Abstract
Context:
Changes in vitamin D binding protein (DBP) concentrations and catabolism of 25-hydroxyvitamin D to 24,25-dihydroxyvitamin D (24,25D) after vitamin D2 supplementation may alter concentrations and bioavailability of circulating 25-hydroxyvitamin D (25D).
Objective:
Examine acute changes in vitamin D metabolism and bioavailability after vitamin D2 supplementation.
Methods:
Study design was secondary analysis of a single-arm interventional study. Thirty consenting volunteers were treated with five 50,000 IU oral doses of ergocalciferol over 2 weeks. Main outcome measures included concentrations of DBP, vitamin D metabolites, and bioavailable 25-hydroxyvitamin D (25D) in pre- and posttreatment serum samples.
Results:
After supplementation, 25D2 (mean ± standard deviation) increased from 1.4 ± 0.9 ng/mL to 45.3 ± 16.5 ng/mL (P < 0.0001), and 25D3 levels decreased from 26.8 ± 9.9 ng/mL to 19.7 ± 8.2 ng/mL (P < 0.0001). Total 25D (25D2 plus 25D3) increased from 28.2 ± 10.0 ng/mL to 65.0 ± 21.1 ng/mL (152.2% ± 102.5%; P < 0.0001). DBP and total 24,25D concentrations increased 39.1% ± 39.4% (165.6 ± 53.8 µg/mL to 222.0 ± 61.1 µg/mL; P < 0.0001) and 31.3% ± 48.9% (3.9 ± 2.0 ng/mL to 4.7 ± 2.1 ng/mL; P = 0.0147), respectively. In contrast to total 25D, bioavailable 25D increased by 104.4% ± 99.6% (from 5.0 ± 2.0 ng/mL to 8.7 ± 2.7 ng/mL; P < 0.001), and 1,25D increased by 32.3% ± 38.8% (from 45.5 ± 10.7 pg/mL to 58.1 ± 13.0 pg/mL; P = 0.0006). There were no changes in calcium or parathyroid hormone (P > 0.05 for both).
Conclusion:
Changes after vitamin D2 supplementation involve acute rise in serum DBP and 24,25D, both of which may attenuate the rise in bioavailable 25D and 1,25D.
Keywords: 24,25-dihydroxyvitamin D; 25-hydroxvitamin D; ergocalciferol supplementation; feedback regulation; vitamin D binding protein
After 2 weeks of treatment with vitamin D2, serum levels of vitamin D binding protein and 24,25-dihydroxyvitamin D increased, which may decrease the bioavailability and half-life of vitamin D metabolites.
Vitamin D deficiency has been associated with several adverse health outcomes, including abnormal bone mineralization, heart disease, and premature mortality [1–8]. Conversely, hypervitaminosis D has been linked with hypercalcemia, tissue calcinosis, and renal injury [9]. In response to variations in exposure (e.g., sunlight, diet) and exogenous supplementation, counter regulatory mechanisms are in place that maintain appropriate concentrations of 1,25-hydroxyvitamin D (1,25D) and its precursor 25-dihydroxyvitamin D (25D) [6, 10, 11]. As with other hormones, feedback loops involve anabolic and catabolic pathways [12–19] that modify levels of bioavailable forms of the hormone [15, 20–25].
Changes in levels of binding proteins and, consequently, changes in bioavailable levels of hormones are well-known endocrine regulatory mechanisms [22, 26–28]. Vitamin D binding protein (DBP) is a circulating binding protein for both 25D and 1,25D [20, 29, 30] and an important determinant of 25D concentrations in the circulation that likely regulates the bioavailability of 25D and 1,25D to target tissues [16]. The determinants of DBP concentrations in humans, however, are incompletely understood. Both 25D and DBP are synthesized and secreted by the liver, and because of the high binding affinity of DBP for 25D, most circulating 25D is tightly bound to DBP [17, 20, 30]. Humans with liver disease demonstrate low blood levels of DBP, and accordingly low total 25D levels, but exhibit normal bioavailable or free serum 25D levels [31]. In contrast, excess DBP results in lower bioavailable levels. For example, in tissue culture models of vitamin D receptor signaling, exogenous addition of DBP to culture media dramatically reduces bioavailability of both 25D and 1,25D [16]. It is unknown, however, whether and to what extent vitamin D supplementation affects DBP levels following routine supplementation.
A second mechanism that allows vitamin D target tissue to modulate vitamin D signaling is by regulating expression of enzymes that convert 25D to it active form (1,25D) and to its inactive catabolite [24,25-dihydroxyvitamin D (24,25D)]. Conversion of circulating 25D to 1,25D occurs primarily in the kidneys and is upregulated during states of vitamin D deficiency [32]. In contrast, 25-hydroxyvitamin D 24-hydroxylase (CYP24A1) is highly expressed in the kidney and converts 1,25D and 25D to the inactive metabolites 1,24,25-trihydroxyvitamin D and 24,25D, respectively [32]. CYP24A1 is thus an efficient suppressor of vitamin D signaling by depleting both the active form of vitamin D (1,25D) and its precursor (25D).
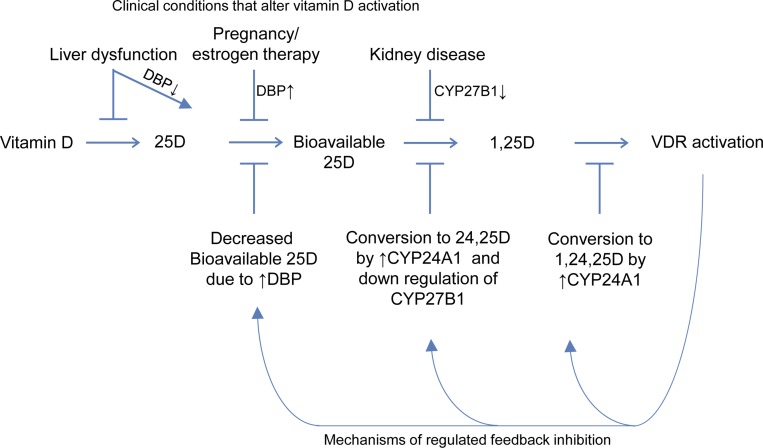
We hypothesized that acute administration of high doses of vitamin D2 would lead to counter regulatory changes in circulating DBP and expression of CYP24A1 and CYP27B1 that, in turn, would lead to sequential alterations in the bioavailability of 25D and its conversion to the active 1,25-dihydroxy form (Fig 1). Given the evidence of multiple mechanisms regulating vitamin D signaling, including the influence of DBP on concentrations of bioavailable 25D, it is likely that there is reciprocal regulation of blood concentrations of DBP after administration of vitamin D supplements, which may be an important feedback mechanism for maintaining appropriate concentrations of 1,25 D. Similar feedback regulatory changes in binding proteins have been described in other hormonal systems (e.g., increases in sex hormone–binding globulin after administration of estrogen) [33, 34]. In this study, we examined the short-term changes of circulating concentrations of DBP, 24,25D, and bioavailable 25D after acute treatment of subjects with high doses of ergocalciferol (vitamin D2).
Figure 1.
Model for regulated sequential conversion of vitamin D to its active form, and clinical conditions known to alter these steps. ↑, increased; VDR, vitamin D receptor.
1. Materials and Methods
A. Study Design and Participants
One hundred healthy adults (age ≥18 years) were recruited using both hard-copy and online advertisements. The study was conducted at the Massachusetts General Hospital from July 2007 to January 2012. Subjects were excluded if they had a known history of kidney disease, nephrolithiasis, or hypercalcemia. Exclusion criteria also included exposure to an active vitamin D analog (e.g., calcitriol, doxercalciferol, or paricalcitol) within 30 days, use of immunomodulatory or immunosuppressive medications, pregnancy, known or suspected active infectious processes, or known granulomatous diseases. Candidates who signed written informed consent underwent screening laboratory testing including serum 25D, calcium, phosphorus, and serum creatinine levels; and urine pregnancy testing. Estimated glomerular filtration rate was calculated using the simplified Modification of Diet in Renal Disease equation [35]. Subjects with serum levels of calcium >10.5 mg/dL and phosphorus >5 mg/dL, a positive urine pregnancy test, or an estimated glomerular filtration rate <60 mL/min were excluded from further participation. All eligible participants with serum 25D level ≤32 ng/mL were offered treatment with 50,000 IU ergocalciferol taken orally every other day for five total doses (250,000 IU) over 2 weeks.
This study represents a secondary analysis of the original aims, which were intended to examine the relationship of vitamin D levels and cathelicidin [36]. The study was conducted in compliance with the Declaration of Helsinki principles, approved by the institutional review boards of Massachusetts General Hospital and the Massachusetts Institute of Technology, and registered with ClinicalTrials.gov (NCT00742235).
B. Data Collection
Subjects were screened for eligibility and, after consent and enrollment, a blood sample was collected before their first dose of ergocalciferol. Subjects were seen again 2 weeks later, after their final dose of ergocalciferol, when a final follow-up blood sample was obtained. For this study, baseline and posttreatment blood samples were analyzed for DBP, 25-hydroxyvitamin D2 (25D2), 25-hydroxyvitamin D3 (25D3), 1,25D, 24,25-dihydroxyvitamin D2 (24,25D2), 24,25-dihydroxyvitamin D3 (24,25D3), parathyroid hormone (PTH), calcium, phosphorous, and albumin concentrations. Vital signs including heart rate, temperature, and blood pressure were measured and recorded at both visits.
C. Biochemical Analyses
Blood samples were drawn into serum separator tubes without anticoagulant, centrifuged at 1430g for 15 minutes, and stored at −80°C for future analysis. To screen subjects for eligibility, baseline blood samples were tested for total 25D using a US Food and Drug Administration-approved immunoassay within a Clinical Laboratory Improvement Amendments-certified hospital laboratory.
To measure pre- and postsupplementation serum concentrations of 25D3, 24,25D3, 25D2, and 24,25D2, serum samples were subsequently tested using liquid chromatography–tandem mass spectrometry (LC-MS/MS). For these analyses, 100 µL of serum was mixed with 25D3–[2H6] and 24,25-(OH)2D3–[2H6] isotopic internal standards dissolved in 5% bovine serum albumin (IsoSciences, King of Prussia, PA), and 25D3, 25D2, 24,25D3, and 24,25D2 were isolated by solid-phase extraction (Strata C-18E 96-well SPE plates; Phenomenex, Torrence, CA), eluted with acetonitrile, and derivatized with 4-phenyl-1,2,4-triazole-3,5-dione. Samples were vacuum lyophilized and redissolved with 100 µL of 50% ethanol. Samples were then analyzed for vitamin D metabolites using reverse-phase chromatography coupled to tandem mass spectrometry in multiple reaction monitoring mode [intra-assay coefficients of variation (CVs): 1.1%, 1.3%, 3.5%, and 4.2% for 25D3, 25D2, 24,25D3, and 24,25D2, respectively]. Assays were calibrated using 25D3 and 25D2 certified reference standards (Cerilliant, Round Rock, TX; accuracy traceable to National Institute of Standards and Technology SRM 2972 reference material), 24R,25-(OH)2D3 standard from Santa Cruz Biotechnology (Santa Cruz, CA), 24R,25-(OH)2D2 standard from Sigma-Aldrich (St. Louis, MO). Complete descriptions of chromatography and mass spectrometer settings have been previously described [37]. Intact serum PTH was measured using the Cobas electrochemiluminescence immunoassay on the Modular Analytics E170 automated analyzer (Roche Diagnostics, Indianapolis, IN; interassay CV, 2.5%).
Serum levels of 1,25D were measured in the 28 subjects with remaining sample by immunoassay on the Diasorin platform (Stillwater, MN; inter- and intra-assay CVs, <7.5%, validated against multiple clinical assay methods as previously described in [38]). Serum albumin and calcium levels were measured by colorimetric assay on automated platforms in a Clinical Laboratory Improvement Amendments-certified hospital laboratory. Serum fibroblast growth factor-23 (FGF23) was measured in pre- and posttreatment samples from the 24 subjects with remaining sample using the second generation human FGF23 (C-term) enzyme-linked immunosorbent assay kit (catalog no. 60-6100) from Immutopics (San Clemente, CA), using the manufacturer’s instructions.
Calculation of bioavailable 25D (defined as the sum of free and albumin-bound 25D) was performed using methods we have previously described [39]. Briefly, total 25D is calculated from the sum of 25D3 and 25D2 concentrations; concentrations of free, DBP-bound, and albumin-bound 25D are calculated based on previously estimated binding affinity constants [20]; and concentrations of bioavailable 25D are calculated by summing the concentrations of free and albumin-bound 25D. To corroborate results obtained by calculated bioavailable 25D measurements, bioavailable 25D was also measured using a direct assay method described in Supplemental Materials (562.5KB, pdf) . The direct assay for bioavailable 25D was calibrated using standards composed of buffered saline, 5% bovine serum albumin, and varying amounts purified vitamin D binding protein (Sigma-Aldrich). Calculated and direct bioavailable assay values were highly correlated with each other (R2 = 0.6901; P < 0.0001; Supplemental Fig 1 (562.5KB, pdf) ).
C-1. Mass spectrometric measurement of DBP
DBP was measured using two different assays, both involving high-performance liquid chromatography and tandem mass spectrometry. Serum from each subject (3 μL) was first mixed with 3 μL of DBP internal standard. The internal standard was composed of bovine DBP purified from fetal calf serum using Cohn ethanol fractionation followed by anion-exchange chromatography.
In the first LC-MS/MS assay method, premixed subject serum and DBP internal standard were diluted into 50 μL of protease digestion mixture composed of 50 μL of 25 mM Tris-HCl, pH 7.4, 10 mM dithiothreitol, and 0.1 mg/mL glutamyl endoproteinase (Worthington Biochemical, Lakewood, NJ), and digested overnight at 37°C. Human and bovine DBPs were then analyzed and quantified by LC-MS/MS. In the second LC-MS/MS assay, which was very similar to a recently published method [40], 3 μL of serum was mixed with DBP internal standard, denatured and reduced with trifluoroethanol and dithiothreitol, and alkylated with iodoacetamide. Denatured samples were then digested overnight at 37°C in 25 mM Tris-HCl, pH 7.4, with 0.1 mg/mL tosyl phenylalanyl chloromethyl ketone-modified trypsin (Worthington Biochemical). After digestion with either Glu-C or trypsin, 10 μL of the resulting digested serum peptides were injected and resolved by reverse-phase chromatography on a Kinetex C18 column, 50 × 3 mm, 5-µm bead diameter (Phenomenex) eluted with 0.1% formic acid and 0% to 100% acetonitrile gradient. Digested peptides specific for the human and bovine DBP were measured using multiple reaction monitoring using settings shown in Supplemental Table 1 (562.5KB, pdf) . Assay characteristics are described in Supplemental Figs 2–4 (562.5KB, pdf) .
The peptides used to quantify DBP for both assay methods are digested peptides common to DBP protein variants Gc1S, Gc1F, and Gc2, and thus quantify total serum DBP concentrations including all major variants. The peptides used to quantify the internal standard for both methods are the homologous peptides liberated by digestion within bovine DBP. Purified DBP calibrators were purchased from Sigma-Aldrich (Gc-globulin, catalog no. G8764). Aliquots of pooled serum frozen at −80°C were used to monitor interassay quality control; the CV of the assay was 6.2%.
All LC-MS/MS assays were performed using an API 5000 triple quadrupole mass spectrometer (SCIEX, Framingham, MA) interfaced with a Shimadzu ultra–high-pressure liquid chromatography system with autosampler (Shimadzu USA, Columbia, MD). Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich.
D. Statistical Analysis
Baseline data were summarized using means, standard deviations, medians, and interquartile ranges. Frequencies were computed for all categorical and ordinal variables. Laboratory parameters were compared using pairwise t tests or Wilcoxon tests, depending upon the normality of the data. Correlation analyses were reported using the Pearson correlation coefficient. The statistical significance was set at a two-sided P < 0.05. All analyses were performed with R version 3.2.2 (https://www.r-project.org/).
2. Results
A. Subject Characteristics
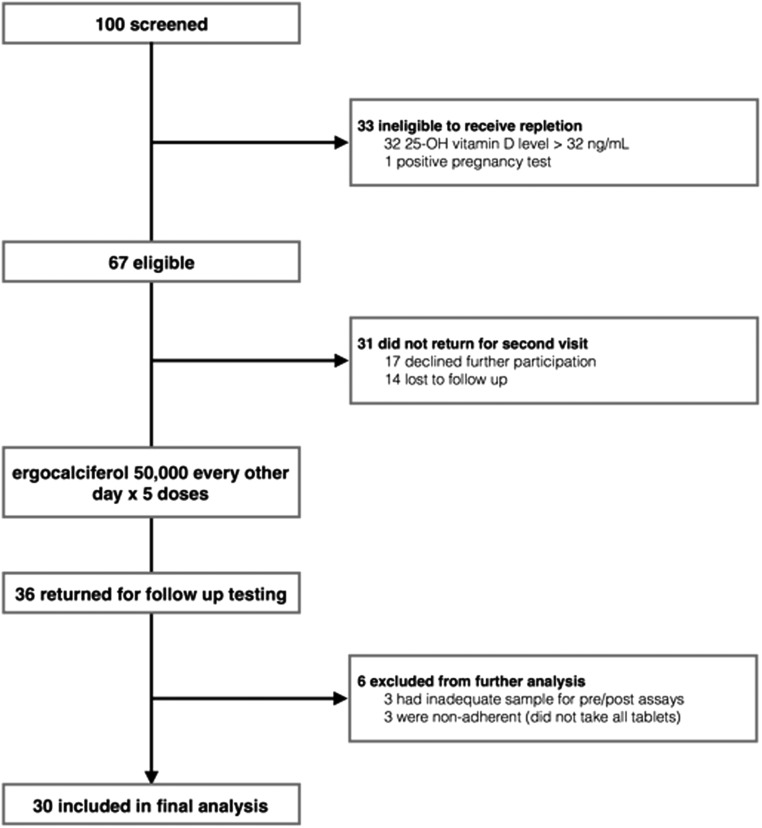
Of the 36 subjects offered oral ergocalciferol supplementation, 30 completed the study; three were excluded due to inadequate sample, and three were excluded due to noncompliance based on the absence of detectable 25D2 in their posttreatment samples (Fig 2). Baseline characteristics of the 30 subjects are shown in Table 1. Most were white, normotensive, and had normal renal function. Baseline levels of serum calcium, parathyroid hormone, and albumin were also within normal limits. As shown in Table 2, the mean baseline serum concentration of vitamin 25D3 was 26.8 ± 9.9 ng/mL (range, 9.6–44.7 ng/mL), mean baseline serum concentration of 25D2 was 1.4 ± 0.9 ng/mL (range, 0.4–4.2 ng/mL), and mean concentration of 24,25D3 was 3.9 ± 2.0 ng/mL (range, 0.81–8.17 ng/mL). The subjects included in the study all had baseline total 25D level <32 ng/mL when tested by immunoassay during screening for eligibility, but a few were found to have baseline values >32 ng/mL when subsequently tested by LC-MS/MS. This is most likely due to the poorer precision and negative bias that have been previously described for clinical immunoassays [41–44]. The mean baseline concentration of serum DBP among subjects was 165.6 ± 53.8 µg/mL (range, 105.7–323.0 µg/mL). Correlations between baseline PTH and total 25D3 and baseline PTH and bioavailable 25D3 were −0.411 (P = 0.02) and −0.413 (P = 0.02), respectively.
Figure 2.
Consort diagram.
Table 1.
Subjects’ Baseline Characteristics
| Characteristic | Data (N = 30 subjects) |
|---|---|
| Age, y | 30.7 (25.9 – 49.4) |
| Male sex | 53 (16) |
| Race | |
| White | 60 (18) |
| Black | 13.3 (4) |
| Asian | 13.3 (4) |
| Other | 13.3 (4) |
| Hispanic ethnicity | 23.3 (7) |
| Never smoked | 46.7 (14) |
| Body mass index, kg/m2 | 24.3 (22.7–28.4) |
Data reported as median (interquartile range) or percentage of total (no.).
Table 2.
Changes in Laboratory Parameters Pre- and Posttreatment With High-Dose Oral Ergocalciferol
| Standard Vitamin D Parameters | Pretreatment* | Posttreatment* | Pa | Average % Change |
|---|---|---|---|---|
| 25D2, ng/mL | 1.4 ± 0.9 | 45.3 ± 16.5 | <0.0001 | 4926 ± 4622 |
| 25D3, ng/mL | 26.8 ± 9.9 | 19.7 ± 8.2 | <0.0001 | −25.0 ± 25.2 |
| Total 25D, ng/mL | 28.2 ± 10.0 | 65.0 ± 21.1 | <0.0001 | 152.2 ± 102.5 |
| Bioavailable 25D, calculated, ng/mL | 5.0 ± 2.0 | 8.7 ± 2.7 | <0.0001 | 104.4 ± 99.6 |
| Bioavailable 25D, direct assay, ng/mL | 3.4 ± 0.2 | 5.6 ± 0.4 | <0.0001 | 99 ± 21 |
| 1,25D, pg/mL | 45.5 ± 10.7 | 58.1 ± 13.0 | 0.0006 | 32.3 ± 38.8 |
| Compensatory responses | ||||
| DBP (GluC method), µg/mL | 165.6 ± 53.8 | 222.0 ± 61.1 | <0.0001 | 39.1 ± 39.4 |
| DBP (trypsin method), µg/mL | 156.2 ± 54.0 | 189.3 ± 52.6 | 0.015 | 26.3 ± 38.9 |
| 24,25D2, ng/mL | 0.05 ± 0.03 | 0.91 ± 0.38 | <0.0001 | 2835.5 ± 2557.7 |
| 24,25D3, ng/mL | 3.8 ± 2.0 | 3.8 ± 1.9 | 0.8042 | 3.3 ± 40.0 |
| Total 24,25D, ng/mL | 3.9 ± 2.0 | 4.7 ± 2.1 | 0.0147 | 31.3 ± 48.9 |
| 24,25D3:25D3 ratio | 0. 14 ± 0.04 | 0.19 ± 0.04 | <0.0001 | 40.6 ± 41.7 |
| 24,25D2:25D2 ratio | 0.021 ± 0.001 | |||
| Minerals and hormones | ||||
| Calcium, mg/dL | 9.4 ± 0.3 | 9.4 ± 0.3 | 0.76 | −0.1 ± 3.2 |
| Phosphate, mg/dL | 3.5 ± 0.5 | 3.6 ± 0.6 | 0.20 | 4.1 ± 14.8 |
| Albumin, g/dL | 4.6 ± 0.2 | 4.6 ± 0.2 | 0.45 | −0.6 ± 5.2 |
| Parathyroid hormone, pg/mL | 47.3 ± 16.7 | 47.6 ± 19.5 | 0.91 | 5.4 ± 40.7 |
| Fibroblast growth factor-23, IU/mL | 36.1 ± 34.4 | 26.5 ± 25.3 | 0.10 | −25 ± 170 |
Data reported as mean ± standard deviation.
P values indicate statistical significance of difference between pre- and posttreatment average values by pairwise t test or pairwise Wilcoxon test, whichever was appropriate.
B. Acute Homeostatic Changes in Vitamin D Metabolites
Table 2 summarizes the changes in levels of standard vitamin D measures, compensatory changes in the vitamin D metabolites, and calcium, phosphate, albumin, PTH, and FGF23 levels after a cumulative dose of 250,000 units of ergocalciferol over 2 weeks (22.3 times the recommended dose of 800 IU per day [45]). The marked rise in levels of 25D2 was reflected in a significant rise in total 25D levels (152%). Changes in concentrations of total 25D within individual subjects are depicted in Supplemental Fig 5 (562.5KB, pdf) . When concentrations of bioavailable 25D were calculated based on measured total 25D, albumin, and DBP concentrations, bioavailable 25D was found to have increased by 104%. When we measured concentrations of bioavailable 25D using a newly developed direct binding assay, bioavailable 25D was found to have increased by 99%, corroborating the results of the calculated bioavailable 25D measurements. Last, concentrations of 1,25D measured by immunoassay (D2 and D3 combined) increased by only 32%.
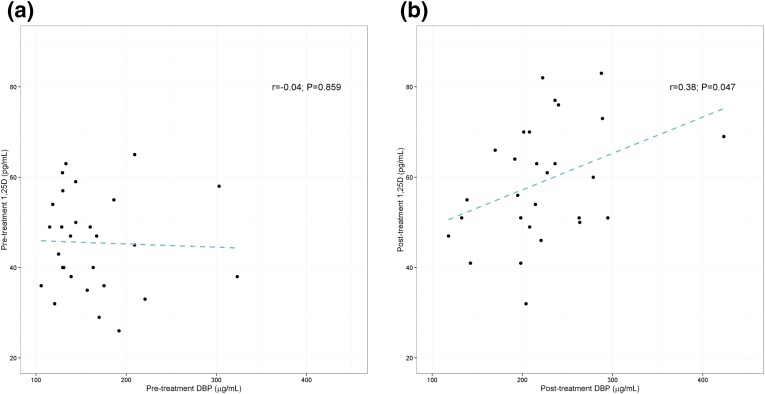
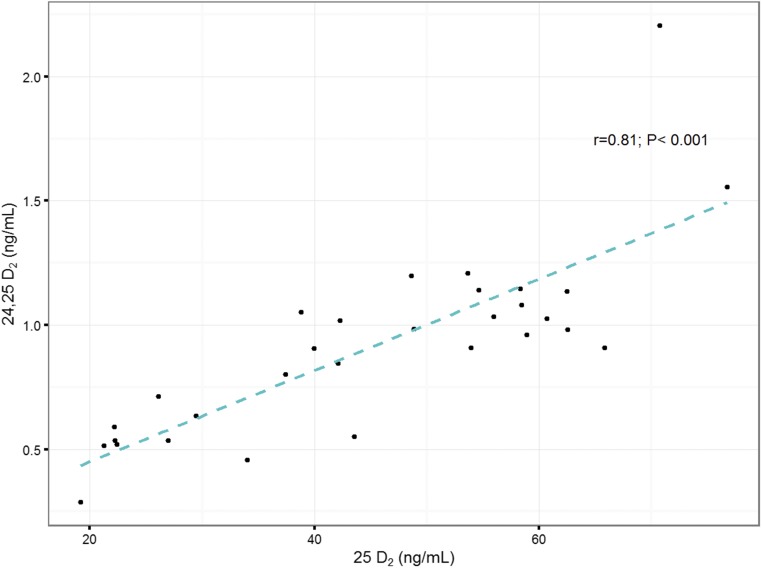
After 2 weeks of supplementation with ergocalciferol, mean circulating DBP concentrations increased approximately 39%, from 165.6 ± 53.8 µg/mL to 222.0 ± 61.1 µg/mL (P < 0.0001). Changes in concentrations of DBP within individual subjects are depicted in Supplemental Fig 6 (562.5KB, pdf) . DBP measured using the second LC-MS/MS assay method also demonstrated a significant increase in DBP concentrations after ergocalciferol supplementation, from 156.2 ± 54.0 µg/mL to 189.3 ± 52.6 µg/mL (P = 0.015). Note that DBP measurements between the two assays were strongly correlated and in close agreement (R2 = 0.8088; Supplemental Fig 4 (562.5KB, pdf) ). There was a significant correlation between serum DBP and 1,25D concentrations after vitamin D2 supplementation, suggesting a relationship between the rise in 1,25D and DBP concentrations (Fig 3). After supplementation, there was a corresponding increase in serum concentrations of the breakdown metabolite 24,25D2 and an overall increase of 24,25D by approximately 31% (Table 2). Furthermore, a strong correlation between 24,25D2 and 25D2 was observed (Fig 4).
Figure 3.
Correlations between serum DBP and 1,25D concentrations before and after vitamin D2 supplementation. (a) Pretreatment. (b) Posttreatment. Pearson correlation coefficient and P values are shown.
Figure 4.
Linear relationships between serum concentrations of 24,25D2 and 25D2 after vitamin D2 supplementation. Linear regression trendlines and Pearson correlation coefficients are shown.
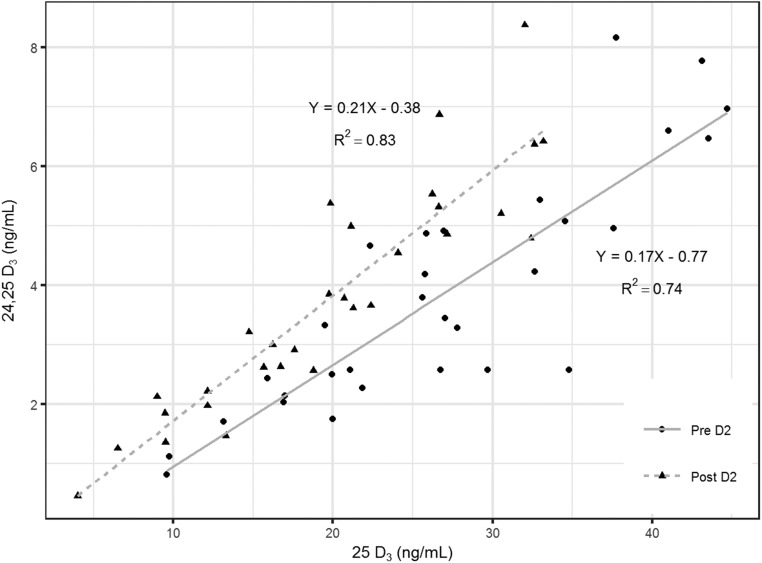
Although there was no change in subjects’ diets or sun exposure during the 2-week study period, concentrations of 25D3 decreased by 25% after supplementation with ergocalciferol. Despite the decrease in concentrations of 25D3, concentrations of its metabolite 24,25D3 did not decrease and, as a consequence, there was a 41% increase in the ratio of 24,25D3 to 25D3. The decrease in 25D3 concentrations relative to 24,25D3 was also reflected by a shift to the left in the linear relationship between 24,25D3 and 25D3 after supplementation (Fig 5). Together, these findings are highly suggestive of an increase in CYP24A1 activity, which would result in increased production of 24,25D3 despite the simultaneous catabolism (and loss) of 25D3.
Figure 5.
Linear relationships between serum concentrations of 24,25D3 and 25D3 before (circles) and after (triangles) vitamin D2 supplementation. Linear regression trendlines and Pearson correlation coefficients are shown.
B-1. Minerals, PTH, and FGF23
Serum levels of calcium, PTH, and albumin were normal at baseline and did not significantly change after supplementation with ergocalciferol (P > 0.05 for all comparisons). Concentrations of FGF23 also did not show any significant changes after ergocalciferol treatment (Table 2).
3. Discussion
Our results suggest that physiologic changes after oral vitamin D2 supplementation involve an acute increase in circulating DBP levels that may attenuate the rise in bioavailable 25D levels, as well as a parallel rise in 24,25D levels, whose production reflects an increased catabolism of 25D by CYP24A1.Upregulation of CYP24A1 would also be predicted to attenuate the rise of the active metabolite 1,25D. This may explain why healthy subjects with relatively normal levels of vitamin D and normal levels of serum calcium do not become hypercalcemic after receiving high doses of vitamin D2.
DBP is the major carrier protein for circulating 25D, with estimated dissociation constants in the low nanomolar range [20, 30, 46, 47]. The high binding affinity of DBP makes it the major carrier for 25D but also results in reduction of the concentrations of free and bioavailable 25D [16, 24], analogous to the effects of thyroid hormone–binding globulin on thyroid hormone bioavailability [22, 26–28]. It has been hypothesized that DBP is essential for delivery of 25D to the kidney via megalin-mediated uptake in the proximal tubules [48]. Cell culture studies, however, confirm that DBP limits uptake of 25D and 1,25D by cells, thus acting as a key regulator of bioavailability of 25D for conversion and bioavailability of 1,25D for signaling [16]. Furthermore, mice genetically deficient for DBP develop profoundly decreased circulating 25D concentrations but are free from any ill effects of vitamin D deficiency while receiving a vitamin D-replete diet, presumably because of adequate bioavailable 25D levels due to the absence of DBP [24]. As a corollary, patients with DBP deficiency secondary to liver disease, as well as women with low DBP concentrations associated with estrogen deficiency after menopause, have low total 25D levels but normal bioavailable or free 25D levels [49, 50]. Our data support the cell culture studies in that the rise in DBP after vitamin D supplementation attenuated the increase in bioavailable 25D and 1,25D levels, compared with the dramatic rise of total 25D (104% and 32%, vs 152%).
After treatment with ergocalciferol, DBP concentrations increased significantly and were also correlated with concentrations of 1,25D. Increases in levels of circulating DBP in patients after vitamin D supplementation have been reported by others. In one study of patients with both hip fracture and vitamin D deficiency, it was found that 3 months of treatment with either cholecalciferol or ergocalciferol produced significant increases in serum DBP concentrations [51]. A similar pattern has been seen in women given estrogens or women during pregnancy; following increases in estrogen, the concentrations of 1,25D increased, and concentrations of DBP increased in parallel with 1,25D [49, 52, 53]. The increase in DBP levels after introduction of exogenous vitamin D and increased 1,25D signaling is analogous to the increase in sex hormone–binding globulin levels in patients treated with exogenous estrogen [33, 34]. Together, these observations suggest that production of DBP may be directly induced by increased activation of vitamin D receptor by 1,25D (Fig 1). Although it may be premature to conclude that our observations offer definitive evidence of a physiologic mechanism for regulation of vitamin D activity, our data suggest that the observed rise in DBP after vitamin D supplementation may be acting to attenuate the rise in 1,25D levels.
In addition to the effects of vitamin D2 supplementation on serum concentrations of DBP, we also observed coordinated changes in 25D catabolism. Although there were no changes in the diet or sun exposure of the subjects, average concentrations of 25D3 decreased significantly among the subjects, while average concentrations of its downstream metabolite 24,25D3 did not decrease. Because production of 24,25D depend on the concentration of its precursor 25D, we would normally expect 24,25D concentrations to decrease proportionally to the decrease in 25D level (this decrease in 24,25D relative to 25D should be further amplified by the fact that the half-life of 25D3 is approximately 55.7-fold longer than that of 24,25D3 [54, 55]). As a consequence of these changes, we observed that the average ratio of 24,25D3 to 25D3 and the linear relationship between 24,25D3 and 25D3 (Fig 5) increased significantly after vitamin D2 supplementation. All these findings suggest a change in the equilibrium between 24,25D3 and its precursor 25D3 after the subjects received vitamin D2 supplements. Together, these findings are most easily explained by an increase in the conversion of 25D to 24,25D by CYP24A1. If we accept this explanation, then the increase in catabolism of 25D3 to 24,25D3 further provides an explanation of why concentrations of 25D3 decreased despite no change in subjects’ sunlight exposure or diet.
These findings have considerable precedence; for example, decreased 25D3 concentration after f4 weeks of supplementation with vitamin D2 have been reported [56]. Furthermore, several studies from our group and others have shown that expression of CYP24A1 is increased by 1,25 D [32, 57–59], that the ratio of 24,25D3 to 25D3 increases disproportionately in subjects with higher 25D3 concentrations [37, 60–65], and that supplementing patients with vitamin D deficiency with vitamin D3 results in increases in the ratio of 24,25D3 to 25D3 similar to the effects we observed in response to vitamin D2 supplementation [62, 45, 66]. One note of significance: A previous study found that 12-week treatment with high-dose ergocalciferol resulted in significant increases in subjects’ FGF23 levels [67], and previous studies have suggested that FGF23 plays a role in regulation of CYP24A1. In our short-term treatment study, however, we observed no statistically significant changes in subjects’ FGF23 levels, thus the changes in 24,25D3 metabolism and 24,25D3 to 25D3 ratio after ergocalciferol treatment could not be explained by changes in FGF23 [68, 69].
This study has several limitations. In contrast to our results (and those of Glendenning et al. [51]), two previous studies of subjects receiving 15 weeks or 1 year of vitamin D supplementation did not report significant changes in DBP concentrations [66, 70]. Reasons for the discrepancy may be due to the fact that our study explored the acute effects of 2 weeks of vitamin D supplementation, whereas the other studies examined changes after longer periods of supplementation, after which feedback inhibitory mechanisms may have already normalized 1,25D signaling. Alternatively, differences may be due to our use of an LC-MS/MS assay to measure DBP, whereas these previous reports used various immunoassays. Second, after supplementation with vitamin D2, there were increases in concentrations of 1,25D, 25D, and 24,25D. Although we hypothesize that the changes in DBP are in response to increased activation of vitamin D receptor by 1,25D, it is possible that other vitamin D metabolites (e.g., 25D or 24,25D) may be influencing DBP expression, because there is growing evidence that 24,25D may have its own physiologic activities [71]. It is also possible that a portion of the increase in 1,25D is due to the increase in DBP concentrations and not the reverse. To clarify these issues, future studies are needed to investigate whether supplementation with 1,25D directly causes similar changes in DBP expression. Third, the number of subjects in our study was relatively small, and the study design did not include placebo controls. Thus, although the effects seen in our subjects were substantial, the strength of our conclusions would be fortified by additional studies with larger numbers of subjects and the inclusion of placebo controls. It should also be noted that baseline average concentrations of DBP in our healthy subjects were lower than the DBP concentrations reported by others [40, 72, 73]. The fact that we used a different calibrator for DBP than these past studies may explain these differences; furthermore, these previous studies used a polyclonal immunoassay that has been shown to produce measurements significantly higher than other validated assay methods [73]. Furthermore, because our study focused on changes in DBP concentrations within individuals after treatment, and not on differences in absolute DBP concentrations, differences in DBP concentrations compared with other assay methods should not alter the interpretation or significance of our results.
In conclusion, after supplementation with high doses of ergocalciferol (29.7 times the daily recommended supplement of 600 IU) among otherwise healthy subjects, a marked rise in 25D2 concentrations and a 152% increase in total 25D concentrations were accompanied by a 39% rise in blood levels of DBP and a 36% rise in 24,25D levels. Together, the increases in DBP levels and CYP24A1 activity may serve as regulatory mechanisms meant to actively prevent excessive signaling and vitamin D receptor activation, and maintain minerals and respective hormones in homeostatic balance.
Acknowledgments
We thank Dr. Kathryn Lucchesi for critical reading and helpful editorial suggestions.
Acknowledgments
A.B. is supported by Grant K08 HL121801 from the National Institutes of Health (NIH) and by American Diabetes Association Innovation Grant 1-15-IN-02. R.T. is supported by NIH Grants DK094872 and DK094486.
Author contributions: A.H.B., I.B., C.P., S.A.K., and R.I.T. designed the study and collected data. A.H.B., I.B., C.P., S.A.K., D.X., and R.I.T. analyzed and interpreted the data, and drafted the manuscript. A.H.B., I.B., S.A.K., and R.I.T. approved the final manuscript. A.H.B., I.B., and R.I.T. are accountable for all aspects of the work and are jointly responsible to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Clinical trial registry: ClinicalTrials.gov no. NCT00742235 (registered 25 August 2008).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 1,25D
- 1,25-dihydroxyvitamin D3 plus 1,25-dihydroxyvitamin D2
- 24,25D
- 24,25-dihydroxyvitamin D
- 24,25D2
- 24,25-dihydroxyvitamin D2
- 24,25D3
- 24,25-dihydroxyvitamin D3
- 25D
- 25-dihydroxyvitamin D
- 25D2
- 25-hydroxyvitamin D2
- 25D3
- 25-hydroxyvitamin D3
- CYP24A1
- 25-hydroxyvitamin D 24-hydroxylase
- DBP
- vitamin D binding protein
- FGF23
- fibroblast growth factor-23
- LC-MS/MS
- liquid chromatography–tandem mass spectrometry
- PTH
- parathyroid hormone.
References and Notes
- 1.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC, Lee JS, Jackson RD, Robbins JA, Wu C, Stanczyk FZ, LeBoff MS, Wactawski-Wende J, Sarto G, Ockene J, Cummings SR. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149(4):242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginde AA, Liu MC, Camargo CA Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169(6):626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Jackson RD, Johnson KC, Judd H, Kooperberg CL, Kuller LH, LaCroix AZ, Lane DS, Langer RD, Lasser NL, Lewis CE, Limacher MC, Manson JE; Women’s Health Initiative Investigators . Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354(7):684–696. [DOI] [PubMed] [Google Scholar]
- 5.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, Finkelstein JS. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777–783. [DOI] [PubMed] [Google Scholar]
- 7.Heaney RP, Weaver CM. Calcium and vitamin D. Endocrinol Metab Clin North Am. 2003;32(1):181–194, vii–viii (vii-viii). [DOI] [PubMed] [Google Scholar]
- 8.Villareal DT, Civitelli R, Chines A, Avioli LV. Subclinical vitamin D deficiency in postmenopausal women with low vertebral bone mass. J Clin Endocrinol Metab. 1991;72(3):628–634. [DOI] [PubMed] [Google Scholar]
- 9.Blank S, Scanlon KS, Sinks TH, Lett S, Falk H. An outbreak of hypervitaminosis D associated with the overfortification of milk from a home-delivery dairy. Am J Public Health. 1995;85(5):656–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004; 80(6, Suppl):1678S–1688S. [DOI] [PubMed] [Google Scholar]
- 11.Powe CE, Evans MK, Wenger J, Zonderman AB, Berg AH, Nalls M, Tamez H, Zhang D, Bhan I, Karumanchi SA, Powe NR, Thadhani R. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams JS, Rafison B, Witzel S, Reyes RE, Shieh A, Chun R, Zavala K, Hewison M, Liu PT. Regulation of the extrarenal CYP27B1-hydroxylase. J Steroid Biochem Mol Biol. 2014;144(Pt A):22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranch D, Zhang MY, Portale AA, Perwad F. Fibroblast growth factor 23 regulates renal 1,25-dihydroxyvitamin D and phosphate metabolism via the MAP kinase signaling pathway in Hyp mice. J Bone Miner Res. 2011;26(8):1883–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akeno N, Saikatsu S, Kawane T, Horiuchi N. Mouse vitamin D-24-hydroxylase: molecular cloning, tissue distribution, and transcriptional regulation by 1alpha,25-dihydroxyvitamin D3. Endocrinology. 1997;138(6):2233–2240. [DOI] [PubMed] [Google Scholar]
- 15.Bikle DD, Gee E. Free, and not total, 1,25-dihydroxyvitamin D regulates 25-hydroxyvitamin D metabolism by keratinocytes. Endocrinology. 1989;124(2):649–654. [DOI] [PubMed] [Google Scholar]
- 16.Chun RF, Lauridsen AL, Suon L, Zella LA, Pike JW, Modlin RL, Martineau AR, Wilkinson RJ, Adams J, Hewison M. Vitamin D-binding protein directs monocyte responses to 25-hydroxy- and 1,25-dihydroxyvitamin D. J Clin Endocrinol Metab. 2010;95(7):3368–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddad JG., Jr Transport of vitamin D metabolites. Clin Orthop Relat Res. 1979;(142):249–261. [PubMed] [Google Scholar]
- 18.Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523(1):9–18. [DOI] [PubMed] [Google Scholar]
- 19.Shinki T, Jin CH, Nishimura A, Nagai Y, Ohyama Y, Noshiro M, Okuda K, Suda T. Parathyroid hormone inhibits 25-hydroxyvitamin D3-24-hydroxylase mRNA expression stimulated by 1 alpha,25-dihydroxyvitamin D3 in rat kidney but not in intestine. J Biol Chem. 1992;267(19):13757–13762. [PubMed] [Google Scholar]
- 20.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63(4):954–959. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter TO, Zhang JH, Parra E, Ellis BK, Simpson C, Lee WM, Balko J, Fu L, Wong BY, Cole DE. Vitamin D binding protein is a key determinant of 25-hydroxyvitamin D levels in infants and toddlers. J Bone Miner Res. 2013;28(1):213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, Hewison M. Vitamin D and DBP: The free hormone hypothesis revisited. J Steroid Biochem Mol Biol. 2014;144(Pt A):132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlsson T, Osmancevic A, Jansson N, Hulthén L, Holmäng A, Larsson I. Increased vitamin D-binding protein and decreased free 25(OH)D in obese women of reproductive age. Eur J Nutr. 2014;53(1):259–267. [DOI] [PubMed] [Google Scholar]
- 24.Safadi FF, Thornton P, Magiera H, Hollis BW, Gentile M, Haddad JG, Liebhaber SA, Cooke NE. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103(2):239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz JB, Lai J, Lizaola B, Kane L, Weyland P, Terrault NA, Stotland N, Bikle D. Variability in free 25(OH) vitamin D levels in clinical populations. J Steroid Biochem Mol Biol. 2014;144(Pt A):156–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosner W. Sex steroids and the free hormone hypothesis. Cell. 2006;124(3):455–456, author reply 456–457. [DOI] [PubMed] [Google Scholar]
- 27.Adams JS. “Bound” to work: the free hormone hypothesis revisited. Cell. 2005;122(5):647–649. [DOI] [PubMed] [Google Scholar]
- 28.Midgley JE. The free thyroid hormone hypothesis and measurement of free hormones. Clin Chem. 1993;39(6):1342–1344. [PubMed] [Google Scholar]
- 29.Bikle DD, Siiteri PK, Ryzen E, Haddad JG. Serum protein binding of 1,25-dihydroxyvitamin D: a reevaluation by direct measurement of free metabolite levels. J Clin Endocrinol Metab. 1985;61(5):969–975. [DOI] [PubMed] [Google Scholar]
- 30.Arnaud J, Constans J. Affinity differences for vitamin D metabolites associated with the genetic isoforms of the human serum carrier protein (DBP). Hum Genet. 1993;92(2):183–188. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz JB, Lai J, Lizaola B, Kane L, Markova S, Weyland P, Terrault NA, Stotland N, Bikle D. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab. 2014;99(5):1631–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res. 2014;55(1):13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moutos D, Smith S, Zacur H. The effect of monophasic combinations of ethinyl estradiol and norethindrone on gonadotropins, androgens and sex hormone binding globulin: a randomized trial. Contraception. 1995;52(2):105–109. [DOI] [PubMed] [Google Scholar]
- 34.Thijssen JH. Hormonal and nonhormonal factors affecting sex hormone-binding globulin levels in blood. Ann N Y Acad Sci. 1988;538:280–286. [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461–470. [DOI] [PubMed] [Google Scholar]
- 36.Bhan I, Camargo CA Jr., Wenger J, Ricciardi C, Ye J, Borregaard N, Thadhani R. Circulating levels of 25-hydroxyvitamin D and human cathelicidin in healthy adults. J Allergy Clin Immunol. 2011;127(5):1302–1304 e1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berg AH, Powe CE, Evans MK, Wenger J, Ortiz G, Zonderman AB, Suntharalingam P, Lucchesi K, Powe NR, Karumanchi SA, Thadhani RI. 24,25-Dihydroxyvitamin d3 and vitamin D status of community-dwelling black and white Americans. Clin Chem. 2015;61(6):877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valcour A, Zierold C, Podgorski AL, Olson GT, Wall JV, DeLuca HF, Bonelli F. A novel, fully-automated, chemiluminescent assay for the detection of 1,25-dihydroxyvitamin D in biological samples. J Steroid Biochem Mol Biol. 2016;164:120–126. [DOI] [PubMed] [Google Scholar]
- 39.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res. 2011;26(7):1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henderson CM, Lutsey PL, Misialek JR, Laha TJ, Selvin E, Eckfeldt JH, Hoofnagle AN. Measurement by a novel LC-MS/MS methodology reveals similar serum concentrations of vitamin D-binding protein in blacks and whites. Clin Chem. 2016;62(1):179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Enko D, Fridrich L, Rezanka E, Stolba R, Ernst J, Wendler I, Fabian D, Hauptlorenz S, Halwachs-Baumann G. 25-hydroxy-Vitamin D status: limitations in comparison and clinical interpretation of serum-levels across different assay methods. Clin Lab. 2014;60(9):1541–1550. [DOI] [PubMed] [Google Scholar]
- 42.Farrell C, Soldo J, Williams P, Herrmann M. 25-Hydroxyvitamin D testing: challenging the performance of current automated immunoassays. Clin Chem Lab Med. 2012;50(11):1953–1963. [DOI] [PubMed] [Google Scholar]
- 43.Ong L, Saw S, Sahabdeen NB, Tey KT, Ho CS, Sethi SK. Current 25-hydroxyvitamin D assays: do they pass the test? Clin Chim Acta. 2012;413(13-14):1127–1134. [DOI] [PubMed] [Google Scholar]
- 44.Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets. 2011;12(1):19–28. [DOI] [PubMed] [Google Scholar]
- 45.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouillon R, van Baelen H, de Moor P. Comparative study of the affinity of the serum vitamin D-binding protein. J Steroid Biochem. 1980;13(9):1029–1034. [DOI] [PubMed] [Google Scholar]
- 47.Chun RF, Peercy BE, Adams JS, Hewison M. Vitamin D binding protein and monocyte response to 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D: analysis by mathematical modeling. PLoS One. 2012;7(1):e30773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96(4):507–515. [DOI] [PubMed] [Google Scholar]
- 49.Bikle DD, Gee E, Halloran B, Haddad JG. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest. 1984;74(6):1966–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pop LC, Shapses SA, Chang B, Sun W, Wang X. Vitamin D-binding protein in healthy pre- and postmenopausal women: relationship with estradiol concentrations. Endocr Pract. 2015;21(8):936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Glendenning P, Chew GT, Inderjeeth CA, Taranto M, Fraser WD. Calculated free and bioavailable vitamin D metabolite concentrations in vitamin D-deficient hip fracture patients after supplementation with cholecalciferol and ergocalciferol. Bone. 2013;56(2):271–275. [DOI] [PubMed] [Google Scholar]
- 52.Bouillon R, Van Assche FA, Van Baelen H, Heyns W, De Moor P. Influence of the vitamin D-binding protein on the serum concentration of 1,25-dihydroxyvitamin D3. Significance of the free 1,25-dihydroxyvitamin D3 concentration. J Clin Invest. 1981;67(3):589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Hoof HJ, de Sévaux RG, van Baelen H, Swinkels LM, Klipping C, Ross HA, Sweep CG. Relationship between free and total 1,25-dihydroxyvitamin D in conditions of modified binding. Eur J Endocrinol. 2001;144(4):391–396. [DOI] [PubMed] [Google Scholar]
- 54.Jones KS, Assar S, Harnpanich D, Bouillon R, Lambrechts D, Prentice A, Schoenmakers I. 25(OH)D2 half-life is shorter than 25(OH)D3 half-life and is influenced by DBP concentration and genotype. J Clin Endocrinol Metab. 2014;99(9):3373–3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar R, Wiesner R, Scott M, Go VL. Physiology of 24,25-dihydroxyvitamin D3 in normal human subjects. Am J Physiol. 1982;243(5):E370–E374. [DOI] [PubMed] [Google Scholar]
- 56.Itkonen ST, Skaffari E, Saaristo P, Saarnio EM, Erkkola M, Jakobsen J, Cashman KD, Lamberg-Allardt C. Effects of vitamin D2-fortified bread v. supplementation with vitamin D2 or D3 on serum 25-hydroxyvitamin D metabolites: an 8-week randomised-controlled trial in young adult Finnish women. Br J Nutr. 2016;115(7):1232–1239. [DOI] [PubMed] [Google Scholar]
- 57.Chow EC, Quach HP, Vieth R, Pang KS. Temporal changes in tissue 1α,25-dihydroxyvitamin D3, vitamin D receptor target genes, and calcium and PTH levels after 1,25(OH)2D3 treatment in mice. Am J Physiol Endocrinol Metab. 2013;304(9):E977–E989. [DOI] [PubMed] [Google Scholar]
- 58.Seth-Vollenweider T, Joshi S, Dhawan P, Sif S, Christakos S. Novel mechanism of negative regulation of 1,25-dihydroxyvitamin D3-induced 25-hydroxyvitamin D3 24-hydroxylase (Cyp24a1) transcription: epigenetic modification involving cross-talk between protein-arginine methyltransferase 5 and the SWI/SNF complex. J Biol Chem. 2014;289(49):33958–33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petkovich M, Melnick J, White J, Tabash S, Strugnell S, Bishop CW. Modified-release oral calcifediol corrects vitamin D insufficiency with minimal CYP24A1 upregulation. J Steroid Biochem Mol Biol. 2015;148:283–289. [DOI] [PubMed] [Google Scholar]
- 60.de Boer IH, Sachs MC, Chonchol M, Himmelfarb J, Hoofnagle AN, Ix JH, Kremsdorf RA, Lin YS, Mehrotra R, Robinson-Cohen C, Siscovick DS, Steffes MW, Thummel KE, Tracy RP, Wang Z, Kestenbaum B. Estimated GFR and circulating 24,25-dihydroxyvitamin D3 concentration: a participant-level analysis of 5 cohort studies and clinical trials. Am J Kidney Dis. 2014;64(2):187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaufmann M, Gallagher JC, Peacock M, Schlingmann KP, Konrad M, DeLuca HF, Sigueiro R, Lopez B, Mourino A, Maestro M, St-Arnaud R, Finkelstein JS, Cooper DP, Jones G. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J Clin Endocrinol Metab. 2014;99(7):2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wagner D, Hanwell HE, Schnabl K, Yazdanpanah M, Kimball S, Fu L, Sidhom G, Rousseau D, Cole DE, Vieth R. The ratio of serum 24,25-dihydroxyvitamin D(3) to 25-hydroxyvitamin D(3) is predictive of 25-hydroxyvitamin D(3) response to vitamin D(3) supplementation. J Steroid Biochem Mol Biol. 2011;126(3-5):72–77. [DOI] [PubMed] [Google Scholar]
- 63.Burild A, Frandsen HL, Jakobsen J. Simultaneous quantification of vitamin D3, 25-hydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in human serum by LC-MS/MS. Scand J Clin Lab Invest. 2014;74(5):418–423. [DOI] [PubMed] [Google Scholar]
- 64.Cashman KD, Hayes A, Galvin K, Merkel J, Jones G, Kaufmann M, Hoofnagle AN, Carter GD, Durazo-Arvizu RA, Sempos CT. Significance of serum 24,25-dihydroxyvitamin D in the assessment of vitamin D status: a double-edged sword? Clin Chem. 2015;61(4):636–645. [DOI] [PubMed] [Google Scholar]
- 65.Edouard T, Husseini A, Glorieux FH, Rauch F. Serum 24,25-dihydroxyvitamin D concentrations in osteogenesis imperfecta: relationship to bone parameters. J Clin Endocrinol Metab. 2012;97(4):1243–1249. [DOI] [PubMed] [Google Scholar]
- 66.Cashman KD, Hayes A, O’Donovan SM, Zhang JY, Kinsella M, Galvin K, Kiely M, Seamans KM. Dietary calcium does not interact with vitamin D3 in terms of determining the response and catabolism of serum 25-hydroxyvitamin D during winter in older adults. Am J Clin Nutr. 2014;99(6):1414–1423. [DOI] [PubMed] [Google Scholar]
- 67.Burnett-Bowie SA, Leder BZ, Henao MP, Baldwin CM, Hayden DL, Finkelstein JS. Randomized trial assessing the effects of ergocalciferol administration on circulating FGF23. Clin J Am Soc Nephrol. 2012;7(4):624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bai X, Miao D, Xiao S, Qiu D, St-Arnaud R, Petkovich M, Gupta A, Goltzman D, Karaplis AC. CYP24 inhibition as a therapeutic target in FGF23-mediated renal phosphate wasting disorders. J Clin Invest. 2016;126(2):667–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dusso AS, Rodriguez M. Enhanced induction of Cyp24a1 by FGF23 but low serum 24,25-dihydroxyvitamin D in CKD: implications for therapy. Kidney Int. 2012;82(10):1046–1049. [DOI] [PubMed] [Google Scholar]
- 70.Sollid ST, Hutchinson MY, Berg V, Fuskevag OM, Figenschau Y, Thorsby PM, Jorde R. Effects of vitamin D binding protein phenotypes and vitamin D supplementation on serum total 25(OH)D and directly measured free 25(OH)D. Eur J Endocrinol. 2016;174(4):445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van der Meijden K, Lips P, van Driel M, Heijboer AC, Schulten EA, den Heijer M, Bravenboer N. Primary human osteoblasts in response to 25-hydroxyvitamin D3, 1,25-dihydroxyvitamin D3 and 24R,25-dihydroxyvitamin D3. PLoS One. 2014;9(10):e110283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petrone AB, Weir NL, Steffen BT, Tsai MY, Gaziano JM, Djoussé L. Plasma vitamin D-binding protein and risk of heart failure in male physicians. Am J Cardiol. 2013;112(6):827–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lutsey PL, Parrinello CM, Misialek JR, Hoofnagle AN, Henderson CM, Laha TJ, Michos ED, Eckfeldt JH, Selvin E. Short-term variability of Vitamin D-related biomarkers. Clin Chem. 2016;62(12):1647–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Denburg MR, Hoofnagle AN, Sayed S, Gupta J, de Boer IH, Appel LJ, Durazo-Arvizu R, Whitehead K, Feldman HI, Leonard MB, Chronic Renal Insufficiency Cohort study investigators . Comparison of two ELISA methods and mass spectrometry for measurement of vitamin D-binding protein: implications for the assessment of bioavailable vitamin D concentrations across genotypes. J Bone Miner Res. 2016;31:1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]