ABSTRACT
Pseudomonas aeruginosa causes numerous acute and chronic opportunistic infections in humans. One of its most formidable weapons is a type III secretion system (T3SS), which injects powerful toxins directly into host cells. The toxins lead to cell dysfunction and, ultimately, cell death. Identification of regulatory pathways that control T3SS gene expression may lead to the discovery of novel therapeutics to treat P. aeruginosa infections. In a previous study, we found that expression of the magnesium transporter gene mgtE inhibits T3SS gene transcription. MgtE-dependent inhibition appeared to interfere with the synthesis or function of the master T3SS transcriptional activator ExsA, although the exact mechanism was unclear. We now demonstrate that mgtE expression acts through the GacAS two-component system to activate rsmY and rsmZ transcription. This event ultimately leads to inhibition of exsA translation. This inhibitory effect is specific to exsA as translation of other genes in the exsCEBA operon is not inhibited by mgtE. Moreover, our data reveal that MgtE acts solely through this pathway to regulate T3SS gene transcription. Our study reveals an important mechanism that may allow P. aeruginosa to fine-tune T3SS activity in response to certain environmental stimuli.
IMPORTANCE The type III secretion system (T3SS) is a critical virulence factor utilized by numerous Gram-negative bacteria, including Pseudomonas aeruginosa, to intoxicate and kill host cells. Elucidating T3SS regulatory mechanisms may uncover targets for novel anti-P. aeruginosa therapeutics and provide deeper understanding of bacterial pathogenesis. We previously found that the magnesium transporter MgtE inhibits T3SS gene transcription in P. aeruginosa. In this study, we describe the mechanism of MgtE-dependent inhibition of the T3SS. Our report also illustrates how MgtE might respond to environmental cues, such as magnesium levels, to fine-tune T3SS gene expression.
KEYWORDS: ExsA, GacAS, MgtE, Pseudomonas aeruginosa, RsmA, gene regulation, magnesium, posttranscriptional, type III secretion
INTRODUCTION
The Gram-negative bacterium Pseudomonas aeruginosa is implicated in a wide range of opportunistic infections in humans (1, 2). A major virulence factor used by P. aeruginosa to initiate acute infections is a type III secretion system (T3SS) (3, 4). This macromolecular apparatus spans the bacterial cell envelope and acts like a syringe, injecting several toxins directly into host cells (5). This leads to actin cytoskeleton rearrangement, host cell rounding, and cell death (5, 6). These actions promote tissue damage and decrease phagocytic clearance (7–9). In addition to acute infections, P. aeruginosa is also able to establish chronic infections through formation of biofilms, most notably in the airways of cystic fibrosis (CF) patients (9, 10). During P. aeruginosa biofilm formation, altered gene regulation typically leads to a reduction in T3SS gene expression (9, 11–14). Additionally, isolates from chronically colonized CF patients usually contain mutations that decrease T3SS production (9, 15). Thus, during both acute and chronic infections, P. aeruginosa appears to tightly regulate T3SS gene expression in response to environmental conditions.
P. aeruginosa T3SS gene expression is controlled by the master transcription factor ExsA, which is responsible for activating transcription of all T3SS genes, including exsA itself (16). Under noninducing conditions, ExsA is bound by the antiactivator protein ExsD and is unable to bind to its target promoters to initiate gene transcription. Two other proteins important for T3SS regulation, ExsC and ExsE, form a separate complex. Under inducing conditions (contact of P. aeruginosa with host cells, the presence of serum, or low-Ca2+ conditions), ExsE is secreted through the T3SS apparatus, thus permitting ExsC to sequester ExsD. ExsA, released from ExsD, subsequently activates the T3SS regulon. This mechanism has been referred to as “intrinsic regulation” (16).
In addition to the ExsDCE network, several other pathways also control exsA expression and/or synthesis (9, 16). These pathways work concurrently but distinctly from “intrinsic regulation” to further control T3SS gene expression and are referred to as “extrinsic regulation.” One example of extrinsic regulation is the RsmA/RsmY/RsmZ signaling cascade. RsmA is an RNA binding protein belonging to the CsrA family (17). CsrA family members regulate gene expression at the posttranscriptional level by binding to target mRNAs at conserved sequence motifs and impacting their stability and/or translation (17). RsmA appears to control T3SS gene expression by increasing exsA translation through an undetermined mechanism (18). This activity depends upon the concentration of free RsmA in the cell and is controlled by two noncoding RNAs, RsmY and RsmZ (18, 19). RsmY and RsmZ function by directly sequestering RsmA from target mRNA (18, 20–22) and are thus negative regulators of ExsA synthesis. Transcription of rsmYZ is directly controlled by the GacAS two-component system (TCS) (17, 18, 23, 24). The environmental signals governing RsmY and RsmZ expression are poorly understood but include two additional sensor kinases, LadS and RetS. Both GacS and LadS are able to phosphorylate the GacA response regulator to enhance rsmY and rsmZ transcription (25, 26). In contrast, RetS inhibits GacA-mediated rsmY and rsmZ transcription by forming a heterodimer with GacS and preventing GacA phosphorylation (27). Though this pathway controls production of ExsA, the availability of ExsA to regulate T3SS gene expression is still dependent on the intrinsic regulation described above.
Previous studies found that the P. aeruginosa inner membrane magnesium transporter MgtE inhibits T3SS gene expression (28). Whereas an mgtE mutant demonstrates enhanced T3SS gene expression, mgtE overexpression inhibits the T3SS (28). The mechanism by which mgtE inhibits the T3SS was not elucidated in these prior studies, although the effect of MgtE on T3SS gene expression is distinct from its role as an Mg2+ transporter in P. aeruginosa (28). Additionally, deletion of both mgtE and exsA results in negligible T3SS activity (28), indicating that MgtE acts through ExsA to regulate T3SS gene expression. In the present study, we show that mgtE expression inhibits ExsA translation by increasing rsmY and rsmZ transcription. We also demonstrate that mgtE acts exclusively through the RsmA/RsmY/RsmZ signaling pathway to inhibit ExsA-mediated T3SS gene transcription. Because mgtE transcription is significantly upregulated by growth under low-Mg2+ conditions and in the presence of some antibiotics (29, 30), this pathway may provide a mechanism for P. aeruginosa to modulate T3SS gene expression in response to signals encountered during infections.
RESULTS
MgtE inhibits T3SS gene expression at the posttranscriptional level.
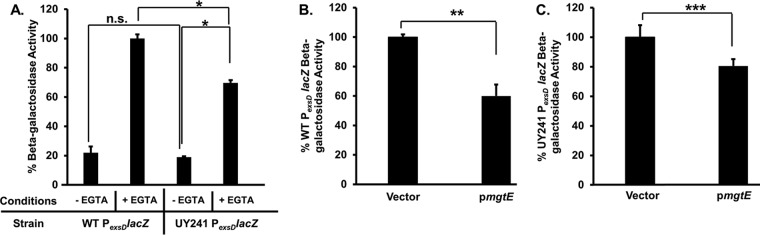
Previous studies found that mgtE expression inhibits T3SS gene transcription by acting through ExsA (28). We considered three possibilities to account for the inhibitory effect of mgtE expression: (i) reduced exsA transcription, (ii) reduced ExsA synthesis, and/or (iii) impaired ExsA function. Because exsA regulates its own transcription (by acting at the PexsC promoter to control transcription of the exsCEBA operon) (31), it was necessary to uncouple exsA transcription from its own control to analyze potential mgtE effects on exsA transcription. To this end, we used the previously described P. aeruginosa UY241 strain (32), in which the ExsA-dependent PexsC promoter has been replaced with a constitutive variant of the PlacUV5 promoter (Pcon). Removal of the native promoter should uncouple exsCEBA transcription from activity of regulatory molecules that naturally bind to the PexsC promoter. Indeed, UY241 has been shown to display constitutive exsA transcription (32). As a control, PexsD-lacZ reporter activity (as a marker for ExsA-dependent transcription) was measured in wild-type (WT) PA103 and the UY241 strain following growth under noninducing (high-calcium [–EGTA]) and inducing (low-calcium [+EGTA]) conditions for T3SS gene expression (33–35). Whereas ExsA is sequestered by ExsD in the WT strain under noninducing conditions and PexsD-lacZ reporter activity is low, EGTA stimulation results in the release of ExsA from ExsD and induction of PexsD-lacZ reporter activity (Fig. 1A) (36). Strain UY241 also demonstrates EGTA-dependent induction of PexsD-lacZ reporter activity, but the overall level of activity is reduced due to the lack of ExsA autoregulation at the PexsC promoter (Fig. 1A). We next expressed mgtE in the WT (Fig. 1B) and UY241 strains (Fig. 1C) and measured PexsD-lacZ reporter activity. In both the WT and UY241 strains, mgtE expression resulted in a significant reduction in PexsD-lacZ reporter activity. Because native transcriptional control has been lost in the UY241 strain, these data suggest that MgtE inhibits T3SS gene expression by acting on ExsA at a posttranscriptional level. mgtE expression appears to lead to less T3SS inhibition in UY241 compared to wild-type PA103 (Fig. 1B and C). We attribute this to ExsA autoregulation in the wild type; uninhibited ExsA levels are higher in the wild type than in UY241 (leading to the higher T3SS gene expression seen in Fig. 1A), and so mgtE-mediated inhibition has an apparent greater effect. Importantly, we found that mgtE expression in PA103 resulted in an approximately 100-fold increase in mgtE transcript abundance compared to the vector control (see Fig. S1A in the supplemental material). This increase is concordant with transcript levels seen in physiologically relevant concentrations of antibiotics and magnesium (29, 30). Similar results were obtained with P. aeruginosa strain PA14 (Fig. S1B).
FIG 1.
mgtE inhibits T3SS gene expression at the posttranscriptional level. (A) PA103 PexsD-lacZ (WT) and UY241 were assayed under either T3SS-noninducing (–EGTA) or -inducing (+EGTA) conditions and assayed for β-galactosidase activity from the PexsD-lacZ reporter construct. The percentage of activity was calculated considering the PexsD-lacZ activity in EGTA-treated WT as 100%. *, P < 0.0005; n.s., not significant. (B) PA103 PexsD-lacZ (WT) with either the vector control or pmgtE was assayed under T3SS-inducing conditions, and β-galactosidase activity from the PexsD-lacZ construct was measured. The percentage of activity was calculated considering the PexsD-lacZ activity in the WT with blank vector as 100%. **, P < 0.05. (C) Strain UY241 with either the vector control or pmgtE was assayed under T3SS-inducing conditions, and β-galactosidase activity from the PexsD-lacZ construct was measured. The percentage of activity was calculated considering the PexsD-lacZ activity in UY241 with blank vector as 100%. ***, P < 0.005.
ExsA translation is repressed by mgtE expression.
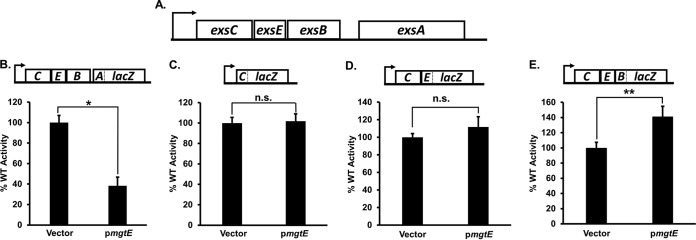
We next tested the hypothesis that mgtE expression inhibits ExsA translation. We introduced the mgtE expression vector (pmgtE) into a panel of WT PA103 strains carrying lacZ translational reporters within the exsCEBA operon, wherein lacZ is fused at exsC codon 15 (exsC′-′lacZ), exsE codon 15 (exsCE′-′lacZ), exsB codon 2 (exsCEB′-′lacZ), or exsA codon 77 (exsCEBA′-′lacZ) (18); for reference, the full exsCEBA operon is diagrammed in Fig. 2A. Each reporter is integrated in single copy on the chromosome at the CTX phage attachment site and transcribed from a constitutive PlacUV5 promoter. Whereas plasmid-expressed mgtE reduced exsCEBA′-′lacZ translational reporter activity by almost 3-fold (Fig. 2B), mgtE had no negative impact on the exsC′-′lacZ (Fig. 2C), exsCE′-′lacZ (Fig. 2D), or exsCEB′-′lacZ (Fig. 2E) reporter activities. We found a similar decrease in exsCEBA′-′lacZ reporter activity in P. aeruginosa strain PA14 (see Fig. S2 in the supplemental material). These data suggest that mgtE expression inhibits ExsA translation and that this activity is specific to exsA in the exsCEBA operon. For reasons that are unclear, the exsCEB′-′lacZ reporter showed a significant increase upon mgtE expression.
FIG 2.
mgtE expression specifically represses exsA translation. (A) Diagram of the exsCEBA operon. (B to E) Translational exsA (exsCEBA′-′lacZ) (B), exsC (exsC′-′lacZ) (C), exsE (exsCE′-′lacZ) (D), and exsB (exsCEB′-′lacZ) (E) reporter strains, with either the vector control or pmgtE, were assayed under T3SS-inducing (+EGTA) conditions for β-galactosidase activity. The percentage of activity was calculated considering the lacZ activity from the respective strains with the blank vector as 100%. The reporter constructs were transcribed from a constitutive PlacUV5 promoter. The particular translational fusion tested in each panel is diagrammed above each graph. *, P < 0.005; **, P < 0.05; n.s., not significant.
The small intergenic region between exsB and exsA contains a minor, Vfr-dependent promoter (PexsA) that controls exsA transcription (37). Because the PexsA promoter is present in the exsCEBA′-′lacZ translational reporter, we considered the possibility that the observed reduction in exsCEBA′-′lacZ activity (Fig. 2A) resulted from MgtE transcriptional inhibition of PexsA promoter activity. To investigate this hypothesis, we monitored the effect of mgtE expression on a cyclic AMP (cAMP)-Vfr signaling (CVS) reporter (38). The CVS reporter consists of lacZ fused to the cAMP- and CRP/Vfr-dependent lacP1 promoter from Escherichia coli. As shown in Fig. S3A in the supplemental material, mgtE expression had no significant effect on CVS reporter activity. This result is further supported by the finding that mgtE expression in a vfr mutant still inhibits PexsD-lacZ activity (Fig. S3B). We infer from these data that mgtE does not alter transcription from the PexsA proximal promoter, which is consistent with the conclusion that mgtE affects ExsA at a posttranscriptional level.
It is also possible that mgtE decreases exsA mRNA stability rather than specifically inhibiting exsA translation. To distinguish between these two possibilities, we isolated mRNA from UY241, carrying either a vector control or pmgtE, and performed quantitative reverse transcription-PCR (qRT-PCR) using primer pairs designed to amplify a central region of exsA (see Materials and Methods). Intriguingly, mgtE expression increased exsA transcript levels (Fig. 3). The same effect was also observed in the strain carrying the exsCEBA′-′lacZ translational reporter used for the experiment in Fig. 2 (see Fig. S4 in the supplemental material). These findings suggest that mgtE might have positive effects on exsA mRNA stability, but more importantly, they strongly suggest that the posttranscriptional inhibition of ExsA synthesis by mgtE is due to repression of ExsA translation as opposed to impaired exsA mRNA stability.
FIG 3.
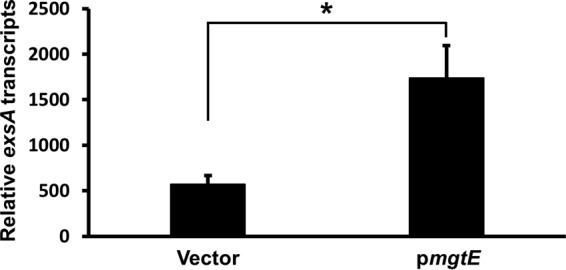
exsA mRNA remains stable upon mgtE expression. mRNA was isolated from P. aeruginosa UY241 containing either the vector control or pmgtE, and exsA transcript stability was analyzed by qRT-PCR. exsA transcript abundance was normalized to that of the control transcript fbp. *, P < 0.05.
mgtE upregulates rsmY and rsmZ transcription in a GacAS dependent manner.
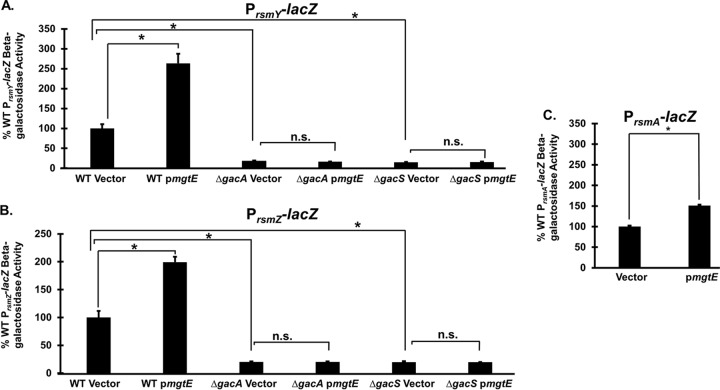
We next examined possible mechanisms for the mgtE-mediated exsA inhibition. We reasoned that since mgtE is an inner membrane protein (28), its translation-repressive effects on exsA were likely indirect—possibly by stimulating one of the established signaling pathways that control ExsA translation. For this reason, we investigated whether mgtE affected RsmA/RsmY/RsmZ signaling (18). To explore this hypothesis, we measured PrsmY-lacZ, PrsmZ-lacZ, and PrsmA-lacZ transcriptional reporter activity (18) and found that mgtE expression significantly upregulates rsmY and rsmZ transcription by approximately 2.5-fold and 2-fold, respectively (Fig. 4A and B). In PA14 strains containing these constructs, mgtE expression also significantly enhanced rsmY and rsmZ transcription (see Fig. S5 in the supplemental material). Interestingly, mgtE expression also stimulated rsmA transcription (Fig. 4C), but to a smaller degree than measured for rsmY and rsmZ. Because the GacAS two-component system is essential for rsmY and rsmZ transcription (17, 18, 23, 24), we hypothesized that the mgtE effect requires GacAS. As evident from Fig. 4A and B, mgtE expression failed to stimulate PrsmY-lacZ and PrsmZ-lacZ reporter activities in the absence of either gacA or gacS, thus supporting a role for GacAS in mgtE-mediated RsmYZ regulation.
FIG 4.
mgtE expression affects Rsm signaling by a GacAS-dependent mechanism. Transcriptional rsmY (PrsmY-lacZ) (A), rsmZ (PrsmZ-lacZ) (B), and rsmA (PrsmA-lacZ) (C) reporter strains in the WT, ΔgacA, and ΔgacS backgrounds with either the vector control or pmgtE were assayed under T3SS-inducing (+EGTA) conditions for β-galactosidase activity. The percentage of activity was calculated considering the lacZ activity from the respective WT reporter strains with the blank vector as 100%. *, P < 0.01; n.s., not significant (ANOVA).
mgtE expression in an rsmY rsmZ mutant fails to inhibit T3SS gene expression.
Since our data suggest that mgtE affects RsmA/RsmY/RsmZ signaling to inhibit exsA translation, we next tested whether the effect of MgtE functions solely through RsmA/RsmY/RsmZ signaling to inhibit ExsA-dependent transcription by expressing mgtE in an rsmY rsmZ double mutant. Consistent with a previous report (16), the rsmY rsmZ mutant demonstrates increased PexsD-lacZ reporter activity compared to the WT (Fig. 5). Whereas mgtE expression significantly inhibited PexsD-lacZ reporter activity in the WT background, reporter activity was unaffected in the rsmY rsmZ mutant (Fig. 5). These data suggest that mgtE works solely through the RsmA/RsmY/RsmZ signaling cascade to inhibit exsA translation.
FIG 5.
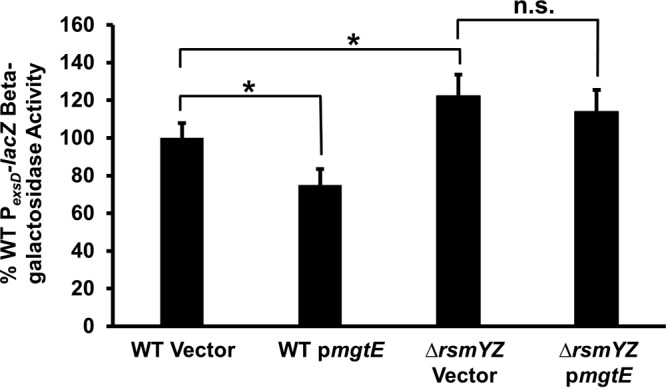
RsmYZ are required for MgtE-mediated inhibition of T3SS gene transcription. PA103 PexsD-lacZ and ΔrsmYZ PexsD-lacZ strains with either the vector control or pmgtE were assayed under T3SS-inducing (+EGTA) conditions for β-galactosidase activity from the PexsD-lacZ reporter construct. The percentage of activity was calculated considering the PexsD-lacZ activity in WT with blank vector as 100%. *, P < 0.01; n.s., not significant (ANOVA).
Previous studies identified an interaction between mgtE and algR during T3SS transcriptional regulation, though the nature of this interaction was unclear (30). Among other activities, the AlgZR two-component system inhibits T3SS gene expression (30, 39); one mechanism by which this occurs is by enhancing rsmY and rsmZ transcription, thus inhibiting ExsA translation (18). Therefore, we considered the possibility that MgtE could additionally stimulate rsmY and rsmZ transcription through AlgZR. However, mgtE expression inhibited PexsD-lacZ reporter activity, even in an algZR deletion mutant (Fig. 6), suggesting that mgtE-mediated T3SS repression does not work directly through AlgZR.
FIG 6.
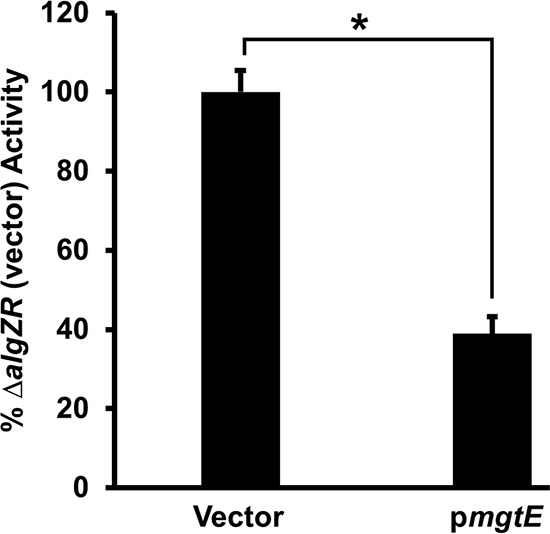
mgtE inhibits T3SS gene transcription in a ΔalgZR background. ΔalgZR strains with either the vector control or pmgtE were assayed under T3SS-inducing (+EGTA) conditions for β-galactosidase activity from the PexsD-lacZ reporter construct. The percentage of activity was calculated considering the PexsD-lacZ activity in the ΔalgZR strain with blank vector as 100%. *, P < 0.0005.
DISCUSSION
The central role of ExsA as the primary regulator of P. aeruginosa T3SS makes it an attractive target for therapeutic development (16). Defining signaling networks that control exsA expression, synthesis, and activity is critical to realizing that goal. Previous work found that the MgtE magnesium transporter inhibits T3SS gene expression (28); mgtE gene expression inhibits, while mgtE deletion enhances, T3SS. In this study, we demonstrate that MgtE accomplishes this activity by inhibiting exsA translation (Fig. 7). Considering the function of MgtE, it is intriguing to speculate that magnesium concentration fluctuations could mediate toxicity changes. However, as magnesium has pleiotropic effects on P. aeruginosa (40), this signaling is likely complex.
FIG 7.
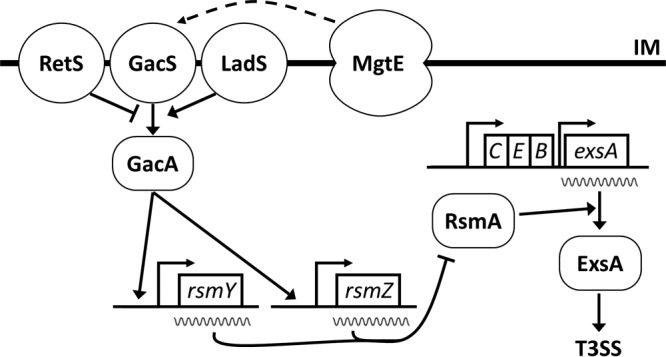
A model for mgtE-mediated control of T3SS. MgtE affects signaling through GacAS, by an unknown mechanism, to stimulate transcription of rsmY and rsmZ. Increased rsmY or rsmZ levels, in turn, sequester RsmA, preventing it from enhancing translation of exsA, thus inhibiting T3SS gene transcription. IM, inner membrane.
MgtE-mediated inhibition of exsA translation appears to occur through the RsmA/RsmY/RsmZ pathway (Fig. 4 and 5). Increased RsmY and RsmZ levels sequester RsmA, resulting in decreased exsA translation. Importantly, we found that mgtE expression does not decrease exsA transcript levels (Fig. 3), further supporting the conclusion of a specific effect on translation. Additionally, lack of involvement of Vfr in MgtE-mediated T3SS gene transcription inhibition strongly indicates that exsA translation inhibition is not the result of decreased transcription activity from the PexsA promoter located on translational fusion constructs (see Fig. S3 in the supplemental material). The fact that MgtE does not inhibit other genes in the exsCEBA operon (Fig. 2) suggests that there may be regulatory sequences specific to exsA translational control. However, it is also formally possible that the lack of additional coding sequences for these other genes masks any potential effects MgtE exerts on downstream motifs. There does seem to be a stronger inhibition by mgtE in the Δvfr strain compared to vfr-competent strains (Fig. 1 versus Fig. S3B). The reasons for this effect are unclear, but it could be the result of competing effects on T3SS expression through the Vfr/CVS and Rsm pathways, an as-yet-unidentified Vfr pathway, or simply stochastic variation.
Overexpression of mgtE also enhances rsmA transcription (Fig. 4C), although to a much lesser degree than rsmY and rsmZ (Fig. 4A and B). As was proposed in another study, it is plausible that the net result from increased rsmA, rsmY, and rsmZ transcription is reduced RsmA availability (18). First, RsmA positively regulates rsmY and rsmZ transcription, which likely plays a role in maintaining homeostasis (18, 41). Second, RsmA binds to its own mRNA to repress translation (42). Therefore, the mgtE-dependent increase in rsmA, rsmY, and rsmZ expression could result in reduced RsmA availability and decreased exsA translation (Fig. 2).
MgtE lacks helix-turn-helix or other DNA binding motifs (43, 44), which would be needed to directly impact transcription. Therefore, it seems that the positive effect of MgtE on rsmY and rsmZ transcription is indirect. This is indeed supported by our data that mgtE expression fails to affect rsmY and rsmZ transcription in both a gacA mutant and a gacS mutant (Fig. 4A and B). These results also establish that MgtE influences rsmY and rsmZ transcription through GacAS. As a membrane protein, MgtE could be involved in direct or indirect binding interactions with GacS, LadS, RetS, or a novel membrane protein that affects signaling through GacS. Because MgtE signals through GacA and GacS, it would be interesting to investigate whether MgtE expression leads to higher phosphorylated states of GacA. It is important to note that RetS was found to regulate biofilm formation in response to magnesium limitation (45), a condition that also enhances mgtE transcription (29). Additionally, a recent report showed that AlgR influences rsmY and rsmZ activity through an unknown mechanism (18). However, we found that mgtE expression still repressed T3SS transcription in a ΔalgZR background (Fig. 6), indicating that MgtE affects RsmY and RsmZ levels solely through GacAS. Future work will investigate the mechanism by which mgtE affects GacAS signaling.
It is noteworthy that mgtE transcription is significantly upregulated under low-Mg2+ and high-antibiotic conditions (29, 30), which are commonly found during host infections like in the CF lung environment (46–52). We expressed mgtE from a plasmid to simulate the effects of high mgtE expression (Fig. S1), such as could occur during host infection. Thus, our study describes a mechanism that might allow P. aeruginosa to respond to the host environment and optimize T3SS gene expression. Additionally, because MgtE signals through GacAS, our results indicate two environmental signals encountered by P. aeruginosa during infection (low Mg2+ concentrations and high antibiotic concentrations during infection) that potentially affect the GacAS signaling pathway. Thus, it is possible that MgtE serves as a sensor, altering T3SS expression in response to changes in the extracellular environment (i.e., magnesium levels and antibiotics). Because the effects on exsA levels and T3SS gene expression are modest (Fig. 1, 2, and 5) (28), we propose that MgtE “fine-tunes” the T3SS response in accordance with the chemical environment, rather than acting as a binary on/off switch.
MgtE is important for the pathogenesis of other microorganisms, such as Aeromonas hydrophila and Campylobacter jejuni (53, 54). A. hydrophila, in particular, has both a T3SS and an RsmA homologue (55, 56). An intriguing avenue of future research will be to investigate whether MgtE homologs in other pathogens inhibit T3SS through a conserved mechanism of action (i.e., modulation of RsmA activity). Similar to MgtE in P. aeruginosa, the housekeeping Mg2+ transporter CorA, found in numerous bacteria, is reported to transport Mg2+ and modulate virulence as two distinct functions (57). Future work will investigate whether CorA signaling is similar to that of P. aeruginosa MgtE.
Taken together, the results of our present study describe the mechanism by which MgtE influences T3SS in P. aeruginosa: by inhibiting exsA translation, which, in turn, leads to downstream effects on ExsA-mediated T3SS gene transcription. This signaling cascade is one mechanism used by P. aeruginosa to respond to Mg2+ scarcity and high-antibiotic conditions. Additional characterization of upstream events of this signaling cascade (i.e., how MgtE affects GacAS signal transduction) would further increase our understanding of the mechanism used by P. aeruginosa to orchestrate signaling pathways in response to the host environment.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table 1. Expression of mgtE was accomplished by transforming the indicated strains with plasmid pmgtE (28), which contains the full-length mgtE gene immediately downstream from the ParaBAD promoter on vector pMQ72 (58). This promoter is leaky in P. aeruginosa, and we have previously found mgtE expression in the absence of arabinose induction (28). Plasmids were maintained in Escherichia coli S17 (28) cultured on LB agar plates or LB containing 10 μg/ml gentamicin; we used Miller's LB (10 g/liter tryptone, 5 g/liter yeast extract, 10 g/liter NaCl). Plasmids were isolated from E. coli using a QIAprep spin miniprep kit (Qiagen) according to the manufacturer's instructions and electroporated into the appropriate P. aeruginosa strains. Transformed P. aeruginosa cells were cultured in Vogel Bonner minimal (VBM) medium (18, 59) agar plates with 60 μg/ml gentamicin, and the presence of the respective plasmids was confirmed by PCR with primers p729 (5′-CAGACCGCTTCTGCGTTCTG-3′) and p730 (5′-GCAACTCTCTACTGTTTCTCC-3′) (30). These primers bind to sequences on vector pMQ72 that flank the mgtE insertion site. For β-galactosidase assays, P. aeruginosa strains were cultured overnight on VBM agar plates with gentamicin. Cells were subcultured the next day to a starting concentration at an optical density at 600 nm (OD600) of 0.1 in Trypticase soy broth (TSB) supplemented with 100 mM monosodium glutamate and 1% glycerol (18). EGTA (2 mM) was added to the medium to activate T3SS gene expression, through induction of the intrinsic regulatory cascade (i.e., secretion of ExsE, leading to desequestration of the T3SS activator ExsA) (33, 60).
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant characteristic(s) | Reference(s) or source |
|---|---|---|
| PA103 PexsD-lacZ | WT strain with ExsA-dependent PexsD-lacZ reporter chromosomally integrated at CTX site | 4, 10, 18, 64 |
| UY241 | Constitutive transcription of exsCEBA in PA103 PexsD-lacZ background | 32 |
| PA14 | WT strain | 65 |
| PA103 PlacUV5-exsCEBA′-′lacZ | PlacUV5-driven exsA translational reporter integrated at CTX site | 18 |
| PA103 PlacUV5-exsCEB′-′lacZ | PlacUV5-driven exsB translational reporter integrated at CTX site | 18 |
| PA103 PlacUV5-exsCE′-′lacZ | PlacUV5-driven exsE translational reporter integrated at CTX site | 18 |
| PA103 PlacUV5-exsC′-′lacZ | PlacUV5-driven exsC translational reporter integrated at CTX site | 18 |
| PA14 PlacUV5-exsCEBA′-′lacZ | PlacUV5-driven exsA translational reporter integrated at CTX site | This study |
| PA103 CVS | PlacP1-lacZ reporter for cAMP-Vfr signaling | 38 |
| PA103 PexsD-lacZ Δvfr | Isogenic deletion of vfr in PA103 PexsD-lacZ background | 18 |
| PA103 PexsD-lacZ ΔalgZR | Isogenic deletions of algZ and algR in PA103 PexsD-lacZ background | 18 |
| PA103 PexsD-lacZ ΔrsmYZ | Isogenic deletions of rsmY and rsmZ in PA103 PexsD-lacZ background | 18 |
| PA103 PrsmY-lacZ | rsmY transcriptional reporter chromosomally integrated at CTX site | 18 |
| PA103 PrsmZ-lacZ | rsmZ transcriptional reporter chromosomally integrated at CTX site | 18 |
| PA103 PrsmA-lacZ | rsmA transcriptional reporter chromosomally integrated at CTX site | 18, 66 |
| PA103 PrsmY-lacZ ΔgacA | Isogenic deletion of gacA in PA103 PrsmY-lacZ background | 18 |
| PA103 PrsmY-lacZ ΔgacS | Isogenic deletion of gacS in PA103 PrsmY-lacZ background | 18 |
| PA103 PrsmZ-lacZ ΔgacA | Isogenic deletion of gacA in PA103 PrsmZ-lacZ background | 18 |
| PA103 PrsmZ-lacZ ΔgacS | Isogenic deletion of gacS in PA103 PrsmZ-lacZ background | 18 |
| PA14 PrsmY-lacZ | rsmY transcriptional reporter chromosomally integrated at CTX site | This study |
| PA14 PrsmZ-lacZ | rsmZ transcriptional reporter chromosomally integrated at CTX site | This study |
Genetic manipulations.
PA14 PlacUV5-exsCEBA′-′lacZ was constructed by conjugation of plasmid p3UY51 (18) from Escherichia coli strain S17 into PA14. PA14 PrsmY-lacZ and PA14 PrsmZ-lacZ were constructed by conjugation of plasmids mini-CTX-PrsmY-lacZ and mini-CTX-PrsmY-lacZ (18), respectively, into PA14. Exconjugants were selected on VBM plates with tetracycline, as described above.
β-Galactosidase assays.
P. aeruginosa was grown to an OD600 of 1.0, and β-galactosidase activity was measured as reported earlier (18). ortho-Nitrophenyl-β-d-galactopyranoside (ONPG) was used as the substrate for β-galactosidase in all the β-galactosidase assays involving transcriptional reporters; chlorophenol red–β-d-galactopyranoside (CPRG) was used as the substrate in assays involving translational reporters (18, 61). Plasmid pmgtE (28) and its empty backbone vector pMQ72 (58) were used to assess the effect of mgtE expression on transcription and translation.
RNA isolation and real-time qRT-PCR.
P. aeruginosa strains were cultured as described above for β-galactosidase assays and harvested at an OD600 of 1.0, whereupon the pellet was washed with phosphate-buffered saline (PBS). This was followed by RNA isolation using the RNeasy Plus kit (Qiagen) according to the manufacturer's instructions. A few modifications were made to the protocol, as described earlier (30). Briefly, the RNA was subjected to on-column DNase digestion prior to elution. Additionally, after elution, a second DNase digestion was performed, followed by the RNA cleanup procedure. These digestions result in negligible DNA contamination of the final isolated RNA sample (30). cDNA was synthesized from the RNA using the Superscript III first-strand synthesis system for RT-PCR (Invitrogen), according to the manufacturer's guidelines (30). DNA contamination of the RNA preparations was tested in control reactions by performing cDNA synthesis in the absence of reverse transcriptase. Real-time quantitative reverse transcription-PCR (qRT-PCR) was performed as previously reported (30) using the following primers: exsARTfor (5′-GCTGATGCTCTTCGCGTTCAGTCC-3′) and exsARTrev (5′-TGGGCATAGAGGATTCTCCGCTCG-3′), which amplify exsA from nucleotides 436 to 676; mgtERTforNewest (5′-AAGCAAGTGCTGGAAGTCATGG-3′) and mgtERTrevNewest (5′-ATGTTGAGGACTTCGCTTTCGC-3′), which amplify mgtE from nucleotides 332 to 587; and lacZRTfor (5′-CAACTGTTTACCTTGTGGAG-3′) and lacZRTrev (5′-TATGAACGGTCTGGTCTTTG-3′), which amplify the lacZ transcript from nucleotides 2271 to 4800. Samples were normalized to the fbp transcript using primers PA5110for (5′-CCTACCTGTTGGTCTTCGACCCG-3′) and PA5110rev (5′-GCTGATGTTGTCGTGGGTGAGG-3′) (28, 30, 62, 63).
Statistical analyses.
At least three independent experiments were performed for each assay. A two-sample Student t test was used to determine statistical significance (P < 0.05). For multiple comparisons, a one-way analysis of variance (ANOVA) with Tukey's honestly significant difference (HSD) post hoc test was used to determine statistical significance (P < 0.05).
Supplementary Material
ACKNOWLEDGMENTS
We thank A. J. Shih and members of the Yahr laboratory for helpful insights.
This work was supported by 16GRNT30230012 from the American Heart Association to G.G.A. and AI055042 and AI097264 from the National Institutes of Health to T.L.Y.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00268-17.
REFERENCES
- 1.Turner KH, Everett J, Trivedi U, Rumbaugh KP, Whiteley M. 2014. Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS Genet 10:e1004518. doi: 10.1371/journal.pgen.1004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yahr TL, Greenberg EP. 2004. The genetic basis for the commitment to chronic versus acute infection in Pseudomonas aeruginosa. Mol Cell 16:497–498. doi: 10.1016/j.molcel.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto K. 2004. Role of bacterial proteases in pseudomonal and serratial keratitis. Biol Chem 385:1007–1016. doi: 10.1515/BC.2004.131. [DOI] [PubMed] [Google Scholar]
- 4.Barbieri JT, Sun J. 2004. Pseudomonas aeruginosa ExoS and ExoT. Rev Physiol Biochem Pharmacol 152:79–92. doi: 10.1007/s10254-004-0031-7. [DOI] [PubMed] [Google Scholar]
- 5.Galle M, Carpentier I, Beyaert R. 2012. Structure and function of the type III secretion system of Pseudomonas aeruginosa. Curr Protein Pept Sci 13:831–842. doi: 10.2174/138920312804871210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coburn B, Sekirov I, Finlay BB. 2007. Type III secretion systems and disease. Clin Microbiol Rev 20:535–549. doi: 10.1128/CMR.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee VT, Smith RS, Tummler B, Lory S. 2005. Activities of Pseudomonas aeruginosa effectors secreted by the type III secretion system in vitro and during infection. Infect Immun 73:1695–1705. doi: 10.1128/IAI.73.3.1695-1705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vance RE, Rietsch A, Mekalanos JJ. 2005. Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect Immun 73:1706–1713. doi: 10.1128/IAI.73.3.1706-1713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauser AR. 2009. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol 7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain M, Ramirez D, Seshadri R, Cullina JF, Powers CA, Schulert GS, Bar-Meir M, Sullivan CL, McColley SA, Hauser AR. 2004. Type III secretion phenotypes of Pseudomonas aeruginosa strains change during infection of individuals with cystic fibrosis. J Clin Microbiol 42:5229–5237. doi: 10.1128/JCM.42.11.5229-5237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zolfaghar I, Angus AA, Kang PJ, To A, Evans DJ, Fleiszig SM. 2005. Mutation of retS, encoding a putative hybrid two-component regulatory protein in Pseudomonas aeruginosa, attenuates multiple virulence mechanisms. Microbes Infect 7:1305–1316. doi: 10.1016/j.micinf.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Laskowski MA, Osborn E, Kazmierczak BI. 2004. A novel sensor kinase-response regulator hybrid regulates type III secretion and is required for virulence in Pseudomonas aeruginosa. Mol Microbiol 54:1090–1103. doi: 10.1111/j.1365-2958.2004.04331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furukawa S, Kuchma SL, O'Toole GA. 2006. Keeping their options open: acute versus persistent infections. J Bacteriol 188:1211–1217. doi: 10.1128/JB.188.4.1211-1217.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasamiravaka T, Labtani Q, Duez P, El Jaziri M. 2015. The formation of biofilms by Pseudomonas aeruginosa: a review of the natural and synthetic compounds interfering with control mechanisms. Biomed Res Int 2015:759348. doi: 10.1155/2015/759348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D'Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A 103:8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz MR, King JM, Yahr TL. 2011. Intrinsic and extrinsic regulation of type III secretion gene expression in Pseudomonas aeruginosa. Front Microbiol 2:89. doi: 10.3389/fmicb.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brencic A, Lory S. 2009. Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA. Mol Microbiol 72:612–632. doi: 10.1111/j.1365-2958.2009.06670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Intile PJ, Diaz MR, Urbanowski ML, Wolfgang MC, Yahr TL. 2014. The AlgZR two-component system recalibrates the RsmAYZ posttranscriptional regulatory system to inhibit expression of the Pseudomonas aeruginosa type III secretion system. J Bacteriol 196:357–366. doi: 10.1128/JB.01199-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burrowes E, Baysse C, Adams C, O'Gara F. 2006. Influence of the regulatory protein RsmA on cellular functions in Pseudomonas aeruginosa PAO1, as revealed by transcriptome analysis. Microbiology 152:405–418. doi: 10.1099/mic.0.28324-0. [DOI] [PubMed] [Google Scholar]
- 20.Pessi G, Williams F, Hindle Z, Heurlier K, Holden MT, Camara M, Haas D, Williams P. 2001. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J Bacteriol 183:6676–6683. doi: 10.1128/JB.183.22.6676-6683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorger-Domenigg T, Sonnleitner E, Kaberdin VR, Blasi U. 2007. Distinct and overlapping binding sites of Pseudomonas aeruginosa Hfq and RsmA proteins on the non-coding RNA RsmY. Biochem Biophys Res Commun 352:769–773. doi: 10.1016/j.bbrc.2006.11.084. [DOI] [PubMed] [Google Scholar]
- 22.Heurlier K, Williams F, Heeb S, Dormond C, Pessi G, Singer D, Camara M, Williams P, Haas D. 2004. Positive control of swarming, rhamnolipid synthesis, and lipase production by the posttranscriptional RsmA/RsmZ system in Pseudomonas aeruginosa PAO1. J Bacteriol 186:2936–2945. doi: 10.1128/JB.186.10.2936-2945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burrowes E, Abbas A, O'Neill A, Adams C, O'Gara F. 2005. Characterisation of the regulatory RNA RsmB from Pseudomonas aeruginosa PAO1. Res Microbiol 156:7–16. doi: 10.1016/j.resmic.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Davies JA, Harrison JJ, Marques LL, Foglia GR, Stremick CA, Storey DG, Turner RJ, Olson ME, Ceri H. 2007. The GacS sensor kinase controls phenotypic reversion of small colony variants isolated from biofilms of Pseudomonas aeruginosa PA14. FEMS Microbiol Ecol 59:32–46. doi: 10.1111/j.1574-6941.2006.00196.x. [DOI] [PubMed] [Google Scholar]
- 25.Ventre I, Goodman AL, Vallet-Gely I, Vasseur P, Soscia C, Molin S, Bleves S, Lazdunski A, Lory S, Filloux A. 2006. Multiple sensors control reciprocal expression of Pseudomonas aeruginosa regulatory RNA and virulence genes. Proc Natl Acad Sci U S A 103:171–176. doi: 10.1073/pnas.0507407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chambonnier G, Roux L, Redelberger D, Fadel F, Filloux A, Sivaneson M, de Bentzmann S, Bordi C. 2016. The hybrid histidine kinase LadS forms a multicomponent signal transduction system with the GacS/GacA two-component system in Pseudomonas aeruginosa. PLoS Genet 12:e1006032. doi: 10.1371/journal.pgen.1006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman AL, Merighi M, Hyodo M, Ventre I, Filloux A, Lory S. 2009. Direct interaction between sensor kinase proteins mediates acute and chronic disease phenotypes in a bacterial pathogen. Genes Dev 23:249–259. doi: 10.1101/gad.1739009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson GG, Yahr TL, Lovewell RR, O'Toole GA. 2010. The Pseudomonas aeruginosa magnesium transporter MgtE inhibits transcription of the type III secretion system. Infect Immun 78:1239–1249. doi: 10.1128/IAI.00865-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffey BM, Akhand SS, Anderson GG. 2014. MgtE is a dual-function protein in Pseudomonas aeruginosa. Microbiology 160:1200–1213. doi: 10.1099/mic.0.075275-0. [DOI] [PubMed] [Google Scholar]
- 30.Redelman CV, Chakravarty S, Anderson GG. 2014. Antibiotic treatment of Pseudomonas aeruginosa biofilms stimulates expression of the magnesium transporter gene mgtE. Microbiology 160:165–178. doi: 10.1099/mic.0.070144-0. [DOI] [PubMed] [Google Scholar]
- 31.Brutinel ED, Vakulskas CA, Brady KM, Yahr TL. 2008. Characterization of ExsA and of ExsA-dependent promoters required for expression of the Pseudomonas aeruginosa type III secretion system. Mol Microbiol 68:657–671. doi: 10.1111/j.1365-2958.2008.06179.x. [DOI] [PubMed] [Google Scholar]
- 32.Brutinel ED, Vakulskas CA, Yahr TL. 2009. Functional domains of ExsA, the transcriptional activator of the Pseudomonas aeruginosa type III secretion system. J Bacteriol 191:3811–3821. doi: 10.1128/JB.00002-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCaw ML, Lykken GL, Singh PK, Yahr TL. 2002. ExsD is a negative regulator of the Pseudomonas aeruginosa type III secretion regulon. Mol Microbiol 46:1123–1133. doi: 10.1046/j.1365-2958.2002.03228.x. [DOI] [PubMed] [Google Scholar]
- 34.Dasgupta N, Ashare A, Hunninghake GW, Yahr TL. 2006. Transcriptional induction of the Pseudomonas aeruginosa type III secretion system by low Ca2+ and host cell contact proceeds through two distinct signaling pathways. Infect Immun 74:3334–3341. doi: 10.1128/IAI.00090-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vakulskas CA, Brutinel ED, Yahr TL. 2010. ExsA recruits RNA polymerase to an extended −10 promoter by contacting region 4.2 of sigma-70. J Bacteriol 192:3597–3607. doi: 10.1128/JB.00129-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urbanowski ML, Lykken GL, Yahr TL. 2005. A secreted regulatory protein couples transcription to the secretory activity of the Pseudomonas aeruginosa type III secretion system. Proc Natl Acad Sci U S A 102:9930–9935. doi: 10.1073/pnas.0504405102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marsden AE, Intile PJ, Schulmeyer KH, Simmons-Patterson ER, Urbanowski ML, Wolfgang MC, Yahr TL. 2016. Vfr directly activates exsA transcription to regulate expression of the Pseudomonas aeruginosa type III secretion system. J Bacteriol 198:1442–1450. doi: 10.1128/JB.00049-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fulcher NB, Holliday PM, Klem E, Cann MJ, Wolfgang MC. 2010. The Pseudomonas aeruginosa Chp chemosensory system regulates intracellular cAMP levels by modulating adenylate cyclase activity. Mol Microbiol 76:889–904. doi: 10.1111/j.1365-2958.2010.07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okkotsu Y, Little AS, Schurr MJ. 2014. The Pseudomonas aeruginosa AlgZR two-component system coordinates multiple phenotypes. Front Cell Infect Microbiol 4:82. doi: 10.3389/fcimb.2014.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guina T, Wu M, Miller SI, Purvine SO, Yi EC, Eng J, Goodlett DR, Aebersold R, Ernst RK, Lee KA. 2003. Proteomic analysis of Pseudomonas aeruginosa grown under magnesium limitation. J Am Soc Mass Spectrom 14:742–751. doi: 10.1016/S1044-0305(03)00133-8. [DOI] [PubMed] [Google Scholar]
- 41.Perez-Martinez I, Haas D. 2011. Azithromycin inhibits expression of the GacA-dependent small RNAs RsmY and RsmZ in Pseudomonas aeruginosa. Antimicrob Agents Chemother 55:3399–3405. doi: 10.1128/AAC.01801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marden JN, Diaz MR, Walton WG, Gode CJ, Betts L, Urbanowski ML, Redinbo MR, Yahr TL, Wolfgang MC. 2013. An unusual CsrA family member operates in series with RsmA to amplify posttranscriptional responses in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 110:15055–15060. doi: 10.1073/pnas.1307217110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeda H, Hattori M, Nishizawa T, Yamashita K, Shah ST, Caffrey M, Maturana AD, Ishitani R, Nureki O. 2014. Structural basis for ion selectivity revealed by high-resolution crystal structure of Mg2+ channel MgtE. Nat Commun 5:5374. doi: 10.1038/ncomms6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hattori M, Tanaka Y, Fukai S, Ishitani R, Nureki O. 2007. Crystal structure of the MgtE Mg2+ transporter. Nature 448:1072–1075. doi: 10.1038/nature06093. [DOI] [PubMed] [Google Scholar]
- 45.Mulcahy H, Lewenza S. 2011. Magnesium limitation is an environmental trigger of the Pseudomonas aeruginosa biofilm lifestyle. PLoS One 6:e23307. doi: 10.1371/journal.pone.0023307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanders NN, Franckx H, De Boeck K, Haustraete J, De Smedt SC, Demeester J. 2006. Role of magnesium in the failure of rhDNase therapy in patients with cystic fibrosis. Thorax 61:962–968. doi: 10.1136/thx.2006.060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta A, Eastham KM, Wrightson N, Spencer DA. 2007. Hypomagnesaemia in cystic fibrosis patients referred for lung transplant assessment. J Cyst Fibros 6:360–362. doi: 10.1016/j.jcf.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Palmer KL, Aye LM, Whiteley M. 2007. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol 189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stutman HR, Shalit I, Marks MI, Greenwood R, Chartrand SA, Hilman BC. 1987. Pharmacokinetics of two dosage regimens of ciprofloxacin during a two-week therapeutic trial in patients with cystic fibrosis. Am J Med 82:142–145. [PubMed] [Google Scholar]
- 50.Ilowite JS, Gorvoy JD, Smaldone GC. 1987. Quantitative deposition of aerosolized gentamicin in cystic fibrosis. Am Rev Respir Dis 136:1445–1449. doi: 10.1164/ajrccm/136.6.1445. [DOI] [PubMed] [Google Scholar]
- 51.Geller DE, Pitlick WH, Nardella PA, Tracewell WG, Ramsey BW. 2002. Pharmacokinetics and bioavailability of aerosolized tobramycin in cystic fibrosis. Chest 122:219–226. doi: 10.1378/chest.122.1.219. [DOI] [PubMed] [Google Scholar]
- 52.Wilms EB, Touw DJ, Heijerman HG. 2008. Pharmacokinetics and sputum penetration of azithromycin during once weekly dosing in cystic fibrosis patients. J Cyst Fibros 7:79–84. doi: 10.1016/j.jcf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Merino S, Gavin R, Altarriba M, Izquierdo L, Maguire ME, Tomas JM. 2001. The MgtE Mg2+ transport protein is involved in Aeromonas hydrophila adherence. FEMS Microbiol Lett 198:189–195. doi: 10.1111/j.1574-6968.2001.tb10641.x. [DOI] [PubMed] [Google Scholar]
- 54.Kakuda T, DiRita VJ. 2006. Cj1496c encodes a Campylobacter jejuni glycoprotein that influences invasion of human epithelial cells and colonization of the chick gastrointestinal tract. Infect Immun 74:4715–4723. doi: 10.1128/IAI.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swift S, Karlyshev AV, Fish L, Durant EL, Winson MK, Chhabra SR, Williams P, Macintyre S, Stewart GS. 1997. Quorum sensing in Aeromonas hydrophila and Aeromonas salmonicida: identification of the LuxRI homologs AhyRI and AsaRI and their cognate N-acylhomoserine lactone signal molecules. J Bacteriol 179:5271–5281. doi: 10.1128/jb.179.17.5271-5281.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu HB, Rao PS, Lee HC, Vilches S, Merino S, Tomas JM, Leung KY. 2004. A type III secretion system is required for Aeromonas hydrophila AH-1 pathogenesis. Infect Immun 72:1248–1256. doi: 10.1128/IAI.72.3.1248-1256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papp-Wallace KM, Maguire ME. 2008. Regulation of CorA Mg2+ channel function affects the virulence of Salmonella enterica serovar Typhimurium. J Bacteriol 190:6509–6516. doi: 10.1128/JB.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shanks RM, Caiazza NC, Hinsa SM, Toutain CM, O’Toole GA. 2006. Saccharomyces cerevisiae-based molecular tool kit for manipulation of genes from Gram-negative bacteria. Appl Environ Microbiol 72:5027–5036. doi: 10.1128/AEM.00682-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vogel HJ, Bonner DM. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem 218:97–106. [PubMed] [Google Scholar]
- 60.Kim J, Ahn K, Min S, Jia J, Ha U, Wu D, Jin S. 2005. Factors triggering type III secretion in Pseudomonas aeruginosa. Microbiology 151:3575–3587. doi: 10.1099/mic.0.28277-0. [DOI] [PubMed] [Google Scholar]
- 61.Dasgupta N, Lykken GL, Wolfgang MC, Yahr TL. 2004. A novel anti-anti-activator mechanism regulates expression of the Pseudomonas aeruginosa type III secretion system. Mol Microbiol 53:297–308. doi: 10.1111/j.1365-2958.2004.04128.x. [DOI] [PubMed] [Google Scholar]
- 62.Anderson GG, Moreau-Marquis S, Stanton BA, O'Toole GA. 2008. In vitro analysis of tobramycin-treated Pseudomonas aeruginosa biofilms on cystic fibrosis-derived airway epithelial cells. Infect Immun 76:1423–1433. doi: 10.1128/IAI.01373-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuchma SL, Connolly JP, O'Toole GA. 2005. A three-component regulatory system regulates biofilm maturation and type III secretion in Pseudomonas aeruginosa. J Bacteriol 187:1441–1454. doi: 10.1128/JB.187.4.1441-1454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hauser AR, Cobb E, Bodi M, Mariscal D, Valles J, Engel JN, Rello J. 2002. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med 30:521–528. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G, Villanueva J, Wei T, Ausubel FM. 2006. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci U S A 103:2833–2838. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roy-Burman A, Savel RH, Racine S, Swanson BL, Revadigar NS, Fujimoto J, Sawa T, Frank DW, Wiener-Kronish JP. 2001. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J Infect Dis 183:1767–1774. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.