Abstract
Several lines of evidence implicate microglial activation and abnormal immune response in the etiology of psychosis. Previous positron emission tomography (PET) neuroimaging studies of the translocator protein 18 kDa, TSPO, were limited by low affinity of the first-generation radioligand, low-resolution scanners, and small sample sizes. Moreover, there is a dearth of literature on microglial activation in individuals at clinical high risk (CHR) for psychosis. We used a novel second-generation TSPO radioligand, [18F]FEPPA, to examine whether microglial activation is elevated in the dorsolateral prefrontal cortex (DLPFC) and hippocampus of antipsychotic-naive CHR. Twenty-four CHR (antipsychotic-naive n=22) and 23 healthy volunteers (HV) completed a high resolution [18F]FEPPA PET scan and MRI. The PET data were analyzed using the validated two-tissue compartment model with arterial plasma input function with total volume of distribution (VT) as outcome measure. All analyses were controlled for the TSPO rs6971 polymorphism. We did not observe any significant differences in microglial activation, as indexed by [18F]FEPPA VT, between CHR and HV in either the DLPFC (F(1, 44)=0.41, p=0.52) or the hippocampus (F(1, 44)=2.78, p=0.10). Exploratory associations show that in CHR, [18F]FEPPA VT was positively correlated with apathy (DLPFC: r=0.55, p=0.008; hippocampus: r=0.52, p=0.013) and state anxiety (DLPFC: r=0.60, p=0.003; hippocampus: r=0.48, p=0.024). The lack of significant group differences in [18F]FEPPA VT suggests that microglial activation is not significantly elevated in the clinical high risk state that precedes psychosis.
Introduction
A role of the immune system in the etiology of schizophrenia is supported by convergent evidence from genetic, epidemiology, and preclinical studies (Kirkpatrick and Miller, 2013). Genome-wide association studies have found associations between several inflammation-related genes and risk of schizophrenia (Sekar et al, 2016; Shi et al, 2009; Stefansson et al, 2009), suggesting a link between the immune system and schizophrenia. Supporting these findings, several studies have reported increased level of inflammatory markers (eg, pro-inflammatory cytokines and C-reactive protein) in individuals at elevated clinical risk for schizophrenia (Perkins et al, 2015; Stojanovic et al, 2014) and in patients with psychosis (Fernandes et al, 2016). Moreover, epidemiologic and preclinical studies have suggested an association between early-life infection and risk of schizophrenia (Brown, 2011). Clinical relevance of such links is supported by clinical trials showing a potential role for anti-inflammatory agents in alleviating psychotic symptoms (Khandaker et al, 2015; Sommer et al, 2014).
Microglia, the resident macrophages of the central nervous system, are the key components of the brain’s immune defense system. Following a brain injury they become activated and transform morphologically from ramified to amoeboid form (Perry et al, 2007). An important characteristic of activated microglia is a significant increase in expression of the mitochondrial 18 kDa translocator protein, also known as TSPO, thus making TSPO a suitable target for imaging microglial activation(Venneti et al, 2009).
Several postmortem studies have examined markers of microglial activation including TSPO in patients with schizophrenia, however, there is a wide variability across different studies, as about half of studies showed an increase in microglial activation markers and 40% showed no difference (Trepanier et al, 2016). Given this inconsistency and to avoid limitations of postmortem studies, microglial activation can be quantified in-vivo with positron emission tomography (PET) by using radioligands that target TSPO.
Studies on TSPO have shown a single gene polymorphism in the gene of this protein (rs6971) that affects the binding affinity of second-generation TSPO radioligands such as [18F]FEPPA, [11C]DPA-713 and [11C]PBR28 (Kreisl et al, 2013a; Mizrahi et al, 2012; Owen et al, 2012). On the basis of this polymorphism individuals can be classified as high-affinity binder (HAB), mixed-affinity binder (MAB), or low-affinity binder (LAB).
Thus far, several PET studies have examined microglial activation in schizophrenia whereas a few have examined the disease at very early stages such as first-episode psychosis. Early PET studies of schizophrenia using the prototypical radioligand for TSPO, [11C]PK11195, showed increased binding of this radioligand in treated schizophrenia patients compared to healthy volunteers, respectively, in total gray matter and hippocampus (Doorduin et al, 2009; van Berckel et al, 2008). The interpretation of these studies are, however, limited due to the known technical limitations of [11C]PK11195 (Vivash and O'Brien, 2016). These limitations promoted the development of second-generation TSPO radioligands with greater advantages for quantifying TSPO expression in vivo (Doorduin et al, 2009; van Berckel et al, 2008). The first study using a second-generation TSPO radioligand, [11C]DAA1106, found no significant difference in binding between chronic medicated schizophrenia and healthy volunteers; however, [11C]DAA1106 binding in schizophrenia patients was significantly correlated with duration of illness and severity of positive psychotic symptoms (Takano et al, 2010). Recently, Coughlin and colleagues (Coughlin et al, 2016) using PET and another second-generation TSPO radioligand, [11C]DPA-713, also found no difference in microglial activation between recent-onset schizophrenia (n=12) and healthy volunteers (n=14). Our study in treated chronic schizophrenia (n=16), and more recently in untreated first-episode psychosis (total n=19, n=14 antipsychotic naive) using [18F]FEPPA did not observe significant group differences between patients and matched healthy volunteers or significant associations between [18F]FEPPA binding and length of illness, severity of symptoms, or neuropsychological measures when controlling for multiple testing (Hafizi et al, 2016; Kenk et al, 2015). Most recently, Collste et al (2017) using another second-generation TSPO radioligand, [11C]PBR28, reported significantly lower binding of [11C]PBR28 in antipsychotic-naive first-episode psychotic patients (n=16) as compared to matched healthy volunteers.
To date there is only one other study that examined microglial activation in CHR (n=14) which reported elevated [11C]PBR28 distribution volume ratio (DVR) in the gray matter compared to healthy volunteers (n=14; Bloomfield et al, 2015). In the same study and in a separate cohort they reported an elevated DVR in the gray matter of chronic treated schizophrenia (n=14) as compared to matched healthy volunteers. However, using DVR as outcome measure poses several important limitations (Narendran and Frankle, 2016). In fact, when the [11C]PBR28 data of Bloomfield et al are analyzed using the outcome measure, total distribution volume (VT) with the validated two tissue compartment model (2TCM) (Fujita et al, 2008; Owen et al, 2012), there were no significant group differences between CHR and healthy volunteers or schizophrenia patients versus matched healthy volunteers.
In the current study, we investigated microglial activation in the largest CHR sample so far (n=25, n=22 antipsychotic naive), using the gold standard [18F]FEPPA VT and a high-resolution research tomograph (HRRT). We also examined associations between microglial activation and severity of symptoms in addition to neuropsychological measures.
Materials and methods
Subjects
Twenty-five CHR and 24 matched healthy volunteers were initially enrolled and scanned in this study. One healthy volunteer and one CHR were excluded from all the analyses due to having the low-affinity binder genotype that did not allow [18F]FEPPA PET quantification. Most of the participants in the CHR group were antipsychotic-naive (n=22). Fifteen healthy volunteers of the total of 24 have been included in our previous cohorts (Hafizi et al, 2016), whereas none of the CHR individuals have been previously reported.
To be eligible, CHR individuals had to meet the following inclusion criteria: fulfillment of diagnostic criteria for prodromal syndrome as per the Criteria of Prodromal Syndromes (Miller et al, 2002) with no current Axis I disorders, as determined with the Structured Clinical Interview for DSM-IV (SCID; First et al, 1995), such as depression which was shown to be associated with microglial activation(Setiawan et al, 2015). Healthy volunteers did not have any history of psychiatric illness, psychoactive drug use, and/or first-degree relatives with a major mental disorder. Participants were excluded for any of the following: current or past history of diagnosis of substance abuse or a positive urine drug screen; pregnancy or current breastfeeding; clinically significant medical illness; and the presence of metal implants precluding an MRI scan. In CHR, clinical status and severity of symptoms (eg, psychosis-risk symptoms) were assessed with the structured interview for psychosis-risk syndromes (SIPS), scale of psychosis-risk symptoms (SOPS; Miller et al, 2002), Calgary Depression Scale (depression scale), Snaith-Hamilton Pleasure Scale (pleasure scale), Global Assessment of Functioning scale (global functioning), state-trait anxiety inventory (anxiety scale) and Apathy Evaluation Scale (apathy scale). Neurocognitive performance was assessed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; Randolph, 1998; Wilk et al, 2004). Assessments are described in detail in the method section of the Supplementary Material.
This study was approved by the Research Ethics Board at the Centre for Addiction and Mental Health. All subjects provided written informed consent after being informed of all study procedures.
PET and MRI Data Acquisition and Analysis
Details of PET and MRI data acquisition have been described elsewhere (Kenk et al, 2015) and are summarized below and in the method section of the Supplementary Material. Proton density-weighted (PD) brain MRI scan was obtained for each subject using a 1.5T General Electric Signa scanner (General Electric Medical Systems, Milwaukee, WI, USA) for four healthy volunteers and two CHR. For the remaining 23 CHR and 20 healthy volunteers, PD MRI images were acquired using a 3T MR-750 scanner (General Electric Medical Systems). All [18F]FEPPA PET scans were performed using a high-resolution neuro-PET camera system (HRRT, Siemens Molecular Imaging, Knoxville, TN, USA) for 125 min following an intravenous bolus injection of 183.74±12.14 (mean±SD) MBq of [18F]FEPPA. Arterial blood samples were collected automatically using an automatic blood sampling system (Model PBS-101, Veenstra Instruments, Joure, Netherlands) for the first 22.5 min after radiotracer injection at a rate of 2.5 ml/min and manually at −5, 2.5, 7, 12, 15, 20, 30, 45, 60, 90, and 120 min to measure radioactivity in blood and determine the relative proportion of radiolabeled metabolites. Dispersion- and metabolite-corrected plasma input function was generated as previously described (Rusjan et al, 2011).
Image processing and calculation of total distribution volumes (VT)
Time-activity curves were extracted for dorsolateral prefrontal cortex (DLPFC), hippocampus, medial prefrontal cortex, temporal cortex, total gray matter, and whole brain using validated in-house imaging pipeline ROMI (Rusjan et al, 2006). All regions of interest were delineated using individual proton density (PD) MRI (Rusjan et al, 2006). Kinetic parameters of [18F]FEPPA were derived from the time-activity curves using the two-tissue compartment model (2TCM) and plasma input function to obtain the total distribution volume (VT) for each region of interest, which has been validated for [18F]FEPPA quantification and described elsewhere (Kenk et al, 2015; Rusjan et al, 2011). PET images were also corrected for partial volume effect using the Muller-Gartner approach (Muller-Gartner et al, 1992), and the results are presented in the Supplementary Materials (Supplementary Figure 1 in the Supplementary Material).
For exploratory purposes, we investigated the difference between clinical groups using DVR as an outcome measure. DVR is defined as regional VT normalized by VT in the cerebellum, gray matter, or whole brain (DVR=VT _Region/VT _k, where k represents cerebellum, gray matter, or whole brain). The results of DVR analyses are presented in details in the Supplementary Materials.
Voxel-based PET image analysis
Parametric images of [18F]FEPPA VT were generated using the Logan graphical analysis method, to examine voxel-wise group comparisons of VT between groups. More details are provided in the method section of the Supplementary Material.
rs6971 Polymorphism Genotyping
The participants were categorized based on the TSPO rs6971 as high-(C/C), mixed-(C/T), and low-affinity (T/T) binders, as described elsewhere (Mizrahi et al, 2012; Owen et al, 2012). Details of genotyping procedures are provided in the Supplementary Material.
Statistical Analysis
Demographic measures were examined for any group differences using analysis of variance (ANOVA, continuous variables) or chi-square tests (categorical variables). Multivariate analysis of variance (MANOVA), with regional VTs as the dependent variables, group (CHR individuals vs healthy volunteers) as the independent variable, and the TSPO genotype (rs6971) as a covariate were carried out to test for differences in [18F]FEPPA VTs between clinical groups. Partial correlations controlling for the effects of TSPO rs6971 polymorphism were used to explore the association between [18F]FEPPA VTs and clinical and neuropsychological measures. All statistical analyses were performed using SPSS (version 22.0; IBM, Armonk, NY, USA), with p<0.05 two-tailed considered significant. Bonferroni correction was used to correct for multiple comparisons in regions we set out to test (ie dorsolateral prefrontal cortex and hippocampus). For descriptive purposes, we also report differences in medial prefrontal cortex, temporal cortex, total gray matter, and whole brain, with VT data.
Results
Demographics and Injection Parameters
Demographic and clinical characteristics of groups are presented in Table 1. Most of the participants in the CHR group were antipsychotic naive (n=22). Three out of 24 CHR individuals were taking antipsychotic medications at a very low dose: risperidone 0.5 mg, 1 mg and aripiprazole 5 mg, respectively. Six CHR individuals were taking antidepressants (SNRI or SSRI). Compared to the healthy volunteers, CHR received significantly lower specific activity (F=4.74, p=0.035). All other PET radiotracer injection parameters and demographics did not differ between the two groups (all p>0.05).
Table 1. Demographic Characteristics of the Participants and Radioligand Injection Parameters.
| Demographics | HV (n=23) | CHR (n=24) | ||
|---|---|---|---|---|
| Age (years) | 23.04±3.20 | 21.21±3.35 | F=3.69, P=0.061 | |
| Gender | Male/Female | 9/14 | 11/13 | χ2=0.22, P=0.64 |
| Genotypea | HAB/MAB/LAB | 19/4/1 | 18/6/1 | χ2=0.41, P=0.52 |
| Drugb (current use) | ||||
| Nicotine | 0 | 5 | ||
| Cannabis | 0 | 0 | ||
| Lifetime recreational drug use history (>10 times) | ||||
| Cannabis | 0 | 10 | ||
| MDMA | 0 | 1 | ||
| Anti-psychotic usec | 0 | 3 | ||
| PET measures | Amount injected (mCi) | 4.87±0.37 | 5.06±0.26 | F=3.94, P=0.053 |
| Specific activity (mCi/μmol) | 3536.16±3896.46 | 1675.07±1508.41 | F=4.74, P=0.035 | |
| Mass injected (μg) | 1.25±0.94 | 1.86±1.40 | F=3.003, P=0.090 | |
| Depression scale | 6.50±4.20 | |||
| Apathy scale | 38.88±11.55 | |||
| Pleasure scale | 3.75±3.23 | |||
| Global functioning | 53.83±7.38 | |||
| SOPS | Total | 33.58±10.80 | ||
| RBANS | Total | 88.79±16.06 | ||
| Anxiety scaled | ||||
| State | 46.04±14.20 | |||
| Trait | 55.65±10.81 | |||
Abbreviations: Anxiety scale, state-trait anxiety inventory; Apathy scale, Apathy Evaluation Scale; Depression scale, Calgary Depression Scale; HAB, high-affinity binder; LAB, low-affinity binder; Global functioning, Global assessment of functioning; MAB, mixed-affinity binder; PET, positron emission tomography; Pleasure scale, Snaith-Hamilton Pleasure Scale; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; SOPS, Scale of Psychosis-risk Symptoms.
Two low-affinity binders (one in each diagnostic group) were excluded from all the analyses listed in the table.
All the participants in both HV and CHR groups had negative urine drug screening test for ethanol, methadone, benzodiazepines, opiate, cannabis, and cocaine.
Three CHR individuals were taking antipsychotic medications at a very low dose: risperidone 0.5 mg, 1 mg and aripiprazole 5 mg, respectively.
Anxiety scores were not available for one clinical high risk individual.
Differences in [18F]FEPPA VT between CHR and Healthy Volunteers
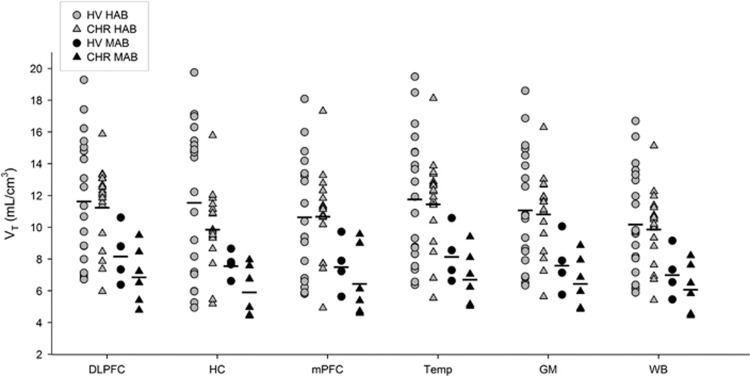
After controlling for the rs6971 polymorphism, no significant effect of clinical group (healthy volunteer vs CHR) was detected on [18F]FEPPA VTs (Figure 1; F(2, 43)=2.70, p=0.08; Hippocampus: F(1, 44)=2.78, p=0.10, 15.61% higher in healthy volunteers than CHR; DLPFC: F(1, 44)=0.41, p=0.52, 5.27% higher in healthy volunteers than CHR). The lack of group effect was not altered after controlling for age, tobacco, and/or anti-depressant use, excluding the CHR individuals that were on antidepressants and also with the correction for partial volume effects (Supplementary Figure 1). These results were consistent with other exploratory regions of interest (Supplementary Table 1). However, after removing an outlier (a CHR participant with hippocampus VT value 2 standard deviation above the mean), we found a trend toward significance (F(2, 42)=3.12, p=0.054; Hippocampus: F(1, 43)=3.83, p=0.057, 18.13% higher in healthy volunteers than CHR; DLPFC: F(1, 43)=0.78, p=0.38, 7.19% higher in healthy volunteers than CHR; Supplementary Table 9, and Figure 3). The CHR outlier was drug-naive, medically healthy and did not have any comorbidity.
Figure 1.
Total distribution volume of [18F]FEPPA (VT) in CHR and healthy volunteers across different ROIs. DLPFC, hippocampus, mPFC, Temporal cortex, total gray matter, and whole brain.
In addition, we found no significant effect of clinical group with any of the DVR methods used. Results of the DVR method obtained before and after correction for partial volume effects and also other brain regions are reported in Supplementary Tables 2–4.
Voxel-Based Analyses
In line with results of the region of interest analyses, we did not find any group differences using the ROI-independent voxel-based analyses, confirming the lack of difference in [18F]FEPPA VT between CHR and healthy volunteers (Supplementary Figure 2).
Association between [18F]FEPPA VT and Severity of Symptoms, Clinical and Neuropsychological Measures in CHR
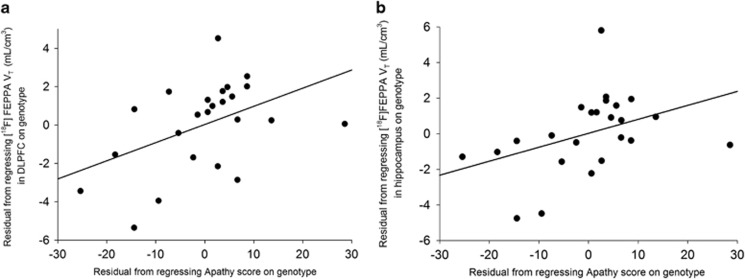
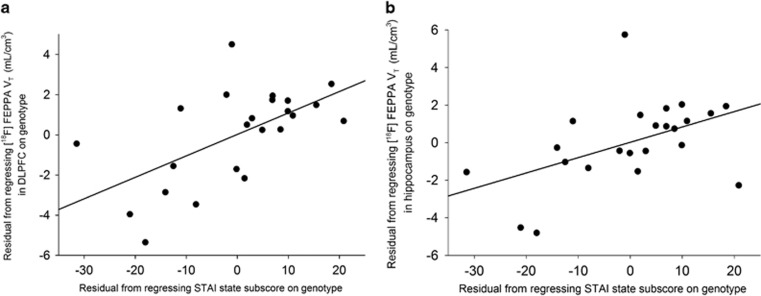
There were no significant correlations between [18F]FEPPA VT (before and after partial volume correction) and severity of psychosis-risk symptoms as measured by the SOPS, cognitive function as measured by RBANS, depression as measured by Calgary depression scale (depression scale), anhedonia as measured by Snaith-Hamilton pleasure scale (pleasure scale), and global functioning as measured by Global Assessment of Functioning (global functioning) (Supplementary Tables 5). Interestingly, apathy scores as measured by Apathy Evaluation Scale were significantly correlated with [18F]FEPPA VT in our primary regions of interest, hippocampus (r=0.52, p=0.013) and DLPFC (r=0.55, p=0.008) (Figure 2), and also in most exploratory brain regions, such that higher [18F]FEPPA VT was associated with higher apathy (Supplementary Table 7). Moreover, we observed a positive correlation between state anxiety score as measured by the state sub-scale of state-trait anxiety inventory and [18F]FEPPA VT in our primary regions of interest, hippocampus (r=0.48, p=0.024) and DLPFC (r=0.60, p=0.003; Figure 3), and also in all the exploratory brain regions, such that higher [18F]FEPPA VT was associated with greater state anxiety (Supplementary Table 8). All the correlations were exploratory and remained after removing the outlier.
Figure 2.
Relationship between apathy score and [18F]FEPPA (VT) in DLPFC (r=0.55, p=0.008) (a) and hippocampus (r=0.52, p=0.013) (b) in CHR.
Figure 3.
Relationship between the state subscore of State-Trait Anxiety Inventory (STAI) and [18F]-FEPPA (VT) in DLPFC (r=0.60, p=0.003) (a) and hippocampus (r=0.48, p=0.024) (b) in CHR.
Discussion
In this study we observed no significant differences in microglial activation in the DLPFC and hippocampus, as indexed by the gold standard [18F]FEPPA VT, between CHR and healthy volunteers. However, after removing an outlier, we observed a trend toward significant lower [18F]FEPPA binding in CHR as compared to healthy volunteers (p=0.054). There was no significant effect of clinical group on [18F]FEPPA VT in other brain regions (ie, the medial prefrontal cortex, the temporal cortex, total gray matter, and the whole brain). The results of voxel wise analyses were consistent with our ROI-based results, suggesting that our findings were not affected by ROI delineation. In addition, we observed positive associations between microglial activation in DLPFC and hippocampus and apathy and anxiety scores in CHR.
Our results in CHR are in line with four recent PET studies that examined microglial activation in psychosis using second-generation TSPO radioligands including a PET study using [11C]DAA1106 (Takano et al, 2010) and our previous study using [18F]FEPPA that found no significant differences between chronic treated schizophrenia and matched healthy volunteers (Kenk et al, 2015). A study using another second-generation TSPO radioligand, reported no significant differences in [11C]DPA-713 binding between recent-onset treated schizophrenia and healthy volunteers (Coughlin et al, 2016). More recently we found similar results in a larger group of untreated (n=14 antipsychotic naive) patients with first-episode psychosis using [18F]FEPPA (Hafizi et al, 2016). These findings along with the results of our current study, suggest that microglial activation is not significantly elevated in CHR or first-episode psychosis relative to healthy volunteers. The trend toward significance that we observed after removing the outlier, is consistent with a recent TSPO PET study using [11C]PBR28 which reported significantly lower TSPO radioligand binding in antipsychotic-naive first-episode psychotic patients as compared to matched healthy volunteers (Collste et al, 2017).
The findings of the present study, however, are in contrast with two early PET studies using [11C]PK11195 that reported higher microglial binding in the total gray matter of recent-onset schizophrenia (van Berckel et al, 2008) and in the hippocampus of treated schizophrenia (Doorduin et al, 2009). Nevertheless, [11C]PK11195 is known to have several limitations such as high non-specific binding, low brain penetration, and low signal-to-noise ratio. Our results are also in apparent contrast with the only other study in CHR that showed higher DVR in CHR and patients with chronic schizophrenia, as compared to healthy volunteers (Bloomfield et al, 2015). However, there are several differences between our study and Bloomfield and colleagues study, particularly the use of an alternative outcome measure, which for radioligands without a reference region is always controversial (Cannon, 2015; Narendran and Frankle, 2016). Notably in the Bloomfield et al [11C]PBR28 study, using the validated VT with 2TCM (Fujita et al, 2008), the authors did not find any significant difference in microglial activation between CHR/ chronic schizophrenia and healthy volunteers. Further, our sample size was substantially larger and we used a high resolution PET scanner (HRRT) with superior sensitivity and resolution.
In CHR, we observed a significant positive association between [18F]FEPPA VT in DLPFC and state anxiety, suggesting that higher microglial activation in the DLPFC is associated with higher anxiety. Preclinical studies support the role of activated microglia and immune mediators in anxiety-like behaviors (Sawada et al, 2014; Wohleb et al, 2013). Clinical studies show a reciprocal relationship between anxiety and immune response, such that inducing an immune response in healthy humans can increase anxiety (Reichenberg et al, 2001) and people with anxiety have impaired immune response (Salim et al, 2012). We also observed a positive association between [18F]FEPPA VT in DLPFC and hippocampus and apathy in CHR. This is in line with a growing body of literature supporting a link between inflammatory markers (eg proinflammatory cytokines) and negative symptoms of schizophrenia (Khandaker et al, 2015; Meyer et al, 2011). The positive association between microglial activation and apathy is also consistent with the current evidence on the role of microglial activation in Alzheimer’s disease (Kreisl et al, 2013b; Suridjan et al, 2015) and the fact that apathy is considered as an early index of inflammatory state in the central nervous system in chronic neurodegenerative diseases (Perry et al, 2007). However, this is speculation at this point and needs to be further studied.
The results of this study should be interpreted considering the limitations that are inherent to neurochemical PET studies. First, in this study CHR received significantly lower specific activity compared to the healthy volunteers. However, there were neither a significant difference in any other PET parameters (Table 1) nor any significant associations between specific activity and VT in any of our regions of interest (p>0.3). Second, while small sample size is a potential limitation in molecular imaging studies, our study is thus far the largest PET study examining microglial activation in clinical populations. Third, although an increase in [18F]FEPPA binding is mostly attributed to microglial activation, studies show that astrocytes and vascular endothelial cells also express TSPO (Notter et al, 2017). Further, TSPO expression may not directly relate with other signs of low-grade inflammation, such as inflammatory cytokines (Notter et al, 2017). However, this would not affect the overall conclusion of this study. Fourth, all the correlations were exploratory in nature, and controlling for potential confounders (ie, age, drug use, and medications) did not alter the outcome. Finally, the variability of VT in our current study, as with other second-generation TSPO radioligands, is relatively high even after controlling for the effect of rs6971 polymorphism, suggesting that larger samples would be needed to find a significant effect between groups. A sample size calculation using the data from the current study showed that to detect group effects between CHR and matched controls in the DLPFC (effect size was 0.19) or the hippocampus (effect size was 0.49), 444 and 67 participants per group, respectively, would be needed (two tailed test at α=0.05 and 80% power). After removing the outlier, a similar sample size calculation revealed that to detect a significant effect of diagnostic group in the DLPFC (effect size=0.26) or hippocampus (effect size=0.58), we would need, respectively, 231 or 49 participants per group. Despite this variability, the binding of [18F]FEPPA was increased during induced inflammation in animals (Zhang et al, 2012), Major Depressive Episode (MDE) patients (Setiawan et al, 2015), meningioma (Ko et al, 2013), and also Alzheimer's disease in humans (Kreisl et al, 2013b; Suridjan et al, 2015; Yasuno et al, 2008). Also, in this study we did not correct VT for the plasma free fraction of the radioligand (fp), as it was previously shown to substantially increase the variability (Hines et al, 2013). More studies are needed to determine whether correcting VT values for fp can improve the ability of [18F]FEPPA to distinguish alterations in microglial activation in CHR. Further, due to this variability in [18F]FEPPA binding, it is also possible that only a subgroup of CHR would present a neuroinflammatory phenotype, or that TSPO imaging cannot accurately capture low-grade inflammatory processes such as those present in psychosis-related disorders (as compared to AD or MDE).
In conclusion, our results showed no evidence of increased microglial activation as quantified with [18F]FEPPA binding, in the DLPFC and the hippocampus of CHR as compared with healthy volunteers.
Funding and disclosure
This work was supported by the National Institutes of Health (NIH) R01 grant MH100043 to Dr Mizrahi. The authors declare no conflict of interest.
Acknowledgments
We thank the excellent staff of the CAMH Research Imaging Centre and the FYPP clinic.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR et al. (2015). Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [11C] PBR28 PET brain imaging study. Am J Psychiatry 173: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS (2011). Further evidence of infectious insults in the pathogenesis and pathophysiology of schizophrenia. Am J Psychiatry 168: 764–766. [DOI] [PubMed] [Google Scholar]
- Cannon TD (2015). Microglial activation and the onset of psychosis. Am J Psychiatry 173: 3–4. [DOI] [PubMed] [Google Scholar]
- Collste K, Plavén-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A et al. (2017). Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C] PBR28. Mol Psychiatry 22: 850–856. [DOI] [PubMed] [Google Scholar]
- Coughlin J, Wang Y, Ambinder E, Ward R, Minn I, Vranesic M et al. (2016). In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [ 11C] DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry 6: e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC (2009). Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 50: 1801–1807. [DOI] [PubMed] [Google Scholar]
- Fernandes B, Steiner J, Bernstein H, Dodd S, Pasco J, Dean O et al. (2016). C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry 21: 554–564. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J (1995) Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCIDI/P. Version 2.0. Biometric Research, New York State Psychiatric Institute: New York. [Google Scholar]
- Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T et al. (2008). Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage 40: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafizi S, Tseng HH, Rao N, Selvanathan T, Kenk M, Bazinet RP et al. (2016). Imaging Microglial Activation in Untreated First-Episode Psychosis: A PET Study With [18F]FEPPA. Am J Psychiatry 174: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines CS, Fujita M, Zoghbi SS, Kim JS, Quezado Z, Herscovitch P et al. (2013). Propofol decreases in vivo binding of 11C-PBR28 to translocator protein (18 kDa) in the human brain. J Nucl Med 54: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G et al. (2015). Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr Bull 41: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB (2015). Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2: 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Miller BJ (2013). Inflammation and schizophrenia. Schizophr Bull 39: 1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Koshimori Y, Mizrahi R, Rusjan P, Wilson AA, Lang AE et al. (2013). Voxel-based imaging of translocator protein 18 kDa (TSPO) in high-resolution PET. J Cereb Blood Flow Metab 33: 348–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL et al. (2013. a). A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab 33: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N et al. (2013. b). In vivo radioligand binding to translocator protein correlates with severity of Alzheimer's disease. Brain 136(Pt 7): 2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Schwarz MJ, Muller N (2011). Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther 132: 96–110. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K et al. (2002). Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry 159: 863–865. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Rusjan PM, Kennedy J, Pollock B, Mulsant B, Suridjan I et al. (2012). Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J Cereb Blood Flow Metab 32: 968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Gartner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP et al. (1992). Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 12: 571–583. [DOI] [PubMed] [Google Scholar]
- Narendran R, Frankle WG (2016). Comment on Analyses and Conclusions of ‘Microglial Activity in People at Ultra High Risk of Psychosis and in Schizophrenia: An [11C] PBR28 PET Brain Imaging Study’. Am J Psychiatry 173: 536–537. [DOI] [PubMed] [Google Scholar]
- Notter T, Coughlin J, Gschwind T, Weber-Stadlbauer U, Wang Y, Kassiou M et al (2017). Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol Psychiatry (in press). [DOI] [PubMed]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A et al. (2012). An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 32: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DO, Jeffries CD, Addington J, Bearden CE, Cadenhead KS, Cannon TD et al. (2015). Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr Bull 41: 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C (2007). Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 7: 161–167. [DOI] [PubMed] [Google Scholar]
- Randolph C (1998) Repeatable Battery for the Assessment of Neuropsychological Status. Psychological Corporation (Harcourt): San Antonio, TX. [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A et al. (2001). Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 58: 445–452. [DOI] [PubMed] [Google Scholar]
- Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F et al. (2006). An automated method for the extraction of regional data from PET images. Psychiatry Res 147: 79–89. [DOI] [PubMed] [Google Scholar]
- Rusjan PM, Wilson AA, Bloomfield PM, Vitcu I, Meyer JH, Houle S et al. (2011). Quantitation of translocator protein binding in human brain with the novel radioligand [18F]-FEPPA and positron emission tomography. J Cereb Blood Flow Metab 31: 1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S, Chugh G, Asghar M (2012). Inflammation in anxiety. Adv Protein Chem Struct Biol 88: 1–25. [DOI] [PubMed] [Google Scholar]
- Sawada A, Niiyama Y, Ataka K, Nagaishi K, Yamakage M, Fujimiya M (2014). Suppression of bone marrow-derived microglia in the amygdala improves anxiety-like behavior induced by chronic partial sciatic nerve ligation in mice. Pain 155: 1762–1772. [DOI] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N et al. (2016). Schizophrenia risk from complex variation of complement component 4. Nature 530: 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G et al. (2015). Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I et al. (2009). Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 460: 753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS (2014). Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull 40: 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D et al. (2009). Common variants conferring risk of schizophrenia. Nature 460: 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovic A, Martorell L, Montalvo I, Ortega L, Monseny R, Vilella E et al. (2014). Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology 41: 23–32. [DOI] [PubMed] [Google Scholar]
- Suridjan I, Pollock BG, Verhoeff NP, Voineskos AN, Chow T, Rusjan PM et al. (2015). In-vivo imaging of grey and white matter neuroinflammation in Alzheimer's disease: a positron emission tomography study with a novel radioligand, [18F]-FEPPA. Mol Psychiatry 20: 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R et al. (2010). Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol 13: 943–950. [DOI] [PubMed] [Google Scholar]
- Trepanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP (2016). Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry 21: 1009–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E et al. (2008). Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 64: 820–822. [DOI] [PubMed] [Google Scholar]
- Venneti S, Wiley CA, Kofler J (2009). Imaging microglial activation during neuroinflammation and Alzheimer's disease. J Neuroimm Pharmacol 4: 227–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivash L, O'Brien TJ (2016). Imaging Microglial Activation with TSPO PET: Lighting Up Neurologic Diseases? J Nucl Med 57: 165–168. [DOI] [PubMed] [Google Scholar]
- Wilk CM, Gold JM, Humber K, Dickerson F, Fenton WS, Buchanan RW (2004). Brief cognitive assessment in schizophrenia: normative data for the Repeatable Battery for the Assessment of Neuropsychological Status. Schizophr Res 70: 175–186. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Powell ND, Godbout JP, Sheridan JF (2013). Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 33: 13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno F, Ota M, Kosaka J, Ito H, Higuchi M, Doronbekov TK et al. (2008). Increased binding of peripheral benzodiazepine receptor in Alzheimer's disease measured by positron emission tomography with [11C]DAA1106. Biol Psychiatry 64: 835–841. [DOI] [PubMed] [Google Scholar]
- Zhang X, Paule MG, Newport GD, Liu F, Callicott R, Liu S et al. (2012). MicroPET/CT Imaging of [18F]-FEPPA in the nonhuman primate: a potential biomarker of pathogenic processes associated with anesthetic-induced neurotoxicity. ISRN Anesthesiol 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.