Abstract
Introduction:
Checkpoint inhibitors have significantly improved the prognosis of patients with advanced melanoma. These cancer immunotherapy drugs have specific endocrine autoimmune toxicity. We describe a case of an adrenal insufficiency secondary to pembrolizumab, an anti-programmed cell death-1 monoclonal antibody. Moreover, this case of polyendocrinopathy resulting from a pembrolizumab as the adrenal insufficiency occurred after a thyroiditis.
Participant:
A 55-year-old female was started on pembrolizumab immunotherapy for a metastatic choroidal melanoma. Five months after initiation, she suffered from thyrotoxicosis. A thyroiditis was diagnosed by iodine-123 thyroid scintigraphy and ultrasonography. Pembrolizumab therapy was maintained. Two weeks later, without any other treatment given, she patient developed hypothyroidism and levothyroxine substitution was started. Pembrolizumab proved to be ineffective and was stopped 9 months after initiation. One month following its discontinuation, the patient was hospitalized in the intensive care unit. Severe hyponatremia (115 mmol/L) associated with hyperkalemia (5.7 mmol/L) led to the early recognition and treatment of an acute adrenal insufficiency. Positive results for adrenal cortex and 21-hydroxylase antibodies were in favor of autoimmune toxicity.
Conclusion:
This case highlights the diversity of potential endocrine toxicity of checkpoint inhibitors. Because acute adrenal crisis may be associated with substantial morbidity and mortality, physicians must be aware of these rare adverse events to allow an early diagnosis.
Keywords: pembrolizumab, polyendocrinopathy, checkpoint inhibitors, adrenal insufficiency, thyroiditis
We present a case of adrenal insufficiency secondary to pembrolizumab and of polyendocrinopathy secondary to pembrolizumab.
Checkpoint inhibitors have transformed the prognosis for patients with advanced melanoma [1]. These immunomodulators restore the activity of cytotoxic T lymphocytes inhibited by cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) as well as programmed cell death protein 1 (PD-1) receptor and its ligands, PD-L1 and PD-L2. They are divided into two categories of agents: CTLA-4 inhibitors (ipilimumab and tremelimumab) and PD-1 inhibitors (nivolumab and pembrolizumab) [2]. Their mechanism of action induces specific autoimmune toxicity. These immune-related adverse events are mainly gastrointestinal, hepatic, dermatologic, and endocrinologic. The exact risk and mechanism of these side effects remain incompletely understood. Ipilimumab is often responsible for pituitary dysfunction, affecting up to 18% of patients in a phase 3 study [3], whereas nivolumab and pembrolizumab are more often providers of thyroid dysfunction. Hypothyroidism occurs in 1.6% to 8.9% of patients on checkpoint inhibitors and hyperthyroidism occurs in 0.4% to 3.5% of patients [4]. Another more rarely described endocrine adverse effect is adrenal insufficiency. Few cases of CTLA-4 inhibitor-induced adrenal insufficiency have been described in phase 2 and 3 studies [5]. PD-1 inhibitor-induced adrenal insufficiency seems rather rare, but a case of nivolumab-induced primary adrenal failure has recently been described in the literature [6]. Here, we describe a case of polyendocrinopathy resulting from pembrolizumab: a thyroiditis followed by a primary adrenal insufficiency.
1. Case report
A 55-year-old female was started on pembrolizumab immunotherapy for a metastatic choroidal melanoma for which she had already undergone surgery, two different chemotherapy regimens (dacarbazine and fotemustine), and a targeted therapy with a multikinase inhibitor (sorafenib). Before starting pembrolizumab, thyroid function was normal: thyroid-stimulating hormone (TSH) plasma level of 1.8 mIU/L (normal range, 0.4 to 4.0), free thyroxine plasma level of 13.4 pmol/L (normal range, 11.5 to 22.7), and free triiodothyronine plasma level of 4.9 pmol/L (normal range, 3.5 to 6.5). A normal value for serum cortisol (491 nmol/L) was observed in the morning (normal range, 276 to 552).
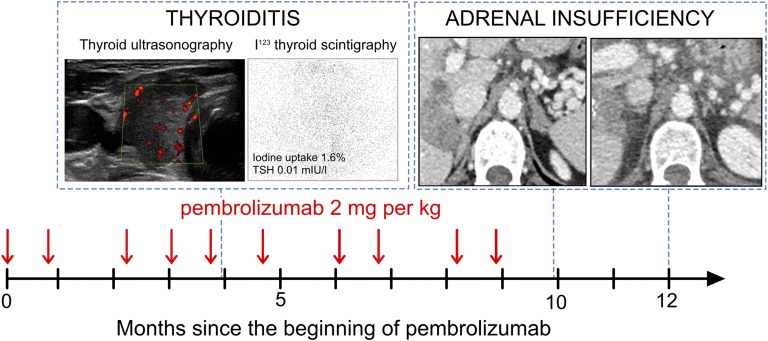
Four months after starting pembrolizumab, she suffered from palpitations and weight loss. Laboratory data showed a low TSH level of 0.01 mIU/L, an elevated free thyroxine level of 91.8 pmol/L, and an elevated triiodothyronine level of 27.2 pmol/L. Antithyroperoxidase, antithyroglobulin, and TSH receptor antibodies were negative. Thyroiditis was diagnosed based on the absence of iodine-123 uptake on thyroid scan. Thyroid ultrasonography showed a heterogeneous and hypoechoic gland (Fig. 1). Two weeks later, without any treatment added, primary hypothyroidism was observed. Levothyroxine was initiated. Pembrolizumab proved to be inefficient after 10 courses at a dose of 2 mg/kg every 3 weeks and was stopped.
Figure 1.
Polyendocrinopathy secondary to pembrolizumab. The dark arrow represents the time in months since the introduction of pembrolizumab. The vertical red arrows represent pembrolizumab injections at a dose of 2 mg/kg. The first CT scan shows the adrenal glands at the time of diagnosis of adrenal insufficiency. The second CT scan shows the adrenal glands 2 months later. I123, iodine-123.
One month after pembrolizumab discontinuation, the patient was hospitalized in the intensive care unit for general physical health deterioration, hypotension at 86/65 mm Hg, hypothermia, and hypoglycemia at 3.6 mmol/L. A blood test showed a severe hyponatremia at 115 mmol/L associated with hyperkaliemia at 5.7 mmol/L and acute renal failure. Acute adrenal crisis was suspected. Treatment with intravenous hydrocortisone was initiated, and the patient rapidly improved. The diagnosis was confirmed by measurement of an undetectable serum cortisol (<14 nmol/L) unresponsive to the Synacthen test (stimulated cortisol remained undetectable at <14 nmol/L). Adrenocorticotropic hormone level was elevated at 88 pmol/L (normal, <13). Other pituitary axes were tested and were normal with prolactin level at 288.8 mIU/L (normal range, 38 to 430), luteinizing hormone at 32.6 UI/L (normal range for menopause, 16 to 54), follicle-stimulating hormone at 56.9 UI/L (normal range for menopause, 23 to 116), and TSH level at 1.7 mIU/L (normal range, 0.4-4.0) under levothyroxine treatment. A growth hormone deficiency was not ruled out by dynamic testing, but growth hormone at 12.6 mIU/L (normal range, 0.26 to 15.0) and insulin-like growth factor-1 at 65 ng/mL (normal range, 65 to 240) were within the normal range. Aldosterone levels were undetectable. Renin level, after a high dose of glucocorticoid replacement, was at 23 µUI/mL (normal range, 9 to 72). Fludrocortisone was then added to hydrocortisone.
To rule out adrenal metastasis, an abdominal computed tomography (CT) scan was undertaken, but no increase in the adrenal gland size was found. The 21-hydroxylase and adrenal cortex antibodies had a positive titer of 1.9 UI/L (normal, <1) and 20 (normal, <0), respectively, which led us to the diagnosis of autoimmune adrenalitis. One month later, 21-hydroxylase antibodies were negative, adrenal cortex antibodies remained positive at 5 (normal, <0), and renin level was elevated at 148 µUI/mL (normal range, 9 to 72), which led us to increase the doses of fludrocortisone. Two months later, CT scan was performed and showed atrophied adrenal glands (Fig. 1).
2. Discussion
Here we report the case of a primary adrenal failure secondary to pembrolizumab. We also report a polyendocrinopathy resulting from pembrolizumab; a transient hyperthyroidism resulting from thyroiditis was followed by this adrenal insufficiency. Establishing the cause of adrenal insufficiency can sometimes be a challenge [7]. In our case, the first step was to distinguish between secondary and primary adrenal insufficiency because there are more cases of hypophysitis than adrenal insufficiency resulting from use of checkpoint inhibitors [4]. Hyperkalemia led us to suspect a primary cause. This hypothesis was confirmed by high adrenocorticotropic hormone levels. The second step was to determine the cause of this primary adrenal insufficiency. Positive antiadrenal antibodies confirmed the autoimmune etiology [7]. The subsequent disappearance of the antibodies may be related to the atrophy of the adrenal cortex observed 2 months later on the CT scan. In the few other cases of primary adrenal insufficiency secondary to immunomodulators described in the literature, anti-21 hydroxylase antibodies and adrenal cortex antibodies were not measured [6, 8, 9]. Thirteen cases have been described secondary to ipilimumab: 11 cases in phase 3 studies and 2 case reports [8, 9]. Four cases have been described secondary to the use of tremelimumab in a phase 3 study [5] and one case was reported with nivolumab [6].
Pembrolizumab has been evaluated in 917 melanoma patients included in phase 2 (n = 316) or 3 (n = 556) trials; no case of adrenal insufficiency has been described [1]. As a consequence, we can assume that it is a very rare adverse effect of pembrolizumab or that it is underdiagnosed. However, the subsequent morbidity and mortality resulting from acute adrenal crisis require patients and physicians to be informed of this particular risk.
Another interesting aspect of this case is the progression of thyroid dysfunction and adrenal insufficiency. Thyroid ultrasonography and iodine-123 thyroid scintigraphy, used when the patient was hyperthyroid, are in favor of inflammatory thyroiditis. This mechanism has already been suggested in an article that described pembrolizumab-induced thyroid dysfunction. Indeed, in this article, thyrotoxicosis was associated with a diffuse increased 18F-fluorodeoxyglucose (FDG) uptake by the thyroid gland, which suggested an inflammatory thyroiditis [10]. In the case report of nivolumab-induced primary adrenal failure, FDG positron emission tomography scan showed uniformly increased FDG activity in both adrenal glands [6]. This suggests a common mechanism of destruction between the two endocrine adverse events.
Another interesting point is that adrenal failure appeared a month after the last injection of pembrolizumab, which suggests that there can be a persistent effect and that endocrine adverse events must be sought even after discontinuation of treatment. These checkpoint inhibitors have specific immune-related adverse effects. It seems difficult to predict which patients are at risk for endocrine toxicity. But, because our patient had thyroiditis before adrenal insufficiency, it seems important to monitor carefully patients who already had an endocrine adverse event.
In conclusion, the mechanisms and risk factors predisposing to endocrine toxicity secondary to checkpoint inhibitors are still unclear. Because of increased use of checkpoint inhibitors, these endocrine immune-related adverse events are going to increase. Physicians must be aware of this endocrine toxicity to avoid morbidity and mortality in these fragile patients.
Acknowledgments
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CT
- computed tomography
- CTLA-4
- cytotoxic T lymphocyte–associated antigen 4
- FDG
- fluorodeoxyglucose
- PD-1
- programmed cell death-1
- TSH
- thyroid-stimulating hormone.
References and Notes
- 1.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S, Ribas A; KEYNOTE-006 investigators . Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 2.Momtaz P, Postow MA. Immunologic checkpoints in cancer therapy: focus on the programmed death-1 (PD-1) receptor pathway. Pharm Genomics Pers Med. 2014;7:357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eggermont AMM, Chiarion-Sileni V, Grob J-J, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, Lebbé C, Ferraresi V, Smylie M, Weber JS, Maio M, Konto C, Hoos A, de Pril V, Gurunath RK, de Schaetzen G, Suciu S, Testori A. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Rahman O, ElHalawani H, Fouad M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: a meta-analysis. Future Oncol. 2016;12(3):413–425. [DOI] [PubMed] [Google Scholar]
- 5.Ribas A, Kefford R, Marshall MA, Punt CJA, Haanen JB, Marmol M, Garbe C, Gogas H, Schachter J, Linette G, Lorigan P, Kendra KL, Maio M, Trefzer U, Smylie M, McArthur GA, Dreno B, Nathan PD, Mackiewicz J, Kirkwood JM, Gomez-Navarro J, Huang B, Pavlov D, Hauschild A. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31(5):616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trainer H, Hulse P, Higham CE, Trainer P, Lorigan P. Hyponatraemia secondary to nivolumab-induced primary adrenal failure. Endocrinol Diabetes Metab Case Rep. 2016;2016. doi:10.1530/EDM-16-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bancos I, Hahner S, Tomlinson J, Arlt W. Diagnosis and management of adrenal insufficiency. Lancet Diabetes Endocrinol. 2015;3(3):216–226. [DOI] [PubMed] [Google Scholar]
- 8.Bacanovic S, Burger IA, Stolzmann P, Hafner J, Huellner MW. Ipilimumab-iduced adrenalitis: a possible pitfall in 18F-FDG-PET/CT. Clin Nucl Med. 2015;40(11):e518–e519. [DOI] [PubMed] [Google Scholar]
- 9.Min L, Ibrahim N. Ipilimumab-induced autoimmune adrenalitis. Lancet Diabetes Endocrinol. 2013;1(3):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, Bravenboer B. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab. 2016;101(11):4431–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]