Abstract
Adaptive optics scanning laser ophthalmoscopy (AO-SLO) has been a promising technique in funds imaging with growing popularity. This review firstly gives a brief history of adaptive optics (AO) and AO-SLO. Then it compares AO-SLO with conventional imaging methods (fundus fluorescein angiography, fundus autofluorescence, indocyanine green angiography and optical coherence tomography) and other AO techniques (adaptive optics flood-illumination ophthalmoscopy and adaptive optics optical coherence tomography). Furthermore, an update of current research situation in AO-SLO is made based on different fundus structures as photoreceptors (cones and rods), fundus vessels, retinal pigment epithelium layer, retinal nerve fiber layer, ganglion cell layer and lamina cribrosa. Finally, this review indicates possible research directions of AO-SLO in future.
Keywords: adaptive optics, scanning laser ophthalmoscopy, retina, fundus imaging
INTRODUCTION
Adaptive Optics, From Starry Sky to Eye
Adaptive optics (AO) is defined as a discipline to improve the performance of an optical system by reducing the effect of wavefront distortions[1]. Wavefront of light is a imaginary surface representing its propagating direction[2]; just like water wave, distortion of wavefront can indicate environment information. The modern AO originated from astronomy in 1950s, when Babcock[3] put forward wavefront information could be used to adjust atmosphere turbulence. This hypothesis was realized in 1977[4] and within another two decades, 10-meter class AO telescopes achieved a precision near diffraction limit[5].
The human eye is also an optical system, in which the counterpart of atmosphere turbulence is wavefront aberration. Aberration is the difference between reference wavefront and the actual wavefront for every point over the pupil[2]. In 19th century, Helmholtz described the human eye as an imperfect optic system, the main reason was the existence of aberration[6]. Aberration of the eye can be described by Zernike polynomials[7], the 2nd order components of which are defocus and astigmatism[8]; the higher order components can also pose an influence on the eye. The ocular aberration limits the resolution of non-AO equipment, such as the scanning laser ophthalmoscopy (SLO) without AO technique.
After its success in astronomy, researchers had tried to apply AO technique in ophthalmology. In 1989, wavefront corrector (an active optical focusing unit at that time) was firstly introduced into fundus imaging, with a considerable improvement on the resolution of SLO[9]. In 1990s, after the great successful introduction of Hartman-Shack wavefront sensor[10] and the deformable mirror in ophthalmology, it was available to perform single-cellular imaging noninvasively in vivo of cone cells[11] with AO flood-illumination ophthalmoscopy (AO-FIO). In 2002, the first AO-SLO was available by introducing AO technique into SLO, which had a higher axial and transverse resolution and better contrast comparing with AO-FIO[12].
Now, many published literatures on AO fundus imaging are done with AO-SLO. This review and update will focus on AO-SLO with the last part to indicate some future research directions.
A COMPARISON OF AO-SLO WITH OTHER FUNDUS IMAGING METHODS
AO-SLO Versus Conventional Fundus Imaging Methods
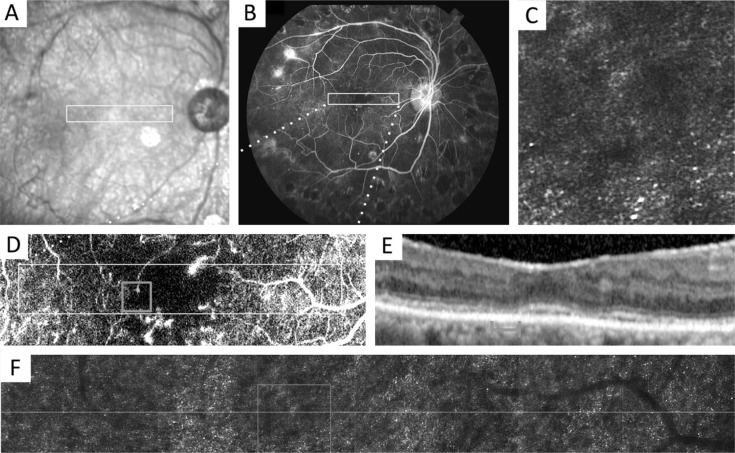
Fundus fluorescein angiography (FFA), indocyanine green angiography (ICGA), optical coherence tomography (OCT) and fundus autofluorescence (FAF) are now widely used methods in fundus imaging. A comparison of these methods with AO-SLO is shown in Table 1[13]–[17]. The main advantage of AO-SLO over the conventional ones is a higher transverse resolution without invasion, which has furthered our observation of retina. An example of multimodal fundus imaging of the same eye with diabetic retinopathy (DR) by FFA, OCT and AO-SLO is given in Figure 1[20].
Table 1. A comparison of AO-SLO with FAF, FFA, ICGA and OCT.
| Methods | Invasive | aTransverse resolution | 1,aField angle | First time available | Applications |
| AO-SLO | 2N | 2.5 µm | 1.5° | 2002[12] | Observing cones, rods, vessel and capillary, nerve fiber layer etc (Table 2). |
| FAF | N | 20 µm | 50° | 31970s[13] | Retinal pseudodrusen, macular edema, choroidal neovascularis[14] choroquine and hydroxychloroquine retinopathy[15] etc. |
| FFA | Y | 20 µm | 50° | 1960[16] | Fundus neovascularization, aneurysms, tumor, telangiectasis, edema, vitreous inflammation[14] etc. |
| ICGA | Y | 20 µm | 50° | 1970 in eye[17] | Choroidal vasculopathy, exudative AMD, inflammation and tumors, central serous chorioretinopathy[15] etc. |
| OCT | N | 420 µm | 45° | 1991[18] | 5Vitreoretinal interface disorders, central serous chorioretinopathy, AMD, diabetic retinopathy[15] etc. |
1Pupil diameter at about 6 mm; 2AO-SLO fluorescein angiography not included; 3Fundus autofluorescence was reported around 1870s[19], but it was not explored for clinical use until 1970s; 4Axial resolution is about 5 µm; 5AO-OCT and angio-OCT not included; aThe approximate data.
Figure 1. Multimodal imaging of a patient's right eye with diabetic retinopathy.
A: Fundus image with location of AO-SLO montage (box); B: Fundus fluorescein angiography (FFA); C: Enlarged 1°×1° AO-SLO image from F; D: Enlarged FFA from B; E: Spectral-domain OCT (SD-OCT) registered to A; F: AO-SLO montage stitched from 2°×2° images with location of SD-OCT (line) and AO-SLO inset (box). This figure is from reference[20] under the permission of cc BY 4.0.
Characteristics of Different Adaptive Optics Equipment
Now three AO fundus imaging methods in research are AO-FIO, AO-OCT and AO-SLO. With AO, all of them can achieve a high transverse resolution; the characteristics of them are as following:
AO-FIO
AO-FIO was invented earlier than AO-SLO and AO-OCT, and is also the only equipment available on market now (rtx1, Imagine Eyes)[21]. AO-FIO is able to observe structures like capillaries and the outer segment of cones[21]; the imaging time of one frame is short with good quality. Moreover, the cost to build an AO-FIO system is usually lower than AO-SLO and AO-OCT as the structure is less complex. However, the axial resolution of AO-FIO is low (about 300 µm) and the observation in axial direction is usually limited.
AO-OCT
As a combination of AO and OCT technique, AO-OCT has a high resolution both transversely and axially, which enables 3D cellular imaging; AO-OCT may play an important role in fundus functional imaging in the future with this advantage[22]. The main limitation of AO-OCT is its inability to detect fluorescent signals, which restricts the imaging of a specific fundus structure[22]. Another limitation of AO-OCT is the slow speed of 3D imaging[23], which may reduce imaging quality with eye movement.
AO-SLO
Comparing with AO-OCT, the axial resolution of AO-SLO is lower (about 5 µm versus about 100 µm[24]). AO-SLO can detect fluorescent signals, which enables imaging methods like AO-SLO fluorescein angiography (AO-SLO FA). Another advantage of AO-SLO is its high recording speed of single frame (about 30 frame per second; FPS)[25].
RESEARCH OF AO-SLO IN OPHTHALMOLOGY
Imaging Methods
Besides the confocol photographing model, many new imaging methods spring up in AO-SLO research, which makes more structures available to be observed and more parameters to be measured.
Fluorescent imaging methods
With an ability to detect fluorescence of SLO, AO-SLO fluorescent imaging methods developed. FFA is a frequently used diagnostic technique in fundus vascular diseases, however, the morphologic information from FFA is incomplete in observing structures like the capillary network[26]. With a higher resolution than FFA, AO-SLO FA can afford more detailed information. Similarly, AO-SLO autofluorescence (AO-SLO AF) and AO-SLO indocyanine green (AO-ICG), as a combination of AO-SLO with AF and ICG imaging respectively, have been applied in photographing fundus structure like retinal pigment epithelium (RPE)[27]–[28]. Another imaging method, two-photon fluorescence AO-SLO (TPF AO-SLO) is able to image a wide variety of structural details in animals, such as ganglion cells, Müller cell processes of the Macaca rhesus[29]. However, with an uncertain safety as using near-infrared exciting light[29]–[31], no research in humans has been published up to now.
Non-confocol imaging method
In split detector AO-SLO, a reflective mask is placed to reflect the confocal signal to a first detector and transmit the multi-scattered light to another two incoherent detectors, thus the confocal signals and the split-detector signals (calculated from non-confocal detectors) can be recorded simultaneously, with a perfect spatial registration[32]. The split detector AO-SLO has been applied in imaging of retinal structure like cones and rods in patients and healthy controls[33]. Using offset pinhole is another method in nonconfocal imaging, which can get structural and perfusion images noninvasively similar to those by AO-SLO FA[34]. With multi-offset pinhole, single neurons in the retinal ganglion cell layer (GCL) are able to be imaged[31], which is of significant difficulty and has only been imaged in animals with TPF AO-SLO before.
Photoreceptors
In 1997, adaptive optics (an AO-FIO) was first successfully[11] applied in ophthalmology to observe cones. AO-SLO has been used in research of photoreceptors for over a decade, in a wide variety of diseases (Table 2). Several parameters of photoreceptor mosaic are studied as following:
Table 2. AO-SLO in fundus imaging.
| Fundus structures | 1Participants |
| RNFL | Normal[47]; Glaucoma[49] |
| Ganglion cell layer | Normal[31] (multi-offset imaging) |
| Cones | Normal (density)[35],[52]–[53]; RP[33]b,[54]; Achromatopsia[55]; Stargardt's disease[56]; APMPPE[57]; Laser maculopathy[58]; DR[36]; AMD[59]; CSC[60]; ERM[61]; AZOOR[62]; Usher syndrome[33]b; AIR[63]; NARP[64]; Pseudodrusen[65]; Type 2 Mac Tel[66] |
| Rods | Normal[67] (density[35] reflectance[68]); RP[33]b; Achromatopsia[55]; Stargardt's[56]; Usher syndrome[33]b |
| RPE | Normal[27]c-[28]; AMD[51]c |
| Lamina cribrosa | Normal[48]; Glaucoma[50] |
| Fundus vessel research | |
| Thickness and ratio | Arteriole wall thickness[40]–[41]; Wall to lumen ratio[69] |
| Blood cells | 2Velocity of leukocyte[70]–[71]; Velocity[42] and aggregation[72] of RBC |
| Parafoveal network | Microvascular density[43]; Foveal avascular zone[44]–[45]; Network and vascular perfusion condition[34],[43]a; Hemodynamics[73]–[74]; Oxygen saturation[75] |
| Diseases | Type 2 diabetes[76]; DR[74],[77]–[78]; Hypertension[41],[69]; MA[46]a; CRVO[79]; Choroideremia (Choroidal vessels)[80] |
AIR: Autoimmune retinopathy; AMD: Age-related macular degeneration; APMPPE: Acute posterior multifocal placoid pigment epitheliopathy; AZOOR: Acute zonal occult outer retinopathy; BVMD: Best vitelliform macular dystrophy; CRVO: Central retinal vein occlusion; CSC: Central serous chorioretinopathy; DR: Diabetic retinopathy; ERM: Epiretinal membrane; MA: Microaneurysms; Mac Tel: Macular telangiectasia; NARP: Neurogenic weakness, ataxia and retinitis pigmentosa syndrome; RBC: Red blood cell; RNFL: Retinal nerve fiber layer; RP: Retinitis pigmentosa; RPE: Retinal pigment epithelium1. 1For research compared patients with healthy controls, only the diseases listed. Special imaging methods: aAO-SLO fluorescein angiography (AO-SLO FA); bSplit-detector AO-SLO; cAO enhanced indocyanine green ophthalmoscopy (AO-ICG). 2Uji et al[71] suggested the moving transparent particles may be leukocytes or plasma gaps.
Density and distribution
Most studies are focused on cones, as imaging of rods is proved to be more difficult[35]. The density of cones is reported to decrease from fovea (about 164 000/mm2) to periphery retina (about 6700/mm2 NR and 5400/mm2 TR at 30°), while density of rods reaches a peak at about 25°NR (about 124 000/mm2) and 20°TR (about 120 000/mm2)[35]. Alteration to photoreceptors has been observed in many different diseases (Table 2)[27]–[80].
Reflectance
As wave-guiding cells, change to reflectance of the photoreceptors is observed in different diseases, such as the dark patches in diabetic retinopathy (DR) patients[36]. However, as the reflectance of photoreceptors in normal people can also be low, it should be careful to evaluate reflectance as an indicator of funds diseases[37]–[38].
Descriptive metrics of mosaic
Besides density, many other metrics have been introduced to describe the photoreceptor mosaic statistically, which may be helpful in auto-analysis of mosaic pattern in the future, such as the spacing metrics like nearest neighbor distance and regularity metrics like nearest neighbor regularity[39]. The regularity metrics are proved to be more sensitive in tracking changes of mosaic like diffuse loss[39].
Fundus Vessels
AO-SLO is able to image fine vessel structures like the capillary and the arteriole wall, and to detect fluoscence with a compatibility with FA, AF and ICG imaging. AO-SLO has been applied in research of different vessel parameters like arteriole thickness[40]–[41], velocity of blood cells in capillary[42], parafoveal capillary density[43] and area of foveal avascular zone[44]–[45]. AO-SLO can also reveal structural details which are difficult to be observed with conventional methods, such as the microscopic features of retinal microaneurysms[46].
Other Structures
Ganglion cell body, retinal nerve fibre layer and lamina cribrosa
All these three structures may play a role in glaucoma study and have been successfully imaged in human noninvasively[31],[47]–[48]. AO-SLO can reveal normal and abnormal retinal nerve bundles in glaucoma patients[49]. AO-SLO can also reveal pores of lamina cribrosa; comparing with normal participants, the mean area in glaucoma patients is significantly larger[50].
Retinal pigment epithelium
RPE can be revealed by AO-ICG and AO-SLO AF[27]. Reflectance imaging of RPE cells with AO-SLO in normal fundus is difficult as the wave-guiding nature of overlying photoreceptors obscures signals from RPE[28]. Imaging of RPE cells with AO-ICG is based on that they can take up injected ICG dye, which can be detected by AO-SLO[27]. AO-SLO AF is able to image RPE mosaic in vivo because it can detect autofluorescence from lipofuscin in RPE cells[28]. In age-related macular degeneration (AMD) patients, research indicates RPE cell morphologies by AO-SLO AF are markedly similar to those seen in postmortem histological studies[51].
FUTURE RESEARCH DIRECTION
Possible research direction of AO-SLO may exist in the following fields:
Imaging
Although AO-SLO has a high transverse resolution to detect single photoreceptors and capillaries, its field angle at one scan is small, or a corresponding retinal area of about 0.085 to 0.34 mm2[81], thus it's sometimes difficult to image the whole area of interest. One method to get an image of larger area is by mosaicking multiple images, which could expand the field angle to over 20°[82]. Another efficient but difficult method is to increase field angle at one scan. Dual-conjugate adaptive optics technique may be one such method, which may increase the field angle to about 7°×7° in one scan[83].
In axial direction, with development of imaging techniques especially non-confocal ones (split detection, offset pinhole, etc), more fundus structures can be observed with AO-SLO, such as ganglion cell body[31] etc. In the near future, structures like choroidal capillaries may be imaged noninvasively.
Eye movement will bring intraframe distortion which affects the imaging quality of AO-SLO, especially in less cooperative patients. Even in eye fixed on a target, there is continuous eye movement[25]. A higher scanning speed or movement compensation may be helpful to achieve a better imaging quality. Lu et al[25] has succeeded to reduce distortion by 50.9% to 79.7% with high imaging speed at 200 FPS.
Automatic algorithms
Processing images from AO-SLO can be time consuming and expert-depended, with requirement of knowledge on both image processing and medicine, which forms a barrier to promote this technique. Therefore some algorithms have been developed for AO-SLO, in automated reference frame selection[84], multiple-images montaging[81] and quantitative analysis of the photoreceptors[53],[85]. However, as far as we know, there is no fully-automatic software available now for the whole procedure from imaging to outputting analytic result, which should be user-friendly and no need of coding.
Clinical practice
Available firstly in 1991[18], OCT was soon applied in research of fundus diseases[86]–[87]. OCT got popular in hospital after entering market in 1996 and its success is dependent on the development of OCT-guided clinical practice. OCT guided diagnosis[88], therapy decision[89] and follow-up[90] has been a daily part of clinic ophthalmology.
Like OCT, AO-SLO is a non-invasive examination. Development of AO-SLO guided clinical practice will improve our understanding and management of ocular diseases and promote this technique concurrently. Future research may focus on the following aspects: 1) Diagnosis. AO-SLO can detect microstructural alterations, such as microvascular damage and morphologic changes of photoreceptors before appearing clinical symptoms, which may be applied in early diagnosis and prevention. In type 2 diabetes without sign of DR, AO-SLO can detect changes of parafoveal capillary network[76]. In patients with retinitis pigmentosa or Usher syndrome, it's reported the visual acuity could be kept in normal range with over 35% fall of cone density[33]. AO-SLO may detect some pathological alterations not seen in OCT[91]. One problem in diagnosis with AO-SLO is lack of reliable reference value, such as the density of photoreceptors, nearly all published studies are with small sample and without a consideration of factors like age, gender and ethnicity[35],[52]–[53]. 2) Treatment evaluation. There are not many published studies to explore AO-SLO in estimating ocular treatment. In autoimmune retinopathy patients, AO-SLO was used to evaluate the density of cones after taking rituximab, which was stable during the period of treatment (but no control group in this study)[63]. Actually, AO-SLO has great potential to assist clinical therapy and one example is gene therapy of monogenetic retinopathy. In retinal degeneration characterized by cones or rods dysfunction, AO-SLO can be applied to monitor morphologic improvements of photoreceptors. AO-SLO endpoints may play a role as structural marker in clinical trial and practice. 3) Follow-up. With a high transverse resolution, AO-SLO guided follow-up may help us understand the structural and functional alterations in ocular diseases. AO-SLO has been reported to follow the longitudinal changes of photoreceptors (AZOOR[62]) and microvessels (DR[78]). More follow-up studies of one or multiple structures may appear in the near future.
Basic medicine research
Combining with fluorescent label, the distribution of a certain protein in retina could be detected with AO-SLO in animals[92], which makes it possible to study a particular fundus structure or cell type in vivo. AO-SLO may also be applied to research fundus physiology, especially in photoreceptors[93] and fundus vessels[94], with a transverse resolution of micron dimension and the convenience to record a video. AO-SLO may help us understand the mechanism of glaucoma better as it can image retinal microvessles, RNFL, GCL and lamina cribrosa in vivo noninvasively[31],[47]–[48].
CONCLUSION
With increasing studies and publications, AO-SLO has been a promising technique. AO-SLO has played an important role in observing microstructures of living human retina with growing popularity. Development of imaging method and progress in clinical application will be made in the coming years, with innovation and cooperation of multiple disciplines from physics to medicine. However, there is still much research work to pave the road for clinical practice of AO-SLO. Thus more high-quality studies are expected on imaging, algorithms and clinical applications in future.
Acknowledgments
Foundation: Supported by National Key Scientific Instrument and Equipment Development Project of China (No.2012YQ12008005).
Conflicts of Interest: Zhang B, None; Li N, None; Kang J, None; He Y, None; Chen XM, None.
REFERENCES
- 1.Wikipedia contributors Adaptive optics. 18 April 2017 16 July 2017; Available at: https://en.wikipedia.org/w/index.php?title=Adaptive_optics&oldid=776097664.
- 2.Venkateswaran K, Romero-Borja F, Roorda A. Design of an Adaptive Optics Scanning Laser Ophthalmoscope. In: Porter J., Queener H. M., Lin J. E., Thorn K., Awwal A., editors. Adaptive Optics for Vision Science: Principles, Practices, Design, and Applications. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2016. [Google Scholar]
- 3.Babcock HW. The possibility of compensating astronomical seeing. Publications of the Astronomical Society of the Pacific. 1953;65(386):229–236. [Google Scholar]
- 4.Hardy JW, Lefebvre JE, Koliopoulos CL. Real-time atmospheric compensation. J Opt Soc Am. 1977;67(3):360–369. [Google Scholar]
- 5.Léna P. Adaptive optics: a breakthrough in astronomy. Experimental Astronomy. 2009;26(1):35–48. [Google Scholar]
- 6.Mrochen M. Is the human eye a perfect optic? Proc Spie. 2001;4245(1):30–35. [Google Scholar]
- 7.Zernike VF. Beugungstheorie des schneidenver-fahrens und seiner verbesserten form, der phasenkontrastmethode. Physica D: Nonlinear Phenomena. 1934;1(7):689–704. [Google Scholar]
- 8.Campbell CE. A new method for describing the aberrations of the eye using Zernike polynomials. Optom Vis Sci. 2003;80(1):79–83. doi: 10.1097/00006324-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Dreher AW, Bille JF, Weinreb RN. Active optical depth resolution improvement of the laser tomographic scanner. Appl Opt. 1989;28(4):804–808. doi: 10.1364/AO.28.000804. [DOI] [PubMed] [Google Scholar]
- 10.Liang J, Grimm B, Goelz S, Bille JF. Objective measurement of wave aberrations of the human eye with the use of a Hartmann-Shack wave-front sensor. J Opt Soc Am A Opt Image Sci Vis. 1994;11(7):1949–1957. doi: 10.1364/josaa.11.001949. [DOI] [PubMed] [Google Scholar]
- 11.Liang J, Williams DR, Miller DT. Supernormal vision and high-resolution retinal imaging through adaptive optics. J Opt Soc Am A Opt Image Sci Vis. 1997;14(11):2884–2892. doi: 10.1364/josaa.14.002884. [DOI] [PubMed] [Google Scholar]
- 12.Roorda A, Romero-Borja F, Donnelly Iii W, Queener H, Hebert T, Campbell M. Adaptive optics scanning laser ophthalmoscopy. Opt Express. 2002;10(9):405–412. doi: 10.1364/oe.10.000405. [DOI] [PubMed] [Google Scholar]
- 13.Liebman PA, Leigh RA. Autofluorescence of visual receptors. Nature. 1969;221(5187):1249–1251. doi: 10.1038/2211249a0. [DOI] [PubMed] [Google Scholar]
- 14.Frampton GK, Kalita N, Payne L, Colquitt JL, Loveman E, Downes SM, Lotery AJ. Fundus autofluorescence imaging: systematic review of test accuracy for the diagnosis and monitoring of retinal conditions. Eye (Lond) 2017;31(7):995–1007. doi: 10.1038/eye.2017.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan Stephen J., Sadda Srinivas R., Hinton David R., Schachat Andrew P. Retina. Fifth Edition ed. Saunders; 2013. [Google Scholar]
- 16.Novotny HR, Alvis DL. A method of photographing fluorescence in circulating blood in the human retina. Circulation. 1961;24(1):82–86. doi: 10.1161/01.cir.24.1.82. [DOI] [PubMed] [Google Scholar]
- 17.Kogure K, David NJ, Yamanouchi U, Choromokos E. Infrared absorption angiography of the fundus circulation. Arch Ophthalmol. 1970;83(2):209–214. doi: 10.1001/archopht.1970.00990030211015. [DOI] [PubMed] [Google Scholar]
- 18.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kühne . On the Photochemistry of the Retina and on Visual Purple. London: Forgotten Books; 2013. [Google Scholar]
- 20.Nesper PL, Scarinci F, Fawzi AA. Adaptive Optics Reveals Photoreceptor Abnormalities in Diabetic Macular Ischemia. PLoS One. 2017;12(1):e0169926. doi: 10.1371/journal.pone.0169926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ra E, Ito Y, Kawano K, Iwase T, Kaneko H, Ueno S, Yasuda S, Kataoka K, Terasaki H. Regeneration of photoreceptor outer segments after scleral buckling surgery for rhegmatogenous retinal detachment. Am J Ophthalmol. 2017;177:17–26. doi: 10.1016/j.ajo.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 22.Jonnal RS, Kocaoglu OP, Zawadzki RJ, Liu Z, Miller DT, Werner JS. A review of adaptive optics optical coherence tomography: technical advances, scientific applications, and the future. Invest Ophthalmol Vis Sci. 2016;57(9):OCT51–68. doi: 10.1167/iovs.16-19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liba O, SoRelle ED, Sen D, de la Zerda A. Contrast-enhanced optical coherence tomography with picomolar sensitivity for functional in vivo imaging. Sci Rep. 2016;6:23337. doi: 10.1038/srep23337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkateswaran K, Roorda A, Romero-Borja F. Theoretical modeling and evaluation of the axial resolution of the adaptive optics scanning laser ophthalmoscope. J Biomed Opt. 2004;9(1):132–138. doi: 10.1117/1.1627775. [DOI] [PubMed] [Google Scholar]
- 25.Lu J, Gu B, Wang X, Zhang Y. High-speed adaptive optics line scan confocal retinal imaging for human eye. PLoS One. 2017;12(3):e0169358. doi: 10.1371/journal.pone.0169358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendis KR, Balaratnasingam C, Yu P, Barry CJ, McAllister IL, Cringle SJ, Yu DY. Correlation of histologic and clinical images to determine the diagnostic value of fluorescein angiography for studying retinal capillary detail. Invest Ophthalmol Vis Sci. 2010;51(11):5864–5869. doi: 10.1167/iovs.10-5333. [DOI] [PubMed] [Google Scholar]
- 27.Tam J, Liu J, Dubra A, Fariss R. In vivo imaging of the human retinal pigment epithelial mosaic using adaptive optics enhanced indocyanine green ophthalmoscopy. Invest Ophthalmol Vis Sci. 2016;57(10):4376–4384. doi: 10.1167/iovs.16-19503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan JI, Dubra A, Wolfe R, Merigan WH, Williams DR. In vivo autofluorescence imaging of the human and macaque retinal pigment epithelial cell mosaic. Invest Ophthalmol Vis Sci. 2009;50(3):1350–1359. doi: 10.1167/iovs.08-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma R, Williams DR, Palczewska G, Palczewski K, Hunter JJ. Two-photon autofluorescence imaging reveals cellular structures throughout the retina of the living primate eye. Invest Ophthalmol Vis Sci. 2016;57(2):632–646. doi: 10.1167/iovs.15-17961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarz C, Sharma R, Fischer WS, Chung M, Palczewska G, Palczewski K, Williams DR, Hunter JJ. Safety assessment in macaques of light exposures for functional two-photon ophthalmoscopy in humans. Biomed Opt Express. 2016;7(12):5148–5169. doi: 10.1364/BOE.7.005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossi EA, Granger CE, Sharma R, Yang Q, Saito K, Schwarz C, Walters S, Nozato K, Zhang J, Kawakami T, Fischer W, Latchney LR, Hunter JJ, Chung MM, Williams DR. Imaging individual neurons in the retinal ganglion cell layer of the living eye. Proc Natl Acad Sci U S A. 2017;114(3):586–591. doi: 10.1073/pnas.1613445114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scoles D, Sulai YN, Langlo CS, Fishman GA, Curcio CA, Carroll J, Dubra A. In vivo imaging of human cone photoreceptor inner segments. Invest Ophthalmol Vis Sci. 2014;55(7):4244–4251. doi: 10.1167/iovs.14-14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun LW, Johnson RD, Langlo CS, Cooper RF, Razeen MM, Russillo MC, Dubra A, Connor TB, Jr, Han DP, Pennesi ME, Kay CN, Weinberg DV, Stepien KE, Carroll J. Assessing photoreceptor structure in retinitis pigmentosa and Usher syndrome. Invest Ophthalmol Vis Sci. 2016;57(6):2428–2442. doi: 10.1167/iovs.15-18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chui TY, Dubow M, Pinhas A, Shah N, Gan A, Weitz R, Sulai YN, Dubra A, Rosen RB. Comparison of adaptive optics scanning light ophthalmoscopic fluorescein angiography and offset pinhole imaging. Biomed Opt Express. 2014;5(4):1173–1189. doi: 10.1364/BOE.5.001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells-Gray EM, Choi SS, Bries A, Doble N. Variation in rod and cone density from the fovea to the mid-periphery in healthy human retinas using adaptive optics scanning laser ophthalmoscopy. Eye (Lond) 2016;30(8):1135–1143. doi: 10.1038/eye.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lammer J, Prager SG, Cheney MC, Ahmed A, Radwan SH, Burns SA, Silva PS, Sun JK. Cone Photoreceptor Irregularity on Adaptive Optics Scanning Laser Ophthalmoscopy Correlates With Severity of Diabetic Retinopathy and Macular Edema. Invest Ophthalmol Vis Sci. 2016;57(15):6624–6632. doi: 10.1167/iovs.16-19537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruce KS, Harmening WM, Langston BR, Tuten WS, Roorda A, Sincich LC. Normal perceptual sensitivity arising from weakly reflective cone photoreceptors. Invest Ophthalmol Vis Sci. 2015;56(8):4431–4438. doi: 10.1167/iovs.15-16547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pallikaris A, Williams DR, Hofer H. The reflectance of single cones in the living human eye. Invest Ophthalmol Vis Sci. 2003;44(10):4580–4592. doi: 10.1167/iovs.03-0094. [DOI] [PubMed] [Google Scholar]
- 39.Cooper RF, Wilk MA, Tarima S, Carroll J. Evaluating descriptive metrics of the human cone mosaic. Invest Ophthalmol Vis Sci. 2016;57(7):2992–3001. doi: 10.1167/iovs.16-19072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arichika S, Uji A, Murakami T, Suzuma K, Gotoh N, Yoshimura N. Correlation of retinal arterial wall thickness with atherosclerosis predictors in type 2 diabetes without clinical retinopathy. Br J Ophthalmol. 2017;101(1):69–74. doi: 10.1136/bjophthalmol-2016-309612. [DOI] [PubMed] [Google Scholar]
- 41.Hillard JG, Gast TJ, Chui TY, Sapir D, Burns SA. Retinal arterioles in hypo-, normo-, and hypertensive subjects measured using adaptive optics. Transl Vis Sci Technol. 2016;5(4):16. doi: 10.1167/tvst.5.4.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bedggood P, Metha A. Direct visualization and characterization of erythrocyte flow in human retinal capillaries. Biomed Opt Express. 2012;3(12):3264–3277. doi: 10.1364/BOE.3.003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinhas A, Razeen M, Dubow M, Gan A, Chui TY, Shah N, Mehta M, Gentile RC, Weitz R, Walsh JB, Sulai YN, Carroll J, Dubra A, Rosen RB. Assessment of perfused foveal microvascular density and identification of nonperfused capillaries in healthy and vasculopathic eyes. Invest Ophthalmol Vis Sci. 2014;55(12):8056–8066. doi: 10.1167/iovs.14-15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chui TY, Zhong Z, Song H, Burns SA. Foveal avascular zone and its relationship to foveal pit shape. Optom Vis Sci. 2012;89(5):602–610. doi: 10.1097/OPX.0b013e3182504227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubis AM, Hansen BR, Cooper RF, Beringer J, Dubra A, Carroll J. Relationship between the foveal avascular zone and foveal pit morphology. Invest Ophthalmol Vis Sci. 2012;53(3):1628–1636. doi: 10.1167/iovs.11-8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubow M, Pinhas A, Shah N, Cooper RF, Gan A, Gentile RC, Hendrix V, Sulai YN, Carroll J, Chui TY, Walsh JB, Weitz R, Dubra A, Rosen RB. Classification of human retinal microaneurysms using adaptive optics scanning light ophthalmoscope fluorescein angiography. Invest Ophthalmol Vis Sci. 2014;55(3):1299–1309. doi: 10.1167/iovs.13-13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takayama K, Ooto S, Hangai M, Arakawa N, Oshima S, Shibata N, Hanebuchi M, Inoue T, Yoshimura N. High-resolution imaging of the retinal nerve fiber layer in normal eyes using adaptive optics scanning laser ophthalmoscopy. PLoS One. 2012;7(3):e33158. doi: 10.1371/journal.pone.0033158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivers KM, Li C, Patel N, Sredar N, Luo X, Queener H, Harwerth RS, Porter J. Reproducibility of measuring lamina cribrosa pore geometry in human and nonhuman primates with in vivo adaptive optics imaging. Invest Ophthalmol Vis Sci. 2011;52(8):5473–5480. doi: 10.1167/iovs.11-7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen MF, Chui TY, Alhadeff P, Rosen RB, Ritch R, Dubra A, Hood DC. Adaptive optics imaging of healthy and abnormal regions of retinal nerve fiber bundles of patients with glaucoma. Invest Ophthalmol Vis Sci. 2015;56(1):674–681. doi: 10.1167/iovs.14-15936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akagi T, Hangai M, Takayama K, Nonaka A, Ooto S, Yoshimura N. In vivo imaging of lamina cribrosa pores by adaptive optics scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2012;53(7):4111–4119. doi: 10.1167/iovs.11-7536. [DOI] [PubMed] [Google Scholar]
- 51.Rossi EA, Rangel-Fonseca P, Parkins K, Fischer W, Latchney LR, Folwell MA, Williams DR, Dubra A, Chung MM. In vivo imaging of retinal pigment epithelium cells in age related macular degeneration. Biomed Opt Express. 2013;4(11):2527–2539. doi: 10.1364/BOE.4.002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chui TY, Song H, Burns SA. Adaptive-optics imaging of human cone photoreceptor distribution. J Opt Soc Am A Opt Image Sci Vis. 2008;25(12):3021–3029. doi: 10.1364/josaa.25.003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cunefare D, Cooper RF, Higgins B, Katz DF, Dubra A, Carroll J, Farsiu S. Automatic detection of cone photoreceptors in split detector adaptive optics scanning light ophthalmoscope images. Biomed Opt Express. 2016;7(5):2036–2050. doi: 10.1364/BOE.7.002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Makiyama Y, Ooto S, Hangai M, Takayama K, Uji A, Oishi A, Ogino K, Nakagawa S, Yoshimura N. Macular cone abnormalities in retinitis pigmentosa with preserved central vision using adaptive optics scanning laser ophthalmoscopy. PLoS One. 2013;8(11):e79447. doi: 10.1371/journal.pone.0079447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Genead MA, Fishman GA, Rha J, Dubis AM, Bonci DM, Dubra A, Stone EM, Neitz M, Carroll J. Photoreceptor structure and function in patients with congenital achromatopsia. Invest Ophthalmol Vis Sci. 2011;52(10):7298–7308. doi: 10.1167/iovs.11-7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song H, Rossi EA, Latchney L, Bessette A, Stone E, Hunter JJ, Williams DR, Chung M. Cone and rod loss in Stargardt disease revealed by adaptive optics scanning light ophthalmoscopy. JAMA Ophthalmol. 2015;133(10):1198–1203. doi: 10.1001/jamaophthalmol.2015.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberts PK, Nesper PL, Onishi AC, Skondra D, Jampol LM, Fawzi AA. Characterizing photoreceptor changes in acute posterior multifocal placoid pigment epitheliopathy using adaptive optics. Retina. 2017 doi: 10.1097/IAE.0000000000001520. [DOI] [PubMed] [Google Scholar]
- 58.Sheyman AT, Nesper PL, Fawzi AA, Jampol LM. Adaptive optics imaging in laser pointer maculopathy. Ophthalmic Surg Lasers Imaging Retina. 2016;47(8):782–785. doi: 10.3928/23258160-20160808-14. [DOI] [PubMed] [Google Scholar]
- 59.Zayit-Soudry S, Duncan JL, Syed R, Menghini M, Roorda AJ. Cone structure imaged with adaptive optics scanning laser ophthalmoscopy in eyes with nonneovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(12):7498–7509. doi: 10.1167/iovs.13-12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vogel RN, Langlo CS, Scoles D, Carroll J, Weinberg DV, Kim JE. High-Resolution Imaging of Intraretinal Structures in Active and Resolved Central Serous Chorioretinopathy. Invest Ophthalmol Vis Sci. 2017;58(1):42–49. doi: 10.1167/iovs.16-20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ooto S, Hangai M, Takayama K, Sakamoto A, Tsujikawa A, Oshima S, Inoue T, Yoshimura N. High-resolution imaging of the photoreceptor layer in epiretinal membrane using adaptive optics scanning laser ophthalmoscopy. Ophthalmology. 2011;118(5):873–881. doi: 10.1016/j.ophtha.2010.08.032. [DOI] [PubMed] [Google Scholar]
- 62.Nakao S, Kaizu Y, Yoshida S, Iida T, Ishibashi T. Spontaneous remission of acute zonal occult outer retinopathy: follow-up using adaptive optics scanning laser ophthalmoscopy. Graefes Arch Clin Exp Ophthalmol. 2015;253(6):839–843. doi: 10.1007/s00417-014-2760-x. [DOI] [PubMed] [Google Scholar]
- 63.Davoudi S, Ebrahimiadib N, Yasa C, Sevgi DD, Roohipoor R, Papavasilieou E, Comander J, Sobrin L. Outcomes in autoimmune retinopathy patients treated with Rituximab. Am J Ophthalmol. 2017;180:124–132. doi: 10.1016/j.ajo.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 64.Gelfand JM, Duncan JL, Racine CA, Gillum LA, Chin CT, Zhang Y, Zhang Q, Wong LJ, Roorda A, Green AJ. Heterogeneous patterns of tissue injury in NARP syndrome. J Neurol. 2011;258(3):440–448. doi: 10.1007/s00415-010-5775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mrejen S, Sato T, Curcio CA, Spaide RF. Assessing the cone photoreceptor mosaic in eyes with pseudodrusen and soft Drusen in vivo using adaptive optics imaging. Ophthalmology. 2014;121(2):545–551. doi: 10.1016/j.ophtha.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Q, Tuten WS, Lujan BJ, Holland J, Bernstein PS, Schwartz SD, Duncan JL, Roorda A. Adaptive optics microperimetry and OCT images show preserved function and recovery of cone visibility in macular telangiectasia type 2 retinal lesions. Invest Ophthalmol Vis Sci. 2015;56(2):778–786. doi: 10.1167/iovs.14-15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dubra A, Sulai Y, Norris JL, Cooper RF, Dubis AM, Williams DR, Carroll J. Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomed Opt Express. 2011;2(7):1864–1876. doi: 10.1364/BOE.2.001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cooper RF, Dubis AM, Pavaskar A, Rha J, Dubra A, Carroll J. Spatial and temporal variation of rod photoreceptor reflectance in the human retina. Biomed Opt Express. 2011;2(9):2577–2589. doi: 10.1364/BOE.2.002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arichika S, Uji A, Ooto S, Muraoka Y, Yoshimura N. Effects of age and blood pressure on the retinal arterial wall, analyzed using adaptive optics scanning laser ophthalmoscopy. Sci Rep. 2015;5:12283. doi: 10.1038/srep12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin JA, Roorda A. Pulsatility of parafoveal capillary leukocytes. Exp Eye Res. 2009;88(3):356–360. doi: 10.1016/j.exer.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uji A, Hangai M, Ooto S, Takayama K, Arakawa N, Imamura H, Nozato K, Yoshimura N. The source of moving particles in parafoveal capillaries detected by adaptive optics scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2012;53(1):171–178. doi: 10.1167/iovs.11-8192. [DOI] [PubMed] [Google Scholar]
- 72.Arichika S, Uji A, Hangai M, Ooto S, Yoshimura N. Noninvasive and direct monitoring of erythrocyte aggregates in human retinal microvasculature using adaptive optics scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2013;54(6):4394–4402. doi: 10.1167/iovs.12-11138. [DOI] [PubMed] [Google Scholar]
- 73.Zhong Z, Song H, Chui TY, Petrig BL, Burns SA. Noninvasive measurements and analysis of blood velocity profiles in human retinal vessels. Invest Ophthalmol Vis Sci. 2011;52(7):4151–4157. doi: 10.1167/iovs.10-6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu Y, Bernabeu MO, Lammer J, Cai CC, Jones ML, Franco CA, Aiello LP, Sun JK. Computational fluid dynamics assisted characterization of parafoveal hemodynamics in normal and diabetic eyes using adaptive optics scanning laser ophthalmoscopy. Biomed Opt Express. 2016;7(12):4958–4973. doi: 10.1364/BOE.7.004958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li H, Lu J, Shi G, Zhang Y. Measurement of oxygen saturation in small retinal vessels with adaptive optics confocal scanning laser ophthalmoscope. J Biomed Opt. 2011;16(11):110504. doi: 10.1117/1.3655354. [DOI] [PubMed] [Google Scholar]
- 76.Tam J, Dhamdhere KP, Tiruveedhula P, Manzanera S, Barez S, Bearse MA, Jr, Adams AJ, Roorda A. Disruption of the retinal parafoveal capillary network in type 2 diabetes before the onset of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(12):9257–9266. doi: 10.1167/iovs.11-8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arichika S, Uji A, Murakami T, Unoki N, Yoshitake S, Dodo Y, Ooto S, Miyamoto K, Yoshimura N. Retinal hemorheologic characterization of early-stage diabetic retinopathy using adaptive optics scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2014;55(12):8513–8522. doi: 10.1167/iovs.14-15121. [DOI] [PubMed] [Google Scholar]
- 78.Chui TY, Pinhas A, Gan A, Razeen M, Shah N, Cheang E, Liu CL, Dubra A, Rosen RB. Longitudinal imaging of microvascular remodelling in proliferative diabetic retinopathy using adaptive optics scanning light ophthalmoscopy. Ophthalmic Physiol Opt. 2016;36(3):290–302. doi: 10.1111/opo.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.P Pinhas A, Dubow M, Shah N, Cheang E, Liu CL, Razeen M, Gan A, Weitz R, Sulai YN, Chui TY, Dubra A, Rosen RB. Fellow eye changes in patients with nonischemic central retinal vein occlusion: assessment of perfused foveal microvascular density and identification of nonperfused capillaries. Retina. 2015;35(10):2028–2036. doi: 10.1097/IAE.0000000000000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morgan JI, Han G, Klinman E, Maguire WM, Chung DC, Maguire AM, Bennett J. High-resolution adaptive optics retinal imaging of cellular structure in choroideremia. Invest Ophthalmol Vis Sci. 2014;55(10):6381–6397. doi: 10.1167/iovs.13-13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen M, Cooper RF, Han GK, Gee J, Brainard DH, Morgan JI. Multi-modal automatic montaging of adaptive optics retinal images. Biomed Opt Express. 2016;7(12):4899–4918. doi: 10.1364/BOE.7.004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu R, Zheng X, Xia M, Lu X, Xuan L. Accurate fixation of adaptive optics fundus imaging field of view based on visual target guidance. Infrared and Laser Engineering. 2015;44(6):1794–1799. [Google Scholar]
- 83.Thaung J, Knutsson P, Popovic Z, Owner-Petersen M. Dual-conjugate adaptive optics for wide-field high-resolution retinal imaging. Opt Express. 2009;17(6):4454–4467. doi: 10.1364/oe.17.004454. [DOI] [PubMed] [Google Scholar]
- 84.Salmon AE, Cooper RF, Langlo CS, Baghaie A, Dubra A, Carroll J. An automated reference frame selection (ARFS) algorithm for cone imaging with adaptive optics scanning light ophthalmoscopy. Transl Vis Sci Technol. 2017;6(2):9. doi: 10.1167/tvst.6.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miyagawa S, Fukuyama H, Hirota M, Yamaguchi T, Kitamura K, Endo T, Kanda H, Morimoto T, Fujikado T. Automated measurements of human cone photoreceptor density in healthy and degenerative retina by region-based segmentation. Clin Ophthalmol. 2017;11:781–790. doi: 10.2147/OPTH.S133070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hee MR, Puliafito CA, Wong C, Duker JS, Reichel E, Schuman JS, Swanson EA, Fujimoto JG. Optical coherence tomography of macular holes. Ophthalmology. 1995;102(5):748–756. doi: 10.1016/s0161-6420(95)30959-1. [DOI] [PubMed] [Google Scholar]
- 87.Puliafito CA, Hee MR, Lin CP, Reichel E, Schuman JS, Duker JS, Izatt JA, Swanson EA, Fujimoto JG. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102(2):217–229. doi: 10.1016/s0161-6420(95)31032-9. [DOI] [PubMed] [Google Scholar]
- 88.Ouyang PB, Duan XC, Zhu XH. Diagnosis and treatment of myopic traction maculopathy. Int J Ophthalmol. 2012;5(6):754–758. doi: 10.3980/j.issn.2222-3959.2012.06.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, Puliafito CA, Davis JL, Flynn HW, Jr, Esquiabro M. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143(4):566–583. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 90.Qi HJ, Li XX, Zhang JY, Zhao MW. Efficacy and safety of ranibizumab for wet age-related macular degeneration in Chinese patients. Int J Ophthalmol. 2017;10(1):91–97. doi: 10.18240/ijo.2017.01.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khanna S, Nesper PL, Koreishi AF, Goldstein DA, Fawzi AA. Visualization of photoreceptors in birdshot chorioretinopathy using adaptive optics scanning laser ophthalmoscopy: a pilot study. Ocul Immunol Inflamm. 2017:1–11. doi: 10.1080/09273948.2017.1298819. [DOI] [PubMed] [Google Scholar]
- 92.Schallek J, Geng Y, Nguyen H, Williams DR. Morphology and topography of retinal pericytes in the living mouse retina using in vivo adaptive optics imaging and ex vivo characterization. Invest Ophthalmol Vis Sci. 2013;54(13):8237–8250. doi: 10.1167/iovs.13-12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Horton JC, Parker AB, Botelho JV, Duncan JL. Spontaneous regeneration of human photoreceptor outer segments. Sci Rep. 2015;5:12364. doi: 10.1038/srep12364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhong Z, Huang G, Chui TY, Petrig BL, Burns SA. Local flicker stimulation evokes local retinal blood velocity changes. J Vis. 2012;12(6):3. doi: 10.1167/12.6.3. [DOI] [PMC free article] [PubMed] [Google Scholar]