Abstract
Context:
Autoimmune thyroid disorders have been linked to vitamin D deficiency, but an effect of vitamin D supplementation is not established.
Objective:
Our objective was to test whether vitamin D compared with placebo could reduce thyroid autoantibodies.
Design:
Predefined additional analyses from a randomized, double-blind, placebo-controlled trial.
Setting:
The study was conducted in different community centers in Oslo, Norway.
Participants:
A total of 251 presumed healthy men and women, aged 18 to 50 years, with backgrounds from South Asia, the Middle East, and Africa were included.
Intervention:
Daily supplementation with 25 µg (1000 IU) vitamin D3, 10 µg (400 IU) vitamin D3, or placebo for 16 weeks.
Outcome Measure:
Difference in preintervention and postintervention antithyroid peroxidase antibody (TPOAb) levels. Additional outcomes were differences in thyroid-stimulating hormone (TSH) and free fraction of thyroxine (fT4).
Results:
There were no differences in change after 16 weeks on TPOAb (27 kU/L; 95% CI, −17 to 72; P = 0.23), TSH (−0.10 mU/L; 95% CI, −0.54 to 0.34; P = 0.65), or fT4 (0.09 pmol/L; 95% CI, −0.37 to 0.55; P = 0.70) between those receiving vitamin D supplementation or placebo. Mean serum 25(OH)D3 increased from 26 to 49 nmol/L in the combined supplementation group, but there was no change in the placebo group.
Conclusion:
Vitamin D3 supplementation, 25 µg or 10 µg, for 16 weeks compared with placebo did not affect TPOAb level in this randomized, double-blind study among participants with backgrounds from South Asia, the Middle East, and Africa who had low vitamin D levels at baseline.
Keywords: vitamin D, autoimmune thyroid disease, thyroid hormone, randomized controlled trial, ethnicity
Vitamin D3 supplementation for 16 weeks compared with placebo did not change the thyroid autoimmunity or thyroid status in this randomized, double-blind, placebo-controlled study among ethnic minorities.
Vitamin D deficiency is widespread, with a high prevalence among ethnic minority populations living in Western countries [1–3]. In Norway vitamin D deficiency is frequently found among ethnic minorities with backgrounds from the Middle East, Asia, and Africa [4, 5]. The main effect of vitamin D is to regulate calcium–phosphate metabolism. However, in recent years numerous studies have indicated that vitamin D may have several other functions, including a possible role in the immune system [6]. In vivo data have suggested beneficial effects of vitamin D on immune function, particularly in the context of autoimmunity. Exposure to vitamin D may lead to a shift from a proinflammatory to a more tolerogenic immune status in animals and humans [7]. Low levels of vitamin D may contribute to the development of autoimmune diseases, including predisposition to autoimmune disorders [8–10].
The thyroid gland is important in regulating the body’s metabolism. The autoimmune thyroid diseases (AITDs) are common worldwide, and ≤10% of the adult population is affected. The prevalence increases with age, and more women than men are affected [11]. Multiple environmental factors and genes are involved in the development of AITDs [12–14]. The AITD may have a gradual, slow or fast onset and may debut after pregnancy, presenting with hypothyroid or hyperthyroid symptoms. Despite elevated levels of antithyroid peroxidase antibody (TPOAb), a marker of autoimmune-mediated destruction of the thyroid tissue, the free fraction of thyroxine (fT4) may be within normal range because of an increased level of thyroid-stimulating hormone (TSH), and the patient can remain without symptoms for a period, being subclinically hypothyroid [15].
Some studies have reported an association between low vitamin D status and AITD, and Kivity et al. reported an association between vitamin D deficiency and thyroid antibodies in 2011 [6, 16]. A meta-analysis of 20 case–control studies found that patients with AITD were more likely to be vitamin D deficient than controls [6]. There are some indications that vitamin D supplementation suppresses the autoimmune reaction and thereby reduces the levels of thyroid autoantibodies [17, 18]. However, this effect is still controversial, and other authors have not found vitamin D deficiency to increase the risk of AITD or to be associated with early stages of AITD [19, 20].
We present here results for the predefined secondary outcomes of our previously reported randomized, double-blind, placebo-controlled trial on the effect of vitamin D supplementation on muscle strength and power in a young adult ethnic minority population living in Norway [21]. Our hypothesis was that 16 weeks of daily vitamin D3 (25 μg/d or 10 μg/d) would reduce the thyroid autoantibodies compared with placebo in this population with unknown thyroid autoantibody status and with presumed high prevalence of low vitamin D levels.
1. Materials and Methods
A. Study Design, Setting, and Participants
This double-blinded, block randomized, placebo-controlled, parallel-group trial was conducted in Oslo, Norway (at latitude 60°N) between January and June 2011. The participants came from an assumed healthy population, men and women, aged 18 to 50 years who were born in or had parents born in the Middle East, Africa, or South Asia. They were recruited through local immigrant organizations, and the study was conducted in these activity centers. Details of the study methods have been described elsewhere (ClinicalTrials.gov identifier NCT01263288) [21]. The exclusion criteria were regular use of vitamin D–containing supplements, ongoing treatment of vitamin D deficiency, use of strong pain killers or medication interfering with vitamin D metabolism (e.g., thiazides, antiepileptic drugs, prednisolone, or hormone replacement therapy), pregnancy, breastfeeding, kidney disease, cancer, tuberculosis, sarcoidosis, osteoporosis, or a recent fracture. None of the participants reported use of thyroid hormone supplements.
B. Intervention, Randomization, and Blinding
Participants who provided consent to participate and fulfilled the eligibility criteria were randomly assigned to one of three equally sized intervention groups given 25 µg of vitamin D3, 10 µg of vitamin D3, or placebo. We included one intervention group with 10 µg of vitamin D3/day because this was the recommended daily intake according to official Norwegian guidelines when the study was performed. The other intervention arm had 25 µg of vitamin D3/day, a dietary supplement dosage approved by authorities in Europe (Denmark and the United Kingdom). The tablets were identical in color, size, taste, and packaging. Group allocations were unknown to participants, research staff, investigators, and data collectors. Randomly varying the block size between three and six (computer-generated) ensured a good balance of gender and different ethnicity in each group. Each participant received a box with 120 tablets (112 tablets correspond to 16 weeks of use). Participants were advised to take two tablets the next day if they had forgotten to take a tablet the previous day and to maintain their usual dietary pattern. To maximize adherence, the participants received brief reminders via mobile phone text messages twice a week during the 16-week trial. Compliance with the supplementation was confirmed by counting the number of tablets in the returned boxes at follow-up.
The number of participants in this study was based on power calculations to investigate the effect of vitamin D supplementation on muscle strength and power (Clinical Trials.gov identifier NCT01263288), and these primary end points have been previously reported [21].
C. Main outcome Variables
The study outcomes were the predefined difference in change during the intervention (T2 − T1) between the combined intervention groups (25 µg and 10 µg) of vitamin D3/day and placebo, in the following thyroid parameters: TPOAb, TSH, and fT4.
D. Blood Sampling and Laboratory Assays
At baseline and at follow-up examination after 16 weeks, blood samples (nonfasting) were collected from the participants. Blood for analyses of serum and plasma was collected and handled in separate tubes and stored at −20°C the same day and within 1 to 2 weeks frozen at −80°C storage. The samples, from baseline and follow-up, were analyzed in one batch at Fürst Medical Laboratory, Oslo, Norway (http://www.furst.no/), accredited by the International Organization for Standardization and part of the Vitamin D Quality Assessment Scheme. Serum 25-hydroxyvitamin D [25(OH)D] was measured with high-pressure liquid chromatography tandem mass spectrometry, able to measure both 25(OH)D3 and 25(OH)D2. Serum 25(OH)D2 was negligible in this population, and 25(OH)D3 was used in the statistical analysis. Serum TPOAb, serum TSH, serum fT4, and plasma parathyroid hormone (PTH) were determined by chemiluminescence method (ADVIA Centaur XP, Siemens) with an interassay coefficient of variation (CV), respectively 6.1%, 4.6%, 6.6%, and 8.2%. The C-reactive protein (CRP) (CV 2.2%), including high-sensitivity CRP for CRP values <11 mg/L, was measured with a latex-reinforced immunoturbidimetric method (ADVIA 2400, Siemens) [22]. The liver enzymes serum aspartate aminotransferase and serum alanine aminotransferase were determined by NADH with pyridoxal phosphate (ADVIA 2400, Siemens) and interassay CV to be 3.3% and 3.7%, respectively. Serum albumin (CV 2.1%) was measured with bromocresol green (ADVIA 2400, Siemens).
E. Statistical Analyses
The generalized estimating equation (GEE) regression method was applied in the analyses of both continuous and binary responses of observations in participants and can compare all participants at inclusion with all participants at follow-up. The outcome variables at the end of the trial were unadjusted and adjusted for gender, age, and baseline values of vitamin D3. Because thyroid function was not the primary endpoint of the trial, the Benjamini–Hochberg procedure was used to control for multiple testing. Correlations were measured with parametric (Pearson) or nonparametric (Spearman) methods. Measured levels of TPOAb were replaced with 24 kU/L if measured as <25 kU/L and with 1301 kU/L if measured as >1300 kU/L. Statistical analyses of the data were performed with SPSS (IBM SPSS Statistic, version 21).
F. Registration and Ethics
The Norwegian Medicine Agency authorized this study as a clinical trial. The study is registered at EudraCT (2010-021114-36). Tablets were manufactured by BioPlus Life Sciences Pvt Ltd, certificated for good manufacturing practice by The Danish Medicines Agency, and the ingredients met the requirements of British Pharmacopé. The study was approved by the Regional Committee for Medical and Health Research Ethics (study code 2010/1982), and all participants gave written informed consent. The participants were advised to contact the study staff by telephone if they had any questions related to the study. The trial was conducted in accordance with national laws and according to the principles of the Declaration of Helsinki (ClinicalTrials.gov identifier NCT01263288).
2. Results
A. Study Sample
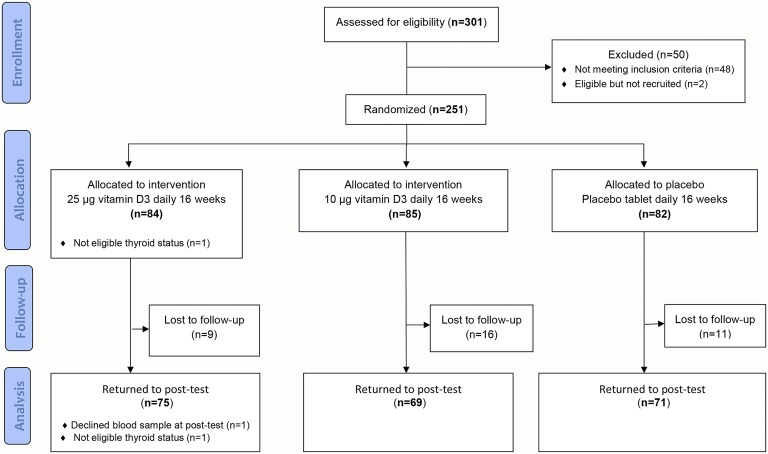
Of 301 people who volunteered for the study, 251 met the inclusion criteria and were randomly assigned to vitamin D3 25 µg/d, vitamin D3 10 µg/d, or placebo. More women (73%) than men were included. After 16 weeks, 215 (86%) attended the follow-up visit (Fig. 1), and nonfasting blood was collected from 214. At the end of the study 1 participant reported use of thyrostatic medication at inclusion and during the study, and this participant was excluded, resulting in a total of 250 participants at baseline and 213 at follow-up in this analysis. Eighty percent of the participants had consumed >80% of the study tablets, and 69% consumed >90% of the study tablets.
Figure 1.
Flow diagram. Effect of vitamin D3 supplementation on thyroid parameters among ethnic minorities in Norway. A randomized, double-blinded, placebo-controlled study.
B. Baseline Characteristics
Median concentration for TPOAb was 49 [interquartile range (IQR) 42 to 59] kU/L (range <25 to >1300 kU/L); 10% (25/250) had levels ≥100 kU/L, 49% (123/250) had levels ≥50 kU/L, and 86% (216/250) had levels ≥35 kU/L. Mean concentration of fT4 was 16.2 (SD 1.7) pmol/L, all within the reference range (9–22 pmol/L) (Table 1). Mean concentration of TSH was 1.9 (SD 1.3) mU/L; 63% (158/250) had levels <2.0 mU/L, and 33% (83/250) had levels between 2.0 and 4.0 mU/L. Seven out of 250 participants (2.8%) had TSH >4.0 mU/L. Five of these participants had subclinical hypothyroidism with slightly elevated TSH (4.3 to 9.1 mU/L) and TPOAb between 52 and >1300 kU/L. The remaining two participants had higher TSH levels (10.0 and 11.0 mU/L) and TPOAb (50 and 746 kU/L). Mean concentration of 25(OH)D3 was 26 (SD 15) nmol/L (range 5 to 87 nmol/L), and only 8% had serum concentration ≥50 nmol/L.
Table 1.
Baseline Characteristics of the Three Allocation Groups Among 250 Participants in the Study of the Effect of Vitamin D3 on Thyroid Parameters. Number (%), Mean (SD), or Median With IQR
| Vitamin D3 25 µg | Vitamin D3 10 µg | Placebo | Alla | |
|---|---|---|---|---|
| Characteristic | n = 83 | n = 85 | n = 82 | n = 250 |
| Women | 57 (69%) | 61 (72%) | 63 (77%) | 181 (72%) |
| Age, y | ||||
| Women | 35 (7.5) | 36 (7.8) | 38 (7.6) | 37 (7.7) |
| Men | 40 (9.1) | 40 (6.6) | 39 (7.8) | 39 (7.8) |
| Ethnic originb | ||||
| South Asia | 31 (37%) | 31 (36%) | 33 (40%) | 95 (38%) |
| Middle East and North Africa | 15 (18%) | 9 (11%) | 12 (15%) | 36 (14%) |
| Sub-Saharan Africa | 37 (45%) | 45 (53%) | 37 (45%) | 119 (48%) |
| TPOAb,c kU/L | 50 (43–60) | 48 (39–58) | 50 (43–59) | 117 (264) |
| Women | 49 (41–60) | 48 (38–56) | 50 (39–59) | 103 (231) |
| Men | 54 (47–60) | 46 (40–61) | 49 (45–60) | 152 (334) |
| TSH, mU/L | 1.7 (1.1–2.5) | 1.6 (1.1–2.1) | 1.7 (1.0–2.6) | 1.9 (1.3) |
| Women | 1.7 (1.1–2.5) | 1.5 (1.2–2.1) | 1.7 (1.1–2.8) | 1.9 (1.2) |
| Men | 1.7 (1.2–2.2) | 1.7 (1.1–2.3) | 1.4 (0.9–2.4) | 1.9 (1.5) |
| fT4, pmol/L | 16.4 (1.9) | 16.2 (1.8) | 15.9 (1.5) | 16.2 (1.7) |
| Women | 16.1 (1.7) | 16.1 (1.8) | 15.8 (1.5) | 16.0 (1.7) |
| Men | 17.3 (1.8) | 16.5 (1.7) | 16.3 (1.5) | 16.8 (1.7) |
| 25(OH)D3, nmol/L | 27 (16) | 26 (15) | 27 (15) | 26 (15) |
| Women | 27 (17) | 25 (15) | 26 (15) | 26 (15) |
| Men | 28 (15) | 27 (15) | 29 (16) | 28 (15) |
| P-PTH,d pmol/L | 7.4 (5.2–9.1) | 6.4 (5.2–8.6) | 7.4 (5.0–9.8) | 7.6 (3.7) |
| Calcium, mmol/L | 2.36 (0.09) | 2.36 (0.08) | 2.36 (0.10) | 2.36 (0.09) |
| CRP, mg/L | 1.7 (0.8–6.0) | 1.8 (0.9–4.0) | 2.0 (0.8–4.0) | 4.4 (6.6) |
| Serum aspartate aminotransferase, U/L | 20 (7) | 21 (7) | 20 (7) | 20 (7) |
| Serum alanine aminotransferase, U/L | 23 (16) | 23 (12) | 23 (13) | 23 (14) |
| Albumin, g/L | 44 (2) | 44 (2) | 44 (2) | 44 (2) |
aAll, characteristics with only number (%) or mean (SD), not with median (IQR).
bNinety-five participants came from South Asia (primarily Sri Lanka, Pakistan, and Afghanistan), 36 participants from the Middle East and North Africa (primarily Morocco, Iraq, and Syria), and 119 participants from sub-Saharan Africa (primarily Somalia and Ethiopia). In this study all the participants had parents from the same geographic area.
cTPOAb <25 kU/L given value 24 (n = 2) and >1300 kU/L given value 1301 (n = 11).
Reference range: TPOAb <100 kU/L, serum TSH, (0.20–4.0 mU/L), serum fT4 (9–22 pmol/L), serum albumin (36–48 g/L).
dMissing 5 values of PTH at baseline.
C. Effect of Supplementation on Vitamin D Status
As reported previously, serum 25(OH)D3 increased after 16 weeks of supplementation by 25 (SD 22) nmol/L, from 27 to 52 nmol/L, in the group receiving 25 µg vitamin D3 (P < 0.001), and increased by 16 (SD 20) nmol/L, from 27 to 43 nmol/L, in the group receiving 10 µg vitamin D3 (P < 0.001) [21]. There was no significant change in 25(OH)D3 in the placebo group, measured to 27 (SD 15) nmol/L at baseline and 25 (SD 12) nmol/L after 16 weeks.
D. Effect of Supplementation on End Point Measures
Sixteen weeks of supplementation with vitamin D3 (combined 25 µg/d or 10 µg/d) compared with placebo had no significant effect on TPOAb (P = 0.23), TSH (P = 0.65), or fT4 (P = 0.70) (Table 2). Analyses of each supplementation group compared with placebo did not show any significant effect on TPOAb, TSH, or fT4 (Table 3). The effect was not changed by adjusting for age, gender, and baseline level of vitamin D3 (Tables 2 and 3). The total number of participants with TPOAb levels <50 kU/L decreased from 51% at baseline to 36% after 16 weeks, odds ratio (OR) = 0.54 (95% CI, 0.39 to 0.75), P < 0.001, but there were no differences between those given supplementation and placebo (P = 0.58). The effect of the end point measures was not changed when we excluded seven participants with elevated TSH >4.0 mU/L at baseline. A sensitivity analysis of only participants with TPOAb <100 kU/L at baseline (n = 225) gave β= 1.1 (95% CI, −3.1 to 5.3), P = 0.60. A separate analysis comparing the change of 25(OH)D3 and the change in TPOAb, independent of group allocation, showed no significant correlation.
Table 2.
Effect of Vitamin D3 Supplementation (25 µg and 10 µg Combined) on Thyroid and Related Parameters at the End of Intervention Compared With Placebo
| Baseline |
After 16 Wk |
Effect (95% CI) of Vitamin D3 Compared With Placeboa |
P | Adjusted Effect (95% CI) of Vitamin D3 Compared With Placebob |
Pb | |
|---|---|---|---|---|---|---|
| Participants, number | 250 | 213 | ||||
| Vitamin D3 | 168 | 142 | ||||
| Placebo | 82 | 71 | ||||
| n (%) | n (%) | OR | OR | |||
| TPOAb <100 kU/L | ||||||
| Vitamin D3 | 153 (91%) | 128 (90%) | 0.82 (0.55 to 1.23) | 0.34 | 0.81 (0.54 to 1.23) | 0.32 |
| Placebo | 72 (88%) | 63 (89%) | ||||
| TPOAb <50 kU/L | ||||||
| Vitamin D3 | 86 (51%) | 49 (35%) | 0.82 (0.40 to 1.67) | 0.58 | 0.80 (0.38 to 1.66) | 0.54 |
| Placebo | 41 (50%) | 27 (38%) | ||||
| Mean (SD) | Mean (SD) | B | B | |||
| TPOAb, kU/L | ||||||
| Vitamin D3 | 111 (255) | 115 (254) | 27 (−17 to 72) | 0.23 | 28 (−17 to 72) | 0.23 |
| Placebo | 128 (282) | 105 (217) | ||||
| TSH, mU/L | ||||||
| Vitamin D3 | 1.8 (1.2) | 2.0 (2.2) | −0.10 (−0.54 to 0.34) | 0.65 | −0.11 (−0.54 to 0.33) | 0.64 |
| Placebo | 2.0 (1.4) | 2.3 (2.4) | ||||
| fT4, pmol/L | ||||||
| Vitamin D3 | 16.4 (1.8) | 16.6 (1.9) | 0.09 (−0.37 to 0.55) | 0.70 | 0.06 (−0.39 to 0.52) | 0.78 |
| Placebo | 15.9 (1.5) | 16.1 (1.8) | ||||
| 25(OH)D3, nmol/L | ||||||
| Vitamin D3 | 26 (16) | 47 (19) | 23 (19 to 27) | <0.001 | 23 (18 to 27) | <0.001 |
| Placebo | 27 (15) | 25 (12) | ||||
| Plasma PTH, pmol/Lc | ||||||
| Vitamin D3 | 7.4 (3.6) | 6.0 (2.3) | −1.54 (−2.56 to −0.51) | 0.003 | −1.50 (−2.51 to −0.49) | 0.004 |
| Placebo | 8.0 (4.0) | 8.2 (4.0) |
The P values (TPOAb, TSH, and fT4) were compared with the Benjamini–Hochberg critical values. There was no difference in the assessment of statistical significance based on a false discovery rate of 10%.
aEffect (calculated with GEE) with the 250 participants at baseline and 213 at follow-up. Placebo is reference.
bAdjusted for gender, age, and vitamin D at baseline.
cMissing 5 values of PTH at baseline and 3 values at follow-up.
Table 3.
Effect of Vitamin D3 Supplementation (25 µg or 10 µg) on Thyroid Parameters at the End of Intervention Compared With Placebo
| Baseline |
After 16 Wk |
Effect (95% CI) of Vitamin D3 Compared With Placeboa |
P | Adjusted Effect (95% CI) of Vitamin D3 Compared With Placeboa,b |
Pb | |
|---|---|---|---|---|---|---|
| Participants, number | 250 | 213 | ||||
| Vitamin D3 25 µg | 83 | 73 | ||||
| Vitamin D3 10 µg | 85 | 69 | ||||
| Placebo | 82 | 71 | ||||
| N(%) | N (%) | OR | OR | |||
| TPOAb <100 kU/L | ||||||
| Vitamin D3 25 µg | 75 (90%) | 64 (88%) | 0.69 (0.46 to 1.06) | 0.09 | 0.69 (0.45 to 1.04) | 0.08 |
| Vitamin D3 10 µg | 78 (92%) | 64 (93%) | 1.05 (0.59 to 1.88) | 0.87 | 1.05 (0.58 to 1.88) | 0.88 |
| Placebo | 72 (88%) | 63 (89%) | ||||
| TPOAb <50 kU/L | ||||||
| Vitamin D3 25 µg | 38 (46%) | 22 (30%) | 0.83 (0.37 to 1.85) | 0.65 | 0.82 (0.36 to 1.86) | 0.63 |
| Vitamin D3 10 µg | 48 (57%) | 27 (39%) | 0.81 (0.35 to 1.85) | 0.61 | 0.78 (0.33 to 1.83) | 0.57 |
| Placebo | 41 (50%) | 27 (38%) | ||||
| Mean (SD) | Mean (SD) | B | B | |||
| TPOAb, kU/L | ||||||
| Vitamin D3 25 µg | 131 (300) | 147 (317) | 40 (−3 to 82) | 0.07 | 39 (−4 to 82) | 0.08 |
| Vitamin D3 10 µg | 92 (202) | 81 (157) | 12 (−39 to 64) | 0.64 | 14 (−37 to 65) | 0.59 |
| Placebo | 129 (282) | 105 (217) | ||||
| TSH, mU/L | ||||||
| Vitamin D3 25 µg | 2.0 (1.5) | 2.2 (2.9) | 0.02 (−0.56 to 0.61) | 0.93 | 0.02 (−0.56 to 0.60) | 0.94 |
| Vitamin D3 10 µg | 1.7 (0.8) | 1.7 (0.8) | −0.25 (−0.64 to 0.14) | 0.21 | −0.25 (−0.64 to 0.14) | 0.21 |
| Placebo | 2.0 (1.4) | 2.3 (2.4) | ||||
| fT4, pmol/L | ||||||
| Vitamin D3 25 µg | 16.5 (1.8) | 16.8 (2.0) | 0.06 (−0.47 to 0.59) | 0.83 | 0.03 (−0.50 to 0.56) | 0.91 |
| Vitamin D3 10 µg | 16.2 (1.8) | 16.5 (1.8) | 0.11 (−0.39 to 0.62) | 0.66 | 0.09 (−0.41 to 0.59) | 0.72 |
| Placebo | 15.9 (1.5) | 16.1 (1.8) |
The P values (TPOAb, TSH, and fT4) were compared with the Benjamini–Hochberg critical values. There was no difference in the assessment of statistical significance using a false discovery rate of 10%.
aEffect (calculated with GEE) with the 250 participants at baseline and 213 at follow-up. Placebo is reference.
bAdjusted for gender, age, and vitamin D at baseline.
E. Additional Analyses
At baseline we found no correlations between 25(OH)D3 and the main outcomes: TPOAb (r = −0.04, P = 0.51), TSH (r = −0.02, P = 0.79), or fT4 (r = 0.05, P = 0.46). TPOAb was positively correlated to TSH (r = 0.33, P < 0.001) but not to fT4 (r = 0.03, P = 0.62) or CRP (r = 0.01, P = 0.93). Albumin at baseline was not correlated to 25(OH)D3 (r = −0.01, P = 0.83), TPOAb (r = 0.08, P = 0.24), TSH (r = −0.01, P = 0.84), or fT4 (r = 0.16, P = 0.013).
3. Discussion
The use of 16 weeks of daily vitamin D3 supplementation (25 µg/d or 10 µg/d) showed no significant effect on thyroid autoimmunity or status compared with placebo in this study among adult, presumed healthy study participants from ethnic minorities living in Norway. The trial had a high prevalence of participants with serum 25(OH)D3 <50 nmol/L at baseline.
A. Strengths and Weaknesses
The study followed CONSORT guidelines (http://www.consort-statement.org) for randomized trials and had a high rate of retention to follow-up (86%). The trial was performed during winter and spring, a time with minimal impact of sun exposure on vitamin D synthesis at this latitude (60°N). The vitamin D status of the participants was not known by inclusion. The blood samples from baseline and follow-up were analyzed in a single batch.
None of the included participants reported use of thyroid hormones, and their TPOAb level was unknown by inclusion. Thus participants in an early stage of an AITD, subclinical hypothyroidism, could have been included. Association studies indicate that vitamin D supplementation can reduce the risk for development and progression of autoimmune diseases [7]. However, a 16-week supplementation period could have been too short to see any effect in an early stage of an AITD, measured by changes in TPOAb or TSH. More women than men were recruited, rendering gender comparisons less efficient, but women have a higher prevalence of AITD than men. Although 16 weeks of supplementation with vitamin D raised the serum levels in the intervention groups, not all participants attained a serum level of 25(OH)D3 ≥50 nmol/L at the end of the trial [21]. However, an association with change in 25(OH)D3 and change in TPOAb, independent of randomization group, was not found. The TPOAb showed a wide range of levels, and the effect of the intervention on TPOAb had a broad 95% CI. A sensitivity analysis of only participants with TPOAb <100 kU/L at baseline (90%) gave a narrow CI but still no effect of intervention. Power calculations to determine the sample size of the study were based on expected changes in muscle strength (results reported elsewhere) and not affected by thyroid function parameters [21].
B. Comparison With Other Studies
B-1. Baseline characteristics and associations
Effraimidis et al. found no association between low vitamin D levels and early stage of thyroid autoimmunity in their cross-sectional part (study A) of a case–control study among euthyroid subjects with genetic susceptibility for AITD [19]. Wang et al. conducted a meta-analysis from published studies up to December 2014 of the association between vitamin D and AITD. They found a higher prevalence of vitamin D deficiency (OR 2.99; 95% CI, 1.88 to 4.74) and lower vitamin D levels in patients with AITD compared with controls [6]. Many of these observational studies did not adjust for confounders and concluded that additional randomized controlled trials were needed to clarify whether reduced vitamin D level is a causal factor in the pathogenesis of AITD or a consequence [6]. Our participants, with a high percentage (92%) of vitamin D deficiency (serum 25(OH)D3 <50 nmol/L) and TPOAb within a wide range, showed no significant baseline correlation between 25(OH)D3 and TPOAb or TSH.
B-2. Effect of vitamin D-supplementation
Few studies have investigated the effect of vitamin D supplementation on thyroid autoimmunity. Effraimidis et al. found that development of TPOAb was not associated with low vitamin D levels in their longitudinal (study B) case–control study [19]. Krysiak et al. found an effect of vitamin D supplementation on thyroid autoimmunity in nonlactating women with postpartum thyroiditis compared with matched healthy postpartum women, in an open, not randomized study [23]. But postpartum, it cannot be excluded that thyroid autoantibodies spontaneously decreased with time and even sometimes return to euthyroid state within 12 months [24]. D’Aurizio et al. found in their review that genetic studies demonstrated an association between AITD and gene polymorphism of vitamin D receptor, vitamin D binding protein, and 1-α-hydroxylase and 25-hydroxylase but that the role of vitamin D in AITD remains controversial [25]. They concluded that more randomized, controlled, prospective trials are needed to demonstrate the causality of vitamin D in AITD and consequently the role of vitamin D supplementation in prevention or improvement of AITD. Chaudhary et al. found a reduction in TPOAb titers among patients with AITD treated with cholecalciferol 60,000 IU/wk for 8 weeks compared with the control group (P = 0.028) in an open-label, randomized controlled trial [18]. In our double-blinded, randomized controlled trial we found that the percentage of participants with low titers of TPOAb (<50 kU/L) decreased after 16 weeks, but there was no difference in the effects of vitamin D supplementation compared with placebo on TPOAb, TSH, or fT4. Links between inflammation and vitamin D and between inflammation and AITD have been found in other studies. Chandler et al. found no effect on CRP in healthy African Americans after 3 months of vitamin D supplementation, although the participants with 25(OH)D <50 nmol/L at baseline had higher CRP levels [26]. We focused on CRP as a possible marker of inflammation in thyroid autoimmunity. However, in our presumed healthy population of immigrants with low levels of 25(OH)D3, we found no correlation between CRP and TPOAb or CRP and 25(OH)D levels at baseline, and as previously reported supplementation with vitamin D did not decrease levels of CRP [22]. Patients with diagnosed AITD and high levels of inflammatory markers may react differently to vitamin D supplementation than our presumed healthy participants. Not all participants in the supplement groups might have been exposed to sufficient 25(OH)D3 levels within a sufficient period of time to affect thyroid status. On the other hand, a study period >16 weeks might have contributed to a greater loss to follow-up and to some impact of the sun (vitamin D–producing UV B rays), and thus blurring the differences between the groups. A higher vitamin D supplementation dosage might have exposed the thyroid autoimmune regulatory system to a higher 25(OH)D level, even though we found no effect of 25-µg vitamin D supplementation on thyroid status compared with placebo. However, we cannot extrapolate our findings to patients with a diagnosis and symptoms of AITD.
C. Conclusion
Daily supplementation of vitamin D3 (25 µg or 10 µg) for 16 weeks compared with placebo had no effect on TPOAb, fT4, or TSH among a population of adult immigrants from the Middle East, Africa, or South Asia living in Norway.
Acknowledgments
The authors thank Eva Kristensen and Morten Ariansen for their help with data collection, Anne Karen Jenum for the biobank support, and Marie Buchmann and Anne-Lise Sund at Fürst Medical Laboratory for facilitating the laboratory analyses. Finally, we extend our gratitude to the study participants who participated in this 16-week trial and the organizations that allowed us to use their venues for recruitment and data collection.
Acknowledgments
The study was sponsored by the Institute of Health and Society, University of Oslo, Norway. The study was also supported by the Norwegian Women’s Public Health Association, Fürst Medical Laboratory, and Nycomed Pharma AS, including free trial drugs. None of the supporting bodies had any influence on the performance of the trial, data analyses, writing, or publication of the results.
Clinical Trial Registry: ClinicalTrials.gov no. NCT01263288.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 25(OH)D
- 25-hydroxyvitamin D
- AITD
- autoimmune thyroid disease
- CRP
- C-reactive protein
- CV
- coefficient of variation
- fT4
- free fraction of thyroxine
- GEE
- generalized estimating equation
- IQR
- interquartile range
- OR
- odds ratio
- PTH
- parathyroid hormone
- TPOAb
- antithyroid peroxidase antibody
- TSH
- thyroid-stimulating hormone.
References and Notes
- 1.Taksler GB, Cutler DM, Giovannucci E, Keating NL. Vitamin D deficiency in minority populations. Public Health Nutr. 2015;18(3):379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prentice A. Vitamin D deficiency: a global perspective. Nutr Rev. 2008;66(10, suppl 2)S153–S164. [DOI] [PubMed] [Google Scholar]
- 3.Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Mølgaard C, Jorde R, Grimnes G, Moschonis G, Mavrogianni C, Manios Y, Thamm M, Mensink GB, Rabenberg M, Busch MA, Cox L, Meadows S, Goldberg G, Prentice A, Dekker JM, Nijpels G, Pilz S, Swart KM, van Schoor NM, Lips P, Eiriksdottir G, Gudnason V, Cotch MF, Koskinen S, Lamberg-Allardt C, Durazo-Arvizu RA, Sempos CT, Kiely M. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103(4):1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holvik K, Meyer HE, Haug E, Brunvand L. Prevalence and predictors of vitamin D deficiency in five immigrant groups living in Oslo, Norway: the Oslo Immigrant Health Study. Eur J Clin Nutr. 2005;59(1):57–63. [DOI] [PubMed] [Google Scholar]
- 5.Eggemoen AR, Knutsen KV, Dalen I, Jenum AK. Vitamin D status in recently arrived immigrants from Africa and Asia: a cross-sectional study from Norway of children, adolescents and adults. BMJ Open. 2013;3(10):e003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Lv S, Chen G, Gao C, He J, Zhong H, Xu Y. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015;7(4):2485–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5(7):2502–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 9.Cantorna MT, Yu S, Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Mol Aspects Med. 2008;29(6):369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007;66(9):1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. [DOI] [PubMed] [Google Scholar]
- 12.Prummel MF, Strieder T, Wiersinga WM. The environment and autoimmune thyroid diseases. Eur J Endocrinol. 2004;150(5):605–618. [DOI] [PubMed] [Google Scholar]
- 13.Medici M, Porcu E, Pistis G, Teumer A, Brown SJ, Jensen RA, Rawal R, Roef GL, Plantinga TS, Vermeulen SH, Lahti J, Simmonds MJ, Husemoen LL, Freathy RM, Shields BM, Pietzner D, Nagy R, Broer L, Chaker L, Korevaar TI, Plia MG, Sala C, Völker U, Richards JB, Sweep FC, Gieger C, Corre T, Kajantie E, Thuesen B, Taes YE, Visser WE, Hattersley AT, Kratzsch J, Hamilton A, Li W, Homuth G, Lobina M, Mariotti S, Soranzo N, Cocca M, Nauck M, Spielhagen C, Ross A, Arnold A, van de Bunt M, Liyanarachchi S, Heier M, Grabe HJ, Masciullo C, Galesloot TE, Lim EM, Reischl E, Leedman PJ, Lai S, Delitala A, Bremner AP, Philips DI, Beilby JP, Mulas A, Vocale M, Abecasis G, Forsen T, James A, Widen E, Hui J, Prokisch H, Rietzschel EE, Palotie A, Feddema P, Fletcher SJ, Schramm K, Rotter JI, Kluttig A, Radke D, Traglia M, Surdulescu GL, He H, Franklyn JA, Tiller D, Vaidya B, de Meyer T, Jørgensen T, Eriksson JG, O’Leary PC, Wichmann E, Hermus AR, Psaty BM, Ittermann T, Hofman A, Bosi E, Schlessinger D, Wallaschofski H, Pirastu N, Aulchenko YS, de la Chapelle A, Netea-Maier RT, Gough SC, Meyer Zu Schwabedissen H, Frayling TM, Kaufman JM, Linneberg A, Räikkönen K, Smit JW, Kiemeney LA, Rivadeneira F, Uitterlinden AG, Walsh JP, Meisinger C, den Heijer M, Visser TJ, Spector TD, Wilson SG, Völzke H, Cappola A, Toniolo D, Sanna S, Naitza S, Peeters RP. Identification of novel genetic Loci associated with thyroid peroxidase antibodies and clinical thyroid disease. PLoS Genet. 2014;10(2):e1004123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultheiss UT, Teumer A, Medici M, Li Y, Daya N, Chaker L, Homuth G, Uitterlinden AG, Nauck M, Hofman A, Selvin E, Völzke H, Peeters RP, Köttgen A. A genetic risk score for thyroid peroxidase antibodies associates with clinical thyroid disease in community-based populations. J Clin Endocrinol Metab. 2015;100(5):E799–E807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumgartner C, Blum MR, Rodondi N. Subclinical hypothyroidism: summary of evidence in 2014. Swiss Med Wkly. 2014;144:w14058. [DOI] [PubMed] [Google Scholar]
- 16.Kivity S, Agmon-Levin N, Zisappl M, Shapira Y, Nagy EV, Dankó K, Szekanecz Z, Langevitz P, Shoenfeld Y. Vitamin D and autoimmune thyroid diseases. Cell Mol Immunol. 2011;8(3):243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simsek Y, Cakır I, Yetmis M, Dizdar OS, Baspinar O, Gokay F. Effects of vitamin D treatment on thyroid autoimmunity. J Res Med Sci. 2016;21:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhary S, Dutta D, Kumar M, Saha S, Mondal SA, Kumar A, Mukhopadhyay S. Vitamin D supplementation reduces thyroid peroxidase antibody levels in patients with autoimmune thyroid disease: an open-labeled randomized controlled trial. Indian J Endocrinol Metab. 2016;20(3):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Effraimidis G, Badenhoop K, Tijssen JG, Wiersinga WM. Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur J Endocrinol. 2012;167(1):43–48. [DOI] [PubMed] [Google Scholar]
- 20.Kmieć P, Sworczak K. Vitamin D in thyroid disorders. Exp Clin Endocrinol Diabetes. 2015;123(7):386–393. [DOI] [PubMed] [Google Scholar]
- 21.Knutsen KV, Madar AA, Lagerløv P, Brekke M, Raastad T, Stene LC, Meyer HE. Does vitamin D improve muscle strength in adults? A randomized, double-blind, placebo-controlled trial among ethnic minorities in Norway. J Clin Endocrinol Metab. 2014;99(1):194–202. [DOI] [PubMed] [Google Scholar]
- 22.Knutsen KV, Madar AA, Brekke M, Meyer HE, Natvig B, Mdala I, Lagerløv P. Effect of vitamin D on musculoskeletal pain and headache: a randomized, double-blind, placebo-controlled trial among adult ethnic minorities in Norway. Pain. 2014;155(12):2591–2598. [DOI] [PubMed] [Google Scholar]
- 23.Krysiak R, Kowalcze K, Okopien B. The effect of vitamin D on thyroid autoimmunity in non-lactating women with postpartum thyroiditis. Eur J Clin Nutr. 2016;70(5):637–639. [DOI] [PubMed] [Google Scholar]
- 24.Sahin M, Corapcioglu D. The effect of vitamin D on thyroid autoimmunity in non-lactating women with postpartum thyroiditis. Eur J Clin Nutr. 2016;70(7):864. [DOI] [PubMed] [Google Scholar]
- 25.D’Aurizio F, Villalta D, Metus P, Doretto P, Tozzoli R. Is vitamin D a player or not in the pathophysiology of autoimmune thyroid diseases? Autoimmun Rev. 2015;14(5):363–369. [DOI] [PubMed] [Google Scholar]
- 26.Chandler PD, Scott JB, Drake BF, Ng K, Manson JE, Rifai N, Chan AT, Bennett GG, Hollis BW, Giovannucci EL, Emmons KM, Fuchs CS. Impact of vitamin D supplementation on inflammatory markers in African-Americans: results of a four-arm, randomized, placebo-controlled trial. Cancer Prev Res (Phila). 2014;7(2):218– 225. [DOI] [PMC free article] [PubMed] [Google Scholar]