the fatty acid composition after measuring total lipid content (%). Fatty acids were methylated using the method by Morrison and Smith (1964). Fatty acid methyl esters (FAMEs) were analyzed by gas chromatography-flame ionization detector (7820A; Agilent, USA). The separation of FAMEs was accomplished on a fused silica capillary column (30 m × 0.25 mm ID, Supelco, USA) with helium as the carrier gas (split ratio = 1:100, flow rate = 1.7 mL/min). Standard polyunsaturated fatty acid (PUFA) No2 analytical standard from Sigma-Aldrich was used to identify each peak.
Lipid peroxidation of the pork loin from each group was determined using thiobarbituric acid (TBA) distillation (Yang et al., 2009). Lipid peroxidation was stimulated in the presence of 50 mM H2O2, and the reaction was terminated by placing homogenates in ice cold water for 10 min. Malondialdehyde contents in the supernatant were measured by TBA reactive substance (TBARS) and expressed as nanomoles per milligram of protein. The absorbance of TBARS was measured at 532 nm using a spectrophotometer (Synergy HT; BioTek, Korea).
Sensory evaluation
For the sensory evaluation of pork loin, 10 panelists were selected and trained in accordance with previous methods (AMSA, 1995; Peachey et al., 2002), and each pork loin sample was evaluated twice. A total of 95 testing sessions were performed with eight samples evaluated per session. Two steaks of 20 mm thickness were cut from each pork loin at 24 h postmortem without visible fat and connective tissue and stored at −20°C until evaluation. The samples were thawed overnight at 4°C and then cooked at 180°C without salt or spices in a humid oven (Hauzen HS-XC364AB; Samsung, Korea). The samples were cooked until an internal temperature of 75°C was reached, as measured by a thermometer (TES-1300; TES Electrical Electronic Co., Taiwan).
The cooked samples were immediately cut into 15 mm cubes and submerged in a water bath (54°C) until they were served to the panelists. Each sample was served in a lidded cup labeled with a three-digit random code. There was a 5-min interval between the evaluations of each sample. The panelists were instructed to cleanse their palate with distilled water (30°C) and salt-free crackers between the samples. Testing took place in individual booths under white light.
The definitions and score distributions for sensory attributes were as follows: juiciness, amount of moisture released after 10 chews (1 = very dry, 5 = very juicy); tenderness, force required to chew 15× for swallowing at a constant rate (1 = very tough, 5 = very tender); flavor, intensity of pork flavor after 20 chews (1 = no pork flavor, 5 = full of pork flavor); off-flavor, intensity of any flavor or after-taste perceived as inappropriate for cooked pork (1 = very weak, 5 = very strong); and overall acceptability and overall satisfaction of juiciness, tenderness, flavor, and off-flavor (1 = very bad, 5 = very good).
Statistical analysis
Comparisons between the two groups were performed using Student’s t-test with the SAS (2013) program. Data are expressed as mean±standard deviation, and statistical significance was defined as p<0.05.
Results and Discussion
Composition analysis of the diets
The chemical composition of CON and FW is shown in Table 1. FW contained slightly more crude ash and crude protein than CON. Water, calcium, and phosphorus contents were similar between the two diets. Amino acid profiles are shown in Table 2. Some amino acids including threonine and valine in FW were higher than those in CON. However, no large differences were found in the contents of major amino acids such as methionine, cysteine, and lysine, which are the first limiting amino acid for pigs (Liao et al., 2015). The fatty acid composition of FW was significantly different than that of CON (Table 3). FW contained higher contents of PUFA and P:S ratio (all p<0.001), whereas lower contents of saturated fatty acid (SFA) (p<0.001) and monounsaturated fatty acid (MUFA) (p<0.05) than CON. Higher contents of PUFA in FW may have been due to a pattern change in the consumption of human food. During the last decade, humans have been consuming PUFAs more frequently than SFAs in most countries by increasing their intake of vegetable and fish oil rather than of animal fat (Micha et al., 2014).
Table 1. Chemical composition of control and food waste diets.
| CON1) | FW2) | |
|---|---|---|
| Water (%) | 12.02 | 13.29 |
| Crude ash (%) | 6.30 | 9.04 |
| Crude protein (%) | 20.21 | 26.59 |
| Calcium (%) | 1.05 | 0.85 |
| Phosphorus (%) | 0.68 | 0.58 |
| Total lipids (%) | 15.67 | 7.33 |
| NaCl (%) | 0.8 | 4.6 |
1)Conventional diet
2)Food waste diet
Table 2. Amino acid profiles of control and food waste diets.
| CON1) | FW2) | |
|---|---|---|
| ASP | 2.44 | 2.42 |
| SER | 0.94 | 1.14 |
| GLU | 4.22 | 4.75 |
| GLY | 0.77 | 1.55 |
| HIS | 0.54 | 0.81 |
| ARG | 1.19 | 1.30 |
| THR | 0.77 | 1.21 |
| ALA | 0.98 | 1.95 |
| PRO | 1.11 | 1.83 |
| TYR | 0.74 | 1.16 |
| VAL | 0.90 | 1.34 |
| LYS | 1.40 | 1.43 |
| ISO | 0.84 | 1.16 |
| LEU | 1.54 | 1.92 |
| PHE | 0.94 | 1.40 |
| MET | 0.40 | 0.52 |
| CYS | 0.37 | 0.34 |
1)Conventional diet
2)Food waste diet
Table 3. Fatty acid composition of control and food waste diets.
| CON1) | FW2) | p-value | |
|---|---|---|---|
| C14:0 | 2.20 ± 0.01 | 2.96 ± 0.06 | 0.002 |
| C16:0 | 23.54 ± 0.04 | 22.33 ± 0.10 | <0.001 |
| C16:1 | 2.49 ± 0.02 | 3.02 ± 0.10 | <0.001 |
| C18:0 | 11.27 ± 0.23 | 8.91 ± 0.10 | <0.001 |
| C18:1n9 | 36.89 ± 0.41 | 34.91 ± 0.03 | 0.014 |
| C18:1n7 | 2.58 ± 0.05 | 2.80 ± 0.04 | 0.004 |
| C18:2n6 | 19.50 ± 0.49 | 20.46 ± 0.20 | 0.035 |
| C18:3n3 | 1.28 ± 0.02 | 2.38 ± 0.04 | <0.001 |
| C20:4n6 | N.D. | N.D. | - |
| C20:5n3 | N.D. | 0.31 ± 0.54 | - |
| C22:6n3 | 0.25 ± 0.22 | 1.93 ± 0.14 | <0.001 |
| SFA | 37.01 ± 0.24 | 34.20 ± 0.11 | <0.001 |
| MUFA | 41.95 ± 0.46 | 40.72 ± 0.14 | 0.011 |
| PUFA | 21.04 ± 0.70 | 25.08 ± 0.24 | <0.001 |
| P:S ratio | 0.57 ± 0.02 | 0.73 ± 0.01 | <0.001 |
| n3 FA | 1.54 ± 0.21 | 4.62 ± 0.40 | <0.001 |
| n6 FA | 19.50 ± 0.49 | 20.46 ± 0.20 | 0.035 |
| n6:n3 ratio | 12.87 ± 1.62 | 4.46 ± 0.41 | <0.001 |
Data are shown as mean ± standard deviation (n=3).
1)Conventional diet
2)Food waste diet
SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; P:S ratio: PUFA/SFA; n3 FA: C18:2n3+C20: 5n3+C22:6n3; n6 FA: C18:2n6+C20:4n6; n6:n3 ratio: n6 FA/n3 FA
Carcass and meat quality characteristics
FW feeding did not affect carcass characteristic including carcass weight and back fat thickness (Table 4). Previous studies similarly showed that there were no carcass quality differences between pigs fed a typical diet and those fed dried FW, regardless of FW content percentage (Chae et al., 2000; Kjos et al., 2000).
Table 4. Carcass characteristics of pigs fed control and food waste diets.
| CON1) | FW2) | p-value | |
|---|---|---|---|
| Carcass weight (kg) | 80.20 ± 5.73 | 79.70 ± 4.40 | 0.829 |
| Back fat thickness (mm) | 21.00 ± 3.30 | 19.10 ± 3.70 | 0.241 |
Data are shown as mean ± standard deviation (n=10 per group).
1)Conventional diet
2)Food waste diet
FW had an influence on the proximate composition of meat and meat quality (Table 5). Pork loins in the FW group contained more NaCl (p<0.05) than those in the control group. This might have been due to the composition of FW. Diets of humans generally contain more sodium than those of livestock.
Table 5. Proximate composition and NaCl concentration of the pork loin from pigs fed control and food waste diets.
| CON1) | FW2) | p-value | |
|---|---|---|---|
| Water (%) | 76.59 ± 0.85 | 76.10 ± 1.28 | 0.339 |
| Crude ash (%) | 1.21 ± 0.21 | 1.24 ± 0.15 | 0.735 |
| Crude protein (%) | 20.04 ± 0.97 | 19.40 ± 0.93 | 0.168 |
| Triglyceride (%) | 3.39 ± 0.60 | 3.12 ± 0.67 | 0.376 |
| NaCl (arbitrary unit) | 1.00 ± 0.10 | 1.14 ± 0.14 | 0.027 |
Data are shown as mean ± standard deviation (n=10 per group).
1)Conventional diet
2)Food waste diet
A high content of NaCl in FW was confirmed in the present study, and it might have an impact on higher NaCl content in pork loins compared with the lower content of NaCl in CON and pork loins in the control group. On the other hand, the requirement of Na in the diets of growingfinishing pigs is only 0.1% (National Research Council, 2012). Therefore, the result in the present study imply that the higher content of NaCl in FW than in CON influenced the resultant high salt content of pork loins in the FW group. The mechanism underlying the relationship between high NaCl intake and high NaCl content in the muscle/meat still remains unclear (Karppanen and Mervaala, 2006).
Meat quality traits are shown in Table 6. Muscle pH at 24 h in the FW group was significantly lower (p<0.05) than that in the control group. Postmortem muscle pH is a very important factor because it has significant impacts on other meat quality characteristics such as meat color and water holding capacity (Apple et al., 2005), showing the relationships between low ultimate muscle pH and inferior meat quality traits (Ryu and Kim, 2005). Based on the results of the present study, lower ultimate muscle pH at 24 h in the FW group seemed to influence other meat quality traits, particularly meat color and water holding capacity, compared with higher ultimate muscle pH in the control group. The pork loins in the FW group showed higher lightness and yellowness (p<0.05) as well as cooking loss (p<0.01). In addition, drip loss and WBs force in the FW group tended to be increased (p<0.1) compared with those in the control group. Values for lightness and drip loss in the FW group met the criteria of pale, soft, and exudative meat (lightness > 50 and drip loss > 5%; Warner et al., 1997). Therefore, pigs fed FW displayed undesirable meat quality characteristics.
Table 6. Meat quality traits of the pork loin from pigs fed control and food waste diets.
| CON1) | FW2) | p-value | |
|---|---|---|---|
| Muscle pH | |||
| pH at 45 min postmortem | 6.05 ± 0.17 | 6.03 ± 0.15 | 0.718 |
| pH at 24 h postmortem | 5.58 ± 0.10 | 5.50 ± 0.05 | 0.043 |
| Meat color | |||
| Lightness (L*) | 49.99 ± 3.18 | 53.15 ± 3.37 | 0.044 |
| Redness (a*) | 4.90 ± 1.05 | 5.78 ± 0.98 | 0.068 |
| Yellowness (b*) | 1.51 ± 0.86 | 2.91 ± 1.37 | 0.018 |
| Water holding capacity | |||
| FFU (mg) | 85.39 ± 26.2 | 94.46 ± 22.2 | 0.415 |
| Drip loss (%) | 5.79 ± 2.69 | 7.84 ± 1.91 | 0.066 |
| Cooking loss (%) | 28.36 ± 1.65 | 31.33 ± 1.91 | 0.002 |
| WBs (N) | 33.69 ± 5.30 | 39.23 ± 7.41 | 0.071 |
Data are shown as mean ± standard deviation (n=10 per group).
1)Conventional diet
2)Food waste diet
FFU, filter-paper fluid uptake; WBs, Warner-Bratzler shear force
Fatty acid composition and TBARS
The fatty acid composition of pork is widely accepted to vary in diets. In monogastric animals, the proportion of certain fatty acids increases linearly as the dietary intake increases (Wood et al., 2008). The result was also confirmed in the present study as the fatty acid composition of loins and back fat showed a positive correlation with diets (Tables 7 and 8). PUFA contents (p<0.001) and P:S ratios (p<0.01) were significantly higher, whereas SFA (p<0.01) and MUFA (p<0.001) contents were significantly lower in loins from the FW group than those from the control group. A similar tendency was observed for fatty acid profiles from back fat in the FW group, except for SFA. With respect to consumers, increased PUFA and decreased SFA contents of meat and back fat are desirable due to their physiological effects such as prevention of cardiovascular diseases. On the other hand, the meat processing industry does not prefer meat with lower contents of SFA, particularly bacon, because a lower degree of saturated fats is closely associated with processing difficulties such as handling and slicing, unlike meat with higher contents of saturated fats (Choe et al., 2015).
Table 7. Fatty acid composition of the pork loin from pigs fed control and food waste diets.
| CON1) | FW2) | p-value | |
|---|---|---|---|
| C14:0 | 1.49 ± 0.13 | 1.41 ± 0.10 | 0.185 |
| C16:0 | 24.97 ± 0.57 | 22.24 ± 2.65 | 0.015 |
| C16:1 | 2.70 ± 0.33 | 2.03 ± 0.33 | <0.001 |
| C18:0 | 13.58 ± 0.62 | 13.38 ± 0.95 | 0.596 |
| C18:1n9 | 38.30 ± 1.47 | 35.47 ± 2.00 | 0.003 |
| C18:1n7 | 3.75 ± 0.11 | 3.47 ± 0.18 | <0.001 |
| C18:2n6 | 12.68 ± 1.09 | 17.91 ± 2.69 | <0.001 |
| C18:3n3 | 0.61 ± 0.09 | 1.17 ± 0.28 | <0.001 |
| C20:4n6 | 1.60 ± 0.32 | 1.77 ± 0.51 | 0.382 |
| C20:5n3 | 0.09 ± 0.03 | 0.44 ± 0.26 | 0.004 |
| C22:6n3 | 0.23 ± 0.04 | 0.71 ± 0.41 | 0.009 |
| SFA | 40.04 ± 0.96 | 37.04 ± 2.68 | 0.010 |
| MUFA | 44.75 ± 1.82 | 40.96 ± 2.01 | <0.001 |
| PUFA | 15.21 ± 1.38 | 22.00 ± 3.90 | <0.001 |
| P:S ratio | 0.38 ± 0.03 | 0.60 ± 0.16 | 0.003 |
| n3 | 0.94 ± 0.12 | 2.32 ± 0.89 | 0.002 |
| n6 | 14.28 ± 1.26 | 19.68 ± 3.10 | <0.001 |
| n6:n3 ratio | 15.34 ± 1.00 | 9.48 ± 3.44 | <0.001 |
Data are shown as mean ± standard deviation (n=3).
1)Conventional diet
2)Food waste diet
SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; P:S ratio: PUFA/SFA; n3 FA: C18:2n3+C20: 5n3+C22:6n3; n6 FA: C18:2n6+C20:4n6; n6:n3 ratio: n6 FA/n3 FA
Table 8. Fatty acid composition of the back fat from pigs fed control and food waste diets .
| CON1) | FW2) | p-value | |
|---|---|---|---|
| C14:0 | 1.73 ± 0.18 | 1.66 ± 0.30 | 0.556 |
| C16:0 | 24.18 ± 2.80 | 21.79 ± 1.63 | 0.039 |
| C16:1 | 2.40 ± 0.24 | 1.93 ± 0.26 | <0.001 |
| C18:0 | 10.30 ± 2.54 | 10.48 ± 1.44 | 0.855 |
| C18:1n9 | 42.81 ± 2.53 | 40.36 ± 1.30 | 0.018 |
| C18:1n7 | 3.58 ± 0.61 | 2.84 ± 0.26 | 0.004 |
| C18:2n6 | 13.65 ± 1.29 | 18.48 ± 2.06 | <0.001 |
| C18:3n3 | 0.91 ± 0.11 | 1.58 ± 0.50 | 0.004 |
| C20:4n6 | 0.24 ± 0.02 | 0.23 ± 0.05 | 0.546 |
| C20:5n3 | 0.04 ± 0.03 | 0.13 ± 0.08 | 0.012 |
| C22:6n3 | 0.17 ± 0.03 | 0.52 ± 0.24 | 0.002 |
| SFA | 36.21 ± 2.97 | 33.92 ± 2.99 | 0.114 |
| MUFA | 48.79 ± 2.74 | 45.13 ± 1.32 | 0.002 |
| PUFA | 15.01 ± 1.26 | 20.95 ± 2.71 | <0.001 |
| P:S ratio | 0.42 ± 0.07 | 0.63 ± 0.13 | <0.001 |
| n3 | 1.12 ± 0.13 | 2.24 ± 0.76 | 0.002 |
| n6 | 13.89 ± 1.29 | 18.71 ± 2.10 | <0.001 |
| n6:n3 ratio | 12.57 ± 1.81 | 9.30 ± 3.34 | 0.015 |
Data are shown as mean ± standard deviation (n=3).
1)Conventional diet
2)Food waste diet
SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; P:S ratio: PUFA/SFA; n3 FA: C18:2n3+C20: 5n3+C22:6n3; n6 FA: C18:2n6+C20:4n6; n6:n3 ratio: n6 FA/n3 FA
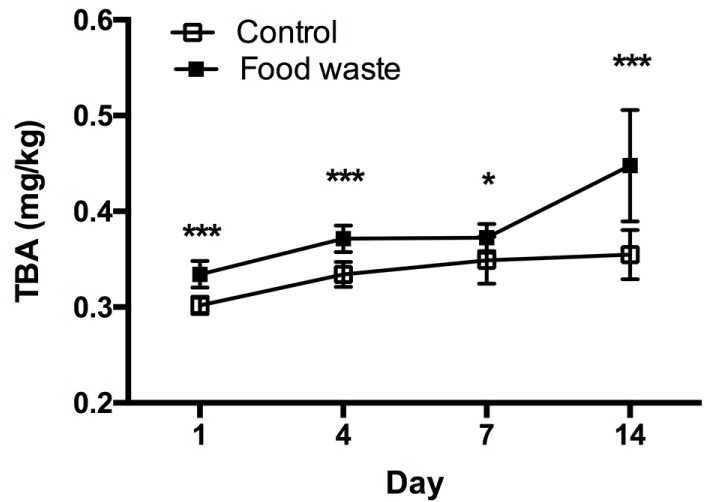
Higher content of PUFA in meat is closely related to shorter storage duration due to increased susceptibility to rancidity. Thus, this study measured the TBA content of pork loins to evaluate the susceptibility to lipid peroxidation. TBA contents in the FW group were significantly higher (p<0.05) than those in the control group during the entire storage period (Fig. 1). These results were contrary to those in a previous study (Kjos et al., 2000) that reported no differences in TBA contents for back fat, though both groups indicated no fresh values. These results suggest that pork loins from pigs fed diets containing FW are highly susceptible to rancidity; hence, they are not suitable for long-time storage.
Fig. 1. Lipid peroxidation of the pork loin from pigs fed control and food waste containing feed.
Sensory evaluation
The sensory evaluations of pork loin from pigs fed CON and FW are shown in Table 9. The off-flavor of pork from the FW group was significantly higher than that from the control group (p<0.05); however, tenderness, juiciness, flavor, and overall acceptability had no significant differences. These findings were contrary to those by Kwak and Kang (2006) who reported no effects of FW on pork flavor at a 50% inclusion level in pig rations. Westendorf et al. (1998) also reported no difference in flavor for pork produced from pigs fed FW and produced from those fed a conventional diet. Therefore, it might be important to consider the composition of FW used in different studies.
Table 9. Sensory evaluation of the pork loin from pigs fed control and food waste containing feed.
| CON1) | FW2) | p-value | |
|---|---|---|---|
| Tenderness | 2.89 ± 0.44 | 2.75 ± 0.44 | 0.4840 |
| Juiciness | 2.29 ± 0.35 | 2.54 ± 0.49 | 0.2077 |
| Flavor | 2.72 ± 0.16 | 2.63 ± 0.21 | 0.2914 |
| Off-flavor | 2.27 ± 0.27 | 2.63 ± 0.36 | 0.0197 |
| Overall acceptability | 2.69 ± 0.31 | 2.51 ± 0.28 | 0.1098 |
Data are shown as mean ± standard deviation (n=10 per group).
1)Conventional diet
2)Food waste diet
Conclusion
The results of the present study showed similar carcass weight and back fat content in the control and FW groups. However, pork finished on FW was not able to match most meat quality parameters shown in the control group as there were negative impacts on pH, color, drip loss, water holding capacity, and shear force. Pork from FW-fed pigs had a short shelf life and a rapid progression of rancidity, which was likely due to the high contents of PUFA. FW pork also showed off-flavor qualities after sensory evaluations more than the CON pork, which may not be good for consumers. Therefore, the use of FW in pig production remains hard to justify given the negative meat quality characteristics observed in the present study.
Further studies on the quantities of FW to incorporate into conventional diets to attain good quality pork might prove useful in the future if that can be achieved at a lower cost than the use of conventional diets.
References
- AHDB Market Intelligence. [Accessed April. 21, 2017];Pig cost of production in selected countries. Available from: http://pork.ahdb.org.uk/media/74797/cost-of-production-web-2014.pdf .
- AMSA. Research guidelines for cookery, sensory evaluation and instrumental tenderness measurements of fresh meat. American Meat Science Association in cooperation with National Live Stock and Meat Board; Chicago, Illinois: 1995. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International 7th ed. Gaithersburg, Maryland: 2000. [Google Scholar]
- Apple J., Kegley E., Maxwell C., Rakes L., Galloway D., Wistuba T. Effects of dietary magnesium and short-duration transportation on stress response, postmortem muscle metabolism, and meat quality of finishing swine. J. Anim. Sci. 2005;83:1633–1645. doi: 10.2527/2005.8371633x. [DOI] [PubMed] [Google Scholar]
- Aschemann-Witzel J., de Hooge I., Amani P., Bech-Larsen T., Oostindjer M. Consumer-related food waste: Causes and potential for action. Sustain. Dev. 2015;7:6457–6477. [Google Scholar]
- Chae B., Choi S., Kim Y., Kim C., Sohn K. Effects of feeding dried food waste on growth and nutrient digestibility in growing-finishing pigs. Asian-Australas. J. Anim. Sci. 2000;13:1304–1308. doi: 10.5713/ajas.2000.1304. [DOI] [Google Scholar]
- Choe J. H., Yang H. S., Lee S. H., Go G. W. Characteristics of pork belly consumption in South Korea and their health implication. J. Anim. Sci. Technol. 2015;57:22. doi: 10.1186/s40781-015-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folch J., Lees M., Sloane G. H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gonçalves B., Borges O., Costa H. S., Bennett R., Santos M., Silva A. P. Metabolite composition of chestnut (Castanea sativa Mill.) upon cooking: Proximate analysis, fibre, organic acids and phenolics. Food Chem. 2010;122:154–160. doi: 10.1016/j.foodchem.2010.02.032. [DOI] [Google Scholar]
- Honikel K. O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998;49:447–457. doi: 10.1016/S0309-1740(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Karppanen H., Mervaala E. Sodium intake and hypertension. Prog. Cardiovasc. Dis. 2006;49:59–75. doi: 10.1016/j.pcad.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kjos N., Øverland M., Bryhni E. A., Sørheim O. Food waste products in diets for growing-finishing pigs: Effect on growth performance, carcass characteristics and meat quality. Acta Agric. Scand. Sect. A-Anim. 2000;50:193–204. [Google Scholar]
- Kwak W. S., Kang J. S. Effect of feeding food waste-broiler litter and bakery by-product mixture to pigs. Bioresour. Technol. 2006;97:243–249. doi: 10.1016/j.biortech.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Liao S. F., Wang T., Regmi N. Lysine nutrition in swine and the related monogastric animals: Muscle protein biosynthesis and beyond. Springerplus. 2015;4:147. doi: 10.1186/s40064-015-0927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlock R., Terrell R., Savell J., Rhee K., Dutson T. Factors affecting properties of raw-frozen pork sausage patties made with various NaCl/phosphate combinations. J. Food Sci. 1984;49:1363–1366. doi: 10.1111/j.1365-2621.1984.tb14991.x. [DOI] [Google Scholar]
- McGlone J. J. The future of pork production in the world: Towards sustainable, welfare-positive systems. Animals (Basel) 2013;3:401–415. doi: 10.3390/ani3020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micha R., Khatibzadeh S., Shi P., Fahimi S., Lim S., Andrews K. G., Engell R. E., Powles J., Ezzati M., Mozaffarian D. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ Open. 2014;5:e008705. doi: 10.1136/bmjopen-2015-008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison W. R., Smith L. M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride methanol. J. Lipid Res. 1964;5:600–608. [PubMed] [Google Scholar]
- Murto M., Björnsson L., Mattiasson B. Impact of food industrial waste on anaerobic co-digestion of sewage sludge and pig manure. J. Environ. Manage. 2004;70:101–107. doi: 10.1016/j.jenvman.2003.11.001. [DOI] [PubMed] [Google Scholar]
- National Research Council. Nutrient requirements of swine. The National Academis Press; Washington DC: 2012. [Google Scholar]
- Peachey, B. M., Purchas R. W., Duizer L. M. Relationships between sensory and objective measures of meat tenderness of beef m. longissimus thoracis from bulls and steers. Meat Sci. 2002;60:211–218. doi: 10.1016/S0309-1740(01)00123-1. [DOI] [PubMed] [Google Scholar]
- Robinson T., Pozzi F. Mapping supply and demand for animal-source foods to 2030. Food and Agriculture Organization; Washington DC, USA: 2011. [Google Scholar]
- Rohella R., Panda S., Das P. S. Hotel food scraps go to the animals-reduces disposal cost and saves environment. Am. J. Environ. Prot. 2015;3:53–59. [Google Scholar]
- Ryu Y. C., Kim B. C. The relationship between muscle fiber characteristics, postmortem metabolic rate, and meat quality of pig longissimus dorsi muscle. Meat Sci. 2005;71:351–357. doi: 10.1016/j.meatsci.2005.04.015. [DOI] [PubMed] [Google Scholar]
- SAS. SAS/STAT Software for PC. Release 9.4. SAS Institute Inc.; Cary, NC, USA: 2013. [Google Scholar]
- Stein H. H., Connot S., Pedersen C. Energy and nutrient digestibility in four sources of distillers dried grains with solubles produced from corn grown within a narrow geographical area and fed to growing pigs. Asian-Australas. J. Anim. Sci. 2009;22:1016–1025. doi: 10.5713/ajas.2009.80484. [DOI] [Google Scholar]
- Warner R., Kauffman R., Greaser M. Muscle protein changes post mortem in relation to pork quality traits. Meat Sci. 1997;45:339–352. doi: 10.1016/S0309-1740(96)00116-7. [DOI] [PubMed] [Google Scholar]
- Westendorf M., Pas E. Z., Gordon R. Feeding food or table waste to livestock. Prof. Anim. Sci. 1996;12:129–137. [Google Scholar]
- Westendorf M. L., Dong Z. C., Schoknecht P. A. Recycled cafeteria food waste as a feed for swine: Nutrient content digestibility, growth, and meat quality. J. Anim. Sci. 1998;76:2976–2983. doi: 10.2527/1998.76122976x. [DOI] [PubMed] [Google Scholar]
- Wood J., Enser M., Fisher A., Nute G., Sheard P., Richardson R., Hughes S., Whittington F. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008;78:343–358. doi: 10.1016/j.meatsci.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Yang Z., Liu C., Xiang L., Zheng Y. Phenolic alkaloids as a new class of antioxidants in portulaca oleracea. Phytother. Res. 2009;23:1032–1035. doi: 10.1002/ptr.2742. [DOI] [PubMed] [Google Scholar]


