Abstract
We present of a case of severe osteoporosis with thoracic myelopathy secondary to nontraumatic T8 compression fracture managed nonsurgically with 3.5 months of bed rest. Despite treatment with teriparatide starting at initial presentation, 1-year follow-up dual energy x-ray absorptiometry scan revealed a significantly greater than expected 19% reduction in lumbar spine bone mineral density (BMD) and a 6% reduction in total hip density. Daily alcohol consumption, severe osteoporosis at baseline, and immobilization secondary to transient myelopathy treated with strict bed rest all likely contributed to unexpected BMD findings.
Keywords: teriparatide, osteoporosis, bone mineral density, immobilization
This unusual case may raise caution against the use of teriparatide in the setting of immobilization.
Osteoporosis can result in catastrophic physical, financial, and lifestyle consequences, including severely disabling vertebral compression fracture [1, 2]. Teriparatide, recombinant human parathyroid hormone (1-34) [PTH (1-34)], is US Food and Drug Administration–approved for the following osteoporosis populations at high risk of fracture: men (including hypogonadal men), postmenopausal women, and those with osteoporosis associated with sustained glucocorticoid therapy equivalent to prednisone 5 mg/day or greater [3]. High risk for fracture is defined as history of osteoporotic fracture, multiple risk factors for fracture, or patients who have failed with or are intolerant of other available osteoporotic treatments [3]. Bone mineral density (BMD) gain [4–6] and decreased rates of vertebral fracture have been shown in postmenopausal women with teriparatide treatment [1, 5–7]. We report a patient who lost 19% lumbar spine BMD, despite 1 year of teriparatide treatment, in the setting of thoracic myelopathy secondary to nontraumatic T8 compression fracture managed with 3.5 months of bed rest.
1. Case Report
A 79-year-old Caucasian female was referred to the orthopedics clinic with 5 weeks of thoracic back pain, leg paresthesias, and delayed bladder emptying. Body mass index was 22.3 kg/m2 and Babinski sign was positive bilaterally. Magnetic resonance imaging showed T8 compression fracture with thoracic myelopathy. Dual energy x-ray (DXA) confirmed osteoporosis with lowest T-score of −3.7 at the left femoral neck (Table 1).
Table 1.
DXA Scan Reports Obtained at the Time of T8 Compression Fracture and 1 Year Later
| Region | Date | T-Score | BMD | Δ BMD |
|---|---|---|---|---|
| L1-L4 | 9 May 2016 | 0.665 | −0.168 | |
| 8 May 2015 | 0.833 | |||
| L1, L3, and L4 | 9 May 2016 | -4.6 | 0.621 | −0.146 |
| 8 May 2015 | −3.4 | 0.767 | ||
| Left femoral neck | 9 May 2016 | -3.9 | 0.494 | −0.027 |
| 8 May 2015 | −3.7 | 0.521 | ||
| Left total neck | 9 May 2016 | -3.7 | 0.538 | −0.037 |
| 8 May 2015 | −3.4 | 0.575 | ||
| Right femoral neck | 9 May 2016 | -3.7 | 0.536 | −0.004 |
| 8 May 2015 | −3.6 | 0.540 | ||
| Right total neck | 9 May 2016 | -3.8 | 0.528 | −0.037 |
| 8 May 2015 | −3.5 | 0.565 | ||
| Distal 1/3 radius | 9 May 2016 | -4.6 | 0.383 | −0.013 |
| 8 May 2015 | −4.4 | 0.396 |
Boldface text is used to distinguish DXA scan reports obtained 8 May 2015 versus 9 May 2016.
Menopause was at age 51 with a 1-year history of hormone replacement therapy. Corticosteroid exposure included intermittent topical treatment of psoriasis (2011) and steroid injection into the shoulder and wrist (2013). In 2006, she fell from standing height, followed by chronic upper back pain later diagnosed as vertebral compression fracture requiring T12 kyphoplasty in 2009. Severe acute lower back pain, occurring in the process of being seated, was due to another vertebral compression fracture requiring L2 kyphoplasty in 2015. She was a former 40 pack-year smoker. She had not previously received pharmacologic osteoporosis treatment apart from a calcium and vitamin D supplement of unknown dosage.
Laboratory studies did not show additional causes of osteoporosis (Table 2). Complete blood count, liver function testing, thyroid function testing, and serum protein electrophoresis were also normal.
Table 2.
Summary of Laboratory Values
| Study | Reference Range | May 2015 | Aug 2015 | May 2016 |
|---|---|---|---|---|
| Calcium | 8.5-10 mg/dL | 9.2 | 9.6 | 10.2 |
| Phosphorous | 2.5-4.5 mg/dL | 3.3 | 3 | |
| Albumin | 3.4-5.0 g/dL | 3.7 | 4.3 | |
| Creatinine | 0.52-1.04 mg/dL | 0.78 | 0.73 | 0.84 |
| Parathormone | 12-72 pg/mL | 29 | ||
| Alkaline phosphatase | 40-150 U/L | 94 | 114 | |
| 25-hydroxyvitamin D | 20-76 μg/L | 57 | 34 |
All laboratory values were obtained in the afternoon between 1:00 and 6:00 pm.
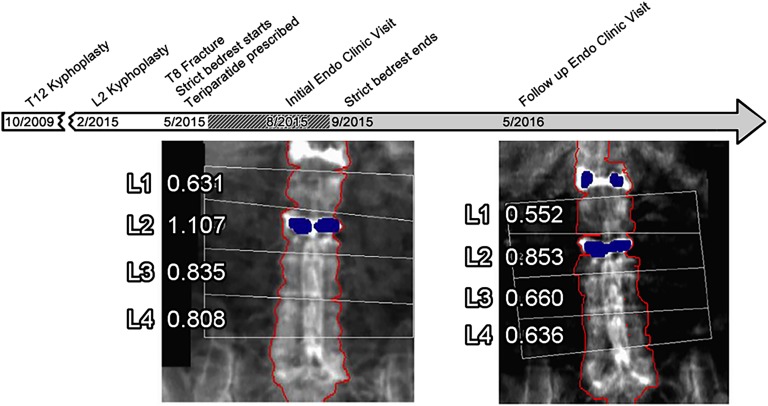
She was treated with strict bed rest and started on teriparatide in anticipation of surgery. She presented to the endocrinology clinic 3 months later on a gurney, confirming adherence to the bed rest order. After 3.5 months of bed rest deconditioning, she was unable to walk and required an additional 3 months of physical therapy. Active myelopathy had resolved at a 6-month orthopedics follow-up. Because of difficult personal logistics, endocrinology follow-up was not until 1 year later, at which time she was in a thoraco-lumbar-sacral orthosis and had returned to many of her past outdoor activities. There were no new fractures. Body mass index remained stable. She, her family, and pharmacy refill records affirmed compliance with teriparatide and supplemental calcium/vitamin D 600 mg/200 IU twice daily. She reported drinking one to three beers per day. Laboratories showed mild hypercalcemia (Table 2) and 24-hour urine calcium 71 mg/24 hours (0.09 mg/mg creatinine; 1.1 mg/kg body weight). Additional steroid exposure had not occurred. Random morning cortisol was 8.9 mg/dL. Other laboratory values were unremarkable (Table 2). Past imaging showed normal appearing adrenals. A 19% loss in areal lumbar and 6% loss in total hip BMD were found on repeat DXA scan (Fig. 1).
Figure 1.
Timeline of major events related to osteoporosis treatment. This includes T12 kyphoplasty, L2 kyphoplasty, endocrine (endo) clinic visits, prescribed bed rest, and lumbar DXA scans demonstrating significant loss in BMD despite treatment with teriparatide over the course of approximately 1 year.
2. Discussion
We present a case of severe, longstanding, and previously untreated osteoporosis with associated 19% loss of lumbar spine BMD during 1 year of teriparatide treatment. Lumbar spine BMD loss is unusual and unexpected in the setting of teriparatide treatment [5, 8]. We hypothesize that immobilization may have decreased and/or reversed the expected BMD gain response to teriparatide.
Immobilization increases bone resorption and bone loss [9]. This has been demonstrated in hospitalized patients [10], those with spinal cord injury [11–13], and healthy subjects subjected to bed rest [14, 15]. However, in these groups, the bone loss has been less than we observed. Specifically, a group of adults lost on average 3.6% (with a range up to 10%) lumbar BMD during short-term bed rest treatment of lumbar intervertebral disc protrusion [10]. Spinal cord injury paralysis has been associated with bone loss below the injury, including up to 4% per month lower limb BMD losses [11], but lumbar spine losses have not been shown [12, 13]. In contrast, healthy individuals on forced bed rest lost an average 2.9% in lumbar spine BMD over 12 weeks [14] and 3.6% over 17 weeks [15]. Adolescent girls lost 15% BMD at the L4 vertebra during 6 weeks of bed rest following operation for scoliosis [16].
The anabolic mechanism and previously reported increases in spine BMD [4–6] made teriparatide an attractive treatment option for our patient. Our patient’s very low baseline BMD (lumbar spine 0.62 g/cm2) may have inflated the unexpected percent BMD loss compared with other studies, where baseline lumbar spine BMD was higher (i.e., 0.81 to 0.82 g/cm2) [4, 6, 8]. Although radial bone loss can be seen with teriparatide treatment [4–6], axial bone loss is rare. An average −6.2% loss in femoral neck BMD was reported in only 10% of postmenopausal women after 1 year of teriparatide in the Fracture Prevention Trial. However, these same women still gained lumbar spine BMD and had reduced vertebral fracture risk [8]. Heany also reported a low rate of BMD loss at the hip, but not the lumbar spine, in teriparatide-treated subjects [17]. More common than bone loss is nonresponse to teriparatide, with lumbar spine nonresponse rates ranging from 2% to 19% [17–20].
Teriparatide-associated loss of areal BMD at the radius, in contrast to axial sites [4–6], is hypothesized to be due in part to its nonweight-bearing status [21]. We were unable to find other reports of teriparatide use in the treatment of advanced baseline osteoporosis during prolonged bed rest. PTH (1-34) prevented bone loss in animal studies of limb immobilization [22, 23] and it synergistically increased bone growth when combined with weight-bearing [23, 24]. In contrast, teriparatide plus mechanical loading was not associated with change in axial BMD in spinal cord injury patients [25].
Fracture risk may not correlate with the BMD loss [8]. Increased bone size, with undermineralized osteoid, might be accompanied by teriparatide-associated improvements in strength [5]. Likewise, in the Fracture Prevention Trial, most of the teriparatide-associated fracture risk reduction was attributed to non-BMD determinants of bone strength, with the greatest impact in the lowest quartile of baseline BMD [26]. Indeed, our patient has not had a fracture while on teriparatide treatment.
Other potential causes of BMD loss in this patient, and limitations of our report, merit consideration. Teriparatide compliance was assessed by historical report and the expected therapy-associated increase in serum calcium, but not bone turnover markers. Although her urine calcium was normal (1.1 mg/kg body weight) [27], it was lower than previously reported on teriparatide (mg/day) [28]. Normal baseline PTH and 25-hydroxyvitamin D (Table 2) argue against coexistent hyperparathyroidism. Still, teriparatide has increased BMD even in subjects with low 25-hydroxyvitamin D [29]. Dexamethasone suppression test was not performed. A bone gain response to teriparatide would be expected, based on studies in glucocorticoid-induced osteoporosis [30, 31] if she had subclinical Cushing syndrome. Impaired response to PTH regulation of bone, or osteoblasts [32, 33], could theoretically cause both severe osteoporosis and teriparatide nonresponse. Finally, alcohol decreased the therapeutic efficacy of PTH (1-34) in animal models [34, 35], leaving open the contribution of her alcohol intake to the BMD loss.
This case highlights an unexpected clinical scenario of teriparatide nonresponse, with significant loss of lumbar spine BMD. Alcohol consumption, severe baseline osteoporosis, and immobilization secondary to myelopathy and bed rest all likely contributed to the BMD losses. This unusual case may raise caution against the use of teriparatide in the setting of immobilization.
Acknowledgments
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- bone mineral density
- DXA
- dual energy x-ray absorptiometry
- MRI
- magnetic resonance imaging
- PTH
- parathyroid hormone.
References and Notes
- 1.Crans GG, Silverman SL, Genant HK, Glass EV, Krege JH. Association of severe vertebral fractures with reduced quality of life: reduction in the incidence of severe vertebral fractures by teriparatide. Arthritis Rheum. 2004;50(12):4028–4034. [DOI] [PubMed] [Google Scholar]
- 2.Ensrud KE, Schousboe JT. Clinical practice. Vertebral fractures. N Engl J Med. 2011;364(17):1634–1642. [DOI] [PubMed] [Google Scholar]
- 3.Teriparatide [package insert]. Highlights of Prescribing Information. http://pi.lilly.com/us/forteo-pi.pdf. Accessed 17 March 2017.
- 4.Leder BZ, Neer RM, Wyland JJ, Lee HW, Burnett-Bowie SM, Finkelstein JS. Effects of teriparatide treatment and discontinuation in postmenopausal women and eugonadal men with osteoporosis. J Clin Endocrinol Metab. 2009;94(8):2915–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodsman AB, Bauer DC, Dempster DW, Dian L, Hanley DA, Harris ST, Kendler DL, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26(5):688–703. [DOI] [PubMed] [Google Scholar]
- 6.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–1441. [DOI] [PubMed] [Google Scholar]
- 7.Genant HK, Siris E, Crans GG, Desaiah D, Krege JH. Reduction in vertebral fracture risk in teriparatide-treated postmenopausal women as assessed by spinal deformity index. Bone. 2005;37(2):170–174. [DOI] [PubMed] [Google Scholar]
- 8.Watts NB, Miller PD, Kohlmeier LA, Sebba A, Chen P, Wong M, Krohn K. Vertebral fracture risk is reduced in women who lose femoral neck BMD with teriparatide treatment. J Bone Miner Res. 2009;24(6):1125–1131. [DOI] [PubMed] [Google Scholar]
- 9.Bauman WA, Cardozo CP. Immobilization Osteoporosis. In: Marcus R, Feldman D, eds. Osteoporosis. Waltham, MA: Elsevier; 2013:1139–1171. 10.1016/B978-0-12-415853-5.00047-9. [Google Scholar]
- 10.Krølner B, Toft B. Vertebral bone loss: an unheeded side effect of therapeutic bed rest. Clin Sci (Lond). 1983;64(5):537–540. [DOI] [PubMed] [Google Scholar]
- 11.Wilmet E, Ismail AA, Heilporn A, Welraeds D, Bergmann P. Longitudinal study of the bone mineral content and of soft tissue composition after spinal cord section. Paraplegia. 1995;33(11):674–677. [DOI] [PubMed] [Google Scholar]
- 12.Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, McWhinney B, Hickman PE. Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab. 1998;83(2):415–422. [DOI] [PubMed] [Google Scholar]
- 13.Jiang S-D, Dai L-Y, Jiang L-S. Osteoporosis after spinal cord injury. Osteoporos Int. 2006;17(2):180–192. [DOI] [PubMed] [Google Scholar]
- 14.Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res. 1998;13(10):1594–1601. [DOI] [PubMed] [Google Scholar]
- 15.Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM. Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res. 1990;5(8):843–850. [DOI] [PubMed] [Google Scholar]
- 16.Hansson TH, Roos BO, Nachemson A. Development of osteopenia in the fourth lumbar vertebra during prolonged bed rest after operation for scoliosis. Acta Orthop Scand. 1975;46(4):621–630. [DOI] [PubMed] [Google Scholar]
- 17.Heaney RP, Watson P. Variability in the measured response of bone to teriparatide. Osteoporos Int. 2011;22(6):1703–1708. [DOI] [PubMed] [Google Scholar]
- 18.Niimi R, Kono T, Nishihara A, Hasegawa M, Kono T, Sudo A. A retrospective analysis of nonresponse to daily teriparatide treatment [published correction appears in Osteroporos Int. 2016;27(9):2887–2888.]. Osteoporos Int. 2016;27(9):2845–2853. [DOI] [PubMed] [Google Scholar]
- 19.Gallagher JC, Rosen CJ, Chen P, Misurski DA, Marcus R. Response rate of bone mineral density to teriparatide in postmenopausal women with osteoporosis. Bone. 2006;39(6):1268–1275. [DOI] [PubMed] [Google Scholar]
- 20.Cohen A, Stein EM, Recker RR, Lappe JM, Dempster DW, Zhou H, Cremers S, McMahon DJ, Nickolas TL, Müller R, Zwahlen A, Young P, Stubby J, Shane E. Teriparatide for idiopathic osteoporosis in premenopausal women: a pilot study. J Clin Endocrinol Metab. 2013;98(5):1971–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleerekoper M, Greenspan SL, Lewiecki EM, Miller PD, Kendler DL, Maricic M, Keaveny TM, Kopperdahl DL, Ruff VA, Wan X, Janos B, Krohn K. Assessing the effects of teriparatide treatment on bone mineral density, bone microarchitecture, and bone strength. J Bone Joint Surg Am. 2014;96(11):e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakai A, Mori T, Sakuma-Zenke M, Takeda T, Nakai K, Katae Y, Hirasawa H, Nakamura T. Osteoclast development in immobilized bone is suppressed by parathyroidectomy in mice. J Bone Miner Metab. 2005;23(1):8–14. [DOI] [PubMed] [Google Scholar]
- 23.Turner RT, Lotinun S, Hefferan TE, Morey-Holton E. Disuse in adult male rats attenuates the bone anabolic response to a therapeutic dose of parathyroid hormone. J Appl Physiol (1985). 2006;101(3):881–886. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, Jee WS, Yuan Z, Wei W, Chen H, Pun S, Liang H, Lin C. Parathyroid hormone and mechanical usage have a synergistic effect in rat tibial diaphyseal cortical bone. J Bone Miner Res. 1999;14(3):439–448. [DOI] [PubMed] [Google Scholar]
- 25.Gordon KE, Wald MJ, Schnitzer TJ. Effect of parathyroid hormone combined with gait training on bone density and bone architecture in people with chronic spinal cord injury. PM R. 2013;5(8):663–671. [DOI] [PubMed] [Google Scholar]
- 26.Chen P, Miller PD, Delmas PD, Misurski DA, Krege JH. Change in lumbar spine BMD and vertebral fracture risk reduction in teriparatide-treated postmenopausal women with osteoporosis. J Bone Miner Res. 2006;21(11):1785–1790. [DOI] [PubMed] [Google Scholar]
- 27.Pak CY, Oata M, Lawrence EC, Snyder W. The hypercalciurias. Causes, parathyroid functions, and diagnostic criteria. J Clin Invest. 1974;54(2):387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller PD, Bilezikian JP, Diaz-Curiel M, Chen P, Marin F, Krege JH, Wong M, Marcus R. Occurrence of hypercalciuria in patients with osteoporosis treated with teriparatide. J Clin Endocrinol Metab. 2007;92(9):3535–3541. [DOI] [PubMed] [Google Scholar]
- 29.Dawson-Hughes B, Chen P, Krege JH. Response to teriparatide in patients with baseline 25-hydroxyvitamin D insufficiency or sufficiency. J Clin Endocrinol Metab. 2007;92(12):4630–4636. [DOI] [PubMed] [Google Scholar]
- 30.Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest. 1998;102(8):1627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saag KG, Shane E, Boonen S, Marín F, Donley DW, Taylor KA, Dalsky GP, Marcus R. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357(20):2028–2039. [DOI] [PubMed] [Google Scholar]
- 32.Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40(6):1434–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lombardi G, Di Somma C, Rubino M, Faggiano A, Vuolo L, Guerra E, Contaldi P, Savastano S, Colao A. The roles of parathyroid hormone in bone remodeling: prospects for novel therapeutics. J Endocrinol Invest. 2011;34(7, Suppl):18–22. [PubMed] [Google Scholar]
- 34.Iwaniec UT, Trevisiol CH, Maddalozzo GF, Rosen CJ, Turner RT. Effects of low-dose parathyroid hormone on bone mass, turnover, and ectopic osteoinduction in a rat model for chronic alcohol abuse. Bone. 2008;42(4):695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaddini GW, Turner RT, Grant KA, Iwaniec UT. Alcohol: a simple nutrient with complex actions on bone in the adult skeleton. Alcohol Clin Exp Res. 2016;40(4):657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]