Abstract
Context:
Severe obesity is one of the major features of Bardet-Biedl syndrome (BBS) and causes reduced life expectancy. Bariatric surgery is an effective treatment of morbid obesity. Data on the effect of bariatric surgery for monogenic obesity is essentially lacking. We present the clinical and metabolic 3-year follow-up of sleeve gastrectomy in a BBS patient.
Case Description:
A 37-year-old obese woman with BBS (body mass index, 40 kg/m2) was referred to our clinic for uncontrolled diabetes, dyslipidemia, hypertension, and nonalcoholic fatty liver disease (NAFLD). After sleeve gastrectomy, progressive weight loss was observed, with a 32% total weight loss at 3-year follow-up. Glycemic control and NAFLD improved significantly. Blood pressure normalized, and treatment was discontinued 3 months after surgery.
Conclusions:
Laparoscopic sleeve gastrectomy can be a safe and effective treatment of morbid BBS-related obesity in adult patients. Significant and sustained weight loss leads to the improvement of several obesity-related comorbidities such as diabetes, hypertension, and NAFLD, as in polygenic obesity. Further data are needed to confirm the long-term efficacy and safety of bariatric surgery in BBS.
Keywords: sleeve gastrectomy, obesity, Bardet Biedl syndrome, BBS1
We present the 3-year follow-up after sleeve gastrectomy in a BBS patient. Significant weight loss improved several obesity-related comorbidities. No major complication was observed.
Bardet-Biedl syndrome (BBS) is a rare autosomal recessive form of syndromic obesity. Causal mutations have been identified in >20 genes involved in primary cilia formation and function [1, 2]. Major features include retinitis pigmentosa, truncal obesity, postaxial polydactyly, urogenital abnormalities, and cognitive impairment [1]. Lifestyle changes and pharmacotherapy have very limited efficacy in BBS. Emerging data suggest that bariatric surgery can achieve sustained weight loss by altering weight-regulatory signaling pathways. However, the existing literature on bariatric surgery in BBS patients is extremely limited [3]. We describe here the 3-year follow-up of a BBS patient after sleeve gastrectomy.
1. Case Description
A 37-year-old woman was referred for morbid obesity and uncontrolled diabetes. She was of Tunisian origin, born to a family with a high degree of consanguinity. She had undergone surgeries for postaxial polydactyly on her left hand and uterine and vaginal septum. At age 23, she developed rod-cone dystrophy with gradual vision loss. She developed diabetes, nonalcoholic fatty liver disease (NAFLD), dyslipidemia, hypertension, and kidney dysfunction. Genetic screening revealed a homozygous p.Met390Arg mutation in BBS1.
She progressively gained weight since early childhood, reaching 108 kg for 163-cm height. Dietary evaluation revealed hyperphagia, with an estimated intake of 4000 kcal/d comprising three large meals rich in dietary fat and carbohydrates, many daily snacks, and around twice-weekly binge eating. Intensification of diabetes treatment did not result in adequate glycemic control; after insulin initiation, glycosylated hemoglobin (HbA1c) was still 7.8%. Hypertension required triple therapy. A statin was initiated for dyslipidemia. The patient asked if bariatric surgery could be envisaged. Having been informed of the paucity of data and possible postoperative complications, she opted for surgery.
2. Materials and Methods
Body composition was assessed by dual-energy x-ray absorptiometry (Hologic DXA Scan; Zaventem, Belgium). Abdominal visceral and subcutaneous fat areas were determined on single-axial, 5-mm-thick computed tomography (CT) images acquired at L3. Subcutaneous fat area was calculated by subtracting visceral from total abdominal fat area.
The patient underwent a 2-hour, 75-g oral glucose tolerance test to assess glucose tolerance, insulin sensitivity, and insulin secretion. Insulin sensitivity and secretion were calculated using the homeostasis model assessment 2 (HOMA-2) calculator (www.dtu.ox.ac.uk/homacalculator) and Matsuda model (http://mmatsuda.diabetes-smc.jp/MIndex.html).
Respiratory gas exchanges were measured by indirect calorimetry (Deltatrac; Datex, Helsinki, Finland). Resting energy expenditure (REE) was calculated from the ventilation rates (liters) of carbon dioxide, oxygen and 24-hour urinary nitrogen output using standard equations. It was plotted against fat-free mass, which is recognized as its best predictor, to eliminate the differences related to sex, age, and degree of obesity. The patient’s predicted REE was calculated by linear regression against a control population.
3. Results
Presurgical characteristics of the patient are reported in Table 1. Abdominal CT revealed hepatomegaly and steatosis, with a liver volume of 3210 mL and a density of 19 Hounsfield units. FibroScan indicated fibrosis. The patient underwent sleeve gastrectomy without perioperative complications.
Table 1.
Clinical and Biological Characteristics Before Surgery and at the Indicated Years of Follow-Up
| Duration of Follow-Up | Baseline | 6 Months | 1 Year | 1.5 Years | 2 Years | 3 Years |
|---|---|---|---|---|---|---|
| Body weight and body composition | ||||||
| Body weight (kg) | 108.5 | 86.4 | 81.7 | 71.8 | 80.8 | 73.0 |
| BMI (kg/m2) | 40.8 | 32.5 | 30.8 | 27.0 | 30.4 | 27.5 |
| Fat-free mass (kg) | 57.2 | 48.8 | 47.2 | 43.1 | 45.2 | — |
| Fat mass (kg) | 51.3 | 37.6 | 34.5 | 28.7 | 35.6 | — |
| Fat mass (% body weight) | 47.3 | 43.5 | 42.2 | 40.0 | 44.0 | — |
| Subcutaneous fat area (cm2) | 526 | — | 407 | 297 | — | — |
| Visceral fat area (cm2) | 362 | — | 183 | 186 | — | — |
| Glucose homeostasis | ||||||
| HbA1c (%) | 7.8 | 6.2 | 6.9 | 6.9 | 9.8 | 7.1 |
| Fasting plasma glucose (mg/dL) | 234 | 144 | 118 | 124 | 172 | 148 |
| Fasting insulin (μUI/mL) | 73 | 34 | 20 | 21 | 28 | — |
| Fasting C-peptide (ng/mL) | 9.9 | 5.9 | 4.7 | 4.6 | 6.1 | — |
| HOMA (%B) | 98 | 118 | 138 | 125 | 92 | — |
| HOMA (%S) | 10 | 20 | 27 | 27 | 18 | — |
| Matsuda index | 0.43 | 1.00 | 1.87 | 1.62 | — | — |
| Insulinogenic index | 0 | 0 | 0.24 | 0.27 | — | — |
| Resting energy expenditure | ||||||
| Measured value (kcal/24 h) | 1853 | 1145 | 1281 | — | — | — |
| Percentage predicted (%) | 114 | 78 | 93 | — | — | — |
| Activity (kcal/24 h) | 172 | 232 | — | — | — | — |
| Total cholesterol (mg/dL) | 176 | 147 | 136 | 157 | 184 | 180 |
| HDL cholesterol (mg/dL) | 41 | 42 | 30 | 36 | 34 | 52 |
| LDL cholesterol (mg/dL) | 89 | 65 | 79 | 80 | 108 | 103 |
| Triglycerides (mg/dL) | 229 | 196 | 135 | 203 | 211 | 129 |
| Creatinine (mg/dL) | 1.3 | 1.0 | 1.2 | 1.0 | 1.3 | 1.2 |
| Creatinine clearance (mL/min) | 43 | 68 | 79 | 82 | 68 | — |
| Liver volume (mL) | 3210 | 1967 | 1916 | 1467 | — | — |
| Liver density (HU) | 19 | 53 | 45 | 55 | — | — |
| Fibroscan (kPa) | 11.7 | 4.6 | 5.8 | — | — | — |
| GGT (U/L) | 89 | 44 | 69 | 38 | 34 | 43 |
| Clinical features | ||||||
| Systolic BP (mm Hg) | 140 | 130 | 100 | 100 | 120 | 110 |
| Diastolic BP (mm Hg) | 90 | 85 | 60 | 60 | 80 | 70 |
Abbreviations: BP, blood pressure; BW, body weight; GGT; Gamma Glutamyl Transferase; HDL, high-density lipoprotein; HOMA (%B), Homeostasis Model Assessment of beta cell function as percentage of a normal reference population; HOMA (%S), Homeostasis Model Assessment of insulin sensitivity as percentage of a normal reference population; HU, Hounsfield units; kPa, kilopascal; LDL, low-density lipoprotein; U/L, units per liter.
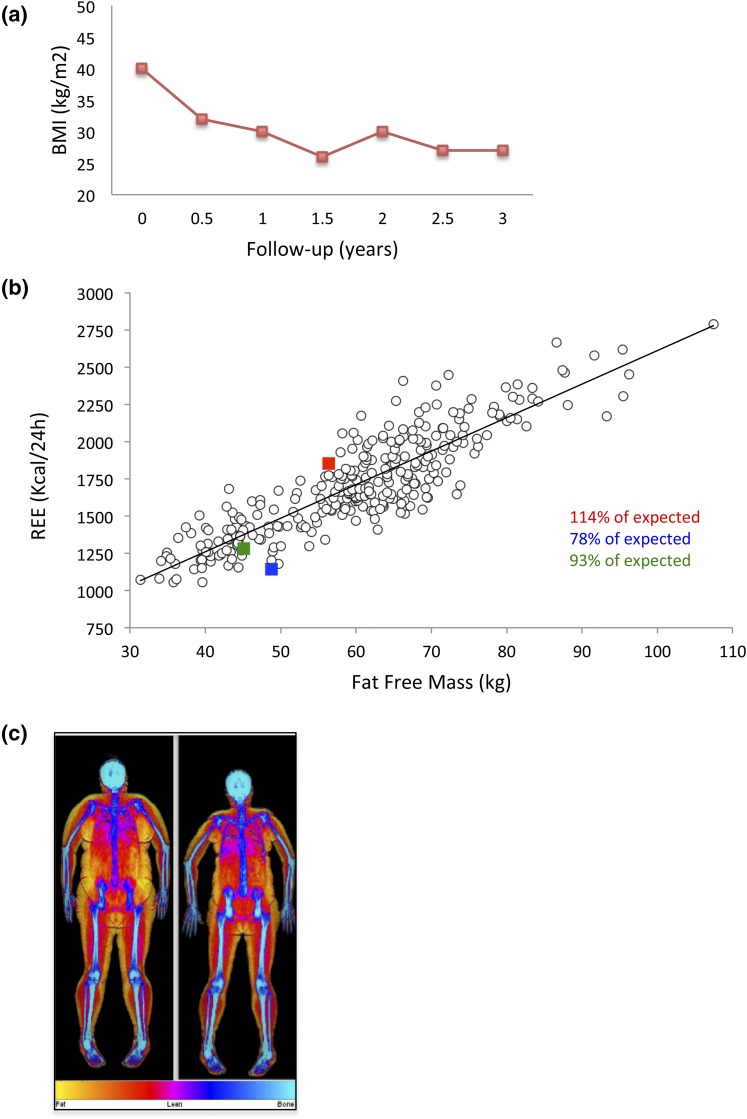
Progressive weight loss was observed in the first 18 months of follow-up, reaching 36-kg weight loss [Fig. 1(a)]. Fat mass decreased to 39% of body mass (Table 1). Subcutaneous and visceral fat areas decreased by 44% and 49%. At 2-year follow-up, she regained 8 kg after reduced physical activity and increased caloric intake. By 3 years, body weight again decreased and stabilized at 73 kg. At baseline, her measured REE was slightly higher than that predicted. As expected, REE was lower than predicted for fat-free mass at 6-month follow-up during rapid weight loss. At 1-year follow-up, when weight was stabilizing, REE returned to predicted values [Fig. 1(b)]. REE was not evaluated afterward due to reduced compliance. The patient’s persistent hunger sensation disappeared, and her estimated intake decreased to 1200 kcal/d at 6 months, reaching 1800 kcal/d at 2 years and becoming more variable in the third year. She had three meals daily with lower calorie content, compared with the presurgery period, and reduced snacking. Episodes of binge eating were fewer and easier to control.
Figure 1.
(a) Evolution of BMI after bariatric surgery. (b) REE before surgery (red symbol) and at 6-month (blue symbol) and 1-year follow-up (green symbol), compared with 297 control subjects. (c) Body composition before surgery and at 18-month follow-up, assessed by dual-energy x-ray absorptiometry.
The patient’s insulin sensitivity, estimated by HOMA-2 and Matsuda index, improved progressively in the first year and then stabilized (Table 1). This and an improved insulinogenic index, a measure of insulin secretion, resulted in improved glucose homeostasis. At 18-month follow-up, HbA1c was 6.9% after withdrawal of all diabetes medications (Table 1). At 2-year follow-up, weight regain transiently deteriorated glycemic control, but after weight loss and metformin and gliquidone initiation, HbA1c was 7.1% at 3-year follow-up. At 6 months, atorvastatin was discontinued. A relapse of hypercholesterolemia was observed 3 months later, and treatment was reinitiated. The abdominal CT showed that liver steatosis rapidly regressed (liver density between 45 and 55 Hounsfield units), and the FibroScan showed reduced liver stiffness, suggestive of regression of fibrosis (Table 1). By 1.5 years, liver volume had normalized. Blood pressure normalized, and antihypertensive treatment was discontinued 3 months after surgery.
4. Discussion
BBS is a pleiotropic genetic disorder characterized by severe early-onset obesity [1]. The mechanisms underlying obesity remain unclear. Preclinical studies suggest a crucial role of primary cilia in nutritional status signaling. BBS patients are hyperleptinemic and obese at very young age, suggesting leptin resistance [2].
Growing evidence suggests that improved hypothalamic signaling is key to the effectiveness of bariatric surgery. However, the existing literature on surgery in hypothalamic obesity is scarce [3]. Wijnen et al. showed sustained weight loss after Roux-en-Y gastric bypass (RYGB) in craniopharyngioma-related obese patients [4]. Studies in Prader-Willi syndrome are contradictory, raising concern about the safety of bariatric surgery in these patients [3, 5]. Patients with heterozygous MC4R mutations are responsive to RYGB [6], but gastric banding in a patient homozygous for a MC4R mutation was unsuccessful [7].
Alqahtani et al. provided data on sleeve gastrectomy in 24 pediatric patients with monogenic forms of obesity. An average loss of 59% of excess body mass index (BMI) was reported, without complications [8]. These data suggest that sleeve gastrectomy could be a safe and effective procedure in managing monogenic forms of obesity.
RYGB in a 16-year-old boy with BBS (BMI, 52 kg/m2) reduced BMI by 33% after 3 years [9]. Mujahid et al. reported on two BBS cases [10]. The first was a 35-year-old patient (BMI, 53 kg/m2) who lost 12% weight 6 months after gastric banding and had improved diabetes control. At 2-year follow-up, she regained weight and glucose control deteriorated. The second case was a 33-year-old woman (BMI, 50 kg/m2) who underwent sleeve gastrectomy, resulting in reduced appetite and 24% weight loss at 12-month follow-up.
In our case, sleeve gastrectomy was chosen based on the patient’s eating profile and surgical expertise. A weight reduction of 32% was observed at 3-year follow-up, consistent with the results of sleeve gastrectomy in polygenic obese patients. It has been suggested that BBS patients have alterations in REE, resulting in disrupted energy homeostasis [3]. Reduced REE has been reported in patients with hypothalamic damage [11]. However, patients had higher BMI than controls, and REE was correlated to body weight, which leads to underestimation of REE for fat-free mass. Bbs1 gene deletion in mice reduced energy expenditure [12]. On the other hand, no difference in REE was found between 20 BBS patients and 20 controls matched for body weight and composition, suggesting the absence of specific energy metabolism abnormalities [13]. Our data are consistent with this hypothesis, with a predicted REE comparable to the REE of a control population [Fig. 1(b)].
After surgery, fat mass and visceral adiposity decreased, and insulin resistance indices improved, as did glucose homeostasis (Table 1). Weight and metabolic fluctuations 2 years after surgery showed that the patient remained vulnerable to weight regain with glycemic deterioration. Diet and drug therapy restored glycemic control, showing the need for long-term follow-up.
In conclusion, sleeve gastrectomy can be a safe and effective treatment of morbid BBS-related obesity in adults. Weight loss improves diabetes, hypertension, and NAFLD, similar to effects observed in polygenic obese patients. Further data are needed to confirm the long-term efficacy and safety of bariatric surgery in BBS patients.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BBS
- Bardet-Biedl syndrome
- BMI
- body mass index
- CT
- computed tomography
- HOMA
- homeostasis model assessment
- NAFLD
- nonalcoholic fatty liver disease
- REE
- resting energy expenditure
- RYGB
- Roux-en-Y gastric bypass.
References and Notes
- 1.Forsythe E, Beales PL. Bardet-Biedl syndrome. Eur J Hum Genet. 2013;21(1):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee EB, Mattson MP. The neuropathology of obesity: insights from human disease. Acta Neuropathol. 2014;127(1):3–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bingham NC, Rose SR, Inge TH. Bariatric surgery in hypothalamic obesity. Front Endocrinol (Lausanne). 2012;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wijnen M, Olsson DS, van den Heuvel-Eibrink MM, Wallenius V, Janssen J, Delhanty PJ, van der Lely AJ, Johannsson G, Neggers S. Efficacy and safety of bariatric surgery for craniopharyngioma-related hypothalamic obesity-A matched case-control study with two years of follow-up. Int J Obes. 2017;41(2):210–216. [DOI] [PubMed] [Google Scholar]
- 5.Scheimann AO, Butler MG, Gourash L, Cuffari C, Klish W. Critical analysis of bariatric procedures in Prader-Willi syndrome. J Pediatr Gastroenterol Nutr. 2008;46(1):80–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aslan IR, Campos GM, Calton MA, Evans DS, Merriman RB, Vaisse C. Weight loss after Roux-en-Y gastric bypass in obese patients heterozygous for MC4R mutations. Obes Surg. 2011;21(7):930–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslan IR, Ranadive SA, Ersoy BA, Rogers SJ, Lustig RH, Vaisse C. Bariatric surgery in a patient with complete MC4R deficiency. Int J Obes. 2011;35(3):457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alqahtani AR, Elahmedi M, Alqahtani YA. Bariatric surgery in monogenic and syndromic forms of obesity. Semin Pediatr Surg. 2014;23(1):37–42. [DOI] [PubMed] [Google Scholar]
- 9.Daskalakis M, Till H, Kiess W, Weiner RA. Roux-en-Y gastric bypass in an adolescent patient with Bardet-Biedl syndrome, a monogenic obesity disorder. Obes Surg. 2010;20(1):121–125. [DOI] [PubMed] [Google Scholar]
- 10.Mujahid S, Huda MS, Beales P, Carroll PV, McGowan BM. Adjustable gastric banding and sleeve gastrectomy in Bardet-Biedl syndrome. Obes Surg. 2014;24(10):1746–1748. [DOI] [PubMed] [Google Scholar]
- 11.Holmer H, Pozarek G, Wirfält E, Popovic V, Ekman B, Björk J, Erfurth EM. Reduced energy expenditure and impaired feeding-related signals but not high energy intake reinforces hypothalamic obesity in adults with childhood onset craniopharyngioma. J Clin Endocrinol Metab. 2010;95(12):5395–5402. [DOI] [PubMed] [Google Scholar]
- 12.Guo DF, Cui H, Zhang Q, Morgan DA, Thedens DR, Nishimura D, Grobe JL, Sheffield VC, Rahmouni K. The BBSome controls energy homeostasis by mediating the transport of the leptin receptor to the plasma membrane. PLoS Genet. 2016;12(2):e1005890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. doi: 10.1038/sj.ijo.0802420. Grace C, Beales P, Summerbell C, Jebb SA, Wright A, Parker D, Kopelman P. Energy metabolism in Bardet-Biedl syndrome. Int J Obes. 2003;27:1319–1324. [DOI] [PubMed] [Google Scholar]