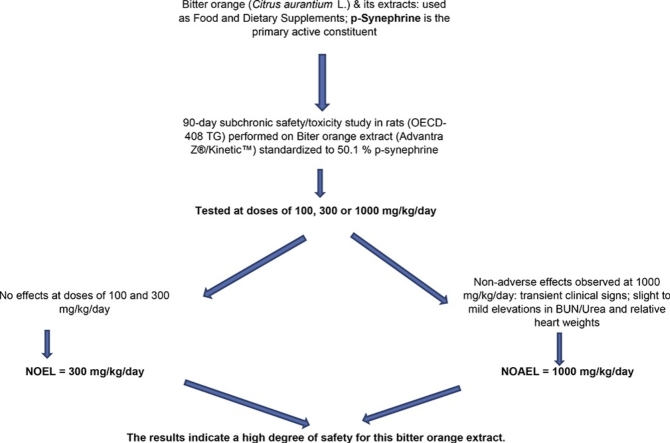
Graphical abstract
Keywords: Citrus aurantium, Bitter orange, p-Synephrine, Subchronic toxicity, No-observed-adverse-effect-level (NOAEL), No-observed-effect-level (NOEL)
Highlights
-
•
The LD50 of an extract standardized to 50% p-synephrine is greater than 5000 mg/kg in rats.
-
•
This study assessed the oral safety of this extract at doses of 100, 300 and 1000 mg/kg/day for 90 days in rats of both sexes.
-
•
No effect at 100 mg/kg and 300 mg/kg. No-observed-effect–level (NOEL) of extract was 300 mg/kg.
-
•
No-observed-adverse-effect-level (NOAEL) of the extract was 1000 mg/kg.
-
•
The results indicate a high degree of safety for this bitter orange extract. Transcient clinical signs including BUN.urea and relative heart weights.
Abstract
Bitter orange (Citrus aurantium L.) extracts are widely used in dietary supplements and bitter oranges are used in various juices and food products. p-Synephrine, the primary active constituent, comprises approximately 90% of total protoalkaloids. This study, performed per OECD 408 guidance, examined the 90-day subchronic safety/toxicity of an extract standardized to 50% p-synephrine at doses of 100, 300 and 1000 mg/kg/day to male and female rats. No adverse effects were observed with respect to any of the observed parameters of clinical signs, functional observations of sensory reactivity, grip strength and motor activity, ophthalmology, body weights, hematology, food consumption, urinalysis, organ weights, as well as gross and microscopic pathology at termination at any of the doses in either sex. Treatment at 1000 mg/kg body weight/day of the extract resulted in non-adverse effects including fully reversible signs of repetitive head burrowing in the bedding material and piloerection for short periods of time in both sexes immediately after administration, which gradually disappeared by treatment day-81. A slight and reversible elevation of BUN and urea levels in male rats, and slight to mild increase in the relative but not absolute heart weights of male and female rats was observed. Based on these results, the no-observed-effect-level (NOEL) for this bitter orange extract standardized to 50% p-synephrine was 300 mg/kg, while the no-observed-adverse-effect-level (NOAEL) was 1000 mg/kg. The results indicate a high degree of safety for this bitter orange extract.
1. Introduction
p-Synephrine is the primary active constituent in bitter orange extracts, comprising approximately 90% of the total protoalkaloids [1]. The extracts are derived from the dried, unripe fruits of Citrus aurantium L. by extraction with water and ethanol [1], [2]. Various preparations of bitter oranges have been widely used for hundreds of years in foods and folk medicine [2], [3]. Although bitter orange extracts are now extensively used in dietary supplements and p-synephrine occurs in various Citrus juices and food products, subchronic safety studies on this extract and p-synephrine have not been conducted. It has been previously shown that the oral LD50 of a bitter orange extract standardized to 50% p-synephrine exceeded 5000 mg/kg in female rats [4], indicating a high degree of safety. Oral administration of this extract at 2000 mg/kg to female rats for four consecutive days did not produce any overt signs of toxicity [4].
In a repeated dose 14-day study, rats were treated orally with a 50% p-synephrine-containing bitter orange extract at doses of 0, 250, 500, 1000 and 2000 mg/kg/day [4]. No significant effects were observed at any dose with respect to body weights, relative and absolute organ weights, clinical chemistry and hematological parameters, and gross pathological findings or food intake. One male and one female rat died after receiving four daily doses of 2000 mg/kg while a second male rat died after 8 days at this dose. All animals that died exhibited gastrointestinal impaction with ingesta at necropsy. In the second week of administration of 1000 mg/kg and 2000 mg/kg, the rats exhibited transient signs of repetitive burrowing of their heads in the bedding material over periods of about 15 and 45 min, respectively. The no-observed-effect-level (NOEL) for the bitter orange extract in this study was 500 mg/kg. The maximum tolerated dose (MTD) of bitter orange extract standardized to 50% p-synephrine in rats was determined to be 1000 mg/kg body weight/day, not withstanding the transient and reversible clinical signs that were observed at 1000 mg/kg [4].
The objective of this repeated dose 90-day oral toxicity study was to assess the safety and toxicological profile of Citrus aurantium extract standardized to 50% p-synephrine when administered to male and female rats daily by oral gavage. Daily doses of the extract were administered to rats at 100 mg/kg, 300 mg/kg and 1000 mg/kg. This study was performed to obtain information on the possible health hazards if any likely to arise from repeated exposure to the extract over a prolonged period of time covering post-weaning maturation and growth well into adulthood. This study was performed in the OECD-GLP certified test facilities of INTOX Private LTD.75, Urawade, Tal. Mulshi, Maharashtra, INDIA.
2. Materials and methods
2.1. Test article and formulation
Bitter orange extract (Advantra Z®/Kinetic™) standardized to 50.1% p-synephrine was provided to the test facility by Novel Ingredients LLC, East Hanover, NJ 07936 USA. The amount of p-synephrine present in the extract was confirmed by an independent testing laboratory. The standardized extract is derived from the dried immature (green) fruits of Citrus aurantium (bitter orange). In addition to p-synephrine, the other primary constituents of the extract are carbohydrates (approximately 40–45% and ash (approximately 5–8%). The extract was suspended in analytical grade water which contained 0.50% w/v carboxymethyl cellulose (CMC) as a suspending agent to prepare dosing formulations with the desired test concentrations. The dosing formulations were prepared fresh daily, approximately one hour prior to initiation of dose administration by gavage, and agitated prior to each dosing. Control animals received the vehicle composed of CMC in water.
2.2. Animals
Female and male Sprague Dawley rats were purchased from Taconic Biosciences, Inc., USA through its vendor Vivo Bio Tech Ltd., Telangana, India. The female rats were non-pregnant and nulliparous. In order to ensure that the animals were in good health prior to experimentation, all animals were subjected to veterinary examination.
The animals used for the 90 day subchronic study were between the ages of 6–8 weeks at the beginning of treatment with mean body weights of 298 and 218 g for males and females, respectively. Animals were housed in groups of two or three of the same sex per cage suspended in polypropylene, solid bottom, sterilized cages with stainless steel grill tops, and clean sterilized corn-cob bedding.
The animal facility temperature was maintained between 19–25 °C with the relative humidity between 30–70%, and a 12 h light/dark cycle. The room was supplied with 100% fresh, filtered air with 10–15 air changes per hour. The animals were fed ad libitum Altromin brand extruded pelleted rat chow obtained from M/s Altromin Spezialfutter GmbH & Co. KG, Germany. Drinking water was provided ad libitum through sterilized bottles with stainless steel sipper tubes. Prior to being made available to the animals, the water was passed through an Aquaguard water filter and subjected to ultra violet irradiation. The animals were acclimated to their environment for five to six days prior to experimentation. Each animal was assigned an individual tail number, weighed and randomized.
2.3. Experimental design
This 90-day subchronic toxicity study was conducted in compliance with INTOX Study Plan No. P/16458/SOR-90/16, and was approved by the Institutional Animal Ethics Committee (IAEC) of INTOX. This study plan incorporated the recommendations made in the following guidelines:
-
1.
OECD guideline for Testing of Chemicals, Repeated Dose 90-day Oral Toxicity Study in Rodents, No. 408, Adopted on 21 September 1998.
-
2.
U.S. FDA, Guidance for Industry Botanical Drug Products, U.S. Department of Health and Human Services, Center for Drug Evaluation and Research (CDER), June 2004.
-
3.
International Conference on Harmonization (ICH) Tripartite Guideline; ICH Topic M3(R2), Non-Clinical Safety Studies for the Conduct of Human Clinical Trials and Marketing Authorization for Pharmaceuticals, Current Step 4 version dated 11 June 2009.
-
4.
Guidelines for Toxicity Investigation of Herbal Medicines, Research guidelines for evaluating the safety and efficacy of Herbal Medicines, World Health Organization, 1993.
-
5.
The principles of Good Laboratory Practice as set forth in: OECD, 1998; OECD Series on Principles of Good Laboratory Practice and Compliance Monitoring, Number 1, ‘OECD Principles on Good Laboratory Practice’ ENV/MC/CHEM (98) 17 (as revised in 1997).
-
6.
Guidelines for Laboratory Animal Facility, published in The Gazette of India, December 15, 1998, and Guide for the Care and Use of Laboratory Animals, National Research Council, Institute of Laboratory Animal Resources, 2011, National Academy Press, Washington, DC.
In this study, groups (G) of 10 rats of each sex were administered the bitter orange extract standardized to 50% p-synephrine daily by oral gavage for 90 consecutive days at the dose levels of 100 mg/kg (G2), 300 mg/kg (G3), and 1000 mg/kg (G4). Concurrent control groups (G1) of 10 rats of both sexes were treated with the vehicle (0.5% carboxymethyl cellulose in analytical grade water).
A typical maximum dose of 100 mg/day of p-synephrine in a 70 kg human is equivalent to approximately 9 mg/kg/day for rats when based on body surface area. The corresponding human equivalent dose (HED) for the 50% p-synephrine-containing bitter orange extract is therefore 18 mg/kg/day. Choice of the highest dose of 1000 mg/kg/day dose was based on its being identified as a maximum tolerated dose (MTD) in a 14-day dose range finding study where groups of five rats of each sex were treated orally with 250, 500, 1000 and 2000 mg/kg/day of the 50% p-synephrine-containing bitter orange extract. The 1000 mg/kg/day dose which is approximately a 50 times multiple of the HED of the test extract in rats, is specified by OECD test guideline No. 408 and by other referred guidelines as a ‘limit dose’[4]. The lowest dose of 100 mg/kg used in the current study is approximately a five times multiple of the HED of the test extract in rats. Choice of the middle dose of 300 mg/kg (30X multiple of the HED) ensured the desired three-fold interval between the selected doses. The dosage volumes were 5 ml/kg for all doses administered as a single daily gavage. Additional concurrent groups of five rats of each sex at control [G1(R)] and high dose levels [G4(R)] were treated similarly but, after cessation of treatment, were further observed for reversal of toxicity/delayed toxicity, if any, for a period of 28 days.
2.4. Parameters evaluated
All animals were checked twice daily for deaths or moribundity. The animals were subjected to general cage-side clinical examination after dosing. All signs of ill health, together with any behavioral changes or reaction to treatment were recorded for individual animals. Dated and signed records of appearance, change and disappearance of clinical signs, if any, were maintained on clinical history sheets for individual animals. Detailed clinical examinations were performed weekly outside the cage during the 90-day treatment period (G1–G4) as well as the 28-day recovery period [G1(R) and G4(R)]. The signs that were checked included, but were not limited to changes in fur, skin, mucous membranes, eyes, occurrence of excretions and secretions, autonomic activity such as lacrimation, piloerection and pupil size, and unusual respiratory pattern. Changes in posture, gait and response to handling as well as the presence of tonic or clonic movements, and sterotypies or bizarre behavior were assessed.
Ophthalmoscopic examinations were performed on all rats prior to initiation of the experiment (pretest, Day-0). On day-89 of the treatment period, ophthalmological examinations were performed on animals from control and high dose (1000 mg/kg; G4) groups. Ophthalmological examinations were not performed on animals at lower dosage levels and the reversal groups due to the absence of any significant differences observed between the control and high dose groups. Eyes of the rats were examined by means of a direct ophthalmoscope. After initial examination of eyes for pupillary reflexes, the pupils were dilated using a 1% tropicamide ophthalmic solution (Tropicamet®, manufactured by Sun Ways Pvt. Ltd., Mumbai, India) to facilitate examination of fundus.
During the 12th week of treatment, all animals were examined for assessment of sensory reactivity, grip strength and motor activity using a functional observational battery [5]. These neurological examinations were comprised of the following in home-cage and in open field examinations: posture/movement, respiration, palpebral closure, lacrimation, salivation, skin and hair coat, urination, defecation, locomotor activity, rearing and gait. The following manipulative examinations and assessment of responses to stimuli were also examined: tactile (touch) response, response to nociceptive stimuli (tail pinch), pupil response to light, proprioception–righting reflex, auditory response, head shaking, landing foot splay and grip strength.
During the 12th week of treatment, the rats were also assessed for their locomotor activity by placing them in a ‘multiple unit open field enclosure’ made of Perspex and having a black, non-reflective surface (Orchid Scientific and Innovative India Ltd., India). The open field enclosure has four chambers, each with inner dimensions of 19.5 inches (length) X 19.5 inches (width) X 15 inches (height). At a given time, up to four rats were placed inside the open field enclosure, one in each chamber. A video camera was placed at about 2 m above the enclosure and sent signals to a duly validated software system − Anymaze® (Stoelting Co., 620 Wheat Lane, Wood Dale, IL 60191, USA). Movements of the rats were tracked by the video camera for a period of 10 min (600 s) per rat, while the Anymaze® software analyzed the same for different parameters and generated graphical and numeric reports for each of the rats. The following locomotor assessments were recorded: total distance travelled ﴾m﴿, average speed ﴾m/s﴿, absolute turn angle ﴾°﴿, rotations of the animal's body and absolute head turn angle ﴾°﴿. In all the assessments, vehicle control group rats were included concurrently to enable a fair comparison.
Food consumed by rats in each cage was recorded weekly, and food intake per rat per day was calculated based upon the amount of food offered to each cage, the amount left in each cage at end of assessment period, and the number of rats per cage. Chow was offered to the rats in the form of firm, steam extruded food pellets which resulted in minimal spillage, and therefore the food consumption was not corrected for spillage.
Body weights of all animals were recorded individually on a weekly basis, and at necropsy on day-90 (fasting body weights). Body weights of all animals of the recovery groups were also recorded at weekly intervals and at necropsy (day-119 − fasting body weights). For organ weight determinations liver, lungs, kidneys (right and left), testes (right and left), ovaries (right and left), epididymides (right and left), heart, brain, pancreas, adrenal glands (right and left), spleen and thyroid gland were carefully dissected and trimmed to remove fat and other contiguous tissue. The tissues were weighed immediately to minimize the effects of drying on organ weight, and preserved in 10% buffered formalin. Relative organ weights as a percent of necropsy body weights were also calculated.
Blood samples were collected on day-90 or after the 28-day recovery period prior to necropsy from all animals which had been fasted overnight. Sampling of blood was conducted under light CO2 asphyxiation through the orbital sinus of the animals, and the samples were deposited in K2EDTA-containing tubes for hematology, and heparin-containing tubes for clinical chemistry.
The following hematological parameters were determined on blood samples using an Abbott Cell Dyn 3700 Hematology Analyser (Abbott Park, IL 60064, USA): hemoglobin (Hb), hematocrit (PCV), total erythrocyte count (total RBC), total leukocyte count (total WBC), total platelet count (platelets) and differential leukocyte (WBC) count. Leucocytes were differentiated as neutrophils (N), lymphocytes (L), eosinophils (E), monocytes (M) and basophils (B). The following erythrocyte indices were calculated: mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), and mean corpuscular hemoglobin concentration (MCHC). In addition, the coagulation parameters prothrombin time and activated partial thromboplastin time were determined using a semi-automated coagulation analyzer.
Plasma samples were analyzed for clinical chemistry parameters using a Dimension Xpand Plus Clinical Chemistry System (Siemens Healthcare Diagnostics Inc. Newark, U.S.A.). Commercially available diagnostic kits manufactured by Siemens Healthcare Diagnostics Inc. were used to perform the analyses. The following parameters were measured: aspartate aminotransaminase (AST), alanine aminotransaminase (ALT), alkaline phosphatase (ALP), gamma glutamyl transpeptidase (GGT), total protein, glucose, creatinine, blood urea nitrogen (BUN), albumin, globulin (calculated), albumin/globulin (A/G) ratio (calculated), total bile acid, total bilirubin, sodium, calcium, potassium, phosphorus, total cholesterol, and triglycerides.
Urinalyses were performed on day-88 of the treatment period on animals from the control and high dose groups. Urinalyses were not performed on the lower dose levels and recovery groups in the absence of any significant differences observed between the control and high dose groups. Urine samples were collected using specially designed stainless steel urine collection cages. Each rat was housed in a cage, and a urine sample was collected over a period of about 4 h. No food or water was offered during this period.
The urine samples were subjected to qualitative/semi-quantitative and microscopic evaluation of following parameters: color, appearance, specific gravity, pH, protein, glucose, ketones, volume (timed), bilirubin, urobilinogen, nitrite, leukocytes, occult blood. Tests were performed using Multistix® 10 SG multiple reagent diagnostic strips (Siemens Healthcare Diagnostics Pty. Ltd., Bayswater Victoria 3153, Australia). The results were read using ‘Clinitek Status’ Urine Analyser’ (Siemens Medical Solutions Diagnostics).
The urine samples were centrifuged, and a drop of centrifuged (2500 rpm) urine sample was spread on a microscope slide. The slides were examined microscopically for the presence of the following: epithelial cells, casts, crystals, and other abnormal constituents.
On completion of the 90 days of treatment or the 28-day recovery period, rats were sacrificed by exsanguination from the abdominal aorta under CO2 asphyxiation and subjected to gross necropsy which included the examination of the external surfaces, orifices, cranial, thoracic and abdominal cavities, and their contents, as well as the organs described above.
For histopathological examination, the following tissues and organs were fixed in 10% neutral buffered formalin, embedded in paraffin wax, sectioned at about 5 μm thickness, stained with haematoxylin and eosin, and examined microscopically: brain (cerebrum, cerebellum, midbrain/pons), pons), spinal cord (at three levels: cervical, mid-thoracic and lumbar), pituitary, thyroid gland, parathyroid gland, adrenal glands, thymus, salivary glands, oesophagus, stomach, small and large intestines (including Peyer’s patches, duodenum, jejunum, terminal ileum, colon, rectum), liver, pancreas, kidneys, spleen, heart, lungs, aorta, testes/ovaries, epididymides, prostate, mammary gland in females, urinary bladder, trachea, mesenteric lymph node, axillary lymph node, peripheral nerve (sciatic), and bone marrow from femur (fresh bone marrow aspirate-smear).
Microscopic examinations were carried out on all listed organs and tissues of all animals of control and high dose groups, sacrificed at termination of the treatment period. Since treatment related adverse effects were not observed in any tissues/organs at the high dose level, the low dose (100 mg/kg; G2) group and mid-dose (300 mg/kg; G3) group as well as the recovery groups [G1(R) and G4(R)] were not examined.
The microscopic findings were allocated grades of severity using the following convention adopted at the Test Facility. These grades of severity are based on both gross and microscopic tissue alterations.
| DESCRIPTION |
|---|
| Minimal − Very small amount of change, <10% involvement of organ |
| Mild − Lesions is easily identified but of limited severity, 11–25% involvement of organ |
| Moderate − Lesion is prominent, 26–70% involvement of organ |
| Severe − 70–100% involvement of organ |
2.5. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistical Software (version 23). The data regarding neurological examinations, body weights, hematology, clinical chemistry and organ weights were subjected to Levene’s test for homogeneity. Normality of distribution for the locomotor activity data was ascertained by employing Kolmogorov-Simov’s test and also the Shapiro-Wilk test. The data were log transformed wherever required. The data with homogeneous intra-group variances were subjected to one-way analysis of variance (ANOVA). When ‘F' values were significant, Dunnett's multiple comparison of means of treated groups with control mean was employed as a post-hoc test.
Independent t Test (following Levene’s test) was employed for comparing the data from the two recovery groups generated during and at termination of the recovery period and also for comparing the urinalysis parameters between control and high dose groups. The variance was evaluated at 5% level of significance.
3. Results
3.1. Survival
The daily oral administration of the C. aurantium (bitter orange) extract containing 50% p-synephrine for 90 consecutive days at and up to the dose of 1000 mg/kg (G4) did not have any adverse effect on the survival of the male and female rats in this study. During the 90-day treatment period, there was no incidence of any mortality among the treated male and female rats (G2-G4). Similarly, all male and female rats from recovery groups survived through the 28-day post-treatment recovery period [G1(R) and G4(R)].
3.2. General examination
The daily general clinical examinations and the weekly detailed clinical examinations of rats conducted during the 90-day treatment period and the 28-day recovery period revealed that except for the mild and transient signs of discomfort and piloerection exhibited by rats treated at the highest dose of 1000 mg/kg body weight (G4) and to which the rats eventually adapted (see ensuing paragraphs), the C. aurantium (bitter orange) extract containing 50% p-synephrine did not induce any remarkable and abnormal clinical signs indicative of systemic toxicity in rats of either sex. Treatment of male and female rats with the extract at doses of 100 mg/kg (G2) and 300 mg/kg (G3) body weight did not induce any abnormal clinical signs throughout the 90-day treatment period of the study or the 28-day recovery period.
Daily treatment with the extract at the highest dose (1000 mg/kg body weight; G4) resulted in mild, transient signs of discomfort in rats immediately after their gavage administration. These male and female rats did not exhibit any abnormalities during the initial 9–10 days of the treatment period. However, thereafter, following dose administration the rats began exhibiting signs of repetitive burrowing of their heads in the bedding material (corn-cob). Such behavior, suggestive of some kind of discomfort, was initially observed in rats for about 5–10 min after dose administration when they kept their heads inside the bedding in episodes of 1–2 min each. Durations of these signs gradually waned until about the 81 st day of dosing, thereafter such signs completely disappeared.
Similarly, in the 6th week of the study (from day 35) both male and female rats treated with the extract at the dose of 1000 mg/kg body exhibited signs of piloerection 5–10 min after dose administration which lasted for about one hour. The period of piloerection gradually decreased to about 30 min between weeks 8–10, and thereafter to 10–15 min until week 12 after which this response disappeared with no piloerection visible after about day 81 of the study in animals of both sexes.
3.3. Ophthalmological observations
Daily treatment with the extract containing 50% p-synephrine at and up to the dose of 1000 mg/kg body weight did not induce any ophthalmological abnormalities in male and female rats. The ophthalmological examinations, carried out on all rats prior to start of the study, and on rats from control and high dose groups at termination of the study, did not reveal any remarkable and treatment related incidence of any ophthalmic abnormalities (data not shown).
3.4. Functional observations
Treatment of male and female rats with the 50% p-synephrine-containing C. aurantium extract at and up to the dose of 1000 mg/kg did not induce any remarkable and abnormal alterations in qualitative and quantitative parameters of their sensory reactivity, grip strength and motor activity. This ‘functional observational battery’ was carried out during the 12th week of the study (Table 1). No differences were noted for animals of either sex at any dose of the extract relative to the control groups with respect to posture, movement, respiration, lacrimation, salivation, skin and hair coat, and gait.
Table 1.
Summary of Functional Observations.
| Male Rats − Day 85/86 | |||||
|---|---|---|---|---|---|
| Group | G1 & G1 (R) | G2 | G3 | G4 & G4 (R) | |
| Treated with | Vehicle |
Citrus aurantium Extract 50% p-Synephrine |
|||
| Dose (mg/kg/day) | 0 | 100 | 300 | 1000 | |
| No. of animals in the group | 15 | 10 | 10 | 15 | |
| Findings | Incidence (No. of animals with findings) | ||||
| Home Cage & Open Field | |||||
| Posture/Movement | Normal | 15 | 10 | 10 | 15 |
| Respiration | Normal | 15 | 10 | 10 | 15 |
| Palpebral closure | Normal | 15 | 10 | 10 | 15 |
| Lacrimation | Normal | 15 | 10 | 10 | 15 |
| Salivation | Normal | 15 | 10 | 10 | 15 |
| Skin & hair coat | Normal | 15 | 10 | 10 | 15 |
| Locomotor activity | Normal | 15 | 10 | 10 | 15 |
| Gait | Normal | 15 | 10 | 10 | 15 |
| Urination frequency (in 3 min) # | Mean | 2.20 | 1.80 | 2.00 | 2.13 |
| ± S.D. | 0.68 | 0.79 | 0.67 | 0.64 | |
| Defecation Frequency (in 3 min) # | Mean | 2.20 | 1.60 | 2.10 | 1.67 |
| ± S.D. | 0.68 | 0.52 | 0.74 | 0.72 | |
| Rearing Frequency (in 3 min) # | Mean | 1.93 | 2.50 | 2.00 | 2.00 |
| ± S.D. | 0.80 | 0.53 | 0.82 | 0.76 | |
| Manipulative | |||||
| Touch response | Normal | 12 | 7 | 8 | 10 |
| Exaggerated | 3 | 3 | 2 | 5 | |
| Tail pinch response | Normal | 7 | 6 | 5 | 10 |
| Exaggerated | 8 | 3 | 5 | 5 | |
| Slow | – | 1 | – | – | |
| Pupil constriction in response to light | Normal | 15 | 10 | 10 | 15 |
| Righting reflex | Normal | 15 | 10 | 10 | 15 |
| Auditory response | Normal | 15 | 10 | 10 | 15 |
| Head shaking | Normal | 15 | 10 | 10 | 15 |
| Landing foot splay (cm) # | Mean | 6.39 | 5.88 | 6.09 | 6.25 |
| ± S.D. | 0.26 | 0.84 | 0.39 | 0.25 | |
| Grip strength − Fore limb | Mean | 506.22 | 518.20 | 461.71S− | 526.12 S+ |
| ± S.D. | 37.97 | 27.02 | 32.27 | 60.70 | |
| Female Rats − Day 87/89/90 | |||||
|---|---|---|---|---|---|
| Group | G1 & G1 (R) | G2 | G3 | G4 & G4 (R) | |
| Treated with | Vehicle |
Citrus aurantium Extract 50% p-Synephrine |
|||
| Dose (mg/kg/day) | 0 | 100 | 300 | 1000 | |
| No. of animals in the group | 15 | 10 | 10 | 15 | |
| Findings | Incidence (No. of animals with findings) | ||||
| Home Cage & Open Field | |||||
| Posture/Movement | Normal | 15 | 10 | 10 | 15 |
| Respiration | Normal | 15 | 10 | 10 | 15 |
| Palpebral closure | Normal | 15 | 10 | 10 | 15 |
| Lacrimation | Normal | 15 | 10 | 10 | 15 |
| Salivation | Normal | 15 | 10 | 10 | 15 |
| Skin & hair coat | Normal | 15 | 10 | 10 | 15 |
| Locomotor activity | Normal | 15 | 10 | 10 | 15 |
| Gait | Normal | 15 | 10 | 10 | 15 |
| Urination frequency (in 3 min) # | Mean | 2.07 | 2.20 | 2.20 | 2.07 |
| ± S.D. | 0.59 | 0.63 | 0.63 | 0.70 | |
| Defecation Frequency (in 3 min) # | Mean | 1.67 | 1.60 | 1.60 | 1.80 |
| ± S.D. | 0.62 | 0.52 | 0.52 | 0.56 | |
| Rearing Frequency (in 3 min) # | Mean | 1.60 | 1.80 | 1.80 | 1.93 |
| ± S.D. | 0.74 | 0.79 | 0.79 | 0.70 | |
| Manipulative | |||||
| Touch response | Normal | 12 | 9 | 9 | 14 |
| Exaggerated | 2 | 1 | 1 | 1 | |
| Tail pinch response | Normal | 8 | 5 | 7 | 11 |
| Exaggerated | 7 | 5 | 3 | 4 | |
| Pupil constriction in response to light | Normal | 15 | 10 | 10 | 15 |
| Righting reflex | Normal | 15 | 10 | 10 | 15 |
| Auditory response | Normal | 15 | 10 | 10 | 15 |
| Head shaking | Normal | 15 | 10 | 10 | 15 |
| Landing foot splay (cm) # | Mean | 5.67 | 5.92 | 5.97 | 5.85 |
| ± S.D. | 0.48 | 0.39 | 0.50 | 0.53 | |
| Grip strength − Fore limb # | Mean | 382.53 | 362.26 | 381.23 | 367.88 |
| ± S.D. | 16.67 | 18.44 | 16.97 | 18.14 | |
Vehicle −0.5% (w/v) Carboxymethyl Cellulose, aqueous.
# Measured parameters of treated rats do not differ significantly (P > 0.05) from those of the control group.
S+/S-: Group mean value is significantly (P<0.05) higher/lower than the respective value of the vehicle control group.
The values of quantitative parameters (frequencies of urination, defecation and rearing, the landing foot splay and the grip strength) of the treatment group rats of both sexes did not differ significantly (P > 0.05) from those of the vehicle control group rats during the 12th week of the study (Table 1).
Furthermore, treatment of male and female rats with the extract at and up to the dose of 1000 mg/kg did not induce any remarkable and abnormal alterations in quantitative parameters of their locomotor activity as were assessed in week 12 of treatment by placing them in an open field and tracking their movements individually for a period of 10 min (600 s) per rat by means of a video camera connected to a validated software system (Anymaze®) (Table 2). The values of quantitative parameters including total distance travelled ﴾m﴿, average speed ﴾m/s﴿, absolute turn angle ﴾°﴿, rotations of the animal's body, and absolute head turn angle ﴾°﴿ of the treatment group rats of both sexes did not differ significantly (P > 0.05) from those of the vehicle control group rats.
Table 2.
Summary of Locomotor Activity Data.
| Male Rats | |||||||
|---|---|---|---|---|---|---|---|
| Group | Dose mg/kg bw/day | Total distance travelled (m) | Average speed (m/s) | Absolute turn angle (°) | Rotations of the animal's body | Absolute head turn angle (°) | |
| Vehicle Control − (0.5% (w/v) Carboxymethyl Cellulose, aqueous) | |||||||
| G1 & G1 (R) | 0 | Mean | 8.784 | 0.015 | 6930 | 6.8 | 10040 |
| ± S. D. | 5.201 | 0.009 | 4409 | 4.9 | 4882 | ||
| n | 15 | 15 | 15 | 15 | 15 | ||
| Test Item: Citrus aurantium Extract 50% p-Synephrine | |||||||
| G2 | 100 | Mean | 11.355 | 0.019 | 8332 | 8.0 | 10306 |
| ± S. D. | 4.820 | 0.008 | 2528 | 4.2 | 4294 | ||
| n | 10 | 10 | 10 | 10 | 10 | ||
| G3 | 300 | Mean | 11.224 | 0.019 | 11111 | 9.4 | 15295S+ |
| ± S. D. | 2.782 | 0.005 | 4160 | 3.3 | 6098 | ||
| n | 10 | 10 | 10 | 10 | 10 | ||
| G4 & G4 (R) | 1000 | Mean | 8.925 | 0.015 | 8640 | 7.5 | 12580 |
| ± S. D. | 6.591 | 0.011 | 5030 | 5.8 | 5489 | ||
| n | 15 | 15 | 15 | 15 | 15 | ||
| S+/S-: Group mean value is significantly (P < 0.05) higher/lower than the respective value of the vehicle control group. Animals were assessed on days 87 and 88. The duration of the test was 600 s. | |||||||
| Female Rats | |||||||
|---|---|---|---|---|---|---|---|
| Group | Dose mg/kg bw/day | Total distance travelled (m) | Average speed (m/s) | Absolute turn angle (°) | Rotations of the animal's body | Absolute head turn angle (°) | |
| Vehicle Control − (0.5% (w/v) Carboxymethyl Cellulose, aqueous) | |||||||
| G1 & G1 (R) | 0 | Mean | 17.928 | 0.030 | 16103 | 15.7 | 20126 |
| ± S. D. | 3.716 | 0.006 | 2638 | 3.8 | 1888 | ||
| n | 15 | 15 | 15 | 15 | 15 | ||
| Test Item: Citrus aurantium Extract 50% p-Synephrine | |||||||
| G2 | 100 | Mean | 18.849 | 0.031 | 15821 | 15.4 | 20266 |
| ± S. D. | 1.506 | 0.002 | 2345 | 3.0 | 1081 | ||
| n | 10 | 10 | 10 | 10 | 10 | ||
| G3 | 300 | Mean | 17.441 | 0.029 | 15796 | 12.6 | 19290 |
| ± S. D. | 2.460 | 0.004 | 5978 | 2.0 | 1401 | ||
| n | 10 | 10 | 10 | 10 | 10 | ||
| G4 & G4 (R) | 1000 | Mean | 18.891 | 0.032 | 16646 | 16.5 | 21225 |
| ± S. D. | 5.601 | 0.009 | 3117 | 4.7 | 2612 | ||
| n | 15 | 15 | 15 | 15 | 15 | ||
| Group mean values of treated groups do not differ significantly (P > 0.05) from those of the respective values of the vehicle control group. Animals were assessed on days 87 and 88. The duration of the test was 600 s. | |||||||
3.5. Body weights
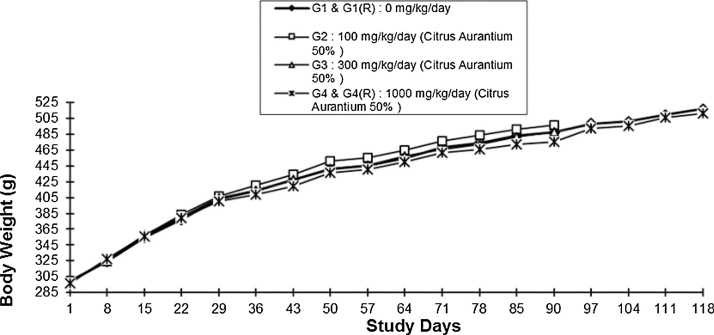
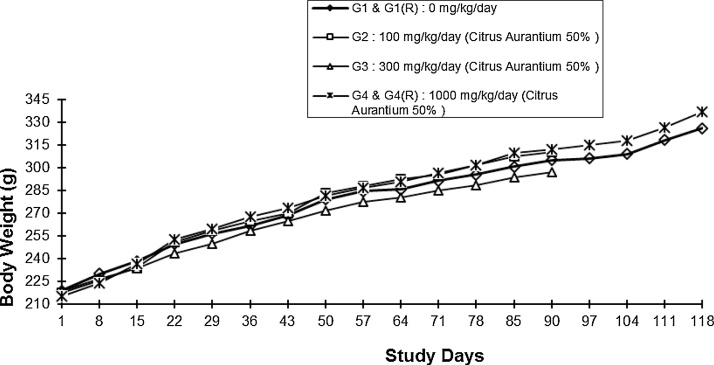
Daily oral administration of extract at and up to the dose of 1000 mg/kg (G4) did not induce any effects on the body weight gain by the male and female rats, during the treatment period of 90 days and the 28-day recovery period [G4(R)]. The values of body weights of treated male and female rats did not differ significantly (P > 0.05) from those of the vehicle control group rats during the 90 days treatment period (G1) and the 28-day recovery period [G1(R)] (Fig. 1, Fig. 2).
Fig. 1.
Body Weights of Male Rats with Time.
Fig. 2.
Body Weights of Female Rats with Time.
3.6. Organ weights
Except for a slight to mild increase in relative but not absolute heart weights in male and female rats treated at 1000 mg/kg, considered a non-adverse alteration, oral administration of the extract containing 50% p-synephrine for 90 days at and up to the dose of 1000 mg/kg did not induce any alterations in the absolute (Table 3) and relative organ weights (Table 4) in the following organs/tissues of treated rats: kidneys, liver, brain, adrenal glands, spleen, testes, epididymides, uterus and ovaries. However, oral administration of the extract daily at a dose of 1000 mg/kg for 90-days induced a small but statistically significant (P < 0.05) increase in the relative heart weights of male and female rats (Table 4), but no significant increase in absolute heart weights was observed (Table 3). Due to an absence of sharp dose dependence, small magnitude, comparability to historical control data, and absence of any correlated histopathology, and absence of any such finding in the recovery groups which suggested reversibility of the effect (Table 5, Table 6), this finding was considered to be of a non-adverse nature.
Table 3.
Summary of Absolute Organ Weights (g) at Termination (Day 91).
| Male Rats | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Dose mg/kg/day | Fasting Body Wt. | Adrenals | Testes | Kidneys | Liver | Brain | Thymus | Heart | Spleen | Epididymides | |
| Vehicle Control − 0.5% (w/v) Carboxymethyl cellulose, aqueous only | ||||||||||||
| G1 | 0 | Mean | 456.00 | 0.055 | 3.90 | 3.14 | 13.22 | 2.41 | 0.43 | 1.63 | 0.83 | 1.55 |
| S. D | 46.68 | 0.007 | 0.31 | 0.35 | 1.83 | 0.16 | 0.10 | 0.17 | 0.13 | 0.19 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| Test Item: Citrus aurantium 50% p-synephrine | ||||||||||||
| G2 | 100 | Mean | 469.20 | 0.050 | 3.93 | 3.12 | 12.93 | 2.38 | 0.53 | 1.66 | 0.85 | 1.59 |
| S. D | 35.12 | 0.012 | 0.33 | 0.36 | 1.46 | 0.13 | 0.17 | 0.16 | 0.08 | 0.22 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 9 | 10 | 10 | 10 | ||
| G3 | 300 | Mean | 462.90 | 0.052 | 3.71 | 3.21 | 13.47 | 2.38 | 0.51 | 1.65 | 0.86 | 1.46 |
| S. D | 18.78 | 0.014 | 0.38 | 0.35 | 1.05 | 0.11 | 0.17 | 0.14 | 0.08 | 0.18 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| G4 | 1000 | Mean | 433.60 | 0.055 | 3.53 | 3.24 | 12.80 | 2.28 | 0.36S− | 1.76 | 1.01 | 1.39 |
| S. D | 38.57 | 0.018 | 0.58 | 0.82 | 1.29 | 0.11 | 0.09 | 0.26 | 0.43 | 0.19 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| Female Rats | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Dose mg/kg/day | Fasting Body Wt. | Adrenals | Ovaries | Kidneys | Liver | Brain | Thymus | Heart | Spleen | Uterus | |
| Vehicle Control − 0.5% (w/v) Carboxymethyl cellulose, aqueous only | ||||||||||||
| G1 | 0 | Mean | 278.60 | 0.064 | 0.127 | 1.87 | 7.53 | 2.18 | 0.39 | 1.10 | 0.69 | 0.77 |
| S. D | 23.30 | 0.017 | 0.016 | 0.20 | 0.79 | 0.11 | 0.09 | 0.11 | 0.10 | 0.33 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| Test Item: Citrus aurantium 50% p-synephrine | ||||||||||||
| G2 | 100 | Mean | 289.00 | 0.074 | 0.134 | 2.05 | 7.76 | 2.16 | 0.38 | 1.12 | 0.66 | 0.72 |
| S. D | 24.34 | 0.026 | 0.027 | 0.15 | 0.51 | 0.13 | 0.08 | 0.09 | 0.07 | 0.17 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| G3 | 300 | Mean | 273.80 | 0.075 | 0.117 | 2.01 | 7.56 | 2.22 | 0.35 | 1.14 | 0.65 | 0.72 |
| S. D | 23.13 | 0.016 | 0.023 | 0.30 | 0.91 | 0.11 | 0.06 | 0.09 | 0.12 | 0.33 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| G4 | 1000 | Mean | 280.30 | 0.076 | 0.130 | 2.06 | 8.13 | 2.19 | 0.39 | 1.19 | 0.69 | 0.76 |
| S. D | 26.92 | 0.016 | 0.034 | 0.30 | 1.06 | 0.13 | 0.08 | 0.13 | 0.11 | 0.30 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
S-: Group mean value is significantly (P < 0.05) lower than the respective value of the vehicle control group.
Group mean values of treated rats do not differ significantly (P > 0.05) from those of the control group.
Table 4.
Summary of Organ Weights Relative to Total Body Weights (%) at Termination (Day 91).
| Male Rats | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Dose mg/kg/day | Fasting Body Wt. (g) | Adrenals | Testes | Kidneys | Liver | Brain | Thymus | Heart | Spleen | Epididymides | |
| Vehicle Control − 0.5% (w/v) Carboxymethyl cellulose, aqueous only | ||||||||||||
| G1 | 0 | Mean | 456.00 | 0.012 | 0.86 | 0.69 | 2.89 | 0.53 | 0.09 | 0.36 | 0.18 | 0.34 |
| S. D | 46.68 | 0.002 | 0.08 | 0.05 | 0.18 | 0.05 | 0.02 | 0.02 | 0.02 | 0.04 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| Test Item: Citrus aurantium 50% p-synephrine | ||||||||||||
| G2 | 100 | Mean | 469.20 | 0.011 | 0.84 | 0.66 | 2.75 | 0.51 | 0.11 | 0.35 | 0.18 | 0.34 |
| S. D | 35.12 | 0.003 | 0.06 | 0.05 | 0.20 | 0.03 | 0.03 | 0.02 | 0.02 | 0.03 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 9 | 10 | 10 | 10 | ||
| G3 | 300 | Mean | 462.90 | 0.011 | 0.80 | 0.70 | 2.91 | 0.51 | 0.11 | 0.36 | 0.19 | 0.32 |
| S. D | 18.78 | 0.003 | 0.08 | 0.09 | 0.17 | 0.02 | 0.04 | 0.03 | 0.02 | 0.04 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| G4 | 1000 | Mean | 433.60 | 0.013 | 0.82 | 0.75 | 2.96 | 0.53 | 0.08 | 0.41S+ | 0.24 | 0.32 |
| S. D | 38.57 | 0.005 | 0.15 | 0.22 | 0.32 | 0.03 | 0.02 | 0.05 | 0.11 | 0.05 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| Female Rats | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Dose mg/kg/day | Fasting Body Wt. (g) | Adrenals | Ovaries | Kidneys | Liver | Brain | Thymus | Heart | Spleen | Uterus | |
| Vehicle Control − 0.5% (w/v) Carboxymethyl cellulose, aqueous only | ||||||||||||
| G1 | 0 | Mean | 278.60 | 0.023 | 0.046 | 0.67 | 2.70 | 0.79 | 0.14 | 0.39 | 0.25 | 0.28 |
| S. D | 23.30 | 0.006 | 0.007 | 0.05 | 0.14 | 0.05 | 0.03 | 0.03 | 0.04 | 0.14 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| Test Item: Citrus aurantium 50% p-synephrine | ||||||||||||
| G2 | 100 | Mean | 289.00 | 0.026 | 0.046 | 0.71 | 2.69 | 0.75 | 0.13 | 0.39 | 0.23 | 0.25 |
| S. D | 24.34 | 0.009 | 0.008 | 0.07 | 0.15 | 0.06 | 0.02 | 0.03 | 0.02 | 0.07 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| G3 | 300 | Mean | 273.80 | 0.028 | 0.043 | 0.74 | 2.76 | 0.82 | 0.13 | 0.42 | 0.24 | 0.26 |
| S. D | 23.13 | 0.006 | 0.010 | 0.11 | 0.29 | 0.07 | 0.02 | 0.03 | 0.04 | 0.12 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| G4 | 1000 | Mean | 280.30 | 0.027 | 0.046 | 0.73 | 2.90 | 0.79 | 0.14 | 0.43S+ | 0.24 | 0.28 |
| S. D | 26.92 | 0.006 | 0.011 | 0.09 | 0.16 | 0.08 | 0.02 | 0.02 | 0.03 | 0.12 | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
S+: Group mean value is significantly (P < 0.05) higher than the respective value of the vehicle control group.
Table 5.
Summary of Absolute Organ Weights (g) at Termination of Recovery Period (Day 119).
| Male Rats | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Dose mg/kg/day | Fasting Body Wt. | Adrenals | Testes | Kidneys | Liver | Brain | Thymus | Heart | Spleen | Epididymides | |
| Vehicle Control (0.5% (w/v) Carboxymethyl Cellulose, aqueous only) | ||||||||||||
| G1 (R) | 0 | Mean | 492.80 | 0.054 | 4.14 | 3.09 | 12.73 | 2.27 | 0.39 | 1.63 | 0.82 | 1.69 |
| S. D | 64.31 | 0.007 | 0.46 | 0.34 | 1.79 | 0.13 | 0.13 | 0.14 | 0.08 | 0.27 | ||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | ||
| Test Item: Citrus aurantium 50% p-synephrine | ||||||||||||
| G4 (R) | 1000 | Mean | 491.00 | 0.06 | 3.82 | 3.18 | 12.48 | 2.24 | 0.42 | 1.57 | 0.77 | 1.71 |
| S. D | 26.68 | 0.01 | 0.36 | 0.86 | 1.47 | 0.10 | 0.15 | 0.15 | 0.11 | 0.20 | ||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | ||
| Female Rats | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Dose mg/kg/day | Fasting Body Wt. | Adrenals | Ovaries | Kidneys | Liver | Brain | Thymus | Heart | Spleen | Uterus | |
| Vehicle Control (0.5% (w/v) Carboxymethyl Cellulose, aqueous only) | ||||||||||||
| G1 (R) | 0 | Mean | 294.20 | 0.075 | 0.114 | 1.87 | 7.12 | 2.26 | 0.34 | 1.11 | 0.61 | 0.67 |
| S. D | 15.45 | 0.007 | 0.033 | 0.27 | 0.58 | 0.07 | 0.07 | 0.12 | 0.05 | 0.15 | ||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | ||
| Test Item: Citrus aurantium 50% p-synephrine | ||||||||||||
| G4 (R) | 1000 | Mean | 330.20 | 0.090 | 0.119 | 1.91 | 8.17 | 2.32 | 0.35 | 1.18 | 0.64 | 0.88 |
| S. D | 35.96 | 0.021 | 0.022 | 0.38 | 1.82 | 0.19 | 0.10 | 0.15 | 0.11 | 0.34 | ||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | ||
| Organ weights of treated rats do not differ significantly (P > 0.05) from those of the control group. | ||||||||||||
Table 6.
Summary of Organ Weights Relative to Total Body Weights (%) at Termination of Recovery Period (Day 119).
| Male Rats | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Dose mg/kg/day | Fasting Body Wt. | Adrenals | Testes | Kidneys | Liver | Brain | Thymus | Heart | Spleen | Epididymides | |
| Vehicle Control (0.5% (w/v) Carboxymethyl cellulose, aqueous only) | ||||||||||||
| G1 (R) | 0 | Mean | 492.80 | 0.011 | 0.84 | 0.63 | 2.58 | 0.47 | 0.08 | 0.33 | 0.17 | 0.35 |
| S. D | 64.31 | 0.001 | 0.04 | 0.05 | 0.08 | 0.07 | 0.03 | 0.03 | 0.01 | 0.05 | ||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | ||
| Test Item: Citrus aurantium 50% p-synephrine | ||||||||||||
| G4 (R) | 1000 | Mean | 491.00 | 0.011 | 0.78 | 0.65 | 2.54 | 0.46 | 0.09 | 0.32 | 0.16 | 0.35 |
| S. D | 26.68 | 0.002 | 0.05 | 0.17 | 0.21 | 0.03 | 0.03 | 0.02 | 0.02 | 0.03 | ||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | ||
| Female Rats | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Dose mg/kg/day | Fasting Body Wt. | Adrenals | Ovaries | Kidneys | Liver | Brain | Thymus | Spleen | Uterus | |
| Vehicle Control (0.5% (w/v) Carboxymethyl cellulose, aqueous only) | |||||||||||
| G1 (R) | 0 | Mean | 294.20 | 0.026 | 0.038 | 0.63 | 2.42 | 0.77 | 0.12 | 0.21 | 0.23 |
| S. D | 15.45 | 0.002 | 0.010 | 0.07 | 0.08 | 0.02 | 0.02 | 0.01 | 0.05 | ||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | ||
| Test Item: Citrus aurantium 50% p-synephrine | |||||||||||
| G4 (R) | 1000 | Mean | 330.20 | 0.028 | 0.037 | 0.58 | 2.48 | 0.71 | 0.11 | 0.20 | 0.27 |
| S. D | 35.96 | 0.008 | 0.009 | 0.11 | 0.51 | 0.11 | 0.03 | 0.03 | 0.13 | ||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | ||
| Organ weights of treated rats do not differ significantly (P > 0.05) from those of the control group. | |||||||||||
A small decrease seen in the absolute thymus weight of male rats treated with 1000 mg/kg of the extract (Table 3) at the end of 90 days was not dose dependent and lacked any histopathological correlations. This decrease was not observed in female rats (Table 3), and the relative thymus weights of rats of both sexes were normal as compared to the control group (Table 4). Furthermore, this difference in thymus weights was not observed in the recovery group (Table 5). As a consequence, this observation with respect to male rats at the highest dose was considered to be of an incidental nature.
3.7. Food consumption
The daily oral administration of the extract at and up to the dose of 1000 mg/kg did not induce any adverse effects on the average daily food consumption by the male and female rats during the 90-day treatment period (G4) and 28-day recovery period [G4(R)]. The values of daily food consumption by the rats, determined weekly, were comparable to those of the vehicle control group rats during the 90-day treatment period (G1) and 28-day recovery period [G4(R)] (data not shown).
3.8. Hematology
Oral administration of the extract to male and female rats for 90 days at and up to the dose of 1000 mg/kg did not induce any alterations in their hematological parameters as apparent from their values determined at termination of the treatment period (G4) (day 91) (Table 7) and at end of the 28-day recovery period [G4(R)] (day 119) (Table 8). The values of all the hematological parameters evaluated in this study of rats treated (G4) with the extract (as listed above in the Materials and Methods section), did not differ significantly from those of the respective vehicle control group (G1) values (P > 0.05) on days 91 and 119. The microscopic evaluation of stained blood smears did not reveal any abnormal and immature cells in animals of either sex.
Table 7.
Summary of Hematological Data at Termination on Day 91. Male Rats.
| Male Rats | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Dose (mg/kg/day) | Hb | PCV | Total RBC | RBC Indices |
Total WBC | Differential WBC Count |
Platelets | PT | APTT | RET | General Blood Picture | |||||||
| MCH | MCV | MCHC | N | L | M | E | B | ||||||||||||
| (g/dl) | (%) | (X106/cmm) | (pg) | (fl) | (g/dl) | (X103/cmm) | % | % | % | % | % | (X103/ cmm) | (Sec) | (Sec) | (%) | ||||
| Vehicle Control: 0.5% (w/v) Carboxymethyl cellulose, aqueous | |||||||||||||||||||
| G1 | 0 | Mean | 16.05 | 46.38 | 8.61 | 18.66 | 53.90 | 34.62 | 6.30 | 21.27 | 74.53 | 0.760 | 3.451 | 0.004 | 853.10 | 17.55 | 13.35 | 1.92 | NAD |
| S. D | 0.66 | 2.21 | 0.47 | 0.45 | 1.06 | 0.34 | 1.24 | 5.72 | 5.82 | 0.333 | 1.184 | 0.009 | 50.88 | 2.67 | 1.86 | 0.45 | – | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| Test Item: Citrus aurantium 50% p-synephrine | |||||||||||||||||||
| G2 | 100 | Mean | 15.93 | 46.18 | 8.64 | 18.46 | 53.52 | 34.51 | 6.31 | 23.89 | 72.97 | 0.590 | 2.544 | 0.009 | 773.70 | 16.98 | 14.10 | 1.66 | NAD |
| S. D | 0.69 | 2.32 | 0.57 | 0.72 | 2.25 | 0.58 | 1.31 | 6.76 | 7.02 | 0.194 | 0.566 | 0.024 | 214.33 | 1.72 | 1.39 | 0.20 | – | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| G3 | 300 | Mean | 15.90 | 45.92 | 8.51 | 18.74 | 54.16 | 34.62 | 5.43 | 28.70 | 67.52 | 0.580 | 3.232 | 0.002 | 862.00 | 19.22 | 14.11 | 1.85 | NAD |
| S. D | 0.86 | 2.30 | 0.66 | 1.21 | 3.91 | 0.63 | 0.79 | 6.67 | 6.15 | 0.226 | 1.301 | 0.008 | 69.27 | 5.46 | 1.83 | 0.42 | – | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| G4 | 1000 | Mean | 15.29 | 44.04 | 8.22 | 18.74 | 53.98 | 34.71 | 6.24 | 22.50 | 72.91 | 0.809 | 3.741 | 0.003 | 893.00 | 18.40 | 18.17 | 2.21 | NAD |
| S. D | 1.00 | 2.68 | 0.82 | 1.86 | 5.47 | 0.55 | 1.55 | 5.03 | 5.56 | 0.650 | 1.186 | 0.007 | 126.52 | 4.06 | 13.80 | 1.15 | – | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| Values of treated rats do not differ significantly (P > 0.05) from those of the respective control group values. | |||||||||||||||||||
| Female Rats | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Dose (mg/kg/day) | Hb | PCV | Total RBC | RBC Indices |
Total WBC | Differential WBC Count |
Platelets | PT | APTT | RET | General Blood Picture | |||||||
| MCH | MCV | MCHC | N | L | M | E | B | ||||||||||||
| (g/dl) | (%) | (X106/cmm) | (pg) | (fl) | (g/dl) | (X103/cmm) | % | % | % | % | % | (X103/ cmm) | (Sec) | (Sec) | (%) | ||||
| Vehicle Control: 0.5% (w/v) Carboxymethyl cellulose, aqueous | |||||||||||||||||||
| G1 | 0 | Mean | 15.42 | 44.86 | 8.02 | 19.25 | 55.99 | 34.38 | 5.57 | 20.06 | 75.45 | 0.48 | 3.91 | 0.10 | 818.70 | 16.60 | 12.86 | 3.90 | NAD |
| S. D | 0.49 | 1.55 | 0.37 | 0.49 | 1.56 | 0.31 | 1.18 | 12.23 | 12.62 | 0.34 | 1.93 | 0.31 | 96.30 | 1.47 | 1.66 | 0.87 | – | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| Test Item: Citrus aurantium 50% p-synephrine | |||||||||||||||||||
| G2 | 100 | Mean | 15.10 | 44.15 | 7.91 | 19.13 | 55.94 | 34.21 | 4.82 | 20.55 | 74.18 | 0.60 | 4.35 | 0.36 | 731.30 | 16.45 | 13.55 | 3.60 | NAD |
| S. D | 0.48 | 1.56 | 0.47 | 0.82 | 2.38 | 0.33 | 0.96 | 8.62 | 9.12 | 0.38 | 1.94 | 1.12 | 125.98 | 1.22 | 1.60 | 0.65 | – | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| G3 | 300 | Mean | 15.36 | 44.76 | 7.88 | 19.50 | 56.83 | 34.32 | 4.73 | 18.75 | 75.48 | 0.96 | 4.79 | 0.00 | 812.70 | 16.49 | 12.56 | 3.66 | NAD |
| S. D | 0.50 | 1.52 | 0.27 | 0.43 | 1.28 | 0.27 | 0.62 | 5.66 | 5.48 | 0.57 | 1.22 | 0.00 | 146.66 | 1.66 | 1.68 | 1.64 | – | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
| G4 | 1000 | Mean | 15.04 | 43.95 | 7.79 | 19.32 | 56.45 | 34.23 | 4.59 | 17.68 | 78.37 | 0.47 | 3.48 | 0.00 | 791.10 | 17.21 | 12.79 | 4.01 | NAD |
| S. D | 0.66 | 2.11 | 0.43 | 0.49 | 1.48 | 0.34 | 0.63 | 2.80 | 3.62 | 0.36 | 1.68 | 0.00 | 89.85 | 1.20 | 1.94 | 1.34 | – | ||
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | ||
Values of treated rats do not differ significantly (P > 0.05) from those of the respective control group values.
Hb-hemoglobin; PCV-hematocrit (packed cell volume); RBC-red blood cells; MCH-mean corpuscular hemoglobin; MCV-mean corpuscular volume; MCHC- mean corpuscular hemoglobin concentration; WBC-white cell count; N-neutrophils; L-leukocytes; M-monocytes; E-eosinophils; B-basphils; PT–prothrombin time; APTT- activated partial thromboplastin time; RET-reticulocyte count.
Table 8.
Summary of Hematology Data at Termination of Recovery Period (Day 119).
| Male Rats | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Dose (mg/kg/day) | Hb | PCV | Total RBC | RBC Indices |
Total WBC | Differential WBC Count |
Platelets | PT | APTT | RET | General Blood Picture | ||||||||
| MCH | MCV | MCHC | L | M | E | B | ||||||||||||||
| (g/dl) | (%) | (X106/cmm) | (pg) | (fl) | (g/dl) | (X103/cmm) | % | % | % | % | % | (X103/ cmm) | (Sec) | (Sec) | (%) | |||||
| Vehicle Control: 0.5% (w/v) Carboxymethyl cellulose, aqueous | ||||||||||||||||||||
| G1 (R) | 0 | Mean | 15.30 | 43.80 | 8.06 | 19.01 | 54.40 | 34.93 | 5.20 | 26.08 | 69.92 | 0.332 | 3.686 | 2.500 | 591.40 | 16.40 | 14.28 | 2.70 | NAD | |
| S. D | 0.58 | 1.72 | 0.54 | 0.71 | 1.59 | 0.42 | 1.36 | 7.24 | 6.79 | 0.162 | 0.921 | 5.590 | 311.74 | 0.87 | 1.69 | 0.28 | – | |||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |||
| Test Item: Citrus aurantium 50% p-synephrine | ||||||||||||||||||||
| G4 (R) | 1000 | Mean | 15.76 | 44.98 | 8.56 | 18.42 | 52.58 | 35.04 | 6.40 | 26.22 | 69.74 | 0.766S+ | 3.262 | 0.000 | 871.00 | 17.44 | 14.10 | 2.45 | NAD | |
| S. D | 0.62 | 1.97 | 0.30 | 0.53 | 1.89 | 0.35 | 1.02 | 6.46 | 6.48 | 0.273 | 0.599 | 0.000 | 40.02 | 0.83 | 2.00 | 0.68 | – | |||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |||
| Female Rats | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Dose (mg/kg/day) | Hb | PCV | Total RBC | RBC Indices |
Total WBC | Differential WBC Count |
Platelets | PT | APTT | RET | General Blood Picture | ||||||||
| MCH | MCV | MCHC | N | L | M | E | B | |||||||||||||
| (g/dl) | (%) | (X106/cmm) | (pg) | (fl) | (g/dl) | (X103/cmm) | % | % | % | % | % | (X103/ cmm) | (Sec) | (Sec) | (%) | |||||
| Vehicle Control: 0.5% (w/v) Carboxymethyl cellulose, aqueous | ||||||||||||||||||||
| G1 (R) | 0 | Mean | 15.54 | 44.14 | 6.91 | 25.39 | 72.05 | 35.21 | 4.52 | 23.92 | 71.74 | 0.285 | 4.042 | 0.017 | 846.80 | 17.14 | 13.30 | 2.32 | NAD | |
| S. D | 0.38 | 1.08 | 2.06 | 12.31 | 34.62 | 0.15 | 0.80 | 3.75 | 4.19 | 0.164 | 1.500 | 0.024 | 82.21 | 0.95 | 2.44 | 1.81 | – | |||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |||
| Test Item: Citrus aurantium 50% p-synephrine | ||||||||||||||||||||
| G4 (R) | 1000 | Mean | 15.46 | 44.72 | 8.13 | 19.07 | 55.07 | 34.61 | 4.57 | 15.20S− | 80.22S+ | 0.508S+ | 4.080 | 0.005 | 714.40 | 17.60 | 13.94 | 1.75 | NAD | |
| S. D | 0.66 | 2.36 | 0.53 | 1.17 | 1.57 | 1.46 | 0.82 | 4.26 | 4.64 | 0.119 | 1.562 | 0.010 | 181.14 | 1.09 | 0.83 | 0.46 | – | |||
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |||
S+/S-: Group mean value is significantly (P < 0.05) higher/lower than the respective control group value.
Hb-hemoglobin; PCV-hematocrit (packed cell volume); RBC-red blood cells; MCH-mean corpuscular hemoglobin; MCV-mean corpuscular volume; MCHC- mean corpuscular hemoglobin concentration; WBC-white cell count; N-neutrophils; L-leukocytes; M-monocytes; E-eosinophils; B-basphils; PT–prothrombin time; APTT- activated partial thromboplastin time; RET-reticulocyte count.
Values of treated rats do not differ significantly (P > 0.05) from those of the respective control group values.
3.9. Clinical chemistry
Except for a slight and reversible (non-adverse) elevation of the BUN and urea levels in the high dose group (G4) of male rats, oral administration of the 50% p-synephrine-containing C. aurantium extract to male and female rats for 90 days at and up to the dose of 1000 mg/kg did not induce alterations in their clinical chemistry parameters as apparent from their values determined at termination of the treatment period (G4) (day-91) (Table 9), and at end of the 28-day recovery period [G4(R)] (day-119) (Table 10). No significant differences occurred between control (G1) and 1000 mg/kg/day (G4) values at termination on day 91 for albumin, enzymes (AST, ALT, GGT, ALP), total bilirubin, cholesterol, creatinine, triglycerides, sodium, potassium, phosphorus, globulin and Albumin/globulin ratio for male and female animals.
Table 9.
Summary of Clinical Chemistry Data at Termination (Day 91).
| Male Rats | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group & Dose (mg/kg/ day) | Test | TP | ALB | ALT | AST | ALP | TBIL | GLU | CHOL | BUN | UREA | CRE | TBA | GGT | TG | Na | K | Ca | PHO | Globulin | A/G |
| Unit | g/dL | g/dL | IU/L | IU/L | IU/L | mg/dL | mg/dL | mg/dL | mg/dL | mg/dL | mg/dL | μmol/L | IU/L | mg/dL | mmol/L | mmol/L | mg/dL | mg/dL | g/dL | g/dL | |
| Vehicle Control : 0.5 % (w/v) Carboxymethyl cellulose, aqueous only | |||||||||||||||||||||
| G1 | Mean | 7.31 | 1.55 | 36.4 | 63.6 | 65.3 | 0.2 | 102.7 | 76.6 | 14.8 | 21.31 | 0.48 | 9.9 | 6.9 | 65.2 | 150 | 4.47 | 10.78 | 6.85 | 5.76 | 0.27 |
| 0 | S. D | 0.34 | 0.11 | 6.96 | 11.65 | 11.84 | 0 | 29.04 | 16.67 | 2.82 | 4.06 | 0.07 | 4.4 | 0.32 | 20.58 | 2.4 | 0.36 | 0.32 | 0.59 | 0.3 | 0.02 |
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Test Item: Citrus aurantium 50% p-synephrine | |||||||||||||||||||||
| G2 | Mean | 7.41 | 1.57 | 41.6 | 71.2 | 75.5 | 0.2 | 93.4 | 70.5 | 16.2 | 23.33 | 0.48 | 14.5 | 6.9 | 78.4 | 149.3 | 4.62 | 10.78 | 7.36 | 5.84 | 0.27 |
| 100 | S. D | 0.32 | 0.09 | 6.17 | 8.65 | 15.4 | 0 | 17.73 | 10.36 | 2.49 | 3.58 | 0.05 | 5.37 | 0.57 | 30.71 | 1.77 | 0.35 | 0.21 | 0.59 | 0.29 | 0.02 |
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| G3 | Mean | 7.44 | 1.56 | 38.3 | 76.5 | 69.2 | 0.14 | 91.8 | 72.2 | 14.3 | 20.59 | 0.47 | 12.22 | 6.5 | 79.2 | 151.6 | 4.45 | 10.68 | 7.19 | 5.88 | 0.27 |
| 300 | S. D | 0.28 | 0.1 | 6.15 | 12.36 | 11.29 | 0.05 | 5.53 | 9.72 | 1.25 | 1.8 | 0.05 | 7.31 | 0.53 | 30.62 | 1.96 | 0.24 | 0.33 | 0.31 | 0.28 | 0.02 |
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| G4 | Mean | 7.42 | 1.57 | 48.1 | 89.1 | 76.4 | 0.14 | 91.4 | 68.3 | 20.10S+ | 28.94S+ | 0.55 | 19.51 | 7.2 | 56.5 | 152.1 | 4.58 | 10.37S- | 7.21 | 5.85 | 0.27 |
| 1000 | S. D | 0.28 | 0.12 | 16 | 26.11 | 15.54 | 0.05 | 9.14 | 10.66 | 6.19 | 8.91 | 0.17 | 15.16 | 0.79 | 20.62 | 3.25 | 0.34 | 0.33 | 0.51 | 0.27 | 0.02 |
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Female Rats | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group & Dose (mg/kg/day) | Test | TP | ALB | ALT | AST | ALP | TBIL | GLU | CHOL | BUN | UREA | CRE | TBA | GGT | TG | Na | K | Ca | PHO | Globulin | A/G Ratio |
| Unit | g/dL | g/dL | IU/L | IU/L | IU/L | mg/dL | mg/dL | mg/dL | mg/dL | mg/dL | mg/dL | μmol/L | IU/L | mg/dL | mmol/L | mmol/L | mg/dL | mg/dL | g/dL | g/dL | |
| Vehicle Control: 0.5% (w/v) Carboxymethyl cellulose, aqueous only | |||||||||||||||||||||
| G1 | Mean | 7.32 | 1.75 | 32.30 | 70.40 | 45.50 | 0.17 | 104.90 | 77.90 | 20.40 | 29.38 | 0.51 | 15.65 | 7.10 | 52.70 | 149.40 | 4.91 | 10.90 | 6.58 | 5.57 | 0.31 |
| 0 | S. D | 0.45 | 0.12 | 8.55 | 13.53 | 13.46 | 0.05 | 11.56 | 18.73 | 2.55 | 3.67 | 0.09 | 6.11 | 0.99 | 21.57 | 3.98 | 0.42 | 0.44 | 0.52 | 0.37 | 0.02 |
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| Test Item: Citrus aurantium 50% p-synephrine | |||||||||||||||||||||
| G2 | Mean | 7.15 | 1.65 | 32.90 | 77.80 | 57.60 | 0.17 | 102.60 | 86.60 | 16.50S− | 23.76S− | 0.49 | 14.90 | 7.20 | 50.70 | 150.60 | 4.90 | 10.41 | 6.18 | 5.50 | 0.30 |
| 100 | S. D | 0.55 | 0.07 | 5.26 | 10.40 | 11.20 | 0.05 | 9.19 | 10.23 | 1.78 | 2.56 | 0.07 | 9.59 | 0.42 | 21.99 | 1.84 | 0.66 | 0.76 | 0.55 | 0.51 | 0.02 |
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| G3 | Mean | 7.37 | 1.74 | 35.30 | 84.30 | 46.30 | 0.18 | 110.00 | 80.00 | 20.60 | 29.66 | 0.52 | 19.80 | 6.50 | 50.50 | 149.10 | 4.78 | 11.03S+ | 6.26 | 5.63 | 0.31 |
| 300 | S. D | 0.21 | 0.08 | 6.27 | 23.77 | 14.93 | 0.04 | 27.86 | 12.53 | 3.03 | 4.36 | 0.06 | 9.93 | 0.53 | 11.58 | 1.97 | 0.29 | 0.44 | 0.44 | 0.23 | 0.02 |
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
| G4 | Mean | 7.18 | 1.61S− | 33.20 | 74.30 | 49.50 | 0.16 | 106.30 | 79.70 | 22.30 | 32.11 | 0.58 | 21.53 | 6.60 | 61.00 | 149.30 | 4.78 | 10.64 | 6.25 | 5.57 | 0.29 |
| 1000 | S. D | 0.32 | 0.10 | 5.87 | 17.05 | 15.00 | 0.05 | 6.83 | 8.47 | 2.71 | 3.90 | 0.06 | 12.81 | 0.84 | 25.08 | 1.42 | 0.35 | 0.23 | 0.69 | 0.31 | 0.02 |
| n | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | |
S+/S-: Group mean value is significantly (P < 0.05) higher/lower than the respective control group value.
TP − Protein (total); ALB- albumin; AST- asparate aminotransferase; ALT- alanine aminotransferase; ALP-alkaline phosphatase; GGT- gamma glutamyl transpeptidase; TBIL- Bilirubin, total; GLU − glucose; CHOL- cholesterol, total; CRE- creatinine; BUN − blood urea nitrogen; TBA- bile acids, total; TG − triglycerides; Na − sodium; K- potassium; Ca − calcium; PHO − phosphorus; A/G Ratio − albumin/globulin ratio.
Table 10.
Summary of Clinical Chemistry Data at Termination of Recovery Period (Day 119).
| Male Rats | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group & Dose (mg/kg/day) | Test | TP | ALB | ALT | AST | ALP | TBIL | GLU | CHOL | BUN | UREA | CRE | TBA | GGT | TG | Na | K | Ca | PHO | Globulin | A/G Ratio |
| Unit | g/dL | g/dL | IU/L | IU/L | IU/L | mg/dL | mg/dL | mg/dL | mg/dL | mg/dL | mg/dL | μmol/L | IU/L | mg/dL | mmol/L | mmol/L | mg/dL | mg/dL | g/dL | g/dL | |
| Vehicle Control: 0.5% (w/v) Carboxymethyl cellulose, aqueous only | |||||||||||||||||||||
| G1 (R) | Mean | 6.54 | 1.42 | 48.40 | 83.00 | 83.40 | 0.10 | 101.20 | 58.40 | 15.00 | 21.60 | 0.46 | 18.30 | 7.20 | 62.40 | 149.20 | 4.60 | 10.18 | 6.86 | 5.12 | 0.28 |
| 0 | S. D | 0.17 | 0.08 | 5.13 | 9.30 | 17.52 | 0.00 | 7.46 | 13.28 | 1.73 | 2.49 | 0.03 | 4.68 | 0.45 | 32.72 | 1.48 | 0.31 | 0.08 | 0.35 | 0.16 | 0.02 |
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Test Item: Citrus aurantium 50% p-synephrine | |||||||||||||||||||||
| G4 (R) | Mean | 6.90 | 1.44 | 44.40 | 80.80 | 94.20 | 0.10 | 91.60 | 61.20 | 17.60 | 25.34 | 0.51 | 20.09 | 7.40 | 61.60 | 147.60 | 4.48 | 10.28 | 7.02 | 5.46 | 0.26 |
| 1000 | S. D | 0.32 | 0.09 | 4.88 | 7.85 | 18.28 | 0.00 | 10.55 | 11.69 | 3.29 | 4.73 | 0.05 | 10.78 | 0.55 | 16.29 | 0.55 | 0.23 | 0.24 | 0.36 | 0.31 | 0.02 |
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Female Rats |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group & Dose (mg/kg/day) | Test | TP | ALB | ALT | AST | ALP | TBIL | GLU | CHOL | BUN | UREA | CRE | TBA | GGT | TG | Na | K | Ca | PHO | Globulin | A/G Ratio |
| Unit | g/dL | g/dL | IU/L | IU/L | IU/L | mg/dL | mg/dL | mg/dL | mg/dL | mg/dL | mg/dL | μmol/L | IU/L | mg/dL | mmol/L | mmol/L | mg/dL | mg/dL | g/dL | g/dL | |
| Vehicle Control: 0.5% (w/v) Carboxymethyl cellulose, aqueous only | |||||||||||||||||||||
| G1 (R) | Mean | 6.68 | 1.36 | 30.60 | 98.60 | 59.80 | 0.10 | 59.20 | 68.20 | 16.60 | 23.90 | 0.58 | 14.05 | 7.20 | 34.00 | 149.40 | 6.20 | 10.12 | 7.64 | 5.32 | 0.26 |
| 0 | S. D | 0.41 | 0.18 | 4.62 | 11.89 | 14.38 | 0.00 | 6.98 | 8.11 | 2.79 | 4.02 | 0.10 | 7.33 | 0.45 | 3.32 | 3.05 | 0.33 | 0.23 | 0.53 | 0.33 | 0.04 |
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Test Item: Citrus aurantium 50% p-synephrine | |||||||||||||||||||||
| G4 (R) | Mean | 6.92 | 1.66S+ | 32.80 | 100.20 | 57.80 | 0.12 | 70.60S+ | 60.60 | 18.60 | 26.78 | 0.61 | 17.03 | 7.00 | 43.60S+ | 146.80 | 6.56 | 10.18 | 7.74 | 5.26 | 0.32S+ |
| 1000 | S. D | 0.34 | 0.11 | 4.55 | 13.16 | 13.99 | 0.04 | 5.68 | 19.42 | 4.51 | 6.49 | 0.07 | 9.74 | 0.71 | 7.83 | 1.30 | 0.23 | 0.33 | 0.91 | 0.35 | 0.03 |
| n | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
S+/S-: Group mean value is significantly (P < 0.05) higher/lower than the respective control group value.
TP − Protein (total); ALB- albumin; AST- asparate aminotransferase; ALT- alanine aminotransferase; ALP- alkaline phosphatase; GGT- gamma glutamyl transpeptidase; TBIL— Bilirubin, total; GLU − glucose; CHOL- cholesterol, total; CRE- creatinine; BUN − blood urea nitrogen; TBA- bile acids, total; TG − triglycerides; Na − sodium; K- potassium; Ca − calcium; PHO − phosphorus; A/G Ratio − albumin/globulin ratio.
Values of treated rats do not differ significantly (P > 0.05) from those of the respective control group values.
The values of BUN, and consequently the calculated urea levels, of high dose (G4) male rats were found to be slightly higher than those of the respective vehicle control group values and the difference was statistically significant (P < 0.05). The alteration was not dose-dependent and the apparently significant levels were well within historical control ranges for BUN and urea for Sprague Dawley rats in the test facility. The finding was not evident at the end of the recovery period and hence noted to be reversible. Although as per histopathology findings in G4 male rats, there was an incidence of minimal lymphocytic Infiltration (2/10) and of solitary cortical cyst (1/10) in kidneys, and also one of these rats had significantly increased kidney weight, no clear correlation could be established. Therefore, although considered to be treatment induced, this finding was considered to be non-adverse in nature.
Small but statistically significant differences were noted for calcium in male (G4) and female rats (G3), and albumin in female rats (G3) (Table 9). These effects were small, not dose dependent, were believed to be incidental, and of no toxicological significance. In the recovery animals (Table 10), calcium values were normal, while small but clinically insignificant differences were noted in female rats (G4 recovered) for albumin, glucose, triglycerides and the albumin/globulin ratio.
3.10. Urinalysis
Treatment of rats with the extract did not induce any alterations in their urinalysis parameters including microscopic appearance of the centrifuged deposits when examined on day 88 of the treatment period. The observations recorded for qualitative urine parameters including color, appearance, and graded quantities of analytes such as protein, glucose, ketones, occult blood and bilirubin were found to be comparable between the high dose (1000 mg/kg) groups of rats of both sexes (G4) and the vehicle control groups (G1) of rats. Group mean values of specific gravity, urobilinogen and pH of urine of male and female rats treated with the extract did not differ significantly (P > 0.05) from those of the vehicle control groups of rats on day 88 of treatment (data not shown).
3.11. Necropsy
Daily oral administration of C. aurantium which contained 50% p-synephrine to male and female rats for 90 days at and up to the dose of 1000 mg/kg, did not induce any remarkable gross abnormalities in their organs/tissues as evidenced during their gross necropsy performed at termination after 90 days of treatment (day 91) or of the 28 days of recovery period (day 119). However, a few isolated (1/10) gross alterations were observed which included an extra-diaphragmatic lobe in liver (male − high dose; G4), undersized adrenals (female − low dose; G2) and a solitary nodule in right horn of uterus (vehicle control group; G1). Considering their solitary incidence and the absence of any dose dependence, these observations were considered to be incidental and unrelated to the treatment.
3.12. Histopathology
Microscopic evaluation of all tissues/organs listed above in all male and female rats from the vehicle control (G1) groups and the high dose (G4) groups treated with the extract at 1000 mg/kg body weight did not reveal incidence of any remarkable histopathological findings which could be attributed to exposure to the extract. A few isolated instances of microscopic findings such as those listed below (incidence pooled for sexes) were considered to be unrelated to treatment with the C. Aurantium extract due to their small incidence with minimal severity, and the comparability of their incidence within the vehicle control group for both sexes.
In kidneys, minimal tubular dilatation was observed 5/20 in G1 group (control) and 4/20 in G4 group (1000 mg/kg), minimal infiltration with lymphocytes 1/20 in G1 and 3/20 in G4, isolated incidences of hyaline cyst 1/10 each in G1 males and G4 females; cortical cyst 1/10 in G4 males, and mineralization 1/10 in G1 males. In lungs, minimal perivascular aggregation of lymphocytes was observed in 2/20 in G1, mild peribronchial lymphoid tissue hyperplasia 1/20 in G1 and 1/20 in G4, and minimal presence of alveolar macrophages 1/20 in G4.
In liver, minimal portal infiltration of lymphocytes was noted in 2/20 in G1 and 2/20 in G4 with an isolated incidence of minimal necrosis in one G4 male rat, and an extra diaphragmatic lobe in liver which was found to be histologically normal as confirmed on microscopic examination. In intestines, isolated instances of minimal sub-mucosal lymphoid hyperplasia in colon 4/20 in G1 and 2/20 in G4 and in rectum 1/20 in G1 and 3/20 in G4 were observed. In stomach, a minimal dilation of glandular portion 6/20 in G1 and 2/20 in G4 occurred. In pituitary, cysts in pars distalis 2/20 in G1 and 1/20 in G4 were noted.
In addition to the above findings, cortical vacuolation occurred in adrenals of one G1 male, minimal lymphocytic infiltration was noted in the prostate in one male rat each from G1 and G4, moderate degeneration occurred in testes of one G4 male, minimal eosinophilic infiltration in uterus was present in one female rat each from G1 and G4, and a solitary incidence of decidual reaction in uterus in one female rat from the G1 control group.
All of the above findings are expected background lesions commonly present in conventionally housed rats of this age. These findings were hence considered to be incidental in nature and not related to treatment with the C. aurantium extract containing 50% p-synephrine.
4. Discussion
In this 90-day subchronic safety/toxicity study daily oral doses of 100 mg/kg, 300 mg/kg and 1000 mg/kg of the C. aurantium (bitter orange) extract standardized to 50% p-synephrine were administered to animals of both sexes. All animals survived the 90 days of treatment and the 28 days of recovery. These doses were selected based on an initial 14-day dose range finding study that employed doses of the bitter orange extract up to 2000 mg/kg/day. At daily oral doses of 100 mg/kg/day and 300 mg/kg/day no effects were observed with respect to ophthalmology, clinical chemistry, hematology, functional activities, urinalysis, locomotor activities, organ weights, body weights, food consumption, gross necropsy and histopathology at termination.
Treatment with 1000 mg/kg body weight/day (G4 groups) of the extract resulted in mild, transient signs of discomfort in rats of both sexes immediately after administration, which gradually disappeared by day 81 of treatment. Repetitive burrowing of their heads in the bedding material and piloerection for short periods of time was observed by animals of both sexes.
The cause of these signs is not clear. These effects could be a pharmacological effect of the extract, and because they were mild, transient, adaptive and fully reversible in nature, were not perceived as adverse effects. It should be noted that these signs were so short lived and clinically insignificant that they did not influence the normal body weight gain and food intake of these rats. Their being of no adverse significance is also supported by the unaltered neuro-behavioral parameters of these rats based on the extensive qualitative/semi-quantitative and quantitative neurological assessments.
The consumption of dietary supplements has increased dramatically in recent years, and concerns are frequently raised regarding the safety of various products, particularly when used in conjunction with conventional drugs [6]. As a consequence, a need exists for the assessment of the safety of dietary supplements when used for long periods of time. Bitter orange products and extracts have been widely used for hundreds of years in Traditional Chinese Medicine [2], [3], [7] as well as various foods as juices, marmalades, and flavoring agents [7], [8]. However, relatively few animal studies have assessed the safety of bitter orange extracts, and no animal studies have previously assessed the long term subchronic toxicity of a bitter orange extract.
Calapai et al. [9] examined the effects of repeated oral administration of 2.5–20 mg/kg of two C. aurantium fruit extracts that had been standardized to 4% and 6% p-synephrine. The effects of these extracts on food intake, body weight gain, arterial blood pressure, electrocardiogram (ECG), and mortality in male rats were examined. Animals were treated for 7 consecutive days, and the various measurements were recorded for 15 consecutive days. Significant dose-dependent decreases in food intake as well as body weight were observed. No significant changes were observed in blood pressure, while the alterations in ECG were significant after 10 days of treatment. Some deaths were noted in the treated animals, although the difference was not statistically significant. Unfortunately, the chemical composition of the bitter orange extract was not reported other than the p-synephrine content. Some of these results must be questioned in light of studies by other investigators [4], [10], [11], [12], [13], [14].
Arbo et al. [10] examined the acute toxicity of a bitter orange extract and p-synephrine in mice. The single oral administration of a bitter orange extract containing 2.5% p-synephrine at doses from 300 to 3000 mg/kg resulted in reduced locomotor activity (hypoactivity). Doses of 150–2000 mg/kg of p-synephrine produced reduced locomotion, piloerection, salivation, gasping, and exophthalmia. All effects were reversible and resolved in 3–4 h. The authors concluded that these effects were due to adrenergic agonist activity. As will be discussed below, p-synephrine is a poor adrenergic agonist, and as a consequence exceedingly high doses are required to produce these effects.
In a 28-day study in mice [11], a bitter orange extract (7.5% p- synephrine) at doses of 400, 2000 or 4000 mg/kg (which corresponds to 30, 150 and 300 mg p-synephrine/kg/day) or with 30 mg or 300 mg p‐synephrine/kg/day were administered orally. No adverse effects were noted regarding biochemical parameters, organ weights, heart rate or blood pressure at any of the doses in the treated mice [11]. A decrease in body weight gain at all doses relative to control animals was observed. Based on body surface area, the 300 mg/kg dose of p-synephrine in mice is approximately 34 times a typical 50 mg human dose for a 70 kg (154 lb) individual.
Both 30 and 300 mg/kg/day of p‐synephrine and the 4000 mg/kg/day of the bitter orange extract that contained 300 mg p-synephrine produced significant increases in hepatic reduced glutathione (GSH) [11], which is an antioxidant and tissue protectant, while the high dose of the bitter orange extract decreased hepatic malondialdehyde content (an indicator of lipid peroxidation and lipid damage). In addition, p‐synephrine increased catalase which eliminates the pro-oxidant hydrogen peroxide. Both 30 and 300 mg/kg/day of p-synephrine as well as 400 mg/kg/day and 2000 mg/kg/day of the bitter orange extract (30 and 150 mg of p-synephrine, respectively) also significantly inhibited glutathione peroxidase activity, thus protecting GSH [11]. In summary, the results of this study demonstrated no adverse effects at very high doses, a desirable weight loss, and antioxidant and tissue protective effects.
The National Center for Toxicological Research in conjunction with the US Food and Drug Administration (FDA) have conducted a several animal studies with respect to the safety of p-synephrine and bitter orange extract [12], [13], [14]. In a study which examined the developmental toxicity of Citrus aurantium in rats, doses of up to 100 mg p-synephrine/kg body weight no developmental toxicity was observed [12]. At up to 100 mg/kg p-synephrine no adverse effects with respect to fetal weight, embryo lethality, or incidence of gross, visceral or skeletal abnormalities were observed. This dose in rats is equivalent to a dose of approximately 1135 mg of p-synephrine in a 70 kg human, which is approximately 23 times greater than a typical 50 mg dose.
The physiological effects of p-synephrine were assessed after administering bitter orange extract as well as isolated p-synephrine to rats for 28-days at doses of up to 50 mg/kg with and without caffeine at 25 mg/kg [13]. No profound or alarming cardiovascular effects were observed. At a 50 mg/kg dose of p-synephrine that was over 11 times greater than a typical dose of 50 mg in humans, a small, clinically insignificant increase of 2–3 mm Hg was observed with respect to systolic and diastolic blood pressure at one hour. A small clinically insignificant decrease in heart rate was observed at one hour under these conditions. Caffeine alone, as expected, and in combination with p-synephrine produced more pronounced but small increases in heart rate and blood pressure [13]. In rats, the 25 mg/kg dose of caffeine is equivalent to approximately a dose of 285 mg in a 70 kg human.
The effects of bitter orange extract and p-synephrine on the cardiovascular system were also examined in exercised rats in the presence and absence of 25 mg/kg caffeine for 28 days [14]. Again, no alarming effects were exhibited. Caffeine alone resulted in 4–5 mm Hg increases in blood pressure, and a small increase in heart rate, while 50 mg/kg p-synephrine exhibited small, clinically insignificant effects on blood pressure. The combination of p-synephrine with caffeine did not produce clinically significant greater effects than caffeine alone [14]. It should again be noted that in a 70 kg human, the dose of 50 mg/kg p-synephrine is approximately 11 times greater than a typical 50 mg dose in combination with approximately 285 mg caffeine.
The safety of p-synephrine has been questioned because it has some structural similarity to ephedrine. p-Synephrine is referred to as a stimulant and is assumed to exhibit cardiovascular activity because of its structural similarity to ephedrine [15], [16], [17], [18], [19], [20], [21]. However, several reviews have summarized over 30 published and unpublished clinical studies involving over 700 subjects who consumed a product containing a bitter orange extract (p-synephrine) alone or in combination with other ingredients including caffeine [3], [22], [23], [24], [25]. These human studies have shown that p-synephrine does not exert cardiovascular effects at commonly used doses, and the results of the current 90 day animal study support these observations in humans. A 100 mg dose of 100 mg p-synephrine consumed daily for 60 days was shown to be devoid of adverse effects in humans [26].
The reason for the paucity of cardiovascular effects of p-synephrine is because it exhibits little or no binding to α-1, α-2, β-1 and β-2 adrenergic receptors based on receptor binding studies. As a consequence p-synephrine does not act as a central nervous system or cardiovascular stimulant producing increases in heart rate or blood pressure at commonly used doses [27]. p-Synephrine binds approximately 10 times more readily to adrenergic receptors from rodents than humans, and therefore responses to p-synephrine in animals cannot be directly extrapolated to humans [28], [29], [30]. Based on relative adrenergic receptor binding, doses of p-synephrine that may be up to 10 times greater would be required to produce a cardiovascular response in humans as compared to rodents. p-Synephrine may be classified as a non-stimulant thermogenic agent in humans [31].
In summary, daily administration of a C. aurantium (bitter orange) extract standardized to 50% p-synephrine to male and female rats for 90 consecutive days by oral gavage at the dose levels of 100 mg/kg, 300 mg/kg or 1000 mg/kg resulted in no incidence of deaths, no incidence of any remarkable abnormal clinical signs, no ophthalmologic alterations and no evidence of neurotoxicity. It induced mild and transient signs of discomfort and of piloerection exhibited by rats at the highest dose of 1000 mg/kg body weight (G4) which were adaptive and fully reversible in nature. These signs disappeared after 80-days of treatment, and hence were considered to be non-adverse in nature.
The treatment had no effect on body weights/body weight gain, no effect on food intake, and induced no remarkable gross pathology or histopathological alterations. Treatment with the bitter orange extract standardized to 50% p-synephrine induced a slight to mild increase in relative but not absolute heart weights in male and female rats at 1000 mg/kg, which was considered to be a non-adverse alteration. The treatment caused no alterations in the absolute and relative weights of other organs of the male and female rats.
Treatment with the bitter orange extract standardized to 50% p-synephrine also induced a slight, reversible and non-adverse elevation of the plasma levels of blood urea nitrogen (BUN) and urea in male rats, but not in female rats, treated at 1000 mg/kg, and it did not exhibit any effects on the other blood chemistry parameters and urinalysis parameters in male and female rats.
5. Conclusions
Based on the findings of this study, following daily oral administration of C. aurantium (bitter orange) extract containing 50% p-synephrine for 90 days to male and female rats, it can be concluded that the extract induced a few non-adverse alterations at the highest tested dose of 1000 mg/kg body weight, while no such alterations were induced at and up to the dose level of 300 mg/kg body weight. It is therefore concluded for C. aurantium (bitter orange) extract containing 50% p-synephrine that its no-observed-effect level (NOEL) is found to be 300 mg/kg, and by considering the observed findings as non-adverse, the no-observed-adverse-effect-level (NOAEL) is found to be greater than 1000 mg/kg body weight.
Conflicts of interest
The study was conducted at INTOX Private LTD., 375, Urawade, Tal. Mulshi, Maharashtra India. SJS is a consultant for Novel Ingredients. NSD, CMC, AK and BS are associated with INTOX Private LTD.
Acknowledgement
The study was conducted under a grant from Novel Ingredients, LLC, East Hanover, NJ 07936 USA which also provided the test product.
References
- 1.Pellati F., Benvenuti S. Chromatographic and electrophoretic methods for the analysis of phenethylamine alkaloids in Citrus aurantium. J. Chromatog. A. 2007;1171(2007):71–88. doi: 10.1016/j.chroma.2007.05.097. [DOI] [PubMed] [Google Scholar]
- 2.Chen J.K., Chen T.T. Chinese Medical Herbology and Pharmacology. Art of Medicine Press; City of Industry, CA USA: 2004. Zhi shi (Fructus aurantii immaturus) pp. 485–487. [Google Scholar]
- 3.Stohs S.J., Shara M. Review of the safety and efficacy of bitter orange (Citrus aurantium) and its primary protoalkaloid, p-synephrine, in weight management. In: Bagchi D., Preuss H.G., editors. Obesity: Epidemiology, Pathophysiology, and Prevention. 2nd ed. CRC Press; Boca Raton, FL, USA: 2007. pp. 535–554. [Google Scholar]
- 4.Deshmukh N.S., Stohs S.J., Magar C.C., Kale A. Citrus aurantium (bitter orange) extract: acute 14-day safety study in rats and the reverse mutation Ames Test. Regul. Toxicol. Pharmacol. 2017;90:318–327. doi: 10.1016/j.yrtph.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 5.Kulig B., Alleva E., Bignami G., Cohn J., Cory-Stechta D., Landa V., O’Donoghue J., Peakall D. Animal behavioral methods in neurotoxicity assessment: SGOMSEC joint report. Environ. Health Persp. 1996;104(Suppl):193–204. doi: 10.1289/ehp.96104s2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margina D., Ilie M., Gradinaru D., Androutsopoulos V.P., Kouretas D., Tsatsakis A.M. Natural products-friends or foes? Toxicol. Lett. 2015;236:154–167. doi: 10.1016/j.toxlet.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Fang Y.S., Shan D.M., Liu J.W., Xu W., Li C.L., Wu H.Z., Ji G. Effects of constituents from Fructus aurantii immaturus and Radix paeoniae alba on gastrointestinal movement. Planta Med. 2009;75:24–31. doi: 10.1055/s-0028-1088342. [DOI] [PubMed] [Google Scholar]
- 8.Stohs S.J., Preuss H.G., Shara M. The safety of Citrus aurantium (bitter orange) and its primary protoalkaloid p-synephrine. Phytother. Res. 2011;25:1421–1428. doi: 10.1002/ptr.3490. [DOI] [PubMed] [Google Scholar]
- 9.Calapai G., Firenzuoli F., Saitta A., Squadrito F., Arlotta M.R., Constantino G., Inferrera G. Anti-obesity and cardiovascular toxic effects of Citrus aurantium extracts in the rat: a preliminary report. Fitoterapia. 1999;70:586–592. [Google Scholar]
- 10.Arbo M.D., Larentis E.R., Linck V.M., Aboy A.L., Pimentel A.L., Henriques A.T., Dallegrave E., Garcia S.C., Leal M.B., Limberger R.P. Concentrations of p-synephrine in fruits and leaves of Citrus species (Rutaceae) and the acute toxicity testing of Citrus aurantium extract and p-synephrine. Food Chem. Toxicol. 2008;46:2770–2775. doi: 10.1016/j.fct.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 11.Arbo M.D., Schmitt G.C., Limberger M.F., Charao M.F., Moro A.M., Ribeiro G.L., Dallegrave E., Garcia S.C., Leal M.B., L.Imberger R.P. Subchronic toxicity of Citrus aurantium L. (Ruttaceae) extract and p-synephrine in mice. Reg. Toxicol. Pharmacol. 2009;54(2009):114–117. doi: 10.1016/j.yrtph.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Hansen D.K., Juliar B.E., White G.E., Pellicore L.S. Developmental toxicity of Citrus aurantium in rats. Birth Defects Res. B. Devel. Reprod. Toxicol. 2011;92:216–223. doi: 10.1002/bdrb.20308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen D.K., George N.I., White G.E., Abdel-Rahman A., Pellicore L.S., Fabricant D. Cardiovascular toxicity of Citrus aurantium in exercised rats. Cardiovasc. Toxicol. 2013;13(2013):208–219. doi: 10.1007/s12012-013-9199-x. [DOI] [PubMed] [Google Scholar]
- 14.Hansen D.K., George N.I., White G.E., Pellicore L.S., Abdel-Rahman A., Fabricant D. Physiological effects following administration of Citrus aurantium for 28 days in rats. Toxicol. Appl. Pharmacol. 2012;261:236–247. doi: 10.1016/j.taap.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Penzak S.R., Jann M.W., Cold J.A., Hon Y.Y., Desai H.D., Seville Gurley B.J. (sour) orange juice: synephrine content and cardiovascular effects in normotensive adults. J. Clin. Pharmacol. 2011;41:1059–1063. doi: 10.1177/00912700122012652. [DOI] [PubMed] [Google Scholar]
- 16.Bent S., Padula A., Neuhaus J. Safety and efficacy of Citrus aurantium for weight loss. Amer. J. Cardiol. 2004;94:1359–1361. doi: 10.1016/j.amjcard.2004.07.137. [DOI] [PubMed] [Google Scholar]
- 17.Fugh-Berman A., Myers A. Citrus aurantium: an ingredient of dietary supplements marketed for weight loss: current status of clinical and basic research. Exptl. Biol. Med. 2004;229:698–704. doi: 10.1177/153537020422900802. [DOI] [PubMed] [Google Scholar]
- 18.Haaz S., Williams K.Y., Fontaine K.R., Allison D.R. Bitter orange. In: Coates P.M., Blackman M.R., Cragg G.M., Levine M., Moss J., White J.D., editors. Encyclopedia of Dietary Supplements. 2nd ed. Marcel Dekker; New York, NY: 2010. pp. 52–59. [Google Scholar]
- 19.Anon Dangerous supplements. What you don’t know about these 12 ingredients could hurt you. Consumer Reports, 2010. Sept. 16–20. [PubMed]
- 20.Natural Medicines Comprehensive Database. Bitter orange, 2016. http://naturaldatabase.therapeuticresearch.com/nd/Search.aspx?pt=100&id=976e.
- 21.Bakhyia N., Ziegenhagen R., Hirsch-Ernst K.I., Dusemund B., Richter K., Schultrich K., Pevny S., Schafer B., Lampen A. Phytochemical compounds in sports nutrition: synephrine and hydroxycitric acid (HCA) as examples for evaluation of possible health risks. Mol. Nutr. Food Res. 2017 doi: 10.1002/mnfr.201601020. [DOI] [PubMed] [Google Scholar]
- 22.Stohs S.J., Preuss H.G., Shara M. A review of the human clinical studies of bitter orange (Citrus aurantium) and its primary protoalkaloid p-synephrine. Int. J. Med. Sci. 2012;9:527–538. doi: 10.7150/ijms.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R. Marles. Synephrine, octopamine and caffeine health risk assessment (HRA) report. Health Canada Natural Health Products Directorate, File No. 172091, 2011, May. p. 1–49. http://www.nutratechinc.com/advz/advz.php?p=2.
- 24.B. Lynch. Review of the safety of p-synephrine and caffeine. Intertek-Cantox Report, 2013, April. pp. 1–20. http://www.novelingredient.com/wp-content/uploads/2017/03/Intertek-Cantox-Apr13.pdf.
- 25.Stohs S.J. Safety, efficacy and mechanistic studies regarding Citrus aurantium (bitter orange) extract and p-synephrine. Phytother. Res. 2017;10 doi: 10.1002/ptr.5879. 1002/ptr.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaats G.R., Miller H., Preuss H.G., Stohs S.J. A 60-day double-blind: placebo-controlled safety study involving Citrus aurantium (bitter orange) extract. Food Chem. Toxicol. 2013;55:358–362. doi: 10.1016/j.fct.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Stohs S.J., Preuss H.G., Shara M. A review of the receptor binding properties of p-synephrine as related to its pharmacological effects. Oxid. Med. Cell. Longevity. 2011 doi: 10.1155/2011/482973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carpene’ C., Galitzky J., Fontana E., Algie C., Lafontan M., Berlan M. Selective activation of beta 3-adrenoreceptors by octopamine: comparative studies in mammalian fat cells. Naunyn Schmiedebergs Arch. Pharmacol. 1999;359:310–321. doi: 10.1007/pl00005357. [DOI] [PubMed] [Google Scholar]
- 29.Carpene’ M.A., Testar X., Carpene’ C. In: High Doses of Synephrine and Octopamine Activate Lipolysis in Human Adipocytes, Indicating That Amines from Citrus Might Influence Adiposity. Hayat Citrus. K., editor. Nova Science Publishers Inc.; 2014. pp. 141–168. Chapter 8. [Google Scholar]
- 30.Mercader J., Wanecq E., Chen J., Carpene C. Isonorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium. J. Physiol. Biochem. 2011 doi: 10.1007/s13105-011-0078-2. [DOI] [PubMed] [Google Scholar]
- 31.Stohs S.J., Badmaev V. A review of stimulant and non-stimulant thermogenic agents. Phytother. Res. 2016;30:733–740. doi: 10.1002/ptr.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]