Abstract
AIM
To elucidate the mechanism of multidrug resistance in retinoblastoma, and to acquire more insights into in vivo drug resistance.
METHODS
Three anticancer drug resistant Y79 human RB cells were generated against vincristine, etoposide or carboplatin, which are used for conventional chemotherapy in RB. Primary cultures from enucleated eyes after chemotherapy (PCNC) were also prepared. Their chemosensitivity to chemotherapeutic agents (vincristine, etoposide and carboplatin) were measured using MTT assay. Western blot analysis was performed to evaluate the expression of p53, Bcl-2 and various multidrug resistant proteins in retinoblastoma cells.
RESULTS
Following exposure to chemotherapeutic drugs, PCNC showed less sensitivity to drugs. No significant changes observed in the p53 expression, whereas Bcl-2 expression was found to be increased in the drug resistant cells as well as in PCNC. Increased expression of P-glycoprotein (P-gp) was observed in drug resistant Y79 cells; however there was no significant change in the expression of P-gp found between primary cultures of primarily enucleated eyes and PCNC. Multidrug resistance protein 1 (Mrp-1) expression was found to be elevated in the drug resistant Y79 cells as well as in PCNC. No significant change in the expression of lung resistance associated protein (Lrp) was observed in the drug resistant Y79 cells as well as in PCNC.
CONCLUSION
Our results suggest that multidrug resistant proteins are intrinsically present in retinoblastoma which causes treatment failure in managing retinoblastoma with chemotherapy.
Keywords: retinoblastoma, chemotherapy, multidrug resistance, multidrug resistance associated proteins
INTRODUCTION
Retinoblastoma is a cancerous growth of immature retinal cells and a disease found exclusively in young children[1]. Of all the pediatric malignancies, retinoblastoma accounts for 4%[2], occurring in one in every 16 000-18 000 live births worldwide[3]. Survival rate for retinoblastoma in India is estimated at 48% owing to metastasis, secondary tumor formation by radiation therapy and late diagnosis[4]. It is caused by both genetic as well as sporadic means. Though leukocoria and strabismus are the initial symptoms for diagnosis, the treatment regime is based on laterality of retinoblastoma, age of the child and stage of the disease. Although, enucleation and external beam radiotherapy are the century old primary treatment options available, the current clinical practice includes chemotherapy[5]. To prevent metastasis and formation of trilateral retinoblastoma, bilateral retinoblastoma cases routinely need chemotherapy[6]. To preserve the vision, the main purpose is to reduce the tumor size, prevent metastasis and exclude central nervous system (CNS) involvement to further facilitate local treatment methods. Moreover, malignancies that invade the CNS may evade the chemotherapeutic drugs become of their inability to cross the blood brain barrier. To date, the standard chemotherapeutic agents used for intraocular retinoblastoma is the combination of vincristine, etoposide and carboplatin (VEC). In 2006, Shields et al[7] used the same VEC formulation against 249 eyes categorized into 4 groups and achieved significant treatment success. However, along with chemoreduction, local treatment methods like thermotherapy or cryotherapy was also used in combination. Combined treatment has earlier shown much better results in retinoblastoma as compared to chemotherapy alone[7]–[8]. But, chemotherapy alone has shown poor efficacy in more advanced tumors and tumor recurrence was seen very often[9]–[10]. One possible reason for this could be the development of drug resistance by the cancer cells owing to expression of multidrug resistant proteins[11]–[14], genomic alterations, in addition to tumor proliferation and deregulation of apoptotic pathways[15]–[19].
Thus, the intrinsic mechanisms by which retinoblastoma resist chemotherapy or acquire drug resistance after treatment remains to be determined. In this study, to elucidate the molecular mechanisms leading to multidrug resistance (MDR) in retinoblastoma, drug resistance retinoblastoma Y79 cells were generated. To acquire more insights into in vivo drug resistance, primary cultures of enucleated eyes after chemotherapy were also prepared. Their chemosensitivity to chemotherapeutic agents (vincristine, etoposide and carboplatin) were measured using MTT assay. A number of proteins (p53, Bcl-2 and MDR associated proteins i.e. P-gp, Mrp-1 and Lrp) involved in the development of drug resistance were analyzed using Western blotting.
MATERIALS AND METHODS
The National Centre for Cell Sciences, Pune, India was contacted for providing Y79 human retinoblastoma cell line. The procured cells were supplemented with heat-inactivated fetal bovine serum (FBS-10%) and penicillin-streptomycin (1%) in tissue culture flasks. Cells were then grown in suspension in RPMI-1640 medium. A constant temperature of 37°C and 5%CO2 was provided for culture growth. For preparing primary cultures, surgically resected tissue specimens from enucleated eyes of retinoblastoma patients were obtained from Dr. Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences, New Delhi, India. All patients were signed an informed consent form. The study complied with the principles of the Declaration of Helsinki. Cultures were maintained in a humidified atmosphere of 5%CO2/95% air at 37°C. Post cells confluence, harvesting and plating for further passages and drug treatment was pursued. To understand the MDR phenomena in retinoblastoma, drug resistant retinoblastoma cells were generated following Zhang et al[20] method. Cultured cells in RPMI medium were exposed (treated) to vincristine, etoposide and carboplatin for the generation of these anticancer drug resistant cells. Initially, each drug with concentration of 1/100 of their 50% growth inhibition (IC50) was given, subsequently followed by subculture in every 10d in RPMI with increasing concentration of drugs. Finally, only those cell lines were chosen for experiments that showed exponential growth at high drug concentrations. Later, experiments were performed in all resistant cells after culturing for at least 2wk in the absence of the anticancer drugs. The drug resistance index (RI) was estimated by MTT assay as the ratio of IC50 of the drug resistant retinoblastoma cells/IC50 of human retinoblastoma Y79 cells and cancer cells with RI of 3 or more were considered chemoresistant. Similarly, to study the MDR in retinoblastoma patients, primary cultures of enucleated eyes after chemotherapy were prepared and effects of drugs on these cells were assessed by MTT assay. Additionally, expression of p53, Bcl-2 and various MDR proteins were also evaluated to further confirm our results. Proteins separated and resolved by SDS-PAGE were transferred to PVDF membranes by electroblotting using Bio-Rad mini gel blot transfer apparatus followed by staining with 0.1% Ponceau S stain and overnight treatment with blocking buffer (5% non-fat milk in PBS) at 4°C. Blots were incubated with primary antibodies for 4h at 37°C followed by PBS-Tween20 washes and incubation with HRP-conjugated secondary antibodies. Finally, the immunoreactive proteins (protein bands) were visualized using ECL kit.
RESULTS
In this study, 3 types of drug resistant Y79 retinoblastoma cells were generated from the Y79 human retinoblastoma cells by stepwise and continuous exposure to vincristine, etoposide and carboplatin each, and named Y79/vincristine cells, Y79/etoposide cells and Y79/carboplatin cells respectively. The IC50 of Y79 cells and 3 resistant retinoblastoma Y79 cells are shown in Table 1.
Table 1. IC50 and RI values of Y79 cells and the drug resistant Y79 cells.
| Drug | IC50 of Y79 retinoblastoma cells (µmol/L) | IC50 of drug resistance Y79 retinoblastoma cells (µmol/L) | Resistance index (RI) |
| Vincristine | 1.65 | 23.2 | 14.06 |
| Etoposide | 1.9 | 19.3 | 10.16 |
| Carboplatin | 3.55 | 26.4 | 7.44 |
The IC50 of Y79 cells to vincristine, etoposide and carboplatin was 1.65 µmol/L, 1.9 µmol/L and 3.55 µmol/L, respectively. In contrast, the IC50 of the 3 resistant Y79 cells, Y79/vincristine, Y79/etoposide and Y79/carboplatin cells was 23.2 µmol/L, 19.3 µmol/L and 26.4 µmol/L against vincristine, etoposide, and carboplatin respectively. The RI was determined as the ratio of the IC50 of the drug resistant cells/ IC50 of parent Y79 cells. RI of Y79/vincristine, Y79/etoposide and Y79/carboplatin cells against vincristine, etoposide, and carboplatin were 14.06, 10.16 and 7.44 respectively. Since all of the RI of the 3 drug resistant cells was more than 3.0, these cells were successfully developed as chemoresistant cancer cells. No apparent morphologic difference was found in each drug resistant cell (Figure 1).
Figure 1. Cell culture of Y79/vincristine cells (B), Y79/etoposide cells (C), Y79/carboplatin cells (D) and compared with Y79 cells (A).
Cells were observed under a phase-contrast microscope (Magnification ×200).
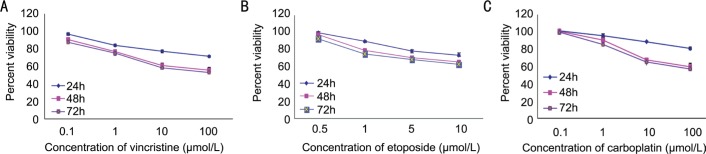
To study the MDR in retinoblastoma patients, primary cultures of enucleated eyes after chemotherapy were prepared and effects of drugs on these cells were assessed by MTT assay (Figure 2).
Figure 2. Effect of vincristine (A), etoposide (B) and carboplatin (C) on cell viability.
Primary cultures of enucleated eyes after chemotherapy were treated with vincristine etoposide and carboplatin respectively in a dose and time dependent manner. Cell viability was determined by MTT assay. Experiments were done twice in triplicate. Values represent mean±SD cell viability as percentage of untreated control samples.
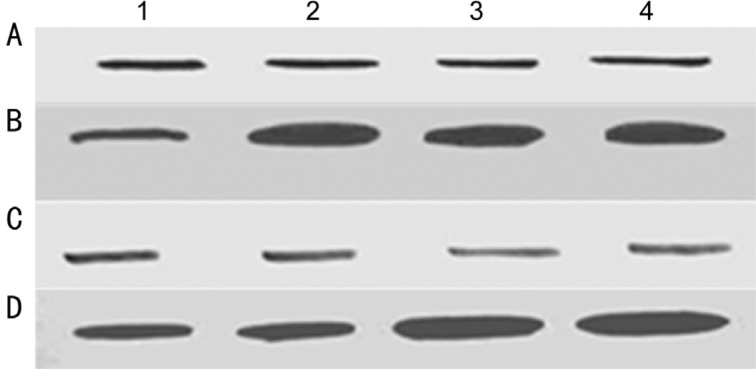
Cells showed less sensitivity to drugs. Western blot analysis was performed to evaluate the expression of p53 and Bcl-2 in drug resistant Y79 cells as well as in primary cultures of enucleated eyes after chemotherapy and compared with Y79 cells and primary cultures of primarily enucleated eyes. No significant changes observed in the p53 expression, whereas Bcl-2 expression was found to be increased in the drug resistant cells as well as in primary cultures of enucleated eyes after chemotherapy (Figure 3).
Figure 3. Western blot analysis of p53 (A) and Bcl-2 (B) in Y79 cells (lane 1) and Y79/vincristine cells (lane 2), Y79/etoposide cells (lane 3), and Y79/carboplatin cells (lane 4); Western blot analysis of p53 (C) and Bcl-2 (D) in primary cultures of primarily enucleated eyes (lanes 1&2) and in primary cultures of enucleated eyes after chemotherapy (lane 3&4).
Cell lysates were resolved by SDS-PAGE, and proteins were immunoblotted and detected using specific antibodies.
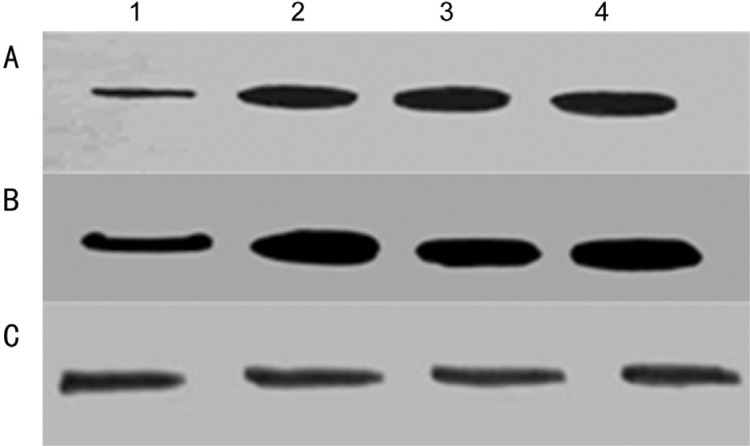
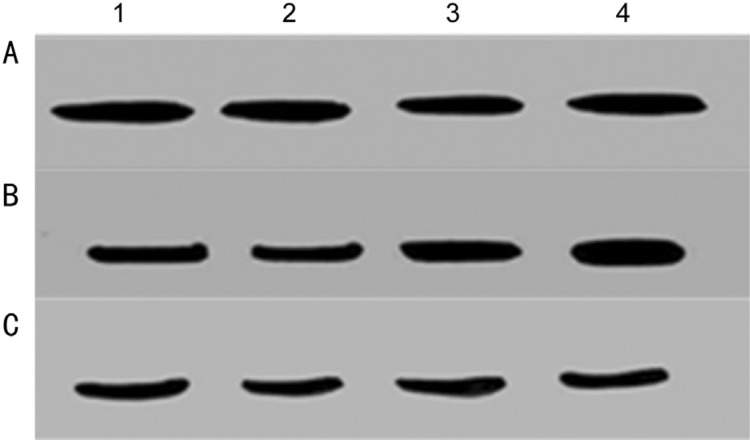
Increased expression of P-gp was observed in drug resistant Y79 cells; however there was no significant change in the expression of P-gp found between primary cultures of primarily enucleated eyes and primary cultures of enucleated eyes after chemotherapy (Figures 4A and 5A). Mrp-1 expression was found to be elevated in the drug resistant Y79 cells as well as in primary cultures of enucleated eyes after chemotherapy (Figures 4B and 5B). No significant change in the expression of Lrp was observed in the drug resistant Y79 cells as well as in the primary cultures of enucleated eyes after chemotherapy (Figures 4C and 5C).
Figure 4. Western blot analysis of P-gp (A), Mrp-1 (B) and Lrp (C) in Y79 cells (lane 1) and Y79/vincristine cells (lane 2), Y79/etoposide cells (lane 3), and Y79/carboplatin cells (lane 4).
Cell lysates were resolved by SDS-PAGE, and proteins were immunoblotted and detected using specific antibodies.
Figure 5. Western blot analysis of P-gp (A), Mrp-1(B) and Lrp (C) in primary cultures of primarily enucleated eyes (lanes 1&2) and in primary cultures of enucleated eyes after chemotherapy (lanes 3&4).
Cell lysates were resolved by SDS-PAGE, and proteins were immunoblotted and detected using specific antibodies.
DISCUSSION
Being the most common intraocular malignant tumor in children, finding the best treatment strategy for retinoblastoma is at the fore. Malignancy in retinoblastoma that progressively involves the extra orbital regions of the eye and later on the CNS compels the patients to go for enucleation which is aesthetically the last option a patient have. As a result, novel studies and research for newer treatment options with minimal side effects and maximum efficacy against retinoblastoma are continuously being searched and conducted. The management of retinoblastoma has evolved over the past decade through the advances in the development of large number of therapeutic agents. Prior to enucleation, chemotherapy provide one such better option for globe salvage. But, drug resistance poses a major barrier in retinoblastoma treatment. To overcome it, investigation of the mechanisms of drug resistance using in vitro MDR cell lines has been performed in the past decades. Our study successfully generated 3 anticancer drug resistant Y79 human retinoblastoma cells with RI greater than 3.0 against the drugs vincristine, etoposide and carboplatin. Moreover, primary cultures of enucleated eyes after chemotherapy were also prepared to understand the mechanism of drug resistance in retinoblastoma. Chemosensitivity to vincristine, etoposide or carboplatin were measured by MTT assay and cells showing less sensitivity to drugs were processed for further studies to understand the mechanism of MDR.
p53 gene mutation have been described earlier as predictors of poor response to chemotherapy in B cell lymphomas patients that reduce complete response rate of the patient. Our study assessed the p53 expression in all the 3 drug resistant Y79 cells as well as in primary cultures of enucleated eyes after chemotherapy to determine whether changes in p53 expression occur during the development of drug resistance. Our results demonstrate no changes in the expression of p53 in drug resistant Y79 cells. No differences were found in the p53 expression between primary cultures of primary enucleated eyes (which showed more sensitivity to drugs) and cultures of enucleated eyes after chemotherapy (which showed less sensitivity to drugs). Our results suggest that changes in p53 expression are not critical for the development of MDR in human retinoblastoma cancer cells. However, this does not exclude the fact that cells with acquired p53 gene mutations are more resistant to chemotherapy and radiation than wild type p53 containing cells, as observed by other groups[21].
Bcl-2 is another protein which is known to inhibit apoptosis and promote cell survival by blocking cell death induced by a variety of stimuli and renders cells resistant to a range of cytotoxic drugs[22]–[23]. Ability of the chemotherapeutic drugs to initiate apoptotic pathways lead us to determine whether deregulated Bcl-2 expression could render cells resistant to drugs commonly used in the treatment of retinoblastoma, including vincristine, etoposide and carboplatin. Overexpression of Bcl-2 confers drug resistance in vitro by inhibiting apoptosis. This study has addressed the possible role of Bcl-2 in the development of drug resistance in resistant Y79 cells as well as in the primary cultures of enucleated eyes after chemotherapy. Increased level of Bcl-2 expression has been observed in drug resistant Y79 cells as well as in the primary cultures of enucleated eyes after chemotherapy. Generally, chemotherapeutic drugs are administered to retinoblastoma patients in cycles of intensive treatment followed by periods of recooperation to reduce their potentially fatal side effects; our findings raise the possibility that at least some malignant cells having deregulated Bcl-2 expression could survive in vivo and begin to proliferate again between cycles of chemotherapy. Another possibility is that Bcl-2's ability to keep cells alive for several days despite drug treatment may create greater opportunities for retinoblastoma cells to acquire additional genetic alterations that could allow the cells to achieve true drug resistance and thus both survive and proliferate in the presence of certain antineoplastic agents.
One of the major causes of chemotherapy failure in human malignancies is MDR, which is a major obstacle in cancer treatment. Besides several other mechanisms, role of two different membrane proteins, P-gp and Mrp-1, belonging to same ATP binding cassette superfamily of transport proteins has been well established in the acquisition of the MDR phenotype[24]–[26]. Scheper et al[27] first discovered Lrp in non P-gp drug resistant cell lines. Clinical studies have reported that Lrp expression predicts drug resistance and poor outcome in patients with acute myelogenous leukaemia, ovarian cancer and other cancers. To overcome MDR, Sreenivasan et al[28] showed in vitro and in silico inhibitory effects of curcumin on MDR associated protein (MRP1) in retinoblastoma cells. Jia et al[29] suggested silencing of ATP-binding cassette, subfamily G, member 2 (ABCG2) by microRNA-3163 to inhibit the MDR in retinoblastoma cancer stem cells (RCSCs). Moreover, an anti-cancer agent piperlongumine (PLGM) was suggested for drug resistant human retinoblastoma cell lines HXO-RB44/VCR and SO-Rb50/CBP[30].
In the present study, Western blotting was performed to see the expression of MDR proteins in the drug resistant Y79 cells as well as in primary cultures of enucleated eyes after chemotherapy (which showed less sensitivity to drugs). Increased P-gp expression was found in all the three drug resistant Y79 human retinoblastoma cells as compared to Y79 retinoblastoma cells. Whereas, no change was found in the expression of P-gp between primary cultures of primarily enucleated eyes and the primary culture of enucleated eyes after chemotherapy. Moreover, increased Mrp-1 expression was seen in both the drug resistant Y79 cells as well as the primary cultures of enucleated eyes after chemotherapy. No changes in the expression of Lrp were found in the drug resistant Y79 retinoblastoma cells as well as in the primary cultures of enucleated eyes after chemotherapy as compared to their respective controls.
Cancer cells are known to express large amounts of P-gp. P-gp reduces the efficacy of the chemotherapeutic agents by ATP dependent efflux, rendering these cancers MDR. In addition, a general antiapoptotic role for P-gp has been proposed. P-gp expression has been studied in different neoplasms and increased expression has been found in several epithelial tumors, such as those of the colon and breast. Association of P-gp with p53 and cell proliferation has been seen in breast carcinomas but not in colorectal carcinomas and hepatocellular carcinoma. Gastric cancer patients without prior chemotherapy showed higher expressions of P-gp, Lrp and Mrp-1, indicating existence of innate drug resistance[31]. Some studies of myeloma, lymphoma, neuroblastoma, acute myelogeneous and acute lymphoblastic leukemia have shown that the presence of P-gp correlated with poor prognosis and the absence of this protein with long term disease free survival[32]–[34].
Overexpression of Mrp-1 protein in cells confers resistance to various anticancer drugs. Activation of PI3K/Akt pathway has been shown to express Mrp-1 and chemoresistance in prostate cancer cell lines and acute myeloid leukemia[35]. Similar over expression pattern has been observed in various other malignancies viz. pancreatic cancer[36], lung cancer[37], breast cancer[38] and glioma[39].
Lrp protein was initially described in non-small cell lung cancer cell lines that lacked P-gp. Also, it was found that Lrp was present in many human cancer cell lines that have not previously been exposed to drugs. Lrp expression of cell lines is associated with resistance to doxorubicin, vincristine, carboplatin, cisplatin, and melphalan. Lrp expression was associated with poor response to chemotherapy and shorter overall survival, which suggests that Lrp is a clinically relevant drug resistance factor in diffuse large B-cell lymphoma[40]. A similar predictive and prognostic value of Lrp expression has been reported for acute myeloid leukemia, multiple myeloma, childhood acute lymphoblastic leukemia and locally advanced bladder cancer[41]–[43]. However, despite several studies having been conducted on the expression of MDR proteins in retinoblastoma, the exact expression pattern and the prognostic value of these proteins in retinoblastoma are not clear so far.
In another study on retinoblastoma cell line HXO-RB, the investigators observed that tumor cell lines expressed both P-gp and Mrp-1[44]. Wilson et al[14] conducted study to elucidate the specific ABC transporters in the treatment of retinoblastoma. They found variable expression of P-gp, Mrp-1, Mrp-2, Mrp-4, and Bcrp in primarily enucleated retinoblastoma. The similar expression of these MDR proteins in tumors treated with chemotherapy and/or external-beam radiotherapy (EBRT), but no significant difference was observed. Furthermore, they found a minor correlation between Mrp-2 and pregnane xenobiotic receptor (PXR).
Kamburoglu et al[13] demonstrated that retinoblastoma intrinsically expresses both P-gp and Mrp-1 and their expressions are not related to differentiation. The expressions of P-gp and Mrp-1 do not seem to be induced by chemotherapy and are not related to the degree of invasion. Li et al[45] demonstrated that the intrinsic MDR of retinoblastoma involves the combined effects of P-gp, Lrp and Mrp-1. A study demonstrates that moxifloxacin modulate the permeability and potentiate antiproliferative activity of anticancer agents (topotecan, etoposide and vinblastine) in retinoblastoma cells[46]. This may suggest that moxifloxacin may modulate the anticancer mediated release of proinflammatory cytokines. Sreenivasan et al[47] showed that curcumin can suppress the MDR1 expression and function, and therefore may be useful as modulators of MDR in retinoblastoma tumor. Selective intra-arterial chemotherapy (IAC) has been adopted by many ocular oncology centers to treat advanced intraocular retinoblastoma. They revealed that possible causes for failure of IAC include poor vitreous penetration, inactive state of tumor seeds within the avascular vitreous cavity, and chemotherapeutic drug resistance[48].
Our results, when compared with previous studies, suggest that retinoblastoma expresses MDR proteins intrinsically. This does not exclude the possible selection of a chemoresistant clone of cells that innately expressed MDR protein as a cause of treatment failure. This conclusion is supported by the previous studies conducted by others in retinoblastoma which shows the presence of MDR proteins in primarily enucleated eyes. We therefore suggest that it is the innate expression of the resistance proteins by the tumor, not the induction of that expression, which results in treatment failure. The urge for overcoming the resistance encountered by the prolonged usage of chemotherapeutic drugs necessitates a deeper understanding of the underlying pathways that favors cell survival and inhibits apoptosis.
Acknowledgments
We sincerely acknowledge the support of Department of Biotechnology, Govt. of India.
Conflicts of Interest: Shukla S, None; Srivastava A, None; Kumar S, None; Singh U, None; Goswami S, None; Chawla B, None; Bajaj MS, None; Kashyap S, None; Kaur J, None.
REFERENCES
- 1.American Cancer Society . Cancer Medicine. Hamilton, Ontario: BC Decker Inc.; 2003. Chapter 85. Neoplasms of the Eye. [Google Scholar]
- 2.Shields CL, Shields JA. Diagnosis and management of retinoblastoma. Cancer Control. 2004;11(5):317–327. doi: 10.1177/107327480401100506. [DOI] [PubMed] [Google Scholar]
- 3.Houston SK, Murray TG, Wolfe SQ, Fernandes CE. Current update on retinoblastoma. Int Ophthalmol Clin. 2011;51(1):77–91. doi: 10.1097/IIO.0b013e3182010f29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swaminathan R, Rama R, Shanta V. Childhood cancers in Chennai, India, 1990–2001: incidence and survival. Int J Cancer. 2008;122(11):2607–2611. doi: 10.1002/ijc.23428. [DOI] [PubMed] [Google Scholar]
- 5.Yanık Ö, Gündüz K, Yavuz K, Taçyıldız N, Ünal E. Chemotherapy in retinoblastoma: current approaches. Turk J Ophthalmol. 2015;45(6):259–267. doi: 10.4274/tjo.06888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Potter P, Shields CL, Shields JA. Clinical variations of trilateral retinoblastoma: a report of 13 cases. J Pediatr Ophthalmol Strabismus. 1994;31(1):26–31. doi: 10.3928/0191-3913-19940101-06. [DOI] [PubMed] [Google Scholar]
- 7.Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, Shields JA. The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology. 2006;113(12):2276–2280. doi: 10.1016/j.ophtha.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Schefler AC, Cicciarelli N, Feuer W, Toledano S, Murray TG. Macular retinoblastoma: evaluation of tumor control, local complications, and visual outcomes for eyes treated with chemotherapy and repetitive foveal laser ablation. Ophthalmology. 2007;114(1):162–169. doi: 10.1016/j.ophtha.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Galindo C, Wilson MW, Haik BG, et al. Treatment of intraocular retinoblastoma with vincristine and carboplatin. J Clin Oncol. 2003;21(10):2019–2025. doi: 10.1200/JCO.2003.09.103. [DOI] [PubMed] [Google Scholar]
- 10.Shields CL, Shelil A, Cater J, Meadows AT, Shields JA. Development of new retinoblastomas after 6 cycles of chemoreduction for retinoblastoma in 162 eyes of 106 consecutive patients. Arch Ophthalmol. 2003;121(11):1571–1576. doi: 10.1001/archopht.121.11.1571. [DOI] [PubMed] [Google Scholar]
- 11.Krishnakumar S, Mallikarjuna K, Desai N, Muthialu A, Venkatesan N, Sundaram A, Khetan V, Shanmugam MP. Multidrug resistant proteins: P-glycoprotein and lung resistance protein expression in retinoblastoma. Br J Ophthalmol. 2004;88(12):1521–1526. doi: 10.1136/bjo.2004.047928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson MW, Fraga CH, Fuller CE, Rodriguez-Galindo C, Mancini J, Hagedorn N, Leggas ML, Stewart CF. Immunohistochemical detection of multidrug-resistant protein expression in retinoblastoma treated by primary enucleation. Invest Ophthalmol Vis Sci. 2006;47(4):1269–1273. doi: 10.1167/iovs.05-1321. [DOI] [PubMed] [Google Scholar]
- 13.Kamburoglu G, Kiratli H, Soylemezoglu F, Bilgiç S. Clinicopathological parameters and expression of P-glycoprotein and MRP-1 in retinoblastoma. Ophthalmic Res. 2007;39(4):191–197. doi: 10.1159/000104680. [DOI] [PubMed] [Google Scholar]
- 14.Wilson MW, Galindo CR, Fraga CH, Hagedorn N, Leggas ML, Stewart C. Expression of the Multidrug resistance proteins and the pregnane X receptor in treated and untreated retinoblastoma. Curr Eye Res. 2009;34(5):386–394. doi: 10.1080/02713680902859621. [DOI] [PubMed] [Google Scholar]
- 15.Vanderwal JE, Hermsen MA, Gille HJ. Comparative genomic hybridisation divides retinoblastomas into a high and a low level chromosomal instability group. J Clin Pathol. 2003;56(1):26–30. doi: 10.1136/jcp.56.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchong MN, Chen D, Corson TW. Minimal 16q genomic loss implicates cadherin-11 in retinoblastoma. Mol Cancer Res. 2004;2(9):495–503. [PubMed] [Google Scholar]
- 17.Mairal A, Pinglier E, Gilbert E. Detection of chromosome imbalances in retinoblastoma by parallel karyotype and CGH analyses. Genes Chromosomes Cancer. 2000;28(4):370–379. [PubMed] [Google Scholar]
- 18.Chen D, Gallie BL, Squire JA. Minimal regions of chromosomal imbalance in retinoblastoma detected by comparative genomic hybridization. Cancer Genet Cytogenet. 2001;129(1):57–63. doi: 10.1016/s0165-4608(01)00427-7. [DOI] [PubMed] [Google Scholar]
- 19.Chakraborty S, Khare S, Dorairaj SK, Prabhakaran VC, Prakash DR, Kumar A. Identification of genes associated with tumorigenesis of retinoblastoma by microarray analysis. Genomics. 2007;90(3):344–353. doi: 10.1016/j.ygeno.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Yashiro M, Qiu H, Nishii T, Matsuzaki T, Hirakawa K. Establishment and characterization of multidrug-resistant gastric cancer cell lines. Anticancer Res. 2010;30(3):915–921. [PubMed] [Google Scholar]
- 21.Reles A, Wen WH, Schmider A, Gee C, Runnebaum IB, Kilian U, Jones LA, El-Naggar A, Minguillon C, Schönborn I, Reich O, Kreienberg R, Lichtenegger W, Press MF. Correlation of p53 mutations with resiistance to platinum based chemotherapy and shortened survival in ovarian cancer. Clin Cancer Res. 2001;7(10):2984–2997. [PubMed] [Google Scholar]
- 22.Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer: Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharma Sci. 2000;11(4):265–283. doi: 10.1016/s0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 23.Beale PJ, Rogers P, Boxall F, Sharp SY, Kelland LR. Bcl-2 family protein expression and platinum drug resistance in ovarian carcinoma. Br J Cancer. 2000;82(2):436–440. doi: 10.1054/bjoc.1999.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambudkar SV, Dey SHCA, Ramachandra M, Pastan I, Gottesman MM. Biochemical. Cellular and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 25.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 26.Tan B, Piwnica-Worms D, Ratner L. Multidrug resistance transporters and modulation. Curr Opin Oncol. 2000;12(5):450–458. doi: 10.1097/00001622-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Scheper RJ, Broxterman HJ, Scheffer GL, Kaaijk P, Dalton WS, van Heijningen TH, van Kalken CK, Slovak ML, de Vries EG, van der Valk P. Overexpression of a M(r) 110,000 vesicular protein in non-P-glycoprotein-mediated multidrug resistance. Cancer Res. 1993;53(7):1475–1479. [PubMed] [Google Scholar]
- 28.Sreenivasan S, Ravichandran S, Vetrivel U, Krishnakumar S. In vitro and In silico studies on inhibitory effects of curcumin on multi drug resistance associated protein (MRP1) in retinoblastoma cells. Bioinformation. 2012;8(1):13–19. doi: 10.6026/97320630008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jia M, Wei Z, Liu P, Zhao X. Silencing of ABCG2 by microRNA-3163 inhibits multidrug resistance in retinoblastoma cancer stem cells. BJ Korean Med Sci. 2016;31(6):836–842. doi: 10.3346/jkms.2016.31.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang XQ, Wang YC, Guo YT, Tang X. Effect of piperlongumine on drug resistance reversal in human retinoblastoma HXO-RB44/VCR and SO-Rb50/CBP cell lines. Int J Clin Exp Pathol. 2015;8(3):2525–2534. [PMC free article] [PubMed] [Google Scholar]
- 31.Hu WQ, Peng CW, Li Y. The expression and significance of P-glycoprotein, lung resistance protein and multidrug resistance-associated protein in gastric cancer. J Exp Clin Cancer Res. 2009;28:144–151. doi: 10.1186/1756-9966-28-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White L. P-glycoprotein expression and prognosis of neuroblastoma. N Eng J Med. 1992;326(17):1162–1163. doi: 10.1056/NEJM199204233261714. [DOI] [PubMed] [Google Scholar]
- 33.Grogan TM, Spier CM, Salmon SE, Matzner M, Rybski J, Weinstein RS, Scheper RJ, Dalton WS. P-glycoprotein expression in human plasma cell myeloma: correlation with prior chemotherapy. Blood. 1993;81(2):490–495. [PubMed] [Google Scholar]
- 34.Wood P, Bugess R, MacGregor A, Liu Yin JA. P-glycoprotein expression on acute myeloid leukemia blast cells at diagnosis predicts response to chemotherapy and survival. Br J Hematol. 1994;87(3):509–514. doi: 10.1111/j.1365-2141.1994.tb08305.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee JT, Steelman LS, McCubrey JA. Phosphatidylinositol 3′-Kinase activation leads to multidrug resistance protein-1 expression and subsequent chemoresistance in advanced prostate cancer cells. Cancer Res. 2004;64(22):8397–8404. doi: 10.1158/0008-5472.CAN-04-1612. [DOI] [PubMed] [Google Scholar]
- 36.O'Driscoll L, Walsh N, Larkin A, Ballot J, Ooi WS, Gullo G, O'Connor R, Clynes M, Crown J, Kennedy S. MDR1/P-glycoprotein and MRP-1 drug efflux pumps in pancreatic carcinoma. Anticancer Res. 2007;27(4B):2115–2120. [PubMed] [Google Scholar]
- 37.Roy S, Kenny E, Kennedy S, Larkin A, Ballot J, Perez De Villarreal M, Crown J, O'Driscoll L. MDR1/P-glycoprotein and MRP-1 mRNA and protein expression in non-small cell lung cancer. Anticancer Res. 2007;27(3A):1325–1330. [PubMed] [Google Scholar]
- 38.Larkin A, O'Driscoll L, Kennedy S, Purcell R, Moran E, Crown J, Parkinson M, Clynes M. Investigation of MRP-1 protein and MDR-1 P-glycoprotein expression in invasive breast cancer: a prognostic study. Int J Cancer. 2004;112(2):286–294. doi: 10.1002/ijc.20369. [DOI] [PubMed] [Google Scholar]
- 39.Calatozzolo C, Gelati M, Ciusani E, Sciacca FL, Pollo B, Cajola L, Marras C, Silvani A, Vitellaro-Zuccarello L, Croci D, Boiardi A, Salmaggi A. Expression of drug resistance proteins Pgp, MRP1, MRP3, MRP5 and GSTpi in human glioma. J Neurooncol. 2005;74(2):113–121. doi: 10.1007/s11060-004-6152-7. [DOI] [PubMed] [Google Scholar]
- 40.Filipits M, Jaeger U, Simonitsch I, Chizzali-Bonfadin C, Heinzl H, Pirker R. Clinical relevance of the lung resistance protein in diffuse large b-cell lymphomas. Oncology. 2000;6(9):3417–3423. [PubMed] [Google Scholar]
- 41.Filipits M, Drach J, Pohl G, Schuster J, Stranzl T, Ackermann J, Königsberg R, Kaufmann H, Gisslinger H, Huber H, Ludwig H, Pirker R. Expression of the lung resistance protein predicts poor outcome in patients with multiple myeloma. Clin Cancer Res. 1999;5(9):2426–2430. [PubMed] [Google Scholar]
- 42.Valera ET, Scrideli CA, Queiroz RG, Mori BM, Tone LG. Multidrug resistant protein (MDR-1), multidrug resistance related protein (MRP) and lung resistant protein (LRP) gene expression in childhood acute lymphoblastic leukemia. Sao Paulo Med J. 2004;122(4):166–171. doi: 10.1590/S1516-31802004000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diestra JE, Condom E, Del Muro XG, Scheffer GL, Pérez J, Zurita AJ, Muñoz-Seguí J, Vigués F, Scheper RJ, Capellá G, Germà-Lluch JR, Izquierdo MA. Expression of multidrug resistance proteins P-glycoprotein, multidrug resistance protein 1, breast cancer resistance protein and lung resistance related protein in locally advanced bladder cancer treated with neoadjuvant chemotherapy: biological and clinical implications. J Urol. 2003;170(4):1383–1387. doi: 10.1097/01.ju.0000074710.96154.c9. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Tang L, Liu X. A gene study on multidrug resistance of retinoblastoma. Zhonghua Yan Ke Za Zhi. 2001;37(4):256–258. [PubMed] [Google Scholar]
- 45.Li B, Gao R, Zhang H, Li LQ, Gao F, Ren RJ. Studies on multidrug resistance associated protein in retinoblastoma. Zhonghua Yan Ke Za Zhi. 2009;45(4):314–317. [PubMed] [Google Scholar]
- 46.Barot M, Gokulgandhi MR, Pal D, Mitra AK. In vitro moxifloxacin drug interaction with chemotherapeutics: implications for retinoblastoma management. Exp Eye Res. 2014;118:61–71. doi: 10.1016/j.exer.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sreenivasan S, Ravichandran S, Vetrivel U, Krishnakumar S. Modulation of multidrug resistance 1 expression and function in retinoblastoma cells by curcumin. J Pharmacol Pharmacother. 2013;4(2):103–119. doi: 10.4103/0976-500X.110882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim J, Do H, Egbert P. Enucleated eyes after failed intra-arterial infusion of chemotherapy for unilateral retinoblastoma: histopathologic evaluation of vitreous seeding. Clin Ophthalmol. 2011;5:1655–1658. doi: 10.2147/OPTH.S24318. [DOI] [PMC free article] [PubMed] [Google Scholar]