Abstract
Beyond their roles in generating nerve impulses, ion channels are important for many facets of cell biology, including swelling and osmotic stress responses. Sabirov and colleagues now report that the prostaglandin transporter SLCO2A1 is a central component of the ubiquitous Maxi‐Cl anion channel. These findings add to a growing number of transporters displaying ion channel activity and not only provide a molecular handle for future Maxi‐Cl physiological and biophysical studies, but also underscore general questions about the principles that underlie such transporter/channel dual‐use behavior.
Subject Categories: Membrane & Intracellular Transport
Despite having had the “parts list” for a cell thanks to genome sequencing, the world of ion channel physiology continues to face the challenge that some well‐known, biophysically characterized membrane currents still lack a molecular identity. In the past few years, a number of exciting discoveries were made in which well‐studied currents such as the calcium‐activated chloride channel (Pedemonte & Galietta, 2014) and the volume‐activated anion channel (VRAC) (Jentsch, 2016) have taken a molecular form. These key advances give ion channel physiologists a target to mutate, delete, and poke in new ways to probe channel biophysical mechanisms and physiological functions. Moreover, given the pervasive lack of good pharmacological tools for most ion channels, the molecular identification step opens a path for developing chemical biology tools for specific ion channels.
One of the enduring channel mysteries has been the molecular identity of “maxi‐anion channels” (MACs). Similar to VRACs, these channels pass chloride ions as well as ATP, are found in nearly every cell type, and can be activated by a variety of diverse inputs such as osmotic, oxidative, and mechanical stresses, as well as temperature, G protein‐coupled receptor activation, and increases in intracellular calcium (Sabirov et al, 2016). In this issue of The EMBO Journal, Sabirov et al (2017) use a battery of approaches, including proteomics, targeted siRNA screening, CRISPR/Cas9 knockouts, and heterologous expression, to arrive at the conclusion that the organic anion transporter, solute carrier organic anion transporter family member 2A1(SLCO2A1) (Fig 1A), is a core component of the Maxi‐Cl channel. SLCO2A1 is better known as a prostaglandin transporter, not a channel (Roth et al, 2012). Key in this new report is the demonstration that the SLCO2A1‐dependent chloride channel activity is sensitive to prostaglandin E2 (PGE2) the normal cargo of SLCO2A1 (Fig 1B) and to inhibition by a prostaglandin transporter inhibitor, bromosulfophthalein (BSP). Further, SLCO2A1 mutations alter one of the core properties associated with an ion channel, the ability to discriminate the types of ions that pass through. Together with other corroborative studies, including the observation that disease mutations associated with SLCO2A1 eliminate channel activity, these new studies make a good case that SLCO2A1 is a central component of the Maxi‐Cl channel.
Figure 1. The transporter‐channel divide.
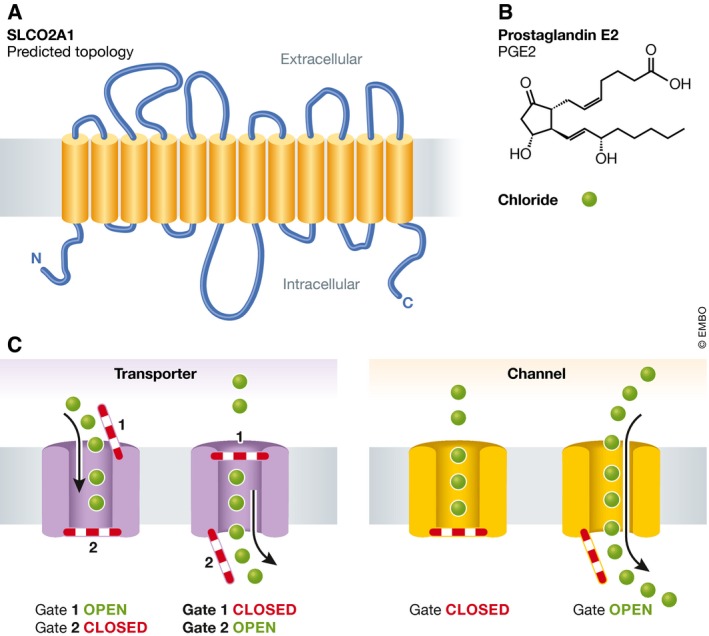
(A) Predicted SLCO2A1 topology. (B) SLCO2A1 substrates, prostaglandin E2, PGE2, and chloride. (C) Schematic comparing generalized transporter (left) and channel (right) mechanisms. Transporters use multiple gates that allow substrate to pass across the membrane through sequential steps such that one gate is always closed, preventing free passage across the membrane. Channels block ion flow with a gate that when opened allows free flow of ions down their electrochemical gradients.
How does a transporter moonlight as a channel? At first glance, transporters and channels appear to be completely different things representing two fundamentally different ways in which cells move ions across membranes (Fig 1C). Transporters use ATP hydrolysis or energy taken from transmembrane gradients to pass ions via mechanisms that involve the sequential substrate movement past a set of barriers or “gates” that ensure that there is never an open path across the membrane (Gadsby, 2009; Drew & Boudker, 2016). The presence of multiple barriers means that there is no unregulated leak of ions across the membrane and that the transport process is very controlled, slow, and has some fixed stoichiometry. By contrast, opening a channel is akin to opening the floodgate on a dam. At rest, the channel is closed by a gate, and no ions can pass. When the gate opens, the ions rush through the hole formed by the channel, down their electrochemical gradients, and only stop once the gate slammed shut.
Despite these mechanistic differences, there are a growing number of transporter proteins that have been found to have channel activity. Strikingly, many of such “dual‐use” scaffolds act as chloride channels. The best known is the cystic fibrosis transmembrane conductance regulator, CFTR. This chloride channel is built from an architecture that is usually used as an ATP‐burning pump that moves substrates against a gradient but that has been reshaped to act as an ATP‐gated channel (Liu et al, 2017). Other cases in which channel activity resides in the heart of a transporter scaffold are found in members of the ClC chloride channel family, some of which function as proton/chloride exchangers (Accardi & Picollo, 2010), the TMEM16 family that includes both calcium‐activated chloride channels and lipid scramblases (Picollo et al, 2015), and the EAAT glutamate transporter family (Cater et al, 2016). None of these protein families share a common architecture, and although each case provides some structural insight on how the architecture for each family can be converted from a transporter to a channel, there are no specific design principles that have emerged for how this type of conversion can be achieved. It is striking that the loss of the multi‐gate barrier in a transporter often leads to chloride currents. The scenario of “broken transporter makes a leaky hole” seems easy to understand with respect to creating a pathway for an ion. However, why such a change should favor anions versus cations is unclear. Given the diverse structures that can act in this dual‐use transporter‐channel mode, the answers may reside in the general biophysical parameters governing the ion–protein or ion–protein–lipid interactions that shape the actual permeation pathway. Further, the polarizability of anions may make them more forgiving with respect to movement along the hydrophobic crevices and passages made by the dual‐use transporter/channel. This new dual‐use example is likely to motivate ion channel biophysicists to pursue further investigation of this recurring phenomenon.
SLCO2A1 is part of large family of transporters that are predicted to have a twelve‐transmembrane topology but for which there is currently no structural information (Roth et al, 2012). The identification of SLCO2A1 as a key component of the Maxi‐Cl channel raises many interesting questions. What sort of structural changes are needed to make this transporter scaffold act as a channel? Where is the pore? Is the fact that PGE2 has a carboxylate related to the ability of the SCLO2A1 to act as part of a chloride channel? Are there other proteins or lipid components that are essential co‐factors to form the native Maxi‐Cl channel? Indeed, although Sabirov et al (2017) show that many of the properties of Maxi‐Cl are recapitulated in reconstitution experiments, the BSP sensitivity is lost, indicating that there are other components in the native channel. Can one find better and more specific small molecules or biologics that could manipulate Maxi‐Cl function? The identification of this new example of a dual‐use scaffold that can serve as transporter and a channel adds to the wave of discoveries that continues to wash away the perceived border between transporters and channels. Such advances make channel surfing fun and are sure to keep ion channel physiologists tuned in.
See also: RZ Sabirov et al (November 2017)
References
- Accardi A, Picollo A (2010) CLC channels and transporters: proteins with borderline personalities. Biochim Biophys Acta 1798: 1457–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cater RJ, Ryan RM, Vandenberg RJ (2016) The split personality of glutamate transporters: a chloride channel and a transporter. Neurochem Res 41: 593–599 [DOI] [PubMed] [Google Scholar]
- Drew D, Boudker O (2016) Shared molecular mechanisms of membrane transporters. Annu Rev Biochem 85: 543–572 [DOI] [PubMed] [Google Scholar]
- Gadsby DC (2009) Ion channels versus ion pumps: the principal difference, in principle. Nat Rev Mol Cell Biol 10: 344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ (2016) VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat Rev Mol Cell Biol 17: 293–307 [DOI] [PubMed] [Google Scholar]
- Liu F, Zhang Z, Csanady L, Gadsby DC, Chen J (2017) Molecular structure of the human CFTR ion channel. Cell 169: 85–95 e8 [DOI] [PubMed] [Google Scholar]
- Pedemonte N, Galietta LJ (2014) Structure and function of TMEM16 proteins (anoctamins). Physiol Rev 94: 419–459 [DOI] [PubMed] [Google Scholar]
- Picollo A, Malvezzi M, Accardi A (2015) TMEM16 proteins: unknown structure and confusing functions. J Mol Biol 427: 94–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M, Obaidat A, Hagenbuch B (2012) OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol 165: 1260–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov RZ, Merzlyak PG, Islam MR, Okada T, Okada Y (2016) The properties, functions, and pathophysiology of maxi‐anion channels. Pflugers Arch 468: 405–420 [DOI] [PubMed] [Google Scholar]
- Sabirov RZ, Merzlyak PG, Okada T, Islam MR, Uramoto H, Mori T, Makino Y, Matsuura H, Xie Y, Okada Y (2017) The organic anion transporter SLCO2A1 constitutes the core component of the Maxi‐Cl channel. EMBO J 36: 3309–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]


