Abstract
AIM
To evaluate the effect of cataract surgery on sleep quality and to compare the difference between ultraviolet-blocking clear intraocular lens (UVB-IOL) and blue-filtering intraocular lens (BF-IOL) implantation.
METHODS
Electronic search was performed of PubMed, MEDLINE, Embase and the Cochrane Library up to January 2016. Studies were eligible when they evaluated the sleep quality before and after cataract surgery by Pittsburgh sleep quality index (PSQI). A random/fixed-effects Meta-analysis was used for the pooled estimate. Heterogeneity was assessed with the I2 test.
RESULTS
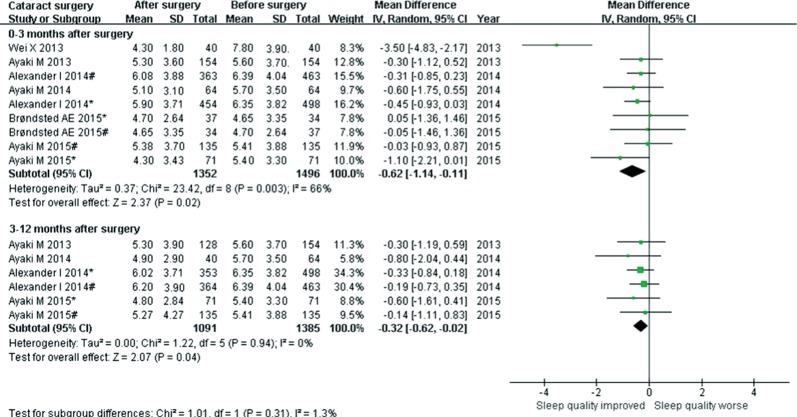
Six studies were selected from 5623 references. Cataract surgery significantly reduced the PSQI scores at postoperative 0-3mo [mean difference (MD) =-0.62, 95%CI: -1.14 to -0.11, P=0.02, I2=66%] and 3-12mo (MD=-0.32, 95%CI: -0.62 to -0.02, P=0.04, I2=0), respectively. Considering different intraocular lens (IOL) implantations, relative post-operative PSQI reduction was found for both UVB-IOL and BF-IOL, but a significant reduction was detected only for UVB-IOL. No significant difference was found with the effect of BF-IOL vs UVB-IOL on sleep quality.
CONCLUSION
This study found that cataract surgery significantly improved the PSQI score-derived subjective sleep quality irrespective of the IOL type implanted. These findings highlight a substantial benefit of cataract surgery on systemic health with photoreceptive restoration in addition to visual acuity improvements.
Keywords: cataract surgery, intraocular lens implantation, sleep quality
INTRODUCTION
Sleep quality is essential for maintaining health; conversely, sleep disorders or disruptions are associated with comorbidity management, medication administration and a personal burden[1]. Cataract development interferes with the spectrum of light transmitted and reduces the amount of light reaching the retina, particularly in the short wavelength range of the visible spectrum (450-490 nm), accounting for the disruption of the human biological rhythm[2]. However, only a few studies with limited subjects and study design have investigated the impact of cataract removal and artificial lens implantation on sleep quality[3]–[13].
There exist two main classes of intraocular lens (IOL) currently implanted, which differ in light transmission properties: the ultraviolet-blocking clear intraocular lens (UVB-IOL) and the blue filtering intraocular lens (BF-IOL). The BF-IOL blocks the short wavelength spectrum blue light more efficiently than the UVB-IOL does, theoretically protecting the retinal pigment epithelium (RPE) from photochemical damage[14]–[15]. However, present studies report conflicting findings about the effect comparison of the two types of IOLs on the prognosis, including sleep quality[3],[5]–[8],[16]–[17].
To our best knowledge, the data regarding the effect of cataract surgery involving UVB-IOLs or/and BF-IOLs on sleep quality have not yet been systematically evaluated and reported. We therefore conducted a systematic review and Meta-analysis to evaluate the effect of cataract surgery on sleep quality and compare the difference between UVB-IOL and BF-IOL implantation.
MATERIALS AND METHODS
Search Strategy and Study Eligibility
This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines[18] for reporting systematic reviews and Meta-analyses. We performed a literature search of the electronic databases PubMed, Embase, and the Cochrane Libraryup to January 2016. We also manually checked the reference lists of all retrieved studies, review articles, and conference abstracts using electronic searches. In our literature search, we included a combination of keywords, such as (cataract OR age-related cataract), (sleep OR circadian rhythm) and (IOL), in the form of title words or medical subject headings. Two reviewers (Zheng L and Wu XH) completed the literature search independently. In addition, these two reviewers further cross-checked the reference lists of all selected articles to identify other relevant studies. When screening discrepancies occurred, consensus was achieved after further discussion.
Inclusion and Exclusion Criteria
We included studies that met the following inclusion criteria: 1) randomized or non-randomized trials focusing on the effect of cataract surgery on sleep quality; 2) the included patients received either an UVB-IOL or a BF-IOL followed by phacoemulsification in surgery; 3) the included patients were at least sixty years of age; 4) the subjects were diagnosed with age-related cataract with nuclear opacification grades of ≥2 according to Lens Opacities Classification System II; 5) the sleep quality of the participants was evaluated using the Pittsburgh sleep quality index (PSQI). The studies were excluded if they were 1) abstracts from conferences, full texts without raw data, duplicate publications, letters, or reviews; 2) the subjects' conditions were in combination with the following (but not restricted to) cardiovascular or cerebrovascular diseases, severe corneal lesions, vitreous hemorrhage, macular edema, age-related macular degeneration (AMD) or glaucoma.
Data Extraction and Outcomes of Interest
Two authors (Zheng L and Wu XH) extracted the data and compared the results; discrepancies were resolved by discussion. We did not contact the authors of the eligible studies for additional data. The primary outcome was defined as a change in sleep quality before and after surgery, as evaluated by the PSQI. The change in the ratio of poor sleepers before and after surgery was analyzed as the secondary outcome.
Assessment of the Risk of Bias
The risk of bias of each trial was assessed according to Cochrane methodology[19], considering six aspects: random sequence generation and allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of the outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). Each domain was graded as low, unclear, and high risk of bias according to the criteria outlined in the Cochrane Handbook for Systematic Review of Interventions. Two authors (Zheng L and Wu XH) assessed each trial independently and resolved disagreements via consensus.
Data Synthesis and Statistical Analysis
The change estimate of the sleep quality (PSQI scores) and ratio of poor sleepers (PSQI score >5.5) were calculated by a Meta-analysis based on the weighted mean differences (WMDs) and odds ratio (OR), respectively. Between-study heterogeneity was assessed using standard χ2 tests and the I2 statistic. I2 values of 50% or more were considered to indicate substantial heterogeneity, and the random-effects model was then used; otherwise, the fixed-effects model was used[20]. All analyses were performed using Review Manager (Version 5.3; The Cochrane Collaboration, 2014, the Nordic Cochrane Centre, Copenhagen, Denmark). Statistical tests were 2-sided and used a significance threshold of P<0.05.
RESULTS
Literature Search and Study Characteristics
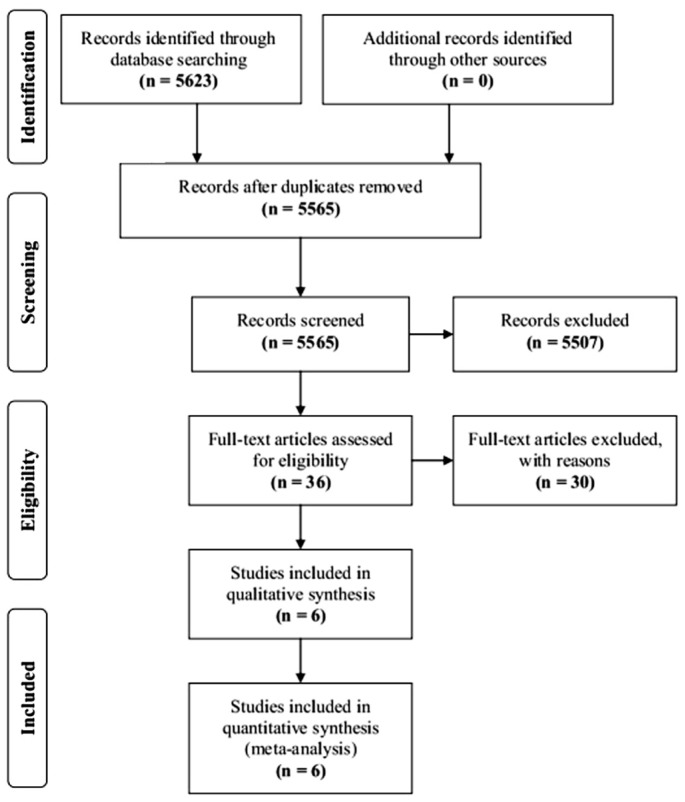
In total, 5623 articles were initially identified. After duplicates and nonrelevant studies were removed, the abstracts of the remaining 5565 studies were reviewed, and 36 articles with potentially relevant studies were further identified in full text. Finally, 6 published studies were determined to be eligible and were included in this Meta-analysis. For details, please refer to Figure 1.
Figure 1. Flowchart of the study selection process.
Among the 6 eligible studies published from 2013 to 2015, three studies were from Japan, 1 from Demark, 1 from the UK, and 1 from China. One of the included studies was a randomized trial, whereas the other five were nonrandomized trials. The sample sizes of the included studies ranged from 40 to 961 subjects, with a combined total of 1509 patients in the pooled estimate. The extracted mean age of the subjects ranged from 73.7 to 76.9 years of age. For more details, refer to Table 1.
Table 1. Characteristics of 6 included studies in the systematic review and Meta-analysis.
| Study | Country | Study type | Participants | Age (a) | M/F | IOL implant | Outcome |
| Brondsted et al, 2015[8] | Denmark | Randomized | 76 | Mean 73.7 (range 50-94) | 35/41 | UVB BF |
PSQI |
| Ayaki et al, 2015[6] | Japan | Nonrandomized | 206 | Mean 74.1 | 83/123 | UVB BF |
PSQI |
| Alexander et al, 2014[3] | UK | Nonrandomized | 961 | 76.94 (5.35) | 412/549 | UVB BF |
PSQI |
| Ayaki et al, 2014[7] | Japan | Nonrandomized | 71 | 74.1 (8.8) | 30/41 | UVB | PSQI |
| Wei et al, 2013[13] | China | Nonrandomized | 40 | Median 74 (range 70-78) | 14/26 | BF | PSQI |
| Ayaki et al, 2013[5] | Japan | Nonrandomized | 155 | 74.8 (8.0) | 62/93 | BF | PSQI |
PSQI: Pittsburgh sleep quality index; UVB: Ultraviolet-blocking clear intraocular lens; BF: Blue-filtering intraocular lens; SD: Standard deviation.
Mean (SD)
Quality of Evidence
According to the Cochrane methodology, the risk of bias of the included studies was assessed by considering adequate sequence generation, allocation concealment, blinding, the evaluation of incomplete outcome data, lack of selective reporting, and lack of other biases (Figure 2A; Table 2).
Figure 2. Risk of bias evaluation of the included studies.
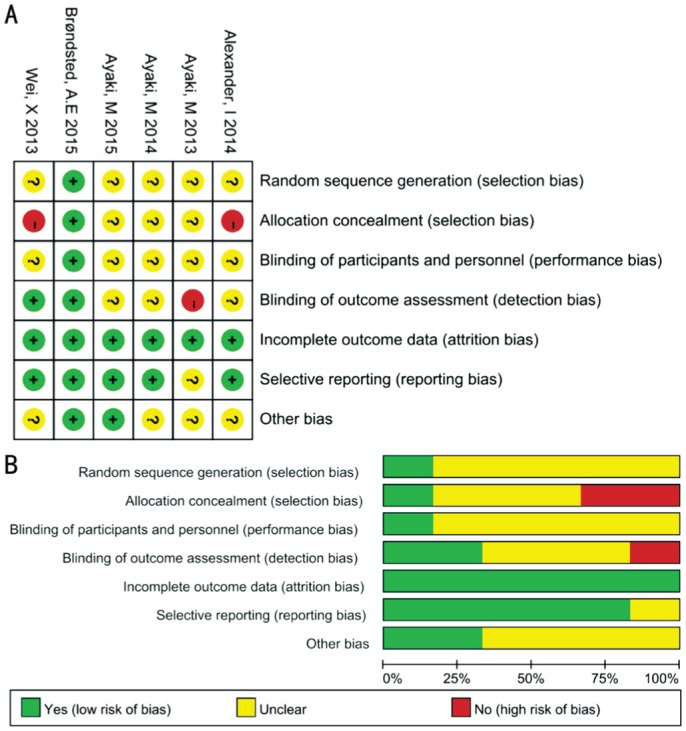
A: Risk of bias summary; B: Risk of bias graph. The green bar: Reported and a low risk of bias; The yellow bar: Unreported and a moderate risk of bias; The red bar: Unreported and a high risk of bias.
Table 2. Risk of bias assessment of included studies.
| Studies | Bias | Authors' judgement | Support for judgment |
| Brøndsted et al, 2015[8] | Random sequence generation (selection bias) | Low risk | Randomization was performed on the day of the surgery using automated, computerized block-randomization lists with a 1:1 allocation ratio and a block size of 9 |
| Allocation concealment (selection bias) | Low risk | The participants were masked to IOL type | |
| Blinding of participants and personnel (performance bias) | Low risk | The IOL type was masked to the participants, but impossible to the investigator | |
| Blinding of outcome assessment (detection bias) | Low risk | Statistical analyses were performed after a complete re-masking of the data post hoc. Masking was not broken before all statistical analyses had been performed | |
| Incomplete outcome data (attrition bias) | Low risk | 72/73 included participants completed the 3-week postoperative visit | |
| Selective reporting (reporting bias) | Low risk | Important outcomes were reported | |
| Other bias | Low risk | Not likely | |
| Ayaki et al, 2015[6] | Random sequence generation (selection bias) | Unclear risk | Nonrandomized trial with consecutive patients enrolled |
| Allocation concealment (selection bias) | Unclear risk | Not reported | |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported | |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported | |
| Incomplete outcome data (attrition bias) | Low risk | All included patients completed the 2mo and 7mo postoperative follow-up visit | |
| Selective reporting (reporting bias) | Low risk | Important outcomes were reported | |
| Other bias | Low risk | Not likely | |
| Alexander et al, 2014[3] | Random sequence generation (selection bias) | Unclear risk | Dual-site study. Patients operated on in Oxford received UVF-IOL (SA60AT); those operated on in Windsor received BF-IOL (SN60AT) |
| Allocation concealment (selection bias) | High risk | Neither investigators nor patients were masked to IOL allocation | |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not likely to blind patients or personnel | |
| Blinding of outcome assessment (detection bias) | Unclear risk | Not reported | |
| Incomplete outcome data (attrition bias) | Low risk | 1482 recruited, 961 completed study; in UVF IOL group, 44 patients (9%) dropped out and in BF-IOL group, 100 patients (22%) dropped out before 1mo postoperation | |
| Selective reporting (reporting bias) | Low risk | Important outcomes were reported | |
| Other bias | Unclear risk | Not reported | |
| Ayaki et al, 2014[7] | Random sequence generation (selection bias) | Unclear risk | Nonrandomized trial, consecutive patients enrolled |
| Allocation concealment (selection bias) | Unclear risk | Not likely | |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not likely | |
| Blinding of outcome assessment (detection bias) | Unclearrisk | Not reported | |
| Incomplete outcome data (attrition bias) | Low risk | All included patients completed the 7mo postoperative follow-up visit | |
| Selective reporting (reporting bias) | Low risk | Important outcomes were reported | |
| Other bias | Unclear risk | Not reported | |
| Wei et al, 2013[13] | Random sequence generation (selection bias) | Unclear risk | Nonrandomized, pre-test/post-test experiment |
| Allocation concealment (selection bias) | High risk | Not likely | |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported | |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators did not tell subjects supposed relationship between IOL and sleep quality | |
| Incomplete outcome data (attrition bias) | Low risk | All included patients completed the 1mo postoperative follow-up visit | |
| Selective reporting (reporting bias) | Low risk | Important outcomes were reported | |
| Other bias | Unclear risk | Not reported | |
| Ayaki et al, 2013[5] | Random sequence generation (selection bias) | Unclear risk | Nonrandomized trial, consecutive patients enrolled |
| Allocation concealment (selection bias) | Unclear risk | Not likely | |
| Blinding of participants and personnel (performance bias) | Unclear risk | Not reported | |
| Blinding of outcome assessment (detection bias) | High risk | Not likely | |
| Incomplete outcome data (attrition bias) | Low risk | All included patients completed the 2mo postoperative follow-up visit | |
| Selective reporting (reporting bias) | Unclear risk | Most important outcomes were reported | |
| Other bias | Unclear risk | Not reported |
Cumulatively, for the six included studies regarding the respective cochrane factors, the studies with a low risk of bias had values (a quantitative index of the risk of bias, range 0-100%) of 16.7%, 16.7%, 16.7%, 33.3%, 100%, 83.3%, and 33.3%; the studies with unreported features and a moderate risk of bias had values of 83.3%, 50.0%, 83.3%, 50.0%, 0, 16.7%, and 66.7%; and the studies at a high risk of bias had values of 0, 33.3%, 0, 13.3%, 0, 0, and 0 (Figure 2B).
Overall Effect of Cataract Surgery on Sleep Quality
Six studies reported the change in sleep quality (PSQI) before and after surgery as the mean difference (MD) at different time points of follow-up. Examination of the forest plots revealed a significant PSQI reduction, namely, the sleep quality improvements during the 0-3mo (MD=-0.62, 95%CI: -1.14 to -0.11, P=0.02, I2=66%) and 3-12mo (MD=-0.32, 95%CI: -0.62 to -0.02, P=0.04, I2=0) follow-up after surgery (Figure 3).
Figure 3. Forest plot estimating the pooled effect of cataract surgery on sleep quality.
Effect of Ultraviolet-blocking Clear Intraocular Lens on Sleep Quality
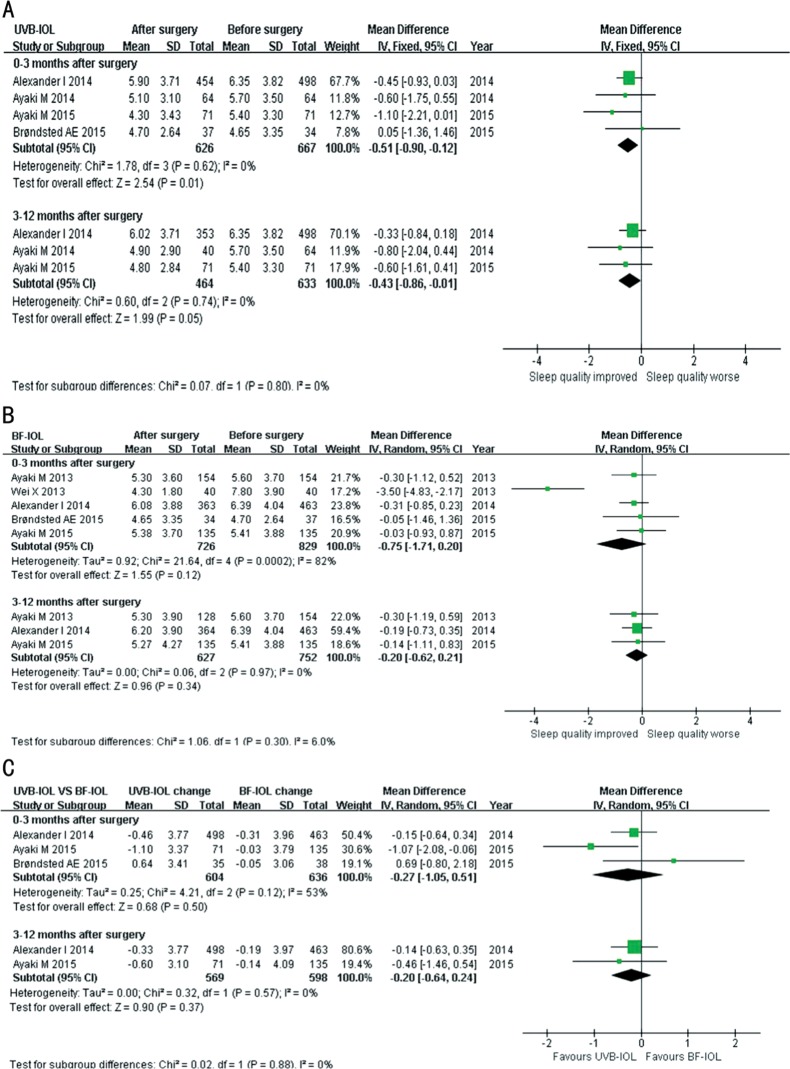
Four studies reported the change in sleep quality (PSQI) before and after surgery as the MD at different time points of follow-up. The Meta-analysis of the fixed-effect model was used for calculating the pooled effect regarding the insignificant heterogeneity (I2<50%). Examination of the forest plots revealed significant PSQI reduction, namely, the sleep quality improvements during the 0-3mo (MD=-0.51, 95%CI: -0.90 to -0.12, P=0.01, I2=0) and 3-12mo (MD=-0.43, 95%CI: -0.86 to -0.01, P=0.05, I2=0) follow-up after surgery (Figure 4A).
Figure 4. Forest plot estimating the pooled effect of cataract surgery on sleep quality, considering different IOL types.
A: Forest plot estimating the pooled effect of cataract surgery with UVB-IOL implantation on sleep quality; B: Forest plot estimating the pooled effect of cataract surgery with BF-IOL implantation on sleep quality; C: Forest plot comparing the pooled effect of cataract surgery with UVB-IOL vs BF-IOL implantation on sleep quality.
Effect of Blue-filtering Intraocular Lens on Sleep Quality
Five studies reported the change in sleep quality (PSQI) before and after surgery as the MD at different time points of follow-up. Meta-analysis of the random effect model (0-3mo after surgery)/fixed-effect model (3-12mo after surgery) was used for calculating the pooled effect regarding the heterogeneity. Examination of the forest plots revealed relative PSQI reductions during the 0-3mo (MD=-0.75, 95%CI: -1.71 to 0.20, P=0.12, I2=82%) and 3-12mo (MD= -0.20, 95%CI: -0.62 to 0.21, P=0.34, I2=0) follow-up after surgery, but the differences were not statistically significant (Figure 4B).
Effect of Ultraviolet-blocking Clear Intraocular Lens vs Blue-filtering Intraocular Lens on Sleep Quality
Three studies provided the results comparing the effect of the two types of IOLs on sleep quality (PSQI) before and after surgery as the MD at different time points of follow-up. Meta-analysis of the random effect model (0-3mo after surgery)/ fixed-effect model (3-12mo after surgery) was used for calculating the pooled effect regarding the heterogeneity. Examination of the forest plots revealed a larger amplitude of PSQI reductions for UVB-IOL compared with BF-IOL during the 0-3mo (MD= -0.27, 95%CI: -1.05 to 0.51, P=0.50, I2=53%) and 3-12mo (MD=-0.20, 95%CI: -0.64 to 0.24, P=0.37, I2=0) follow-up after surgery, but the differences were not statistically significant (Figure 4C).
Effect of Cataract Surgery (Blue-filtering Intraocular Lens) on the Poor-sleepers Ratio
Two studies compared the change in the poor-sleepers (PSQI >5.5) ratio before and after cataract surgery as the OR at the 0-3mo follow-up after surgery. Meta-analysis of the random effect model was used for calculating the pooled effect regarding the substantial heterogeneity. Examination of the forest plots revealed relative reductions in the poor-sleepers ratio during the 0-3mo (MD=0.34, 95%CI: 0.08 to 1.51, P=0.16, I2=79%) after surgery, but the differences were not statistically significant (Figure 5).
Figure 5. Forest plots howing the effect of cataract surgery (BF-IOL) on the poor-sleepers ratio.
DISCUSSION
In this systematic review and Meta-analysis, we evaluated the effect of cataract surgery on sleep quality and compared the difference between the UVB-IOL or BF-IOL implantation. Cataract surgery was found to significantly improve the PSQI score-derived, subjective sleep quality. However, no significant difference was found between the effect of UVB-IOLs and BF-IOLs. The results were in accordance with the theory that replacing the aging lens with an artificial IOL restores the transmitted light reaching the retina and thereby stimulates the activity of all photoreceptors, rods, cones, and the directly photosensitive retinal ganglion cells (pRGCs) and, consequently, the responses to the environmental irradiance, including the sleep systems[21]–[23].
Yet, it remains controversial which type of IOL is better, considering sleep quality improvements for the elderly. Some researchers have argued that it is beneficial to implant the BF-IOL for improving sleep quality based on the theory that the BF-IOL blocks the short wavelength spectrum blue light more efficiently than the UVB-IOL does[15], consequently protecting the RPE from photochemical damage[24]–[27]. However, this study, consistent with several previous studies[3],[5]–[8],[16]–[17], found no significant difference between the UVB-IOL and BF-IOL with respect to sleep quality in senile cataract patients. This finding can be partly explained by the relatively high blue light transmission of 80% and 95%, respectively, compared with 32% in the participants before cataract surgery. Thus, cataract surgery increases the blue light transmission by approximately 250% and 300%, covering the effect of the 15% difference between the UVB-IOL and the BF-IOL[8].
The difference between the effects of the UVB-IOL and the BF-IOL has long been discussed. Ham et al[28] showed that light-induced retina damage from ultraviolet V is associated with the exposure time-span and light intensity. BF-IOLs can prevent part of this light-induced retinal damage and guards against the initiation and development of AMD[29]–[30]. Furthermore, BF-IOLs improve contrast sensitivity, reduce glare under photopic and mesopic conditions[31], and compromise the disturbance of blue color vision[32]–[33]. However, the blue-blocking IOL still maintains a certain amount of blue light transmission to the retina and may improve sleep quality due to the yellow crystal[21]–[22]. Adjusting the lighting to 460-480 nm would possibly minimize any retinal injury while still retaining the most effective short wavelengths of light necessary for circadian entrainment[23].
The findings of the study should be interpreted within the few limitations. First, only a few publications reached our standard. Only one was a semi-randomized trial, and the others were not. The nonrandomized artificial crystal implantation might have caused bias with respect to the study results and might have affected the reliability of the estimate. Second, the participators in the studies came from different areas, even different races, with a varied understanding of and tolerance for sleep disorders when they were asked to complete the PSQI. Additionally, the subjective PSQI method might have produced memorizing bias, interfering with the pooled result. Third, the records of the outcome data among the included studies were not at the same follow-up time point, which possibly affected the reliability of the calculation.
In conclusion, this study found that cataract surgery significantly improved the PSQI score-derived subjective sleep quality irrespective of the intraocular lens type implanted. These findings highlight a substantial benefit of cataract surgery on systemic health with photoreceptive restoration in addition to visual acuity improvements. Further studies with a larger sample size and a randomized study design are expected.
Acknowledgments
Authors' Contributions: Lin HT, Zheng L and Wu XH designed the study. Lin HT and Wu XH collected and analyzed data. Lin HT and Wu XH wrote the manuscript. Lin HT critically revised the manuscript. Zheng L contributed to the funding of the researches, coordinated researches and oversaw the project. All authors reviewed and finally approved the manuscript.
Foundations: Supported by the Key Research Plan for the National Natural Science Foundation of China in Cultivation Project (No.91546101); the Outstanding Young Teacher Cultivation Projects in Guangdong Province (No.YQ2015006); Special Program for Applied Research on Super Computation of the NSFC-Guangdong Joint Fund (the second phase) (No.2016NSFC-GD-05); the Pearl River Science and Technology New Star Project of Guangzhou City (No.2014J2200060); the Guangdong Provincial Natural Science Foundation for Distinguished Young Scholars of China (No.2014A030306030); the Youth Science and Technology Innovation Talents Funds in a Special Support Plan for High Level Talents in Guangdong Province (No.2014TQ01R573); the Young Teacher Top-Support project of Sun Yat-sen University (No.2015ykzd11); Fundamental Research Funds of the State Key Laboratory of Ophthalmology (No.2015QN01).
Conflicts of Interest: Zheng L, None; Wu XH, None; Lin HT, None.
References
- 1.Meuleners LB, Hendrie D, Fraser ML, Ng JQ, Morlet N. The impact of first eye cataract surgery on mental health contacts for depression and/or anxiety: a population-based study using linked data. Acta Ophthalmol. 2013;91(6):e445–e449. doi: 10.1111/aos.12124. [DOI] [PubMed] [Google Scholar]
- 2.Kessel L, Siganos G, Jorgensen T, Larsen M. Sleep disturbances are related to decreased transmission of blue light to the retina caused by lens yellowing. Sleep. 2011;34(9):1215–1219. doi: 10.5665/SLEEP.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander I, Cuthbertson FM, Ratnarajan G, Safa R, Mellington FE, Foster RG, Downes SM, Wulff K. Impact of cataract surgery on sleep in patients receiving either ultraviolet-blocking or blue-filtering intraocular lens implants. Invest Ophthalmol Vis Sci. 2014;55(8):4999–5004. doi: 10.1167/iovs.14-14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asplund R, Ejdervik LB. The development of sleep in persons undergoing cataract surgery. Arch Gerontol Geriatr. 2002;35(2):179–187. doi: 10.1016/s0167-4943(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 5.Ayaki M, Muramatsu M, Negishi K, Tsubota K. Improvements in sleep quality and gait speed after cataract surgery. Rejuvenation Res. 2013;16(1):35–42. doi: 10.1089/rej.2012.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ayaki M, Negishi K, Suzukamo Y, Tsubota K. Color of intra-ocular lens and cataract type are prognostic determinants of health indices after visual and photoreceptive restoration by surgery. Rejuvenation Res. 2015;18(2):145–152. doi: 10.1089/rej.2014.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayaki M, Negishi K, Tsubota K. Rejuvenation effects of cataract surgery with ultraviolet blocking intra-ocular lens on circadian rhythm and gait speed. Rejuvenation Res. 2014;17(4):359–365. doi: 10.1089/rej.2014.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brondsted AE, Sander B, Haargaard B, Lund-Andersen H, Jennum P, Gammeltoft S, Kessel The effect of cataract surgery on circadian photoentrainment: a randomized trial of blue-blocking versus neutral intraocular lenses. Ophthalmology. 2015;122(10):2115–2124. doi: 10.1016/j.ophtha.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 9.Danquah L, Kuper H, Eusebio C, Rashid MA, Bowen L, Foster A, Polack S. The long term impact of cataract surgery on quality of life, activities and poverty: results from a six year longitudinal study in Bangladesh and the Philippines. PLoS One. 2014;9(4):e94140. doi: 10.1371/journal.pone.0094140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser ML, Meuleners LB, Lee AH, Ng JQ, Morlet N. Vision, quality of life and depressive symptoms after first eye cataract surgery. Psychogeriatrics. 2013;13(4):237–243. doi: 10.1111/psyg.12028. [DOI] [PubMed] [Google Scholar]
- 11.Gray CS, Karimova G, Hildreth AJ, Crabtree L, Allen D, O'Connell JE. Recovery of visual and functional disability following cataract surgery in older people: Sunderland Cataract Study. J Cataract Refract Surg. 2006;32(1):60–66. doi: 10.1016/j.jcrs.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 12.Ishii K, Kabata T, Oshika T. The impact of cataract surgery on cognitive impairment and depressive mental status in elderly patients. Am J Ophthalmol. 2008;146(3):404–409. doi: 10.1016/j.ajo.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Wei X, She C, Chen D, Yan F, Zeng J, Zeng L, Wang L. Blue-light-blocking intraocular lens implantation improves the sleep quality of cataract patients. J Clin Sleep Med. 2013;9(8):741–745. doi: 10.5664/jcsm.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sparrow JR, Miller AS, Zhou J. Blue light-absorbing intraocular lens and retinal pigment epithelium protection in vitro. J Cataract Refract Surg. 2004;30(4):873–878. doi: 10.1016/j.jcrs.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 15.Tanito M, Okuno T, Ishiba Y, Ohira A. Transmission spectrums and retinal blue-light irradiance values of untinted and yellow-tinted intraocular lenses. J Cataract Refract Surg. 2010;36(2):299–307. doi: 10.1016/j.jcrs.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 16.Erichsen JH, Brondsted AE, Kessel L. Effect of cataract surgery on regulation of circadian rhythms. J Cataract Refract Surg. 2015;41(9):1997–2009. doi: 10.1016/j.jcrs.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Landers JA, Tamblyn D, Perriam D. Effect of a blue-light-blocking intraocular lens on the quality of sleep. J Cataract Refract Surg. 2009;35(1):83–88. doi: 10.1016/j.jcrs.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorgensen L, Paludan-Muller AS, Laursen DR, Savović J, Boutron I, Sterne JA, Higgins JP, Hróbjartsson A. Evaluation of the Cochrane tool for assessing risk of bias in randomized clinical trials: overview of published comments and analysis of user practice in Cochrane and non-Cochrane reviews. Syst Rev. 2016;5(1):80. doi: 10.1186/s13643-016-0259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones L, Bellis MA, Wood S, Hughes K, McCoy E, Eckley L, Bates G, Mikton C, Shakespeare T, Officer A. Prevalence and risk of violence against children with disabilities: a systematic review and Meta-analysis of observational studies. Lancet. 2012;380(9845):899–907. doi: 10.1016/S0140-6736(12)60692-8. [DOI] [PubMed] [Google Scholar]
- 21.Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47(7):946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herljevic M, Middleton B, Thapan K, Skene DJ. Light-induced melatonin suppression: age-related reduction in response to short wavelength light. Exp Gerontol. 2005;40(3):237–242. doi: 10.1016/j.exger.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Patel AS, Dacey DM. Relative effectiveness of a blue light-filtering intraocular lens for photoentrainment of the circadian rhythm. J Cataract Refract Surg. 2009;35(3):529–539. doi: 10.1016/j.jcrs.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 24.Cugati S, Mitchell P, Rochtchina E, Tan AG, Smith W, Wang JJ. Cataract surgery and the 10-year incidence of age-related maculopathy: the Blue Mountains Eye Study. Ophthalmology. 2006;113(11):2020–2025. doi: 10.1016/j.ophtha.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 25.Klein R, Klein BE, Jensen SC, Cruickshanks KJ. The relationship of ocular factors to the incidence and progression of age-related maculopathy. Arch Ophthalmol. 1998;116(4):506–513. doi: 10.1001/archopht.116.4.506. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Klein BE, Wong TY, Tomany SC, Cruickshanks KJ. The association of cataract and cataract surgery with the long-term incidence of age-related maculopathy: the Beaver Dam eye study. Arch Ophthalmol. 2002;120(11):1551–1558. doi: 10.1001/archopht.120.11.1551. [DOI] [PubMed] [Google Scholar]
- 27.Wang JJ, Klein R, Smith W, Klein BE, Tomany S, Mitchell P. Cataract surgery and the 5-year incidence of late-stage age-related maculopathy: pooled findings from the Beaver Dam and Blue Mountains eye studies. Ophthalmology. 2003;110(10):1960–1967. doi: 10.1016/s0161-6420(03)00816-9. [DOI] [PubMed] [Google Scholar]
- 28.Ham WJ, Jr, Mueller HA, Ruffolo JJ, Jr, Clarke AM. Sensitivity of the retina to radiation damage as a function of wavelength. Photochem Photobiol. 1979;29(4):735–743. doi: 10.1111/j.1751-1097.1979.tb07759.x. [DOI] [PubMed] [Google Scholar]
- 29.Ham WJ, Jr, Mueller HA, Sliney DH. Retinal sensitivity to damage from short wavelength light. Nature. 1976;260(5547):153–155. doi: 10.1038/260153a0. [DOI] [PubMed] [Google Scholar]
- 30.Ham WJ, Jr, Ruffolo JJ, Jr, Mueller HA, Guerry DR., 3rd The nature of retinal radiation damage: dependence on wavelength, power level and exposure time. Vision Res. 1980;20(12):1105–1111. doi: 10.1016/0042-6989(80)90047-4. [DOI] [PubMed] [Google Scholar]
- 31.Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453(7191):102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altimus CM, Guler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, Hattar S. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010;13(9):1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grunert U, Jusuf PR, Lee SC, Nguyen DT. Bipolar input to melanopsin containing ganglion cells in primate retina. Vis Neurosci. 2011;28(1):39–50. doi: 10.1017/S095252381000026X. [DOI] [PubMed] [Google Scholar]