Abstract
Context:
Basal and poststimulation salivary cortisol and cortisone levels can be useful in the diagnosis of adrenal insufficiency. However, little is known about the optimal cutoffs and performance characteristics of these tests.
Objective:
To derive the cutoff values and study the performance characteristics of salivary cortisol and salivary cortisone in the diagnosis of adrenal insufficiency.
Design and Setting:
Prospective study in a regional hospital in Hong Kong from January 2014 to September 2015.
Participants:
Fifty-six Chinese healthy volunteers and 171 patients suspected of having adrenal insufficiency.
Main Outcome Measures:
All participants underwent low-dose short Synacthen test (LDSST) with intravenous injection of 1 μg of tetracosactide (Synacthen 1–24). Serum cortisol, salivary cortisol and cortisone levels were measured at baseline and 30 and 60 minutes afterward.
Results:
Using the reference cutoff (mean − 2 standard deviations of post-LDSST peak serum cortisol) derived from healthy volunteers as the gold standard, receiver operating characteristic analysis of patients’ data revealed that both post-LDSST peak salivary cortisol and cortisone performed better than basal tests. The most optimal cutoff values for serum cortisol as measured by immunoassay and for salivary cortisol and salivary cortisone as measured by liquid chromatography–tandem mass spectrometry (LC-MS/MS) were 376, 8.6, and 33.5 nmol/L, respectively, for post-LDSST peak values, and 170, 1.7, and 12.5 nmol/L, respectively, for basal values.
Conclusions:
We established method-specific reference cutoffs for serum cortisol, salivary cortisol, and salivary cortisone during LDSST. Both post-LDSST peak salivary cortisol and cortisone performed well as diagnostic tests for adrenal insufficiency. Their measurements by LC-MS/MS can alleviate problems associated with serum cortisol immunoassays.
Keywords: salivary cortisol, salivary cortisone, low-dose short Synacthen test, adrenal insufficiency
Reference cutoffs for serum cortisol, salivary cortisol, and salivary cortisone in a short Synacthen test (SST) were established. Both post-SST salivary tests performed well in diagnosing adrenal insufficiency.
An accurate diagnosis of adrenal insufficiency (AI) is important because the condition is potentially life-threatening if missed; however, overdiagnosis may lead to unnecessary replacement of glucocorticoids, leading to decreased quality of life and increased mortality [1]. Various tests, including the insulin tolerance test, the glucagon stimulation test, and the standard (250 μg) and low-dose (1 μg) short Synacthen (tetracosactide) tests (SSTs), have been used to diagnose AI. The SSTs are the most widely used, owing to their convenience, safety, and reasonably good correlation with the insulin tolerance test [2–5]. However, the reference cutoff levels for the SSTs differed in different studies, ranging from 418 to 574 nmol/L, depending on the nature of the study population, the gold standard used to define AI, the dosage of ACTH and time of sampling, the type of statistical analysis used, and the type of cortisol assay used [4, 6–9]. The fluorimetric cortisol assays adopted in older studies also measured corticosterone, thus overestimating the cortisol levels by 20% to 30% [10, 11]. Even among modern immunoassays, different commonly used assays can yield different results [8, 9, 12].
The levels of the binding proteins, cortisol-binding globulin (CBG) and albumin, may also contribute to the variation in cutoff levels and the performance characteristics of the post-SST serum total cortisol [1, 7, 13]. Serum free cortisol has been shown to provide a more accurate measurement of the circulating glucocorticoid status than serum total cortisol [13–15]. However, the direct measurement of serum free cortisol requires laborious laboratory techniques that are not commonly available, and the accuracy of calculated free cortisol [13, 14] is not widely accepted. Salivary cortisol may serve as a better alternative, as it is noninvasive and reflects the level of serum free cortisol [16–19]. Measurement of salivary steroids with liquid chromatography–tandem mass spectrometry (LC-MS/MS) enhances the sensitivity by 10- to 100-fold [19–21], and it improves the specificity by eliminating cross-reactivity among various metabolites [16, 19, 20], thus enabling concomitant measurement of cortisone in addition to cortisol.
The potential utility of salivary cortisone was proposed by Perogamvros et al. [19] in 2009. Salivary cortisone is formed predominantly from serum free cortisol, by the enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD) type 2, as the latter passes through the salivary gland [22]. Diffusion of serum free cortisone into the saliva contributes to a lesser extent [23]. Total and free cortisol levels are higher than total and free cortisone levels in the serum [22–24], yet the ratio is reversed in the saliva, with salivary cortisone to cortisol ratio of 4:1 to 10:1 [19, 24], thus allowing more accurate measurement of cortisone. Both salivary cortisone and cortisol correlate strongly with serum cortisol; however, at low serum total cortisol levels of < 74 nmol/L, when salivary cortisol is undetectable, salivary cortisone can still be detected [24]. In AI patients on hydrocortisone replacement, salivary cortisone can also be used for monitoring, as it is not contaminated by oral hydrocortisone intake [18, 23, 24].
The aims of this study were to derive the cutoff values and study the performance characteristics of salivary cortisol and salivary cortisone, as measured by LC-MS/MS, in the diagnosis of AI.
1. Subjects and Methods
A prospective study was carried out in a regional hospital in Hong Kong between January 2014 and September 2015. The study was approved by the Hospital’s Research and Ethics Committee, and all participants provided written consent for the investigations.
A. Subjects
Healthy subjects were recruited from hospital staff and their relatives. “Healthy” was defined as the absence of any symptoms and the absence of conditions or medications that were known to affect the hypothalamic-pituitary-adrenal axis, the CBG, or the albumin levels. Subjects suspected of having AI from the clinical context constituted the patient group. All subjects with acute hypocortisolism, hemodynamic instability at the time of testing, bleeding inside the oral cavity, pregnancy, or mental incapacity for informed consent were excluded.
B. Test Protocol
Patients receiving chronic hydrocortisone replacement had to stop the drug for at least 3 days before the test (Supplemental Data 1 (14KB, docx) ). A saliva sample for steroid measurement was collected by placing the cotton tubes (Salivette) in the mouth, chewing for 2 to 3 minutes, and then carefully putting the Salivette into a plastic container without touching it with hands. No drinking, eating, using mouthwash, or brushing teeth was allowed within 30 minutes before the collection of saliva samples, and subjects were advised to refrain from smoking or eating liquorice within 24 hours.
All subjects underwent the low-dose SST (LDSST) in the morning. Simultaneous saliva and serum samples were collected at baseline. A 1-μg bolus of Synacthen was then injected intravenously (Supplemental Data 1 (14KB, docx) ). At 30 and 60 minutes after the injection, 2 more pairs of saliva and serum samples were collected. The higher value of the tested parameters, at either 30 or 60 minutes, was regarded as the “peak” value.
C. Laboratory Methods
Serum total cortisol was assayed with the competitive chemiluminescent microparticle immunoassay using the Abbott Architect i2000SR system (Abbott Laboratories, Abbott Park, IL). The assay coefficient of variation (CV) was 4.0% to 6.2% at low levels and 3.3% to 4.3% at high levels. Salivary cortisol and cortisone were assayed with LC-MS/MS using the Waters Xevo TQ MS system (Waters, Milford, MA). The assay CV for salivary cortisol was ~5% to 7% across all ranges; that for salivary cortisone was ~7% to 10% across all ranges. The lower limit of detection was 0.5 nmol/L for both salivary cortisol and cortisone. Serum CBG was measured using a commercial human CBG enzyme-linked immunosorbent assay kit (BioVendor–Laboratorni Medicina, Brno, Czech Republic).
D. Statistical Analysis
The statistical analysis was performed using SPSS version 21.0. When the data were shown to be in a normal distribution by both the Kolmogorov–Smirnov and Shapiro–Wilk tests, the reference range was established by using 2 standard deviations (SDs) above or below the mean (mean ± 2 SDs); otherwise, the 95% reference level was used. Receiver operating characteristic (ROC) curves were drawn to compare the performance of serum cortisol, salivary cortisol, and salivary cortisone during LDSST in the patient group. Continuous data were expressed as mean ± SD and/or range. Correlations among continuous variables were assessed using Pearson correlation coefficients. A P value of <0.05 was considered as statistically significant.
2. Results
Fifty-six healthy Chinese volunteers (28 males and 28 females; mean age, 37 years; age range, 18 to 66 years) were recruited. Two subjects were taking amlodipine for hypertension; 1 subject was taking metformin for diabetes mellitus; and 1 subject was taking amlodipine, losartan, metformin, simvastatin, and aspirin for hypertension, hyperlipidemia, and diabetes. All others had no chronic medical illnesses and required no medication within 1 month of testing. All of them had normal liver, renal, and thyroid function tests. Their mean basal and peak serum total cortisol levels were 252.8 ± 78.5 and 509.2 ± 66.6 (501.6 for males and 516.8 for females) nmol/L, respectively (Table 1); 96.4% (n = 54) of them reached peak cortisol levels at 30 minutes after Synacthen injection. The mean CBG of the male and female volunteers were 28.5 ± 3.3 and 32.9 ± 4.3 μg/mL, respectively. The distribution of serum cortisol and CBG was shown to be normal. The value corresponding to the mean − 2 SDs of the peak serum cortisol in this healthy cohort (376 nmol/L) was used as the gold standard to further evaluate the salivary parameters in the patient group (Table 1).
Table 1.
Basal Characteristics and Results of Normal Subjects
| Range | Mean | SD | Suggested Reference Cut-offa | |
|---|---|---|---|---|
| Age, y | 18–66 | 36.6 | 12.3 | |
| Sex | M = 28; F = 28 | |||
| eGFR,b mL/min/1.73 m2 | 72–158 | 99.7 | 18.1 | |
| Thyroid function test | ||||
| fT4, pmol/L | 11.3–17.6 | 13.7 | 1.4 | |
| TSH, mIU/L | 0.19–3.26 | 1.17 | 0.58 | |
| Serum cortisol, nmol/L | ||||
| Basal (at 0 min) | 95–407 | 252.8 | 78.5 | 95.8 |
| Peak (at 30 or 60 min) | 365–633 | 509.2 | 66.6 | 376.0 |
| Salivary cortisol, nmol/L | ||||
| Basal (at 0 min) | 0.7–12 | 4.3 | 2.6 | 0.7 |
| Peak (at 30 or 60 min) | 10–55 | 23.9 | 8.5 | 10.9 |
| Peak (at 30 or 60 min) (n = 55 after exclusion of outlier) | 10–40 | 23.3 | 7.5 | 8.4 |
| Salivary cortisone, nmol/L | ||||
| Basal (at 0 min) | 8.7–77 | 25.4 | 10.4 | 10.5 |
| Peak (at 30 or 60 min) | 35–112 | 60.3 | 13.3 | 37.1 |
| Peak (at 30 or 60 min) (n = 55 after exclusion of outlier) | 35–85 | 59.4 | 11.4 | 36.5 |
| Serum proteins | ||||
| CBG, ug/mLc | ||||
| Male (n = 28) | 21.1–35.9 | 28.5 | 3.3 | |
| Female (n = 28) | 23.9–40.8 | 32.9 | 4.3 | |
| Albumin, g/L | 41–53 | 46.6 | 2.8 |
For subjects, n = 56 unless otherwise specified. All mean and SD values are rounded to 1 decimal place, except for TSH, which is rounded to 2 decimal places.
Abbreviation: eGFR, estimated glomerular filtration rate.
Mean − 2 SDs for data with normal distribution (bold and underlined); otherwise, the 2.5th percentile was used.
Calculated by the abbreviated modification of diet in renal disease equation: 186 × (Creatinine/88.4)−1.154 × (Age)−0.203 × (0.742 if female).
Divide by 52 to convert μg/mL to μmol/L, as the relative molecular mass of CBG is 52 kDa.
One hundred seventy-one Chinese patients (82 males and 89 females; mean age, 57 years; age range, 19 to 89 years) completed the study. They were referred for investigation of AI due to the following reasons: nonspecific clinical features (n = 55) such as unexplained dizziness and fatigue, electrolyte disturbance (n = 18) such as hyponatremia, diseases or interventions involving the sellar and suprasellar regions (n = 124), previous administration of exogenous glucocorticoids (n = 32), and postadrenalectomy (n = 4) (Table 2). One hundred sixty (93.6%) of them reached their peak cortisol levels at 30 minutes after Synacthen injection. Patients with peak serum cortisol < 376 nmol/L were classified as having AI; the rest were classified as non-AI. The mean peak serum cortisol for the AI group (n = 59) was 203.2 ± 121.6 nmol/L, whereas that for the non-AI group (n = 112) was 486.8 ± 76.7 nmol/L (Table 2).
Table 2.
Basal Characteristics and Test Results of the Patient Group (n = 171)
| Baseline Characteristics | AI (n = 59) | Non-AI (n = 112) |
|---|---|---|
| Age, years | 56.9 ± 14.2 (25–80) | 56.6 ± 12.4 (19–89) |
| Sex | M = 39; F = 20 | M = 43; F = 69 |
| eGFR,a mL/min/1.73m2 | 92.1 ± 29.5 (36–153) | 92.5 ± 25.7 (37–169) |
| Chronic liver disease | 1 | 1 |
| TSH, mIU/L/fT4, pmol/L | 1.43 ± 1.34/13.5 ± 3.4 | 1.28 ± 0.82/13.0 ± 1.8 |
| Clinical feature | 24 (40.7%) | 31 (27.7%) |
| Postural hypotension | 1 | 1 |
| Dizziness/fatigue/weight loss | 19 | 25 |
| Neurologic symptoms | 2 | 1 |
| Gastrointestinal symptoms | 2 | 1 |
| Cushingoid | 0 | 1 |
| Hypoglycemia | 0 | 2 |
| Electrolyte disturbance | 11 (18.6%) | 7 (6.3%) |
| Hyponatremia | 9 | 6 |
| Hyperkalemia | 2 | 1 |
| Suprasellar/sellar disease | 34 (57.6%) | 90 (80.4%) |
| A. Disease | ||
| Nonfunctioning pituitary adenoma | 13 | 48 |
| Acromegaly | 3 | 12 |
| Cushing disease | 1 | 0 |
| Prolactinoma | 2 | 3 |
| Craniopharyngioma | 3 | 3 |
| Pituitary apoplexy | 3 | 5 |
| Empty sellar syndrome | 2 | 5 |
| Nasopharyngeal carcinoma | 1 | 5 |
| Others | 6 | 9 |
| B. Intervention | ||
| Brain surgery | 22 (37.3%) | 66 (58.9%) |
| Radiotherapy | 9 (15.3%) | 16 (14.3%) |
| Gamma knife | 10 (16.9%) | 22 (19.6%) |
| C. Complications | ||
| Visual impairment | 16 (27.1%) | 38 (33.9%) |
| Hypopituitarism other than cortisol | 26 (44.0%) | 41 (36.6%) |
| Cranial diabetes insipidus | 6 (10.2%) | 12 (10.7%) |
| Use of exogenous steroid | 20 (33.9%) | 12 (10.7%) |
| Adrenalectomy for Cushing syndrome | 2 (3.4%) | 2 (1.8%) |
| Addison disease | 1 (1.7%) | 0 (0%) |
| Test results | ||
| Serum cortisol, nmol/L | ||
| Basal (at 0 min) | 106.1 ± 80.7 (<28–320) | 264.1 ± 88.4 (116–532) |
| Peak (at 30 or 60 min) | 203.2 ± 121.6 (<28–366) | 486.8 ± 76.7 (376–682) |
| Salivary cortisol, nmol/L | ||
| Basal (at 0 min) | 2.0 ± 2.1 (<0.5–10) | 4.7 ± 3.0 (1.2–19) |
| Peak (at 30 or 60 min) | 5.9 ± 6.9 (<0.5–33) | 20.9 ± 10.1 (5.3–52) |
| Salivary cortisone, nmol/L | ||
| Basal (at 0 min) | 9.6 ± 9.8 (<0.5–42) | 24.5 ± 10.5 (8.4–61) |
| Peak (at 30 or 60 min) | 18.9 ± 16.2 (<0.5–62) | 54.0 ± 17.3 (17–111) |
| Serum proteins | ||
| CBG, μg /mLb | 29.8 ± 7.6 (10.2–52.3) | 32.0 ± 6.0 (19.4–53.0) |
| Albumin, g/L | 41.8 ± 3.7 (31–49) | 43.6 ± 3.2 (29–51) |
Continuous data are expressed as mean ± SD (range) and are rounded to 1 decimal place, except for TSH, which is rounded to 2 decimal places. Integral values represent number of patients (percentage) unless otherwise specified.
Abbreviation: eGFR, estimated glomerular filtration rate.
Calculated by the abbreviated modification of diet in renal disease equation: 186 × (Creatinine/88.4)−1.154 × (Age)−0.203 × (0.742 if female).
Divide by 52 to convert μg/mL to μmol/L, as the relative molecular mass of CBG is 52 kDa.
The mean basal and peak salivary cortisol levels of the healthy subjects were 4.3 ± 2.6 and 23.9 ± 8.5 nmol/L, respectively (Table 1). The mean basal and peak salivary cortisol levels for the AI patients were 2.0 ± 2.1 and 5.9 ± 6.9 nmol/L, respectively, whereas those for the non-AI patients were 4.7 ± 3.0 and 20.9 ± 10.1 nmol/L, respectively (Table 2).
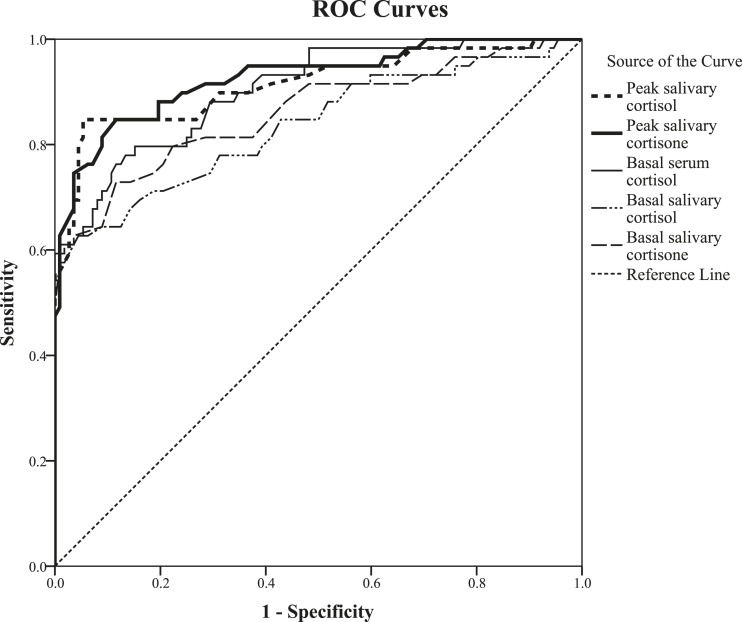
From the ROC curve analysis (Fig. 1), peak salivary cortisol had a larger area under the curve (AUC) (0.914 ± 0.026; estimate ± standard error) than did basal salivary cortisol (0.836 ± 0.036) (Table 3). For the highest accuracy, the cutoff values of peak and basal salivary cortisol were 8.6 nmol/L [sensitivity 84.7%, specificity 94.6%, positive likelihood ratio (LR+) 15.82, negative likelihood ratio (LR−) 0.16] and 1.7 nmol/L (sensitivity 62.7%, specificity 95.5%, LR+ 14.05, LR− 0.39), respectively.
Figure 1.
ROC curves of basal serum cortisol, salivary cortisol, and salivary cortisone, and post-LDSST peak salivary cortisol and cortisone in the patient group (n = 171) when AI (disease) was defined as post-LDSST serum cortisol < 376 nmol/L.
Table 3.
Area Under the Curve (AUC) for the ROC Curves
| Test | AUC (±SE) | Best Cutoff (nmol/L) | Sensitivity (%) | Specificity (%) | LR | |
|---|---|---|---|---|---|---|
| + | − | |||||
| Peak salivary cortisol | 0.914 ± 0.026 (0.863–0.965) | 8.6 | 84.7 | 94.6 | 15.819 | 0.161 |
| Peak salivary cortisone | 0.926 ± 0.022 (0.882–0.970) | 33.5 | 84.7 | 88.4 | 7.301 | 0.173 |
| Basal serum cortisol | 0.903 ± 0.024 (0.856–0.951) | 170 | 76.3 | 88.4 | 6.571 | 0.268 |
| Basal salivary cortisol | 0.836 ± 0.036 (0.766–0.906) | 1.7 | 62.7 | 95.5 | 14.047 | 0.390 |
| Basal salivary cortisone | 0.862 ± 0.033 (0.797–0.926) | 12.5 | 72.9 | 88.4 | 6.279 | 0.307 |
Shown are AUCs for the ROC curves of basal serum cortisol, salivary cortisol, and salivary cortisone, as well as post-LDSST peak salivary cortisol and salivary cortisone with the corresponding best cutoff values and their sensitivity, specificity, and likelihood ratios in the patient group (n = 171) when AI (disease) was defined as post-LDSST serum cortisol < 376 nmol/L.
Abbreviation: SE, standard error.
An alternative approach for establishing the cutoff value for salivary cortisol is to derive it from the results of healthy subjects. The 2.5th percentile of the peak salivary cortisol values in the healthy subjects was 10.9 nmol/L (Table 1). However, the values were not normally distributed due to a subject whose serum cortisol, salivary cortisol, and cortisone (at 0/30/60 minutes) were 236/624/494, 3.6/55/33, and 22/112/88 nmol/L, respectively (i.e., she had a greater response to LDSST than did other subjects). This was a healthy 18-year-old female with an estimated glomerular filtration rate of 102 ml/min/1.73 m2, a normal thyroid function test, an albumin level of 53 g/L, and a CBG of 32.2 μg/mL. After the exclusion of this single outlier [25], the results of the remaining 55 healthy volunteers became normally distributed, and the reference value for peak salivary cortisol (mean − 2 SDs) became 8.4 nmol/L (Table 1), which is very close to the value of 8.6 nmol/L derived from the ROC curves of the patient group using as a reference standard their peak serum cortisol response to LDSST.
The mean basal and peak salivary cortisone levels of the healthy volunteers were 25.4 ± 10.4 and 60.3 ± 13.3 nmol/L, respectively (Table 1). The mean basal and peak salivary cortisone levels for the AI patients were 9.6 ± 9.8 and 18.9 ± 16.2 nmol/L, respectively, whereas the values for the non-AI patients were 24.5 ± 10.5 and 54.0 ± 17.3 nmol/L, respectively (Table 2).
Peak salivary cortisone also had a larger AUC (0.926 ± 0.022) than did basal salivary cortisone (0.862 ± 0.033) from the ROC curve analysis (Fig. 1; Table 3). For the highest accuracy, the cutoff values of peak and basal salivary cortisone were 33.5 nmol/L (sensitivity 84.7%, specificity 88.4%, LR+ 7.30, LR− 0.17) and 12.5 nmol/L (sensitivity 72.9%, specificity 88.4%, LR+ 6.28, LR− 0.31), respectively. As for salivary peak cortisol, an alternative approach to establishing the cutoff value for salivary cortisone is to derive it from the results of the healthy subjects. The 2.5th percentile of the peak salivary cortisone values in all the healthy subjects studied was 37.1 nmol/L (Table 1). However, the values were not normally distributed due to the outlier mentioned previously. After the exclusion of this single outlier, the results of the remaining 55 healthy volunteers became normally distributed, and the reference value for peak salivary cortisol (mean – 2 SDs) became 36.5 nmol/L, which is very close to the 33.5 nmol/L derived from the ROC curves of the patient group.
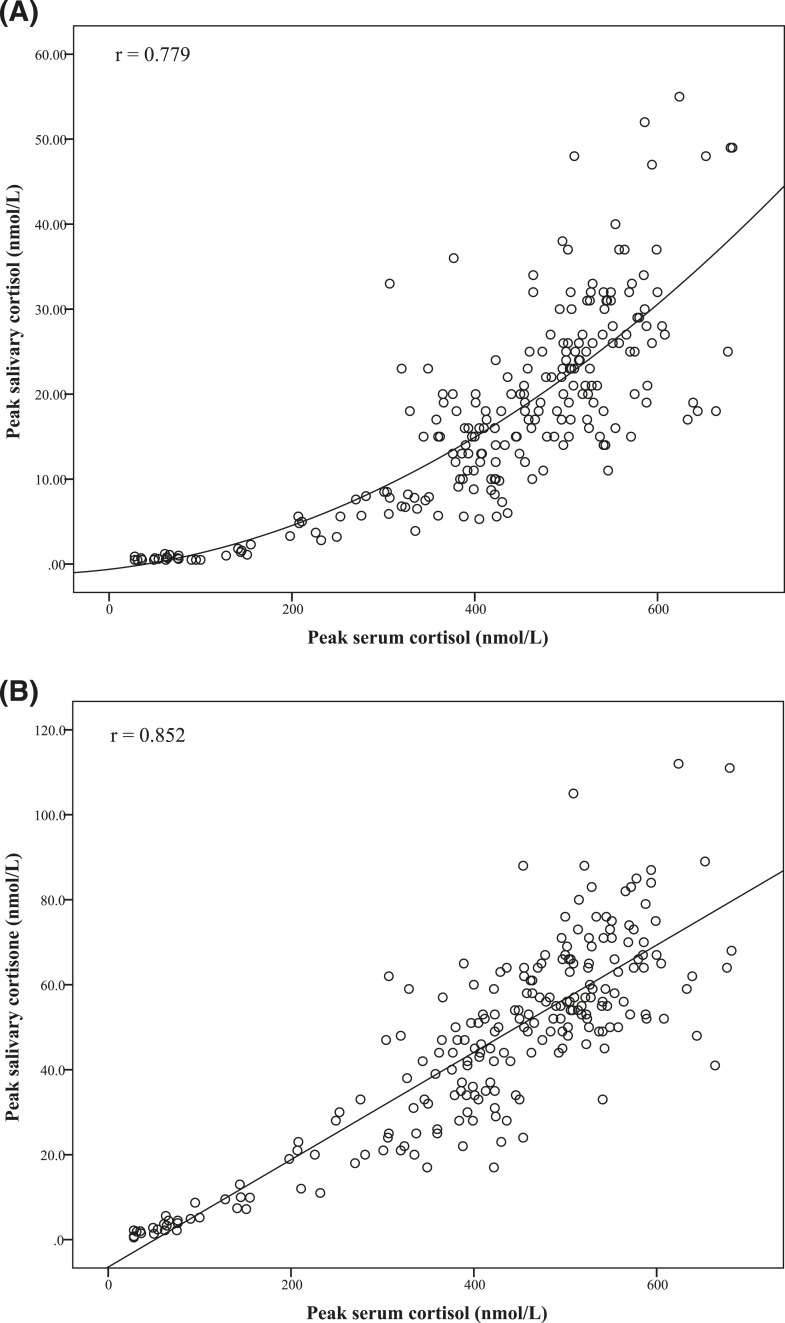
The correlation coefficients (r) between peak salivary cortisol and cortisone with the peak serum total cortisol levels were 0.779 and 0.852, respectively (Fig. 2). Salivary cortisone had a better and more linear correlation with serum total cortisol, whereas salivary cortisol rose exponentially after serum cortisol level reached 400 to 600 nmol/L.
Figure 2.
Correlation between peak serum cortisol with (A) peak salivary cortisol and (B) peak salivary cortisone in all subjects (n = 227) (r = 0.779 and 0.852, respectively; P < 0.01).
Figure 1 and Table 3 illustrated that the ROC for all basal tests yielded significantly smaller AUC with lower sensitivity and specificity at all cutoff values than the peak values. Basal serum cortisol had the highest AUC (0.903 ± 0.024) among all basal tests, with the optimal cutoff value at 170 nmol/L (sensitivity 76.3%, specificity 88.4%, LR+ 6.57, LR− 0.27). The best cutoff values for basal salivary cortisol and basal salivary cortisone were 1.7 nmol/L (sensitivity 62.7%, specificity 95.5%) and 12.5 nmol/L (sensitivity 72.9%, specificity 88.4%), respectively (Table 3).
Eighteen patients (10.5%) were found to have discordant serum and salivary (cortisol and/or cortisone) results: 9 of them were classified as “AI” by the peak serum cortisol criteria (Supplemental Data 2 (18KB, docx) ), and the other 9 “non-AI” (Supplemental Data 3 (18KB, docx) ). Five subjects among the AI patients had low CBG levels of 13.0 to 21.6 μg/mL and non-AI salivary results, and only 1 non-AI subject had a higher CBG level of 45.0 μg/mL and AI salivary results. Five subjects were on oral estrogen therapy. We did not observe any discrepancy between their serum and saliva results (Supplemental Data 4 (14.5KB, docx) ).
3. Discussion
In our study, we established the cutoff value for post-LDSST peak serum cortisol, as assayed by chemiluminescent microparticle immunoassay using the Abbott Architect i2000SR system, from a group of healthy controls. Because the results had a normal distribution, we used mean − 2 SDs to establish this cutoff [3, 6] and found it to be 376 nmol/L. This value appears to be lower than those commonly quoted in the literature. Previously used fluorimetric assays and older generation immunoassays had lower specificity for cortisol, measuring also other glucocorticoid metabolites [10, 11]. A lower dose of Synacthen can also lead to a smaller rise in cortisol. A recent study in which the cortisol values after LDSST and 250 μg of SST [standard dose SST (SDSST)] were compared among 55 Turkish healthy volunteers showed that, for all who had cortisol responses >550 nmol/L during SDSST, the lowest cortisol response achieved during LDSST was only 345 nmol/L [26].
To confirm the validity of this cutoff value, we explored the alternative approach of defining AI with salivary cortisol values of the healthy cohort. We reasoned that because CBG mutations can be completely silent [27], the fact that our “healthy” controls were asymptomatic could not exclude the possibility of their having lower-than-usual CBG and thereby lower total serum cortisol levels. Although we measured their CBG levels, we were unable to find a convincing reference range on CBG either from the literature or from the manufacturer of the CBG assay. Defining AI by using salivary cortisol of the healthy subjects would presumably bypass the problem with the binding proteins. Although the post-LDSST peak salivary cortisol was not distributed normally because of 1 outlier who had a greater-than-usual response to LDSST, the distribution became normal after exclusion of this case, and we could again use the mean − 2 SDs to derive the cutoff, which was found to be 8.4 nmol/L (Table 1). Defining AI as peak salivary cortisol of <8.4 nmol/L, the ROC curve analysis from the patient group showed that the optimal cutoff value for peak salivary cortisone was 33.5 nmol/L (AUC 0.982 ± 0.008, sensitivity 98.1%, specificity 91.5%), and that for peak serum cortisol was 361 nmol/L (AUC 0.960 ± 0.014, sensitivity 88.9%, specificity 92.3%). The value of 361 nmol/L was practically the same as 376 nmol/L when the assay variability was taken into account. This ROC curve also showed that peak serum cortisol levels of 291 and 438 nmol/L gave 100% specificity and sensitivity, respectively (Table 4).
Table 4.
Sensitivity and Specificity of Post-LDSST Tests at Different Cutoff Values
| Test | From ROC Curves of the Patient Group (n = 171) | From Normal Population Mean – 2 SDs | ||
|---|---|---|---|---|
| Cutoff Value | Sensitivitya (%) | Specificitya (%) | ||
| Peak salivary cortisol (nmol/L)b | 8.6 | 85 | 95 | 8.4 (n = 55) |
| 5.2 | 56 | 100 | ||
| 33.5 | 100 | 10 | ||
| Peak salivary cortisone (nmol/L)b | 33.5 | 85 | 88 | 36.5 (n = 55) |
| 15.0 | 44 | 100 | ||
| 62.4 | 100 | 30 | ||
| Peak serum cortisol (nmol/L)c | 361 | 89 | 92 | 376 (n = 56) |
| 291 | 69 | 100 | ||
| 438 | 100 | 64 | ||
The numbers in bold denote the cutoff values as derived from the respective ROC curves, using peak serum cortisol < 376 nmol/L or, for peak serum cortisol, peak salivary cortisol < 8.4 nmol/L, to define disease. The numbers in italics denote the cutoff values as derived from the normal population using mean-2SD.
The sensitivity and specificity (in percentage) were rounded up to the nearest integer in this table.
AI (disease) was defined as post-LDSST serum total cortisol < 376 nmol/L.
AI (disease) was defined as post-LDSST salivary cortisol < 8.4 nmol/L.
The convergence of these 2 approaches convinced us that the method-specific cutoff for peak serum cortisol of 376 nmol/L was a reasonable gold standard to define “AI” and “non-AI” among our patients. Table 2 showed that more subjects in the AI group had clinical features (40.7% in the AI group vs 27.7% in the non-AI group) and electrolyte disturbance (18.6% in the AI group vs 6.3% in the non-AI group) compatible with AI. Additionally, slightly more subjects in the AI group had impairment of other anterior pituitary hormones (44.0% in the AI group vs 36.6% in the non-AI group). We did not perform other objective “confirmatory” tests to obtain independent evidence of hypoadrenalism in the AI group, as our center had previously validated the LDSST against the insulin tolerance test (ITT) in a similar group of patients [4], and the literature evidence on the performance of glucagon stimulation test in diagnosing AI was weak. Moreover, without performing the ITT in our normal controls (we did not think that it was justifiable to request them to undergo this risky test), we were uncertain about what cutoffs to use for this test with the current cortisol assay.
Among 233 patients with nonspecific symptoms, Corbould et al. [28] found that, at cutoff values of 550, 500 and 345 nmol/L, 45%, 26%, and 8% failed the LDSST respectively. The high rates of positive cases at the traditional cutoffs of 500 or 550 nmol/L were up to 50-fold above expected rates [28]. Applying this “reality testing” to our patient population, at the cutoff of 376 nmol/L, 34.5% had AI. Had we defined AI with the traditional cutoff of 500 nmol/L, 70.8% of our patient population would have been considered as having the condition. Many patients with serum cortisol levels between 400 and 500 nmol/L had been followed up by us for >3 years without the need to take regular or stress dose hydrocortisone, and they did not manifest any AI-related features such as hypotension or electrolyte disorder during the follow-up period even when they were exposed to various physical and mental stresses in life. Moreover, 22 (39.3%) of our healthy subjects would have been found to have AI at the cutoff of 500 nmol/L, making the proportion unreasonably high.
Our center participates in the external quality assurance program organized by the Royal College of Pathologists of Australia. We have a 2.4% positive bias from the Abbott analyzer median and a 4.3% positive bias from the medians of all analyzers of different brands. At the cutoff level of 376 nmol/L, the 2.4% bias is ~9 nmol/L, which is probably not significant given the analytical CV of most cortisol immunoassays of 4% to 8%. Centers contemplating adoption of our cutoff should review the difference in bias between their in-use immunoassay method and the Abbott method and adjust accordingly when the bias difference is considered significant.
Using serum cortisol of <376 nmol/L as the gold standard for classifying patients into AI and non-AI groups, we proceeded to derive the optimal cutoff values for peak salivary cortisol and cortisone. From our data, the cutoff value with the highest accuracy was 8.6 nmol/L for peak salivary cortisol. This value is very similar to the peak salivary cortisol reference value of 8.3 nmol/L (LC-MS/MS) established using mean − 2 SDs among 59 subjects with adequate SST or ITT response (serum cortisol > 500 nmol/L, measured by Siemens Centaur immunoassay) in the study by Perogamvros et al. [16]. Cornes et al. [29] also attempted to establish the cutoff value for salivary cortisol (LC-MS/MS) during SST. They defined “normal response” as a peak serum cortisol [measured by electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany) shown by them to give results 20% higher than the Abbott assay] of ≥550 nmol/L after SDSST. Because only 4 of their 36 subjects had abnormal response during the SST, they could only extrapolate the salivary cortisol values from the serum cortisol value of 550 nmol/L, arriving at a cutoff of 15 nmol/L. Two older studies suggested higher cutoff values (20 and 24.3-27.6 nmol/L, respectively), but the number of subjects was small and radioimmunoassays were used to measure salivary cortisol [17, 30].
The ROC curve analysis for peak salivary cortisone showed that its accuracy in diagnosing AI was as high as peak salivary cortisol (Fig. 1; Table 3). The cutoff value associated with the highest accuracy was 33.5 nmol/L for salivary cortisone. The value derived by Cornes et al. [29] in the study mentioned previously was 45 nmol/L. We think that our methodology is a more robust one. To our knowledge, there is otherwise no similar study in the published literature.
We think that serum cortisol, salivary cortisol, and salivary cortisone have their individual roles in defining AI, and together they can give us a more comprehensive picture of the glucocorticoid status of a person, with little additional hardship for the patient and very little additional resources. Serum total cortisol has the advantage of being extensively studied, although the cutoff value is still controversial, making interpretation difficult; additionally, it is affected by variations in the binding proteins. Salivary cortisol theoretically better reflects the serum free cortisol, which is thought to be the active hormone. So far there is more information on salivary cortisol than salivary cortisone, but the latter is interesting in itself: an abnormally high cortisol to cortisone ratio would suggest recent intake of oral hydrocortisone or 11β-HSD type 2 inhibitors such as liquorice [19, 23, 31]. Similar to other investigators [19, 23, 24], our study showed that salivary cortisone had a better and more linear correlation than salivary cortisol with serum total cortisol (on which a large body of literature on tests for AI was based) (Fig. 2), which may be an advantage for salivary cortisone. Salivary cortisone is partly contributed by serum cortisone [23]. Alhough an inert substrate in itself, circulating serum cortisone is converted back to cortisol via local 11β-HSD type 1 enzyme activities, enhancing glucocorticoid action at the tissue level and leading to various metabolic effects [32]. An altered serum cortisone to cortisol ratio may reflect differential activities of the 11β-HSD type 1 and 11β-HSD type 2; however, the physiological or pathological importance of cortisone levels, whether in the serum or in the saliva, still awaits further studies.
In addition to deriving the cutoff values, our data also showed that peak salivary cortisol and cortisone levels of 33.5 and 62.4 nmol/L yielded a sensitivity of 100%, and peak salivary cortisol and cortisone levels of 5.2 and 15 nmol/L yielded a specificity of 100% (Table 4). These levels can be used to more definitively rule out or rule in AI, respectively.
In concordance with other studies [33, 34], we also showed that basal morning serum cortisol, salivary cortisol, or cortisone levels all performed less well in diagnosing AI than did the peak levels (Fig. 1). However, in situations when a stimulation test cannot be performed, our study suggested that cutoff values of 170, 1.7, and 12.5 nmol/L could be used for basal serum cortisol, salivary cortisol, and salivary cortisone, respectively (Table 3). The reported reference values for basal salivary cortisol ranged from 3.2 to 13.3 nmol/L [35–38], due largely to differences in the salivary cortisol assays and methodologies used in various studies [39]. The only study that had explored the basal salivary cortisone cutoff suggested that when AI was defined as post-LDSST serum cortisol of <350 nmol/L (measured by the Siements Immulite 2000 immunoassay), the morning basal salivary cortisone cutoff of 12.5 nmol/L (measured by immunoassay; Salimetrics, Carlsbad, CA) had a negative predictive value of 99.2% and positive predictive value of 30.1% [38]. This value is identical to the basal salivary cortisone cutoff established in our study.
One limitation of our study was that the mean age of the healthy subjects was significantly lower than that of the patient group. We included 4 older subjects with diabetes and hypertension because we were otherwise unable to recruit an adequate number of subjects in the older age range. We think that these conditions and their medications would not affect the hypothalamic-pituitary-adrenal axis and binding protein levels. A previous study [12], however, showed no effect of age on cortisol response to SST. When reanalyzing the data after excluding these 4 subjects with diabetes and/or hypertension (n = 52), we found that the minor change in the reference cutoff values of interest among normal subjects (Supplemental Data 5 (16.7KB, docx) ) did not affect the results from the ROC curve analysis in the patient group (Supplemental Data 6 (16.1KB, docx) ). Some investigators recommended stricter rules in saliva collection, such as avoiding eating and brushing teeth for 2 hours, and mouth rinsing with water 10 to 15 minutes before sample collection [20], but others suggested that 30 minutes of such avoidance should be adequate [24].
Unlike immunoassays, LC-MS/MS enables accurate measurement of specific steroids, and results are less specific assay–dependent and are more comparable among laboratories. We think that LC-MS/MS will soon become the method of choice for measuring steroids; establishing cutoff values using this methodology would be important to facilitate clinical interpretation across different centers. The derivation of cutoff values for salivary cortisol and cortisone would also enable investigators to study the glucocorticoid axis in greater detail, and this would facilitate better understanding of the physiological and pathological roles of cortisone in future studies.
4. Conclusion
Our study showed that peak salivary cortisol and cortisone performed well in the diagnosis of AI, and better than basal values. The cutoffs generated from the ROC curves were 376, 8.6, and 33.5 nmol/L for peak post-LDSST serum cortisol, salivary cortisol, and salivary cortisone, respectively. Those for basal serum cortisol, salivary cortisol, and salivary cortisone were 170, 1.7, and 12.5 nmol/L, respectively. Although we report the precise values generated by the ROC curves, in clinical applications the analytic and biologic variations of all tests should be taken into account, with rounding off of cutoff values appropriate to the degree of variability.
Acknowledgments
This work was supported by Grant KCC/RC/G/1415-A03 from the Hospital Authority, Hong Kong.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 11β-HSD
- 11β-hydroxysteroid dehydrogenase
- AI
- adrenal insufficiency
- AUC
- area under the curve
- CBG
- cortisol-binding globulin
- ITT
- insulin tolerance test
- LC-MS/MS
- liquid chromatography–tandem mass spectrometry
- LDSST
- low-dose short Synacthen test
- LR−
- negative likelihood ratio
- LR+
- positive likelihood ratio
- ROC
- receiver operating characteristic
- SD
- standard deviation
- SDSST
- standard dose short Synacthen test
- SST
- short Synacthen test
References and Notes
- 1.Grossman AB. The diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010;95(11):4855–4863. [DOI] [PubMed] [Google Scholar]
- 2.Simsek Y, Karaca Z, Tanriverdi F, Unluhizarci K, Selcuklu A, Kelestimur F. A comparison of low-dose ACTH, glucagon stimulation and insulin tolerance test in patients with pituitary disorders. Clin Endocrinol (Oxf). 2015;82(1):45–52. [DOI] [PubMed] [Google Scholar]
- 3.Hurel SJ, Thompson CJ, Watson MJ, Harris MM, Baylis PH, Kendall-Taylor P. The short Synacthen and insulin stress tests in the assessment of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol (Oxf). 1996;44(2):141–146. [DOI] [PubMed] [Google Scholar]
- 4.Choi CH, Tiu SC, Shek CC, Choi KL, Chan FK, Kong PS. Use of the low-dose corticotropin stimulation test for the diagnosis of secondary adrenocortical insufficiency. Hong Kong Med J. 2002;8(6):427–434. [PubMed] [Google Scholar]
- 5.Gonzálbez J, Villabona C, Ramón J, Navarro MA, Giménez O, Ricart W, Soler J. Establishment of reference values for standard dose short synacthen test (250 microgram), low dose short synacthen test (1 microgram) and insulin tolerance test for assessment of the hypothalamo–pituitary–adrenal axis in normal subjects. Clin Endocrinol (Oxf). 2000;53(2):199–204. [DOI] [PubMed] [Google Scholar]
- 6.Cho HY, Kim JH, Kim SW, Shin CS, Park KS, Kim SW, Jang HC, Kim SY. Different cut-off values of the insulin tolerance test, the high-dose short Synacthen test (250 μg) and the low-dose short Synacthen test (1 μg) in assessing central adrenal insufficiency. Clin Endocrinol (Oxf). 2014;81(1):77–84. [DOI] [PubMed] [Google Scholar]
- 7.Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Farhan N, Pickett A, Ducroq D, Bailey C, Mitchem K, Morgan N, Armston A, Jones L, Evans C, Rees DA. Method-specific serum cortisol responses to the adrenocorticotrophin test: comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clin Endocrinol (Oxf). 2013;78(5):673–680. [DOI] [PubMed] [Google Scholar]
- 9.Klose M, Lange M, Rasmussen AK, Skakkebaek NE, Hilsted L, Haug E, Andersen M, Feldt-Rasmussen U. Factors influencing the adrenocorticotropin test: role of contemporary cortisol assays, body composition, and oral contraceptive agents. J Clin Endocrinol Metab. 2007;92(4):1326–1333. [DOI] [PubMed] [Google Scholar]
- 10.Moore A, Aitken R, Burke C, Gaskell S, Groom G, Holder G, Selby C, Wood P. Cortisol assays: guidelines for the provision of a clinical biochemistry service. Ann Clin Biochem. 1985;22(Pt 5):435–454. [DOI] [PubMed] [Google Scholar]
- 11.Clayton RN. Diagnosis of adrenal insufficiency. BMJ. 1989;298(6669):271–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark PM, Neylon I, Raggatt PR, Sheppard MC, Stewart PM. Defining the normal cortisol response to the short Synacthen test: implications for the investigation of hypothalamic-pituitary disorders. Clin Endocrinol (Oxf). 1998;49(3):287–292. [DOI] [PubMed] [Google Scholar]
- 13.Ho JT, Al-Musalhi H, Chapman MJ, Quach T, Thomas PD, Bagley CJ, Lewis JG, Torpy DJ. Septic shock and sepsis: a comparison of total and free plasma cortisol levels. J Clin Endocrinol Metab. 2006;91(1):105–114. [DOI] [PubMed] [Google Scholar]
- 14.Fede G, Spadaro L, Tomaselli T, Privitera G, Scicali R, Vasianopoulou P, Thalassinos E, Martin N, Thomas M, Purrello F, Burroughs AK. Comparison of total cortisol, free cortisol, and surrogate markers of free cortisol in diagnosis of adrenal insufficiency in patients with stable cirrhosis. Clin Gastroenterol Hepatol. 2014;12(3):504–512.e8. [DOI] [PubMed] [Google Scholar]
- 15.Limor R, Tordjman K, Marcus Y, Greenman Y, Osher E, Sofer Y, Stern N. Serum free cortisol as an ancillary tool in the interpretation of the low-dose 1-μg ACTH test. Clin Endocrinol (Oxf). 2011;75(3):294–300. [DOI] [PubMed] [Google Scholar]
- 16.Perogamvros I, Owen LJ, Keevil BG, Brabant G, Trainer PJ. Measurement of salivary cortisol with liquid chromatography-tandem mass spectrometry in patients undergoing dynamic endocrine testing. Clin Endocrinol (Oxf). 2010;72(1):17–21. [DOI] [PubMed] [Google Scholar]
- 17.Marcus-Perlman Y, Tordjman K, Greenman Y, Limor R, Shenkerman G, Osher E, Stern N. Low-dose ACTH (1 μg) salivary test: a potential alternative to the classical blood test. Clin Endocrinol (Oxf). 2006;64(2):215–218. [DOI] [PubMed] [Google Scholar]
- 18.Perogamvros I, Aarons L, Miller AG, Trainer PJ, Ray DW. Corticosteroid-binding globulin regulates cortisol pharmacokinetics. Clin Endocrinol (Oxf). 2011;74(1):30–36. [DOI] [PubMed] [Google Scholar]
- 19.Perogamvros I, Owen LJ, Newell-Price J, Ray DW, Trainer PJ, Keevil BG. Simultaneous measurement of cortisol and cortisone in human saliva using liquid chromatography–tandem mass spectrometry: application in basal and stimulated conditions. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(29):3771–3775. [DOI] [PubMed] [Google Scholar]
- 20.Turpeinen U, Hämäläinen E. Determination of cortisol in serum, saliva and urine. Best Pract Res Clin Endocrinol Metab. 2013;27(6):795–801. [DOI] [PubMed] [Google Scholar]
- 21.Inder WJ, Dimeski G, Russell A. Measurement of salivary cortisol in 2012—laboratory techniques and clinical indications. Clin Endocrinol (Oxf). 2012;77(5):645–651. [DOI] [PubMed] [Google Scholar]
- 22.Wood P. Salivary steroid assays—research or routine? Ann Clin Biochem. 2009;46(Pt 3):183–196. [DOI] [PubMed] [Google Scholar]
- 23.Perogamvros I, Keevil BG, Ray DW, Trainer PJ. Salivary cortisone is a potential biomarker for serum free cortisol. J Clin Endocrinol Metab. 2010;95(11):4951–4958. [DOI] [PubMed] [Google Scholar]
- 24.Debono M, Harrison RF, Whitaker MJ, Eckland D, Arlt W, Keevil BG, Ross RJ. Salivary cortisone reflects cortisol exposure under physiological conditions and after hydrocortisone. J Clin Endocrinol Metab. 2016;101(4):1469–1477. [DOI] [PubMed] [Google Scholar]
- 25.Schultheiss OC, Stanton SJ. Assessment of salivary hormones. In: Harmon-Jones E, Beer JS, eds. Methods in Social Neuroscience. 1st ed New York, NY: Guilford Press; 2009:17–44. [Google Scholar]
- 26.Karaca Z, Lale A, Tanriverdi F, Kula M, Unluhizarci K, Kelestimur F. The comparison of low and standard dose ACTH and glucagon stimulation tests in the evaluation of hypothalamo-pituitary-adrenal axis in healthy adults. Pituitary. 2011;14(2):134–140. [DOI] [PubMed] [Google Scholar]
- 27.Simard M, Hill LA, Lewis JG, Hammond GL. Naturally occurring mutations of human corticosteroid-binding globulin. J Clin Endocrinol Metab. 2015;100(1):E129–E139. [DOI] [PubMed] [Google Scholar]
- 28. Corbould A, Jarvis M, Campbell J, Kunde D, Clarkson W, Burns D. Utility of the low-dose short Synacthen test in diagnosis of adrenal insufficiency in outpatients with nonspecific symptoms. Endocrinol Stud. 2012;2(2):e5. [Google Scholar]
- 29.Cornes MP, Ashby HL, Khalid Y, Buch HN, Ford C, Gama R. Salivary cortisol and cortisone responses to tetracosactrin (synacthen). Ann Clin Biochem. 2015;52(Pt 5):606–610. [DOI] [PubMed] [Google Scholar]
- 30.Contreras LN, Arregger AL, Persi GG, Gonzalez NS, Cardoso EM. A new less-invasive and more informative low-dose ACTH test: salivary steroids in response to intramuscular corticotrophin. Clin Endocrinol (Oxf). 2004;61(6):675–682. [DOI] [PubMed] [Google Scholar]
- 31.Raff H, Findling JW. Biomarkers: salivary cortisol or cortisone? Nat Rev Endocrinol. 2010;6(12):658–660. [DOI] [PubMed] [Google Scholar]
- 32.Seckl JR, Walker BR. Minireview: 11β-hydroxysteroid dehydrogenase type 1- a tissue-specific amplifier of glucocorticoid action. Endocrinology. 2001;142(4):1371–1376. [DOI] [PubMed] [Google Scholar]
- 33.Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998;83(7):2350–2354. [DOI] [PubMed] [Google Scholar]
- 34.Raff H. Utility of salivary cortisol measurements in Cushing’s syndrome and adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(10):3647–3655. [DOI] [PubMed] [Google Scholar]
- 35.Deutschbein T, Broecker-Preuss M, Flitsch J, Jaeger A, Althoff R, Walz MK, Mann K, Petersenn S. Salivary cortisol as a diagnostic tool for Cushing’s syndrome and adrenal insufficiency: improved screening by an automatic immunoassay. Eur J Endocrinol. 2012;166(4):613–618. [DOI] [PubMed] [Google Scholar]
- 36.Karpman MS, Neculau M, Dias VC, Kline GA. Defining adrenal status with salivary cortisol by gold-standard insulin hypoglycemia. Clin Biochem. 2013;46(15):1442–1446. [DOI] [PubMed] [Google Scholar]
- 37.Ceccato F, Barbot M, Zilio M, Ferasin S, Occhi G, Daniele A, Mazzocut S, Iacobone M, Betterle C, Mantero F, Scaroni C. Performance of salivary cortisol in the diagnosis of Cushing’s syndrome, adrenal incidentaloma, and adrenal insufficiency. Eur J Endocrinol. 2013;169(1):31–36. [DOI] [PubMed] [Google Scholar]
- 38.Blair J, Lancaster G, Titman A, Peak M, Newlands P, Collingwood C, Chesters C, Moorcroft T, Wallin N, Hawcutt D, Gardner C, Didi M, Lacy D, Couriel J. Early morning salivary cortisol and cortisone, and adrenal responses to a simplified low-dose short Synacthen test in children with asthma. Clin Endocrinol (Oxf). 2014;80(3):376–383. [DOI] [PubMed] [Google Scholar]
- 39.Miller R, Plessow F, Rauh M, Gröschl M, Kirschbaum C. Comparison of salivary cortisol as measured by different immunoassays and tandem mass spectrometry. Psychoneuroendocrinology. 2013;38(1):50–57. [DOI] [PubMed] [Google Scholar]