Abstract
Purpose:
To explore the association between bone mineral density (BMD) and β-cell function.
Methods:
A cross-sectional study was performed in Fujian, China, from 2011 to 2012. The study included 572 elderly men older than age 60 years and 1558 postmenopausal women aged 45 to 86 years, excluding those with diabetes and insulin resistance. Fasting glucose and insulin concentrations were measured. Pancreatic β-cell function was estimated by using the homeostasis model assessment (HOMA-β). Calcaneus BMD was measured by using quantitative ultrasonography. Multiple regression analyses were applied to explore the association.
Results:
Participants with decreased BMD had lower fasting glucose (P < 0.001 in postmenopausal women; P = 0.007 in elderly men) and greater HOMA-β (P = 0.001 in postmenopausal women; P = 0.008 in elderly men) than those with normal BMD, whereas no statistical differences in insulin were seen among categories of BMD. After adjustment for all confounders, HOMA-β was still significantly negatively related to BMD in both groups (all P < 0.001), and remarkable positive relationships were found between BMD and fasting glucose. Furthermore, binary logistic regression presented fully adjusted odds ratios for diabetes in those with osteoporosis vs those with normal BMD: 0.60 [95% confidence interval (CI), 0.38 to 0.94] and 0.66 (95% CI, 0.49 to 0.91) in the original selected population of elderly men (n = 1070) and postmenopausal women (n = 2825), respectively.
Conclusions:
BMD was independently inversely associated with HOMA-β and positively associated with fasting glucose in both elderly men and postmenopausal women, suggesting that bone mass may be a predictor of glucose metabolism. Further research is needed to verify the associations and determine the exact mechanism underlying them.
Keywords: bone mineral density, β-cell function
BMD was independently inversely associated with HOMA-β in both elderly men and postmenopausal women, suggesting that bone mass may be a predictor of glucose metabolism.
Impaired β-cell function and insulin resistance are both involved in the pathogenesis of type 2 diabetes mellitus. It has been reported that, compared with insulin resistance, β-cell dysfunction plays the predominant role in the development of diabetes in some individuals [1, 2]. Most previous studies have shown that insulin resistance is associated with bone mineral density (BMD), and the conclusions are inconsistent. Arikan et al. [3] reported that in patients with type 2 diabetes, remarkable insulin resistance had a negative effect on BMD. Similarly, a cross-sectional study conducted in Korean men demonstrated that bone mass was inversely related to insulin resistance [4]. In contrast, a few studies found that insulin resistance could be a protective factor for bone health [5, 6]. However, to our knowledge, no study to date has examined the association between BMD and pancreatic β-cell function [homeostasis model assessment–estimated β-cell function (HOMA-β)], especially in Asian populations, which have an increasingly higher prevalence of osteoporosis and diabetes compared with Western populations [7, 8].
Therefore, the aim of the current study was to investigate the relationship between BMD and β-cell function by using data from the baseline survey of the REACTION study on Chinese persons in Fujian, China. We restricted our participants to elderly men and postmenopausal women in order to eliminate the effects of sex and hormonal change with different ages and menstrual status.
1. Materials and Methods
A. Study Participants
This cross-sectional study was performed on individuals recruited from two cities (Ningde and Wuyishan) in Fujian, China, from 2011 to 2012. It was part of the baseline survey of the REACTION (Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal) study, which investigated the association between diabetes and risk for cancer. The design and methods of the REACTION study have been described in detail elsewhere [9]. Inclusion criteria were age 60 years or older for men and, among women, cessation of menstruation for at least 1 year. A total of 5083 participants were enrolled in our study. We excluded those with diabetes (n = 1181) diagnosed according to the 1999 World Health Organization criteria [10]. Previous studies [11] have shown that the disposition index, calculated from the product of insulinogenic index and the Matsuda index, is a well-accepted measure of pancreatic β-cell function adjusted for insulin resistance. In contrast, for our study we selected HOMA-β for estimating β-cell function. Therefore, we also excluded persons with insulin resistance (n = 684), defined as homeostasis model assessment–estimated insulin resistance [fasting blood glucose (FBG) × FIns/22.5] > 2.41 [12]. Individuals were also excluded if they met one of the following conditions: (1) self-reported history of cardiovascular, liver, or kidney diseases or malignancies (n = 279); (2) diseases of the thyroid, rheumatoid arthritis, or pituitary disorders (n = 235); and (3) incomplete informations (n = 378). In addition, individuals who took such medications as steroid hormones, bisphosphonates, and other drugs that affect bone and pancreas metabolism were excluded (n = 196). In the end, 572 elderly men and 1558 postmenopausal women were included in the present study. All participants provided written informed consent, and the Ethics Committee of Fujian Provincial Hospital approved the study.
B. Clinical and Anthropometric Measurements
Each participant underwent a face-to-face interview and completed a detailed questionnaire that solicited information on age, sex, lifestyle factors, medical histories, and other relevant social or dietary information. Cigarette smoking and alcohol consumption habits were categorized into three levels: former (those who had consumed previously but had not consumed for ≥1 year), current (those who had consumed in the last year), and never. Physical activity was assessed by using metabolic equivalent (MET) minutes per week according to the Global Physical Activity Questionnaire and thus was classified as low (<600 MET), moderate (≥600 to <3000 MET), and heavy (≥3000 MET). Body weight and height were measured with the participants not wearing shoes and wearing light clothing, to the nearest kilogram and centimeter, respectively. Body mass index (BMI) was calculated as body weight in kilograms divided by height in meters squared. Waist circumference was measured at the middle point between the lower costal margin and the iliac crest. Hip circumference was measured at the level of the greater trochanters. Waist-to-hip ratio (WHR) was defined as the ratio of waist circumference to hip circumference. Blood pressure was measured by using a sphygmomanometer on the right upper arm after 30 minutes of rest. All the procedures were performed by experienced operators.
C. Laboratory Measurements
After an overnight fast for at least 10 hours, venous blood samples were collected. A standardized 75-g oral glucose tolerance test was given to all participants between 8:00 am and 9:00 am. Blood glucose and insulin levels were determined at 0 and 120 minutes after the administration of glucose. The plasma levels of fasting blood glucose were measured by using the glucose oxidase technique (Changchun Huili Biotech Co. Ltd, Changchun, China). Fasting insulin levels were measured by the radioimmunoassay method (Linco Research, St. Charles, MO). Serum total cholesterol, triglycerides, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol were tested with an autoanalyzer (Abbott Laboratories, Lake Bluff, IL). To estimate β-cell function, HOMA was used on the basis of fasting blood glucose and FIns concentrations. HOMA-β was calculated according to the following formula:
D. BMD Measurement
BMD was measured on the dominant calcaneus by quantitative ultrasonography (QUS) using the Achilles Express Ultrasound device (GE Healthcare, Waukesha, WI). All the measurements were performed on the same machine by the same experienced operator in strict accordance with the manufacturer's recommendations. T-scores for BMD were calculated on the basis of the database of a healthy young adult Asian population provided by the manufacturer. Because of the inapplicability of the World Health Organization criteria for QUS, participants were classified into three groups according to revised criteria for QUS [13]: normal BMD (T-score ≥ −0.5), osteopenia (T-score < −0.5 but >−1.8,) osteoporosis (T-score ≤ −1.8).
E. Statistical Analysis
Statistical analyses were performed by using SPSS software, version 17.0 (IBM, Chicago, IL). Variables were presented as means (standard deviations) for normal distribution, as median (interquartile ranges) for nonnormal distribution, or as percentages for categorical variables. Differences in participants among three categories of BMD were examined with one-way analysis of variance, the Kruskal–Wallis test, or the χ2 test as appropriate. Post hoc comparisons were carried by using Bonferroni correction. Multiple linear regression was applied to test for a linear trend, using the natural logarithm–transformed β-cell function (HOMA-β) as the dependent variable. In the multivariate analysis, we used stepwise regression to avoid the multicollinearity within covariates (the criteria of P = 0.10 for a variable to enter and P = 0.15 for a variable to be removed). Two regression models were established to adjust for the confounding factors. In model 1, we adjusted for age and body mass index. In model 2, we further adjusted for low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, serum total cholesterol, triglycerides, WHR, years since menopause (postmenopausal women), smoking status, alcohol consumption, and physical activity. Results were presented as standardized β coefficients and P values.
In addition, for the sake of verifying our results, we used a binary logistic regression model in the original selected population covering those with diabetes and those with insulin resistance (n = 1070 elderly men and n = 2825 postmenopausal women) to evaluate the adjusted odds ratios (ORs) and 95% confidence intervals (CIs), including all confounders in model 2 for the incidence of diabetes across categories of BMD, with normal BMD as the reference. All P values reported were two tailed, and P < 0.05 was considered to represent a statistically significant difference.
2. Results
The baseline characteristics of 572 elderly men and 1558 postmenopausal women with normal BMD, osteopenia, and osteoporosis are shown in Tables 1 and 2, respectively. Both male and female participants with lower BMD were more likely to be older (P < 0.001) and to have lower BMI (P < 0.001) and fasting glucose levels (P < 0.01). With bone mass decreased, individuals were predisposed to have greater pancreatic β-cell function (HOMA-β) (all P values for trend < 0.01). Compared with women who had normal BMD, those with bone loss had lower high-density lipoprotein cholesterol (P = 0.001) and diastolic blood pressure (P = 0.002); this relationship was not seen among men. In addition, alcohol consumption was associated with increased BMD (P < 0.05) in all participants. However, there were no significant differences in fasting insulin, WHR, triglycerides, total cholesterol, low-density lipoprotein cholesterol, and other lifestyle variables (such as smoking and physical activity) among different categories of BMD (all P > 0.05).
Table 1.
Characteristics of Elderly Men With Normal BMD, Osteopenia, and Osteoporosis
| Characteristic | Normal BMD | Osteopenia | Osteoporosis | P Value |
|---|---|---|---|---|
| Participants, n | 275 | 182 | 115 | |
| Age, y | 65.5 (4.6) | 67.1 (5.4)a | 68.9 (6.1)a,b | <0.001 |
| BMI, kg/m2 | 23.4 (2.8) | 22.8 (2.9)a | 22.1 (2.8)a,b | <0.001 |
| HDL cholesterol, mmol/L | 1.34 (1.15–1.65) | 1.33 (1.09–1.86) | 1.40 (1.20–1.61) | 0.528 |
| LDL cholesterol, mmol/L | 2.94 (0.75) | 2.86 (0.81) | 2.89 (0.93) | 0.590 |
| TC, mmol/L | 5.08 (0.93) | 4.92 (1.03) | 4.93 (1.13) | 0.184 |
| TG, mmol/L | 1.17 (0.87–1.74) | 1.13 (0.80–1.58) | 1.07 (0.85–1.58) | 0.086 |
| FBG, mmol/L | 5.53 (5.20–5.99) | 5.49 (5.05–5.90)a | 5.32 (4.95–5.71)a,b | 0.007 |
| FIns, μU/mL | 7.36 (2.24) | 7.65 (2.12) | 7.46 (2.29) | 0.389 |
| HOMA-β | 78.51 (64.31–96.28) | 83.82 (69.56–108.34)a | 88.17 (74.88–114.50)a,b | 0.008 |
| Smoker, % | 0.358 | |||
| Never | 47.3 | 56.1 | 55.6 | |
| Former | 34.5 | 28.0 | 29.6 | |
| Current | 18.2 | 15.9 | 14.8 | |
| Alcohol consumption, % | 0.007 | |||
| Never | 66.9 | 73.6a | 73.9a | |
| Former | 28.7 | 16.5 | 20.9 | |
| Current | 4.4 | 9.9 | 5.2 | |
| Physical activity, % | 0.212 | |||
| Low | 80.7 | 87.4 | 87.8 | |
| Moderate | 8.0 | 3.8 | 5.2 | |
| High | 11.3 | 8.8 | 7.0 | |
| SBP, mmHg | 141.0 (19.6) | 141.5 (21.7) | 136.5 (18.6) | 0.086 |
| DBP, mmHg | 77.6 (10.8) | 76.3 (10.9) | 75.4 (10.2) | 0.153 |
| WHR | 0.89 (0.06) | 0.88 (0.06) | 0.88 (0.06) | 0.677 |
Data are presented as means (standard deviation), median (interquartile ranges), or percentages. One-way analysis of variance or Kruskal–Wallis test was used for continuous data and Pearson χ2 test was used for categorical data. Post hoc tests were performed by using Bonferroni correction.
Abbreviations: DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglycerides; SBP, systolic blood pressure.
P < 0.05 compared with normal BMD group.
P < 0.05 compared with osteopenia group.
Table 2.
Characteristics of Postmenopausal Women With Normal BMD, Osteopenia, and Osteoporosis
| Characteristic | Normal BMD | Osteopenia | Osteoporosis | P Value |
|---|---|---|---|---|
| Participants, n | 732 | 491 | 335 | |
| Age, y | 56.0 (6.5) | 59.0 (6.7)a | 62.3 (8.5)a,b | <0.001 |
| BMI, kg/m2 | 24.0 (2.9) | 23.5 (3.1)a | 23.0 (2.9)a,b | <0.001 |
| HDL cholesterol, mmol/L | 1.46 (0.34) | 1.39 (0.33)a | 1.41 (0.35)a | 0.001 |
| LDL cholesterol, mmol/L | 3.20 (0.81) | 3.15 (0.82) | 3.09 (0.90) | 0.138 |
| TC, mmol/L | 5.43 (1.04) | 5.31 (1.06) | 5.29 (1.17) | 0.065 |
| TG, mmol/L | 1.31 (0.95–1.79) | 1.18 (0.92–1.73) | 1.21 (0.92–1.69) | 0.066 |
| FBG, mmol/L | 5.40 (5.03–5.80) | 5.28 (4.87–5.70)a | 5.22 (4.78–5.61)a,b | <0.001 |
| FIns, μU/mL | 7.82 (2.99) | 7.89 (3.07) | 7.85 (3.15) | 0.843 |
| HOMA-β | 80.08 (62.86–102.42) | 84.41 (64.73–116.83)a | 88.38 (67.15–118.95)a,b | 0.001 |
| Smoker, % | 0.554 | |||
| Never | 99.3 | 99.8 | 99.1 | |
| Former | 0.5 | 0.2 | 0.9 | |
| Current | 0.1 | 0 | 0 | |
| Alcohol consumption, % | 0.045 | |||
| Never | 95.8 | 97.6a | 97.9a | |
| Former | 4.2 | 2.0 | 1.8 | |
| Current | 0 | 0.4 | 0.3 | |
| Physical activity, % | 0.793 | |||
| Low | 91.4 | 92.7 | 92.5 | |
| Moderate | 5.3 | 4.5 | 5.4 | |
| High | 3.3 | 2.8 | 2.1 | |
| SBP, mmHg | 133.1 (19.3) | 133.7 (19.7) | 133.7 (19.7) | 0.849 |
| DBP, mmHg | 76.5 (10.6) | 75.3 (10.5)a | 74.1 (10.1)a | 0.002 |
| YSM, y | 34.0 (31.0–36.0) | 33.0 (31.0–36.0)a | 33.0 (30.0–35.0)a | 0.003 |
| WHR | 0.86 (0.06) | 0.86 (0.06) | 0.87 (0.06) | 0.483 |
Data are presented as means (standard deviation), median (interquartile ranges), or percentages. One-way analysis of variance or Kruskal–Wallis test was used for continuous data and Pearson χ2 test was used for categorical data. Post hoc tests were performed by using Bonferroni correction.
Abbreviations: DBP, diastolic blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglycerides; SBP, systolic blood pressure; YSM, years since menopause.
P < 0.05 compared with normal BMD group.
P < 0.05 compared with osteopenia group.
As presented in Table 3, there were significant negative associations between BMD and logarithm-transformed HOMA-β in both elderly men and postmenopausal women before and after controlling for the effects of various confounding factors (all P < 0.01). Compared with model 1, the correlation results remained significant after adjustment for all potential confounders in model 2, although the strength of the correlations diminished somewhat. In addition, markedly positive associations between BMD and fully adjusted fasting glucose in model 2 were observed (β = 0.199 and P = 0.019 in elderly men; β = 0.140 and P < 0.001 in postmenopausal women).
Table 3.
Multiple Linear Regression Results of Associations Between BMD and HOMA-β
| Variable | LN (HOMA-β)a |
|||
|---|---|---|---|---|
| Elderly Men |
Postmenopausal Women |
|||
| Standardized β | P Value | Standardized β | P Value | |
| BMD unadjusted | −0.131 | 0.002 | −0.115 | <0.001 |
| Model 1b | −0.152 | <0.001 | −0.163 | <0.001 |
| Model 2c | −0.150 | <0.001 | −0.154 | <0.001 |
Values converted to natural logarithmic scale were used as the dependent variables.
Model 1 was adjusted for age and body mass index.
Model 2 was adjusted for low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, total cholesterol, triglyercides, WHR ratio, years since menopause (postmenopausal women), smoking status, alcohol consumption, and physical activity in addition to factors included in model 1.
Table 4 shows adjusted ORs of diabetes across categories of BMD in the original selected population of elderly men (n = 1070) and postmenopausal women (n = 2825) separately, including those with diabetes and insulin resistance. Low BMD was associated with a decreased risk for developing diabetes in both men and women after adjustment for all covariates (for both, P for trend < 0.001). Compared with individuals with normal BMD, individuals with osteoporosis had 40% and 34% lower risk for diabetes development [OR, 0.60 (95% CI, 0.38 to 0.94), P for trend = 0.028 in elderly men; OR, 0.66 (95% CI, 0.49 to 0.91), P for trend = 0.011 in postmenopausal women].
Table 4.
Adjusted ORs (95% CIs) for Incident Diabetes According to Categories of BMD in Primary Selected Population of Elderly Men and Postmenopausal Women
| Category of BMD | Elderly Men (n = 1070) |
Postmenopausal Women (n = 2825) |
||
|---|---|---|---|---|
| Diabetes (%) | Adjusted OR (95% CI) | Diabetes (%) | Adjusted OR (95% CI) | |
| Normal BMD | 14.3 | 1.00 (reference) | 10.5 | 1.00 (reference) |
| Osteopenia | 12.5 | 0.72 (0.48–1.07) | 11.0 | 0.79 (0.57–1.04) |
| Osteoporosis | 10.1 | 0.60 (0.38–0.94) | 11.4 | 0.66 (0.49–0.91) |
| P for trend | — | <0.001 | — | <0.001 |
Covariates included in the model were age, BMI, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, total cholesterol, triglyercides, WHR, years since menopause (postmenopausal women), smoking status, alcohol consumption, and physical activity.
3. Discussion
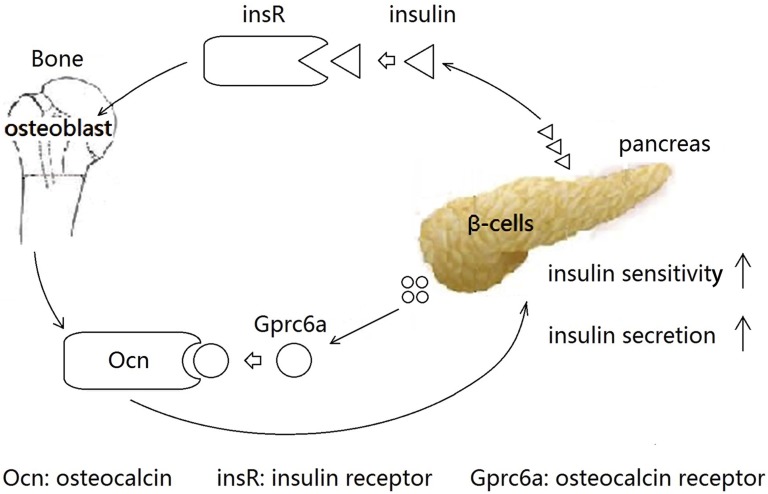
A bone–islet axis exists in the body. Insulin receptor signaling in the osteoblast promotes osteoblast differentiation and simultaneously increases osteocalcin secretion, which leads to regulation of insulin secretion in the pancreas and controls glucose homeostasis [14]. In vivo and in vitro studies demonstrated that Gprc6a, the osteocalcin-sensing receptor, is highly expressed in pancreatic β cells and helps regulate the response of circulating osteocalcin in the bone–pancreas endocrine loop [15]; deletion of this receptor was associated with bone loss, glucose intolerance, and insulin resistance [16]. Moreover, a US study by Pi and Quarles [17] also showed that Gprc6a defined a molecular mechanism linking bone metabolism with metabolic regulation of β cells. All of the previous findings support the existence of the bone–pancreas loop (Fig. 1). However, to date, most studies regarding the connection in the bone–pancreas loop have been conducted in animal models; similar evidence in humans is scarce.
Figure 1.
Interaction between bone and pancreas. Insulin released from pancreatic β-cell activates insulin receptor (insR) in the osteoblast to promote osteoblast differentiation and simultaneously increase osteocalcin (Ocn) secretion. Gprc6a, the osteocalcin-sensing receptor, is highly expressed in the pancreatic β cell. Ocn released from osteoblast targets Gprc6a to increase insulin secretion and promote insulin sensitivity.
In the current study, we observed that BMD were associated inversely with pancreatic β-cell function (HOMA-β) and positively with fasting plasma glucose levels in both elderly men and postmenopausal women, independent of insulin resistance and diabetes. The results were consistent even after adjustment for other potential confounding factors, such as age, BMI, WHR, lipid profiles, and lifestyle change. These findings suggest that bone mass seems to be a predictor of glucose metabolism.
This study investigated the association between BMD and pancreatic β-cell function as assessed by HOMA-β in an Asian population. The exact mechanism responsible for the association remains unclear. It is well documented that in addition to its traditional function, the skeleton is increasingly being recognized as an endocrine organ secreting osteocalcin, an osteoblast-specific hormone. The uncarboxylated form of osteocalcin, but not the carboxylated one, can induce β-cell proliferation, improve insulin sensitivity, and regulate glucose metabolism [18]. However, other studies reported that both uncarboxylated and carboxylated osteocalcin increased glucose transport in a rat model [19] and were associated with blood glucose levels in type 2 diabetes [20]. Analogous to the latter survey, a retrospective cohort study conducted in Chinese individuals also indicated that serum osteocalcin was inversely correlated with fasting plasma glucose levels and positively correlated with homeostasis model assessment of β-cell function (HOMA-β) [21].
Although these studies had inconsistent results, they implied a common viewpoint: Irrespective of its form, osteocalcin contributes to hyperglycemia control and the improvement of pancreatic β-cell function. On the other hand, it has been well established that the level of osteocalcin is significantly higher in postmenopausal osteoporotic women than in nonosteoporotic persons [22]. Similarly, in a cross-sectional study, Melton et al. [23] found that serum osteocalcin was inversely associated with BMD at diverse sites in postmenopausal women without estrogen treatment after adjustment for age. Additionally, other researchers have shown that elevated bone turnover markers, including osteocalcin, are associated with greater bone loss [24–26] and reduced diabetes risk [27] after adjustment for confounding variables in elderly men.
Given these findings, we speculated that the aforementioned statements may be able to primarily explain our observed negative and positive associations between BMD and β-cell function and fasting plasma glucose in our participants, respectively, despite the existence of other mechanisms underlying the associations yet to be detected. Nevertheless, because previous studies have reported that the correlations between bone turnover markers and BMD are weaker in men than in women [24, 26, 28], we therefore postulate that there may exist another main mechanism accounting for the associations between BMD and β-cell function and fasting glucose in elderly men, different from that in postmenopausal women. For example, besides osteocalcin, it is possible that the skeleton extensively produces other hormones that help improve pancreatic β-cell function and glucose homeostasis in elderly bone loss among men. This possibility should be researched further.
In addition, osteotesticular tyrosine phosphatase (OST-PTP), a novel receptor-like protein tyrosine phosphatase, is expressed specifically in bone and testes. In vivo and in vitro experiments demonstrated that mice lacking OST-PTP presented elevated β-cell proliferation, insulin sensitivity, and glucose tolerance [18]. On the basis of this finding, it is hypothesized that there is a link between BMD and the expression of OST-PTP and that OST-PTP can become a mediator for the association between β-cell function and bone mass in both groups of participants, and these may extend our explanations. Future studies focusing on these aspects are warranted.
To verify our results, we also estimated the risk for prevalent diabetes using multivariable logistic regression analysis in the primary selected sample of elderly men (n = 1070) and postmenopausal women (n = 2825) separately and found that a trend toward decreased risk for diabetes was correlated with bone loss; this was analogous to the results of an Australian survey by Yeap et al. [27] revealing that higher bone remodeling rates were regarded as markers for osteoporosis yet were associated with reduced diabetes risk in older men. In our study, compared with participants with normal BMD, those with osteoporosis had lower risk for incident diabetes after controlling for the effects of various confounders. These observations provide better evidence for the inverse correlation between BMD with pancreatic β-cell function.
The current study had several limitations. First, our study had a cross-sectional design, and thus we were unable to ascertain the cause-and-effect relationship between BMD and pancreatic β-cell function. Second, the participants were limited to elderly men and postmenopausal women in China; therefore, the findings may not be generalizable to different ethnic populations. Third, bone status was assessed by using QUS, which is not recognized as an accepted gold standard for BMD measurement. However, it does have several advantages, such as low cost, portability, ease of use, and lack of radiation. Fourth, we did not measure osteocalcin, which might provide direct evidence on and more insight into the association between BMD with β-cell function. Finally, we did not consider other confounders, including vitamin D, adiponectin, leptin, and body composition, which should be involved in future research.
In conclusion, our study found an inverse relationship between BMD and pancreatic β-cell function (HOMA-β) and a positive relationship between BMD and fasting glucose in both elderly men and postmenopausal women. These results suggest that bone mass may be a predictor of glucose metabolism. A longitudinal study is needed to confirm our findings and explore the exact mechanisms in the future.
Acknowledgments
We gratefully acknowledge all participants who were involved in this study.
Acknowledgments
This study was supported by grants from the Chinese Medical Association Foundation and Chinese Endocrine Society (12020240314), National Natural Science Foundation of China (81570706), National Science and technology support program (2013BAI09B13), Natural Science Foundation of Fujian Province (2011J06012, 2012J01322), and Provincial Health and Family Planning Commission of Fujian Province (2013-ZQN-ZD-3). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions: G.C. designed the study and edited the manuscript; S.L. wrote the manuscript; J.L, X.Q., and N.W. collected data and participated in the discussion; H.H., J.Y., K.T., L.C., L. L. (Liantao Li), W.L., H.C., M.L, L.L. (Lixiang Lin), J.L., Y.B., W.W., and G.N. collected data; J.W. reviewed and edited the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- bone mineral density
- BMI
- body mass index
- CI
- confidence interval
- DBP
- diastolic blood pressure
- FBG
- fasting blood glucose
- FIns
- fasting insulin
- HOMA-β
- homeostasis model assessment–estimated β-cell function
- MET
- metabolic equivalent
- OR
- odds ratio
- OST-PTP
- osteotesticular tyrosine phosphatase
- QUS
- quantitative ultrasound
- REACTION, Risk Evaluation of cAncers in Chinese diabeTic Individuals
- a lONgitudinal [study]
- WHR
- waist-to-hip ratio.
References and Notes
- 1.Jensen CC, Cnop M, Hull RL, Fujimoto WY, Kahn SE; American Diabetes Association GENNID Study Group . Beta-cell function is a major contributor to oral glucose tolerance in high-risk relatives of four ethnic groups in the U.S. Diabetes. 2002;51(7):2170–2178. [DOI] [PubMed] [Google Scholar]
- 2.Chailurkit LO, Chanprasertyothin S, Jongjaroenprasert W, Ongphiphadhanakul B. Differences in insulin sensitivity, pancreatic beta cell function and circulating adiponectin across glucose tolerance status in Thai obese and non-obese women. Endocrine. 2008;33(1):84–89. [DOI] [PubMed] [Google Scholar]
- 3.Arikan S, Tuzcu A, Bahceci M, Ozmen S, Gokalp D. Insulin resistance in type 2 diabetes mellitus may be related to bone mineral density. J Clin Densitom. 2012;15(2):186–190. [DOI] [PubMed] [Google Scholar]
- 4.Shin D, Kim S, Kim KH, Lee K, Park SM. Association between insulin resistance and bone mass in men. J Clin Endocrinol Metab. 2014;99(3):988–995. [DOI] [PubMed] [Google Scholar]
- 5.Abrahamsen B, Rohold A, Henriksen JE, Beck-Nielsen H. Correlations between insulin sensitivity and bone mineral density in non-diabetic men. Diab Med. 2000;17(2):124–129. [DOI] [PubMed] [Google Scholar]
- 6.Yüksel O, Dökmetaş HS, Topcu S, Erselcan T, Sencan M. Relationship between bone mineral density and insulin resistance in polycystic ovary syndrome. J Bone Miner Metab. 2001;19(4):257–262. [DOI] [PubMed] [Google Scholar]
- 7.Choi YJ, Oh HJ, Kim DJ, Lee Y, Chung YS. The prevalence of osteoporosis in Korean adults aged 50 years or older and the higher diagnosis rates in women who were beneficiaries of a national screening program: the Korea National Health and Nutrition Examination Survey 2008-2009. J Bone Miner Res. 2012;27(9):1879–1886. [DOI] [PubMed] [Google Scholar]
- 8.Ramachandran A, Snehalatha C, Shetty AS, Nanditha A. Trends in prevalence of diabetes in Asian countries. World J Diabetes. 2012;3(6):110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ning G; Reaction Study Group . Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal (REACTION) study. J Diabetes. 2012;4(2):172–173. [DOI] [PubMed] [Google Scholar]
- 10.Alberti KG, Zimmet PZ.. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1999;15(7):539–553. [DOI] [PubMed] [Google Scholar]
- 11.Pollock NK, Bernard PJ, Gower BA, Gundberg CM, Wenger K, Misra S, Bassali RW, Davis CL. Lower uncarboxylated osteocalcin concentrations in children with prediabetes is associated with beta-cell function. J Clin Endocrinol Metab. 2011;96(7):E1092–E1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu J, Bi Y, Wang T, Wang W, Mu Y, Zhao J, Liu C, Chen L, Shi L, Li Q, Wan Q, Wu S, Qin G, Yang T, Yan L, Liu Y, Wang G, Luo Z, Tang X, Chen G, Huo Y, Gao Z, Su Q, Ye Z, Wang Y, Deng H, Yu X, Shen F, Chen L, Zhao L, Dai M, Xu M, Xu Y, Chen Y, Lai S, Ning G. The relationship between insulin-sensitive obesity and cardiovascular diseases in a Chinese population: results of the REACTION study. Int J Cardiol. 2014;172(2):388–394. [DOI] [PubMed] [Google Scholar]
- 13.Frost ML, Blake GM, Fogelman I. Can the WHO criteria for diagnosing osteoporosis be applied to calcaneal quantitative ultrasound? Osteoporos Int 2000;11(4):321–330. [DOI] [PubMed] [Google Scholar]
- 14.Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, Faugere MC, Aja S, Hussain MA, Brüning JC, Clemens TL. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pi M, Wu Y, Quarles LD. GPRC6A mediates responses to osteocalcin in beta-cells in vitro and pancreas in vivo. J Bone Miner Res. 2011;26(7):1680–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, Christenson L, Li B, Zhang J, Jackson PD, Faber P, Brunden KR, Harrington JJ, Quarles LD. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One. 2008;3(12):e3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pi M, Quarles LD. Multiligand specificity and wide tissue expression of GPRC6A reveals new endocrine networks. Endocrinology. 2012;153(5):2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foresta C, Strapazzon G, De Toni L, Gianesello L, Calcagno A, Pilon C, Plebani M, Vettor R. Evidence for osteocalcin production by adipose tissue and its role in human metabolism. J Clin Endocrinol Metab. 2010;95(7):3502–3506. [DOI] [PubMed] [Google Scholar]
- 20.Kanazawa I, Yamaguchi T, Yamauchi M, Yamamoto M, Kurioka S, Yano S, Sugimoto T. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos Int. 2011;22(1):187–194. [DOI] [PubMed] [Google Scholar]
- 21.Zhou M, Ma X, Li H, Pan X, Tang J, Gao Y, Hou X, Lu H, Bao Y, Jia W. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol. 2009;161(5):723–729. [DOI] [PubMed] [Google Scholar]
- 22.Kalaiselvi VS, Prabhu K, Ramesh M, Venkatesan V. The association of serum osteocalcin with the bone mineral density in post menopausal women. J Clin Diagn Res. 2013;7(5):814–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melton LJ 3rd, Khosla S, Atkinson EJ, O'Fallon WM, Riggs BL. Relationship of bone turnover to bone density and fractures. J Bone Miner Res. 1997;12(7):1083–1091. [DOI] [PubMed] [Google Scholar]
- 24.Szulc P, Montella A, Delmas PD. High bone turnover is associated with accelerated bone loss but not with increased fracture risk in men aged 50 and over: the prospective MINOS study. Ann Rheum Dis. 2008;67(9):1249–1255. [DOI] [PubMed] [Google Scholar]
- 25.Szulc P, Garnero P, Marchand F, Duboeuf F, Delmas PD. Biochemical markers of bone formation reflect endosteal bone loss in elderly men--MINOS study. Bone. 2005;36(1):13–21. [DOI] [PubMed] [Google Scholar]
- 26.Cheng S, Suominen H, Väänänen K, Käkönen SM, Pettersson K, Heikkinen E. Serum osteocalcin in relation to calcaneal bone mineral density in elderly men and women: a 5-year follow-up. J Bone Miner Metab. 2002;20(1):49–56. [DOI] [PubMed] [Google Scholar]
- 27.Yeap BB, Alfonso H, Chubb SA, Gauci R, Byrnes E, Beilby JP, Ebeling PR, Handelsman DJ, Allan CA, Grossmann M, Norman PE, Flicker L. Higher serum undercarboxylated osteocalcin and other bone turnover markers are associated with reduced diabetes risk and lower estradiol concentrations in older men. J Clin Endocrinol Metab. 2015;100(1):63–71. [DOI] [PubMed] [Google Scholar]
- 28.Khosla S, Melton LJ III, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83(7):2266–2274. [DOI] [PubMed] [Google Scholar]