ABSTRACT
The pst gene cluster encodes the phosphate-specific transport (Pst) system. Inactivation of the Pst system constitutively activates the two-component regulatory system PhoBR and attenuates the virulence of pathogenic bacteria. In uropathogenic Escherichia coli strain CFT073, attenuation by inactivation of pst is predominantly attributed to the decreased expression of type 1 fimbriae. However, the molecular mechanisms connecting the Pst system and type 1 fimbriae are unknown. To address this, a transposon library was constructed in the pst mutant, and clones were tested for a regain in type 1 fimbrial production. Among them, the diguanylate cyclase encoded by yaiC (adrA in Salmonella) was identified to connect the Pst system and type 1 fimbrial expression. In the pst mutant, the decreased expression of type 1 fimbriae is connected by the induction of yaiC. This is predominantly due to altered expression of the FimBE-like recombinase genes ipuA and ipbA, affecting at the same time the inversion of the fim promoter switch (fimS). In the pst mutant, inactivation of yaiC restored fim-dependent adhesion to bladder cells and virulence. Interestingly, the expression of yaiC was activated by PhoB, since transcription of yaiC was linked to the PhoB-dependent phoA-psiF operon. As YaiC is involved in cyclic di-GMP (c-di-GMP) biosynthesis, an increased accumulation of c-di-GMP was observed in the pst mutant. Hence, the results suggest that one mechanism by which deletion of the Pst system reduces the expression of type 1 fimbriae is through PhoBR-mediated activation of yaiC, which in turn increases the accumulation of c-di-GMP, represses the fim operon, and, consequently, attenuates virulence in the mouse urinary tract infection model.
IMPORTANCE Urinary tract infections (UTIs) are common bacterial infections in humans. They are mainly caused by uropathogenic Escherichia coli (UPEC). We previously showed that interference with phosphate homeostasis decreases the expression of type 1 fimbriae and attenuates UPEC virulence. Herein, we identified that alteration of the phosphate metabolism increases production of the signaling molecule c-di-GMP, which in turn decreases the expression of type 1 fimbriae. We also determine the regulatory cascade leading to the accumulation of c-di-GMP and identify the Pho regulon as new players in c-di-GMP-mediated cell signaling. By understanding the molecular mechanisms leading to the expression of virulence factors, we will be in a better position to develop new therapeutics.
KEYWORDS: Escherichia coli, Pho regulon, type 1 fimbriae, UPEC, c-di-GMP, phosphate, pst, urinary tract infection
INTRODUCTION
An important aspect of bacterial physiology and virulence is the capacity to sense environmental signals. Sensing the environmental changes leads to adaptation, since genes responding to these changes will be specifically and coordinately regulated. Two-component signal transduction systems (TCSs) are one of the mechanisms by which bacteria respond to environmental signals. TCSs typically comprise an inner membrane histidine kinase sensor protein and a cytoplasmic response regulator (1).
The TCS PhoBR comprises PhoR, which is the sensor histidine kinase, and PhoB, the response regulator, and responds to phosphate limitation, i.e., when the extracellular phosphate concentration falls below 4 μM (2, 3). Thereby, PhoBR regulates genes belonging to the Pho regulon, such as those mediating phosphate transport and metabolism. Genes belonging to the Pho regulon possess PhoB-binding specific DNA sequences, known as Pho boxes, located within their promoter regions (2–5). During phosphate limitation, the TCS PhoBR is activated, and PhoB binds to Pho boxes to induce or repress gene expression (2, 3). The periplasmic alkaline phosphatase (PhoA), which catalyzes the hydrolysis and phosphorylation of a wide variety of phosphate monoesters, and the phosphate-specific transport (Pst) system, an ATP-binding cassette (ABC) transporter specific for inorganic phosphate (Pi), are among members of the Pho regulon (2, 3). In addition to being involved in the transport of Pi, the Pst system negatively regulates the activity of PhoBR, as the disruption of Pst constitutively activates PhoBR regardless of environmental phosphate availability (2, 3). Thus, inactivation of the pst system mimics phosphate-limiting conditions. Moreover, the Pst system is also linked with pathogenesis, as its deletion attenuates the virulence of pathogenic strains (6–9). In uropathogenic Escherichia coli (UPEC), we showed that virulence attenuation of the pst mutant is mainly attributed to the decreased expression of type 1 fimbriae (10).
In UPEC strains, type 1 fimbriae are a key virulence factor and are required to establish infection (11–14). Type 1 fimbriae are expressed in the bladder, and in addition to promoting adhesion to bladder cells and its colonization, they are involved in the invasion of bladder cells (15–18). Type 1 fimbriae are encoded by the fimAICDFGH operon (fim), where fimA encodes the major subunit and fimH encodes the mannose-specific adhesin (19). Expression of the fim operon depends on a promoter located on an invertible element (fimS) (20). The expression of type 1 fimbriae is therefore subjected to phase variation. The expression of type 1 fimbriae is mediated by the switching of fimS between the on and off orientations. The orientation of fimS is mainly controlled by the FimB and FimE recombinases (21). FimB mediates switching in both directions, from phase off to phase on and phase on to phase off, where the on orientation is favored, while FimE promotes switching to the off orientation, i.e., from phase on to phase off (21, 22). In addition to FimB and FimE, UPEC strain CFT073 encodes FimBE-like recombinases IpuA and IpbA (fimX product in UPEC strain UTI89) (23–25). IpuA promotes switching, like FimB, whereas IpbA only promotes the switching to the on position. Furthermore, IpuA and IpbA are sufficient for switching fimS and influencing type 1 fimbria expression in vitro and in vivo (23–25).
Cyclic di-GMP (c-di-GMP) is a bacterial second messenger that controls various processes, such as flagellar motility, biofilm formation, the cell cycle, and virulence of pathogenic bacteria (26, 27). c-di-GMP is synthesized by diguanylate cyclase proteins, which contain GGDEF domains, and is degraded by phosphodiesterase proteins, which contain EAL or HD-GYP domains. c-di-GMP acts via a variety of receptors containing PilZ or I-site domains (28) and acts by influencing transcriptional, translational, and posttranslational regulation (26, 27). Its role in cellular processes is well studied in Salmonella, Vibrio, Yersinia, and Pseudomonas species. In Salmonella, AdrA (homolog of YaiC, also named DgcC [29] in Escherichia coli) is one of the major diguanylate cyclases. By activating the biosynthesis of c-di-GMP, AdrA induces the formation of biofilm by increasing the production of cellulose through the induction of the bcs (bacterial cellulose synthesis) operon (30–33). The expression of adrA is activated by the curli biosynthesis regulator CsgD (30, 34, 35). Cellulose production, along with curli, forms an extracellular matrix favorable to surface adhesion, cellular aggregation, persistence in the environment, and biofilm formation (36).
As mentioned above, deletion of the pst system attenuated virulence of the UPEC CFT073 strain by decreasing the expression of type 1 fimbriae (10). An in silico analysis showed that genes involved in regulation of or in biosynthesis of type 1 fimbriae do not possess Pho boxes in their promoter regions (5). Given that the specific mechanisms by which induction of the Pho regulon (PhoBR) inhibits the expression of type 1 fimbriae are unknown and that the Pho regulon seems to act indirectly, possible mechanisms linking the Pho regulon and type 1 fimbriae were investigated herein by the construction of a transposon library in the pst mutant. In this study, we show that YaiC is one of the important mediators in the pst mutant that contribute to decreased expression of type 1 fimbriae in strain CFT073. Indeed, yaiC is induced in the pst mutant and represses type 1 fimbriae. In the pst mutant strain, yaiC is activated by PhoB due to increased transcription of yaiC from the adjacent phoA-psiF operon, and the increased transcription of yaiC required the PhoB-dependent promoter of the phoA-psiF operon. An accumulation of c-di-GMP was observed in the pst mutant and was concomitant with the increased expression of yaiC. Taken together, our results demonstrate that the induction of yaiC in the pst mutant alters the expression of type 1 fimbriae and contributes to the fitness defect of the pst mutant in the murine model of UTI.
RESULTS
Screening for genes involved in repression of type 1 fimbriae in the pst mutant.
We previously demonstrated that constitutive activation of PhoBR, by disrupting the Pst system, decreased expression and production of type 1 fimbriae and attenuated virulence (10, 37, 38). However, the molecular mechanisms connecting the Pho regulon and regulation of type 1 fimbriae have not yet been determined. Furthermore, an in silico search revealed that the fimB, fimE, and fimA genes do not possess a Pho box(es) in their promoter region (5), which suggests that PhoBR may indirectly affect type 1 fimbrial expression (10). To identify genes that could connect the Pho regulon (Pst system and PhoBR) to the expression of type 1 fimbriae, a transposon library was constructed in the pst mutant. Transposon mutants were screened for an increase in the production of type 1 fimbriae. Inactivation of the pst system constitutively activates PhoBR, which can be monitored by the detection of alkaline phosphatase PhoA activity on lysogeny broth (LB) agar plates supplemented with 5-bromo-4-chloro-3-indolyl phosphate disodium (BCIP). Indeed, the pst mutant appears blue on these plates, whereas the wild-type (WT) strain remains white. Furthermore, as the deletion of phoB in the pst mutant abrogated the activation of the Pho regulon induced by inactivating the pst system (38) and the pst phoB double mutant regained the production of type 1 fimbriae to WT levels (10), we tested our approach by first screening white colonies grown on BCIP-containing plates. Surprisingly, one of the mutants identified in this screen, which demonstrated a regain in type 1 fimbrial expression, did not have a mutation in the phoBR regulatory genes but was found to have a disrupted phoA gene (Fig. 1A). Since phoA encodes a periplasmic enzyme not known to have a regulatory function, we sought to explain the molecular mechanisms linking PhoA and type 1 fimbrial expression in the CFT073 pst mutant.
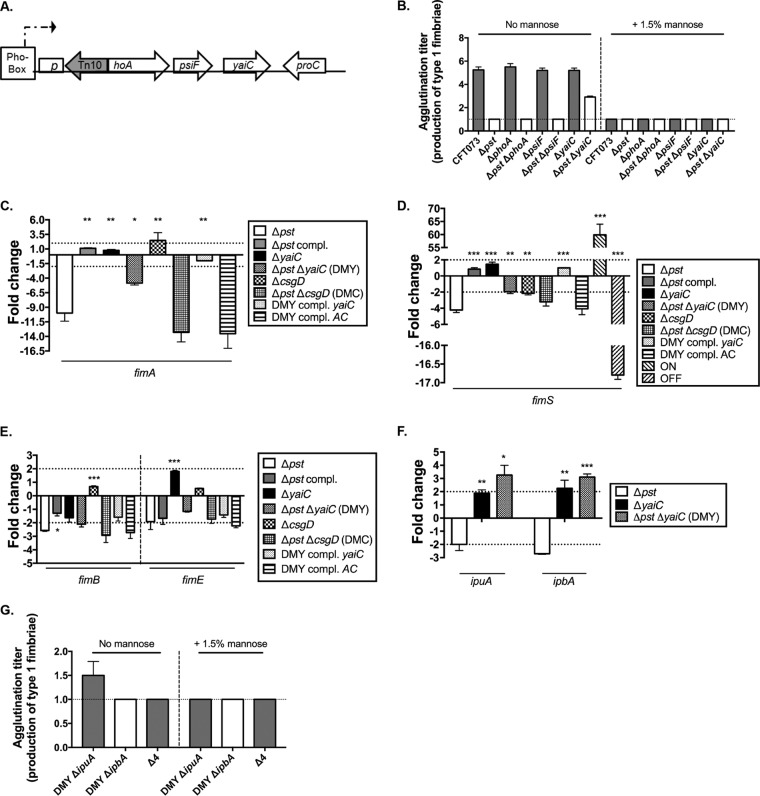
FIG 1.
The yaiC gene alters expression of type 1 fimbriae. (A) Schematic representation of a transposon insertion which caused a regain in expression of type 1 fimbriae. (B) Quantification of type 1 fimbriae by yeast agglutination assay in nonpolar mutants with and without 1.5% mannopyranose. The CFT073 Δfim and Δpst mutant strains were used as negative controls, since they did not agglutinate yeast. The y axis represents the well in which we observed agglutination. Each well corresponds to a 2-fold dilution. For example, an agglutination titer of 2 corresponds to a 2-fold dilution, while a titer of 4 corresponds to an 8-fold dilution. (C) Transcription of fimA in mutant strains compared to the WT strain. (D) On orientation of fimS in the mutant strains compared to the WT strain. (E and F) Expression of fimB and fimE (E) and ipuA and ipbA (F) in mutant strains compared to the WT strain. (G) Quantification of type 1 fimbriae by yeast agglutination assay in mutants with deleted recombinase genes ipuA and/or ipbA with and without mannose. The dotted lines in panels B and G represent the limit of detection that corresponds to the nondiluted sample. The dotted lines in panels C, E, and F correspond to the cutoff of a significant difference in expression, while in panel D, they correspond to the level of on orientation. All results shown are the mean values and standard deviations of the results from four biological experiments. Statistical significance was calculated by the Student t test; *, P < 0.05; **, P < 0.005; ***, P < 0.0001.
The yaiC gene encoding a GGDEF domain protein contributes to the repression of type 1 fimbriae in the pst mutant.
PhoA is a periplasmic enzyme catalyzing the hydrolysis of a wide variety of phosphomonoesters. Given that the transposon was inserted in the opposite orientation of the phoA-psiF operon (Fig. 1A), we hypothesized that the transposon had a polar effect on a gene(s) downstream of phoA by blocking the transcription of psiF and yaiC. This hypothesis is also based on the fact that PhoA does not possess known regulatory domains or functions. To test this possibility, we introduced a nonpolar mutation in both WT and the Δpst mutant strain in phoA, as well as in downstream genes psiF and yaiC. The production of type 1 fimbriae was then evaluated by yeast agglutination on strains cultured to mid-log phase in LB. As expected, mutations within phoA, psiF, or yaiC in the WT strain had no effect on the production of type 1 fimbriae (Fig. 1B), as the Pho regulon is not induced in LB. In contrast, the production of type 1 fimbriae was restored in the pst yaiC double mutant (DMY) (Fig. 1B). The yaiC gene, an ortholog of the diguanylate cyclase gene adrA in Salmonella enterica, encodes a GGDEF domain, is immediately adjacent to the phoA-psiF operon, and is not annotated as being part of this operon (Fig. 1A). To confirm that yeast agglutination was mediated by type 1 fimbriae, the assay was also performed in the presence of 1.5% mannopyranose, which blocks the interaction of the type 1 fimbrial adhesin and yeast and inhibits agglutination. As expected, no yeast agglutination was observed when 1.5% mannopyranose was added to the bacteria (Fig. 1B). To corroborate the yeast agglutination assay, the expression of fimA was quantified by reverse transcription-quantitative PCR (qRT-PCR). Compared to the Δpst mutant strain, the expression of fimA was increased 2-fold in the DMY strain (Δpst ΔyaiC) (Fig. 1C). These results suggest that induction of the Pho regulon in the pst mutant mediates the repression of type 1 fimbriae through the deregulation of yaiC expression, but the phoA gene product itself does not play a role in this process.
The expression of the genes encoding type 1 fimbriae is dependent on an invertible element (fimS), which contains the fim promoter (20). This invertible element alternates between the on and off orientations, which leads to activation and repression of type 1 fimbrial expression, respectively. To correlate increased fim expression in the DMY strain with the orientation of fimS, its orientation was determined by quantitative PCR (qPCR). In a comparison of the on position of strains cultured to mid-log phase in LB, the on orientation is 2.0-fold higher than for the pst mutant (Fig. 1D).
In vitro, we previously demonstrated that repression of fimA in the pst mutant is due to a bias toward the off orientation of fimS, which is mainly linked to the repression of fimB and, possibly, to the downregulation of the Fim-like recombinase genes ipuA and ipbA (10). To correlate the restoration of increased type 1 fimbrial production and fimS orientation with the expression levels of the recombinases, the expression of fimB, fimE, ipuA, and ipbA was quantified by qRT-PCR. Thereby, the deletion of yaiC in the Δpst mutant background had no effect on fimB and fimE expression (Fig. 1E). However, the expression of ipuA and ipbA was restored in the DMY mutant strain, as they were induced 3.9- and 2.9-fold, respectively (Fig. 1E). Since IpuA and IpbA promote the orientation of fimS in the on position, restoration of type 1 fimbrial expression could be attributed to the increase expression of these two Fim-like recombinases. To confirm the contribution of ipuA and ipbA in the regain in the production of type 1 fimbriae in the DMY mutant, we inactivated either ipuA (DMY ΔipuA mutant) or ipbA (DMY ΔipbA mutant) or both genes (Δ4 mutant) in the DMY background. We then determined the production of type 1 fimbriae in these strains by yeast agglutination in the presence and absence of mannopyranose. As expected, the production of type 1 fimbriae was inhibited in all three mutants and restored production to the pst mutant (Fig. 1G). The addition of 1.5% mannopyranose did not affect yeast agglutination, since the production of type 1 fimbriae was inhibited in the mutant strains (Fig. 1G). These data confirm the contribution of ipuA and ipbA in the restoration in the expression of the type 1 fimbriae in the Δpst ΔyaiC double mutant.
Taken together, these results show that yaiC plays a role in decreasing the expression of type 1 fimbriae in the pst mutant. Indeed, its deletion restored the expression of fimA and increased the expression of the ipuA and ipbA recombinases, which in turn promoted orientation of the invertible promoter toward the on position and increased expression of the type 1 fimbriae.
The yaiC gene is induced under phosphate-limiting conditions.
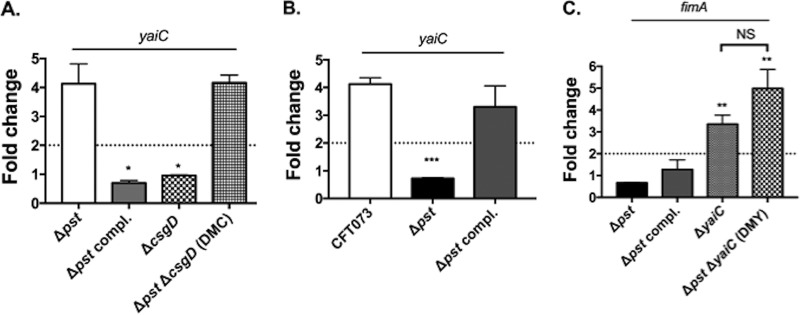
As the deletion of yaiC in the Δpst mutant strain restores the expression/production of type 1 fimbriae, this suggests that yaiC is induced in the pst mutant. To test this possibility, the expression of yaiC was evaluated by qRT-PCR. As shown in Fig. 2A, compared to the WT strain, yaiC was induced 4.4-fold in the pst mutant. Since constitutive induction of the Pho regulon through inactivation of the Pst system induced the expression of yaiC, we tested if physiological activation of the Pho regulon had the same effect. To do so, bacteria were grown in morpholinepropanesulfonic acid (MOPS) low-phosphate (LP) and high-phosphate (HP) broth, and the expression of yaiC was analyzed by qRT-PCR. When grown in LP broth, compared to HP broth, the expression of yaiC was 4.1- and 3.3-fold induced in the WT and Δpst complemented (compl.) mutant strains (Fig. 2B). Since disruption of the pst system constitutively activates the Pho regulon regardless of environmental phosphate availability (3), it was not surprising to observe no difference in yaiC expression between the LP and HP media in the pst mutant.
FIG 2.
Expression of yaiC and fimA under phosphate-limiting conditions. (A) Expression of yaiC in strains grown in LB compared to WT strain. (B) Expression of yaiC in strains grown under low-phosphate (LP) conditions. Expression in LP broth was compared to expression of the corresponding strains grown under high-phosphate (HP) conditions. (C) Expression of fimA in strains grown in LP broth. The expression was compared with those of the WT strain grown in LP broth. The dotted lines correspond to the cutoff of a significant difference in expression. All results shown are the mean values and standard deviations of the results from four biological experiments. Statistical significance was calculated by the Student t test; *, P < 0.05; **, P < 0.005; ***, P < 0.0001; NS, nonsignificant.
We previously showed that growth under phosphate-limiting conditions decreased the expression of type 1 fimbriae (Fig. 2C) (10). This was reflected by similar expression patterns of fimA in the CFT073 and Δpst mutant strains grown in LP broth. As the deletion of yaiC in the pst mutant restored the transcription of fimA in LB, we investigated whether the expression of fimA was restored in the ΔyaiC and the DMY mutants grown in LP broth. Indeed, compared with the WT strain grown in LP broth, fimA was induced 3.4- and 4.0-fold in the CFT073 ΔyaiC and DMY mutants, respectively (Fig. 2C).
These results indicate that induction of the Pho regulon, physiologically or by inactivation of pst, increases the expression of yaiC and consequently represses the expression of type 1 fimbriae. Thereby, these results suggest that yaiC is a repressor of type 1 fimbriae.
Deletion of yaiC restores type 1 fimbria-dependent adhesion of the Pst mutant.
In vitro, we previously demonstrated that the pst mutant adheres to bladder cells as well as the WT strain. However, unlike the WT parent, adhesion of the pst mutant was fim independent and implied that adherence was mediated by other adhesins (10). Nevertheless, the production of other adhesins by the pst mutant was not sufficient to allow efficient colonization of the bladder, as the pst mutant is attenuated in the UTI mouse model (10). Since the deletion of yaiC in the pst mutant restored the expression of type 1 fimbriae, we wondered whether adhesion of the pst yaiC double (DMY) mutant to bladder cells was dependent on type 1 fimbriae. To do so, adhesion to 5637 human bladder epithelial cells was tested in the presence or absence of 1.5% α-d-mannopyranose.
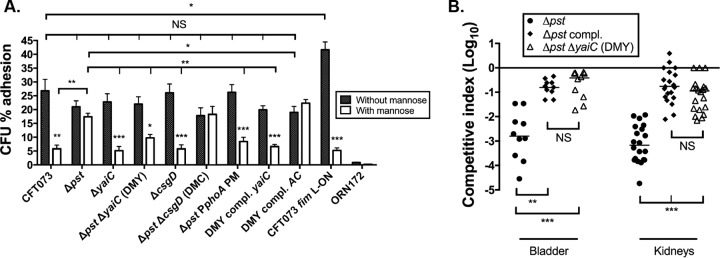
In the absence of mannopyranose, the WT strain adhered to the bladder cells at 26.8% (Fig. 3A). Similarly, the pst and the DMY strains adhered to bladder cells at 21.0 and 22.0%, respectively (Fig. 3A). As previously observed (10), the addition of mannopyranose to the culture medium decreased adherence of the WT strain significantly to 5.8%, whereas it had no significant effect on adhesion of the Δpst mutant strain, which adhered at 17.4% (Fig. 3A). As expected, the deletion of yaiC in the Δpst background restored the type 1 fimbria-dependent adherence to bladder cells, since the presence of mannopyranose decreased the adherence of the DMY strain to 10.7%, which is similar to what is observed in the WT strain (Fig. 3A). Thereby, these results confirm that yaiC is involved in the repression of type 1 fimbriae.
FIG 3.
Adhesion and virulence of yaiC derivative strains. (A) Adherence of strain CFT073 and its derivatives to human 5637 bladder epithelial cells in the presence and absence of 1.5% mannopyranose (mannose). The CFT073 fim-locked-on strain and the E. coli K-12 fim-negative strain ORN172 were used as positive and negative controls, respectively. All results shown are the mean values and standard deviations of the results from four biological experiments. Statistical significance was calculated by the Student t test; *, P < 0.05; **, P < 0.005; ***, P < 0.0001; NS, nonsignificant. (B) CBA/J mice were coinfected with a 1:1 ratio of CFT073 Δlac and either the Δpst mutant or the Δpst ΔyaiC (DMY) mutant strain. Results are presented as the log10 CFU · g−1. Each data point represents a sample from an individual mouse, and the horizontal bars indicate the medians. Each kidney was sampled separately. A Wilcoxon signed-rank test (two-tailed) was used to determine statistical significance; **, P < 0.005; ***, P < 0.0001; NS, nonsignificant.
Deletion of yaiC restored the colonization of the urinary tract in the Pst mutant.
Since YaiC represses the expression of type 1 fimbriae and these fimbriae are important for infection, we tested whether inactivation of yaiC in the pst mutant restores its urinary tract colonization defect. To do so, CBA/J mice were coinfected with a 1:1 ratio of CFT073 Δlac and the Δpst mutant or CFT073 Δlac and the DMY mutant strain. As expected, and as we previously observed (10), at 48 h postinfection (p.i.), the pst mutant was outcompeted 618- and 1,323-fold in the bladder and kidneys, respectively (Fig. 3B). On the other hand, the DMY mutant was only outcompeted 5- and 13-fold in the bladder and kidneys, respectively (Fig. 3B). Although the DMY mutant strain did not colonize the urinary tract at the WT level, it had the same colonization profile as the Δpst compl. mutant strain (Fig. 3B). Thereby, we can conclude that the deletion of yaiC significantly restores the virulence of the pst mutant.
In the pst mutant, expression of yaiC is independent of CsgD.
The transcription of yaiC has been shown to be dependent on CsgD (39). As csgD is regulated by more than 10 transcription factors and several environmental conditions (36, 40, 41), we tested whether induction of the Pho regulon influenced its transcription and, subsequently, yaiC. To do so, qRT-PCR was performed on strains cultured to mid-log phase of growth at 37°C in LB. Interestingly, csgD was not differentially expressed between the WT, the Δpst mutant, and Δpst compl. mutant strains (data not shown). In order to eliminate any potential role of CsgD in yaiC regulation, a nonpolar mutation in csgD was introduced in the pst mutant, and the expression of yaiC was monitored by qRT-PCR. As shown in Fig. 2A, deletion of csgD in the Δpst mutant did not affect yaiC transcription, as it was expressed at the same level as the single Δpst mutant. Furthermore, we tested whether csgD had any effects on type 1 fimbrial expression. As shown in Fig. 1C to E, deletion of csgD in the pst mutant (DMC mutant) had no effect on fimA, fimB, or fimE, or on the orientation of the fimS switch, since their expression and orientation are similar to those of the single pst mutant. These results are also in agreement with the adhesion capacity of the DMC mutant to bladder epithelial cells. Indeed, this mutant adhered to bladder cells in a fim-independent manner, similarly to the single pst mutant (Fig. 3A). Taken together, these results suggest that under conditions in which the Pho regulon is induced, CsgD is not involved in the regulation of type 1 fimbriae. In addition, these results demonstrate that the transcription of yaiC in the pst mutant is independent of CsgD.
In the pst mutant, yaiC is transcribed as part of the phoA-psiF operon and is dependent on the phoA promoter.
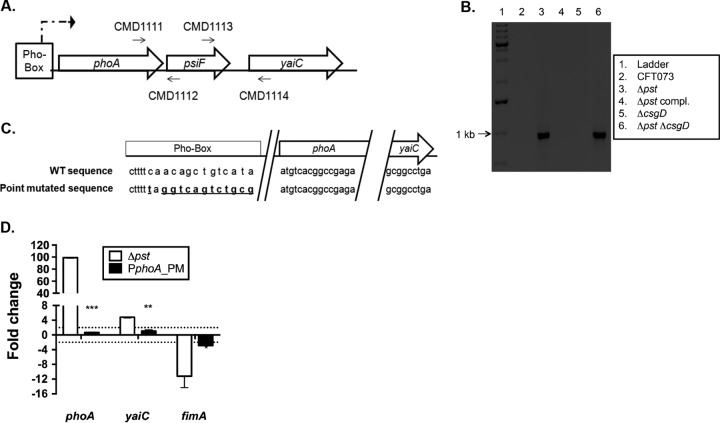
As the expression of yaiC is independent of CsgD in the pst mutant, and as yaiC does not possess a Pho box in its promoter region (5), its induction in the pst mutant and under phosphate starvation conditions seems to be indirectly activated by the Pho regulon. We hypothesized that in the pst mutant, yaiC is cotranscribed as part of the phoA-psiF operon (Fig. 1A). We reasoned this since phoA is positively regulated by PhoB and is strongly induced in a pst mutant (3, 37). Furthermore, an in silico analysis showed that the intergenic region between the phoA-psiF operon and yaiC is only 100 nucleotides. To validate this hypothesis, reverse transcription-PCR (RT-PCR) was performed on the phoA-psiF and yaiC regions from strains cultured to mid-log phase of growth in LB (Fig. 4A and B). The phoA-psiF-yaiC region was reverse transcribed with primers CMD1111 and CMD1114. As expected, no band was observed for the WT, Δpst compl. mutant, or ΔcsgD mutant strains, since the Pho regulon was not induced in these strains under these conditions. However, a 1-kb band, corresponding to the expected product size, was observed for the Δpst and DMC (Δpst ΔcsgD) mutant strains (Fig. 4B), supporting the hypothesis that yaiC is cotranscribed with phoA-psiF.
FIG 4.
yaiC is transcribed as part of the phoA-psiF operon. (A) Schematic representation of how the RT-PCR experiments were performed. The phoA-psiF region was retrotranscribed and amplified with primers CMD1111 and CMD1112, the psiF-yaiC region with primers CMD1113 and CMD1114, and the phoA-yaiC region with primers CMD1111 and CMD1114. (B) Gel electrophoresis of RT-PCR products from amplification of the phoA-psiF region (primers CMD1111 and CMD1114). (C) Schematic representation of the WT and mutated promoter (Pho box) of phoA. The mutated nucleotides are represented by the bold and underlined nucleotides. (D) Expression of phoA, yaiC, and fimA in the pst mutant and its derivative PphoA_PM mutant. The expression was compared to that of the WT strain. The dotted line corresponds to the cutoff of a significant difference in expression. The calculated CT of phoA, yaiC, and fimA was normalized to the CT of the tus gene amplified from the corresponding sample and compared with the WT strain. All results shown are the mean values and standard deviations of the results from four biological experiments. Statistical significance was calculated by the Student t test; *, P < 0.05; **, P < 0.005; ***, P < 0.0001.
As yaiC was cotranscribed with phoA-psiF in the pst mutant, we tested whether its transcription was dependent on the promoter of phoA, which is positively regulated by PhoB. In order to disrupt the expression of phoA-psiF and therefore yaiC, we chromosomally changed 13 nucleotides (see Materials and Methods) (Fig. 4C) in the Pho box of phoA in the pst mutant (PphoA_PM strain). To confirm that the introduced point mutations inhibit phoA expression, qRT-PCR was performed on RNA sampled from the WT, the Δpst mutant, and the PphoA_PM mutant strains cultured to mid-log phase of growth in LB. In the pst mutant, the transcription of phoA was induced 99.0-fold (Fig. 4D). On the other hand, the expression of phoA was abolished in the PphoA_PM mutant strain, as phoA was not differentially expressed compared to the WT strain. Similarly, the expression of yaiC was inhibited in the PphoA_PM mutant strain, whereas it is induced 4.80-fold in the pst single mutant (Fig. 4D). These results strongly suggest that yaiC forms an operon with phoA-psiF and that its transcription is dependent on PhoB.
In addition, the PphoA_PM mutant strain also demonstrated a regain in the expression of type 1 fimbriae. Compared to the WT parent strain, fimA expression is decreased 2.9-fold (Fig. 4D), which is similar to what was observed in the DMY (Δpst ΔyaiC) mutant strain, where the expression of type 1 fimbriae was decreased 4.8-fold (Fig. 1C). Furthermore, adhesion of the PphoA_PM mutant to bladder cells is type 1 fimbria dependent (Fig. 3A). Indeed, adherence of the PphoA_PM mutant strain dropped from 25% to 12.0% in the presence of mannopyranose, which was similar to the type 1 fimbria-dependent adherence of the DMY mutant, which is decreased from 24% to 12.2% upon the addition of mannopyranose (Fig. 3A).
To further demonstrate the role of PphoA-dependent induction of yaiC on expression of type 1 fimbriae in the pst mutant, we introduced either the yaiC gene alone, with its proximal csgD-dependent promoter, or the phoA-psiF-yaiC region, including the PphoA promoter and its Pho box, to the attTn7 site of the DMY mutant strain. As the expression of yaiC in the pst mutant depends on the phoA promoter, the introduction of yaiC alone, from its native promoter, should behave similarly to the DMY (Δpst ΔyaiC) mutant strain. On the other hand, the introduction of phoA-psiF-yaiC in the DMY mutant should resemble the single pst mutant phenotype. To test this hypothesis, the expression of fimA and adhesion properties of these two complemented strains were determined. As expected, the introduction of yaiC in the DMY mutant strain (DMY compl. yaiC) did not affect the expression of fimA, since no difference was observed with the DMY mutant strain (Fig. 1C). However, when the phoA-psiF-yaiC region was introduced in the DMY mutant strain (DMY compl. AC mutant), the expression of fimA was decreased by 13.5-fold, which is similar to the level in the Δpst single mutant (Fig. 1C). The adhesion properties of these two complemented strains to bladder epithelial cells were also distinct. Indeed, the DMY compl. yaiC mutant strain demonstrated type 1 fimbria-dependent adhesion, whereas the DMY compl. AC mutant strain demonstrated type 1 fimbria-dependent adhesion to bladder epithelial cells (Fig. 3A). Thus, adhesion of the DMY compl. AC mutant strain was similar to the adhesion of the single pst mutant.
Taken together, these results demonstrate that under conditions in which the Pho regulon is induced, yaiC is cotranscribed as part of an extended transcriptional unit, which includes phoA-psiF (the phoA-psiF-yaiC operon). Thus, in a pst mutant, yaiC expression is under the control of the phoA promoter and is consequently positively regulated by PhoB.
c-di-GMP influences the expression of type 1 fimbriae.
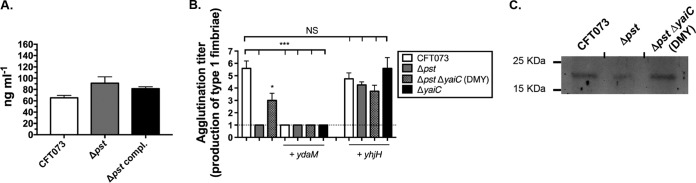
It has been observed that elevated concentrations of c-di-GMP decrease adhesion and invasion and lower the expression of type 1 fimbriae in the adherent-invasive E. coli strain LF82 (42). Since the yaiC gene is involved in biosynthesis of the second messenger c-di-GMP (30, 32) and is induced in the pst mutant, we asked whether the pst mutant produces higher levels of c-di-GMP than the WT strain. To test this hypothesis, we quantified the production of c-di-GMP in different strain backgrounds. As shown in Fig. 5A, the pst mutant produced 91.2 ng · ml−1 c-di-GMP, whereas the WT strain produced 65.4 ng · ml−1. Although the difference was not statistically significant, the pst mutant produced 1.4-fold more c-di-GMP than the WT strain, corresponding to an increase of 25.8 ng · ml−1 of c-di-GMP.
FIG 5.
The c-di-GMP pathway influences the expression of type 1 fimbriae. (A) Production of c-di-GMP in the WT, Δpst mutant, and the Δpst compl. mutant strains. (B) Production of type 1 fimbriae by yeast agglutination assay in strains producing ydaM, from pTRC::ydaM, or yhjH, Each well corresponds to a 2-fold dilution. For example, an agglutination titer of 2 corresponds to a 2-fold dilution, while a titer of 4 corresponds to 8-fold dilution. The dotted line represents the limit of detection that corresponds to the nondiluted sample. (C) Western blot of fimbrial extracts using an anti-FimA serum. All results shown are the mean values and standard deviations of the results from three biological experiments. Statistical significance was calculated by the Student t test; *, P < 0.05; ***, P < 0.0001; NS, nonsignificant.
To confirm that c-di-GMP influences the expression of type 1 fimbriae, the ydaM (GGDEF) and yhjH (EAL) genes were cloned into the inducible pTRC99a vector. Thereby, the production of c-di-GMP by YdaM should decrease the expression of type 1 fimbriae in the WT strain similarly to what is observed in the pst mutant, where the induction of yaiC increased c-di-GMP concentration. On the other hand, the induction of yhjH in the pst mutant will degrade the c-di-GMP and should restore the production of type 1 fimbriae similarly to the WT strain. These plasmid-carried genes were induced in the WT and the Δpst, ΔyaiC, and DMY mutants, and the production of type 1 fimbriae was quantified by a yeast agglutination assay. Thereby, the expression of ydaM in either the WT strain or DMY mutant inhibited the expression of type 1 fimbriae to levels similar to those in the single pst mutant (Fig. 5B). As the pst mutant did not agglutinate yeast, it is not surprising to note that the induction of ydaM had no effect. On the other hand, expression of the yhjH gene in the pst mutant restored the production of type 1 fimbriae to the WT level, while, not surprisingly, it had no effect in the WT and ΔyaiC and Δpst yaiC mutant strains (Fig. 5B).
Since the accumulation of c-di-GMP inhibits the production of type 1 fimbriae, Western blot analysis was performed on the WT and the pst and DMY mutants using an anti-FimA serum. As shown in Fig. 5C, the accumulation of c-di-GMP, through inactivation of pst, inhibits the production of type 1 fimbriae, while the inactivation of yaiC, in the Δpst mutant background, restored the production of type 1 fimbriae to the WT level.
Taken together, these results show that although the increase in c-di-GMP concentration in the pst mutant was not statistically different from that in the WT strain, increased biosynthesis of c-di-GMP through the activation of yaiC in the pst mutant might explain the diminished expression of type 1 fimbriae which results in a decreased capacity to colonize the urinary tract.
DISCUSSION
Inactivation of the Pst system not only constitutively activates the Pho regulon, it also attenuates virulence of pathogenic strains (7, 8). Likewise, in extraintestinal pathogenic E. coli from swine or poultry, attenuation seems to be mainly attributed to alterations in membrane integrity (37, 43–45). In enteropathogenic E. coli, inactivation of the Pst system impairs adhesion to epithelial cells (46, 47). In Vibrio cholerae, the induction of PhoB represses virulence gene expression and induces genes involved in c-di-GMP metabolism (48, 49). In UPEC, we showed that inactivation of Pst attenuates virulence by decreasing the expression of type 1 fimbriae (10). In the current study, we investigated the molecular mechanisms by which activation of the Pho regulon represses the expression of type 1 fimbriae. Herein, we focused on the role of the yaiC-mediated pathway in reducing the expression of type 1 fimbriae.
In the pst mutant, we observed a decrease in the expression of type 1 fimbriae, which is linked to the upregulation of the GGDEF domain-containing gene product of yaiC. By knocking out yaiC in the pst mutant, the expression of type 1 fimbriae was restored. The regain in the expression of type 1 fimbriae in the DMY mutant is demonstrated by the increased type 1 fimbria-dependent adhesion to bladder cells, which was similar to the WT strain. The loss of yaiC in the pst mutant also resulted in a regain in virulence in the UTI mouse model (Fig. 3B). Although the DMY mutant is somewhat outcompeted by the WT strain in the bladder and kidneys, it behaves similarly to the Δpst compl. mutant strain. In the pst mutant, we previously demonstrated that the repression of type 1 fimbriae is linked to the downregulation of fimB, ipuA, and ipbA and to the upregulation of fimE. This differential expression promotes inversion of the fim promoter to the off position (10). As fimB and fimE were not differentially expressed in the DMY mutant strain, the restoration of type 1 fimbrial expression could solely be attributed to the upregulation of ipuA and ipbA (Fig. 1F and G), since these two genes are sufficient for switching the fim promoter and influencing type 1 fimbrial expression (23–25). Since ipuA and/or ipbA are not present in all UPEC or other E. coli strains, regulation of type 1 fimbriae through pst, or through other regulators, may vary among other strains. However, since yaiC is induced in the pst mutant and its deletion restores the expression of type 1 fimbriae, YaiC could be considered a repressor of these fimbriae.
As yaiC does not possess a Pho box in its promoter region and CsgD positively regulates it, we first hypothesized that the Pho regulon may affect yaiC through CsgD. However, we found that under conditions that activate the Pho regulon, the expression of yaiC was dependent on PhoB instead of CsgD. Indeed, the expression of yaiC was dependent on the Pho-regulated promoter of the phoA-psiF operon. Differential regulatory transcription from promoters that are active under distinct conditions has also been observed elsewhere. For instance, in Borrelia burgdorferi, rpoS possesses a short and a long transcript. The short transcript depends on RpoN, whereas the long transcript is dependent on a promoter found 1.5 kb upstream of the rpoS gene (50). The transcription of rpoS from this promoter is via a read-through and includes, in order, the flgI, flgJ, and rpoS genes. Furthermore, in V. cholerae, the acgAB operon is regulated via a read-through transcription from the upstream operon alsDSO (51).
As yaiC is involved in the biosynthesis of c-di-GMP, phosphate starvation seems to be an activating signal of c-di-GMP metabolism. Indeed, cultivation under phosphate-limiting conditions induced the expression of yaiC. This induction was also observed in the pst mutant when cultured in LB. These results are in agreement with what was observed in V. cholerae. Indeed, induction of the Pho regulon activates the transcription of acgAB encoding GGDEF and EAL proteins (49). The expression of acgAB is dependent on PhoB. In the UPEC CFT073 pst mutant, the induction of yaiC increased the intracellular concentration of c-di-GMP (Fig. 5A). Further, the role of c-di-GMP in regulation of type 1 fimbriae was confirmed by expressing the ydaM (GGDEF) or yhjH (EAL) gene in the WT strain and the pst mutant (Fig. 5B). In line with the role of c-di-GMP in the downregulation of type 1 fimbriae, their production was abolished by overexpressing ydaM (GGDEF) in the WT strain; conversely, type 1 fimbriae were restored in the pst mutant by overexpressing yhjH (EAL).
Many groups have reported that increased levels of c-di-GMP attenuate the virulence of pathogenic strains. For example, in Yersinia pestis, inactivation of the EAL gene hmsP increased the production of extracellular polysaccharide (EPS) and decreased virulence (52). In V. cholerae, elevated c-di-GMP levels reduced colonization of the mouse small intestine and decreased expression of the major virulence gene transcriptional activator toxT (53). Furthermore, the phosphodiesterase VieA is necessary for virulence in the mouse infant model and positively regulated the expression of toxT and the cholera toxin ctxAB (54). In E. coli, however, the role of c-di-GMP in virulence is not well studied. In the adherent-invasive E. coli strain LF82, which is associated with Crohn's disease, it has been observed that the c-di-GMP pathway decreased adhesion and invasion of intestinal epithelial cells by repressing the expression of type 1 fimbriae (42). Furthermore, in a clinical UPEC strain, it has been observed that the production of cellulose decreased adhesion to bladder cells and decreased kidney colonization (55). Accordingly, Raterman et al. (56) showed that deregulation of the YfiN diguanylate cyclase in UPEC strain CFT073 can induce the synthesis of curli and cellulose production, which decreased competitive colonization of the murine urinary tract. In our current report, we demonstrated, for the first time, that the regulation of type 1 fimbriae by the Pho regulon implicates the metabolism of c-di-GMP, and that the repression of type 1 fimbriae through c-di-GMP production underlies one of the mechanisms leading to virulence attenuation of the pst mutant in the UTI mouse model (Fig. 3B) (10).
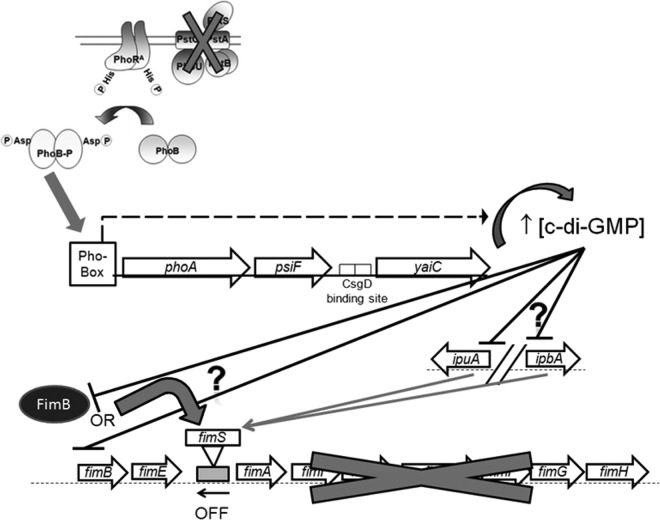
According to the results shown in this study, under conditions in which the Pho regulon is activated, PhoB binds to the Pho box promoter of phoA, leading to the transcription of the phoA-psiF operon. Transcription from this promoter extends through to yaiC. Increased expression of yaiC leads to an elevated intracellular concentration of c-di-GMP. The accumulation of c-di-GMP represses transcription of the fimB, ipuA, and ipbA Fim recombinase genes, leading to increased orientation of the fim promoter in the off position, repressed expression of type 1 fimbriae (Fig. 6), and consequently, decreased urinary tract colonization. As yaiC is transcribed from the phoA promoter and is positively regulated by PhoB, yaiC could be considered a new member of the Pho regulon and a regulator of type 1 fimbriae. It remains to be determined how yaiC (c-di-GMP gene) specifically influences expression of the recombinase genes fimB, ipuA, and ipbA, the fim promoter switch, and type 1 fimbriae (Fig. 6).
FIG 6.
Model illustrating the interactions between the Pho regulon, yaiC, and type 1 fimbriae. Arrows represent an activation/promotion, while ⊥ represents an inhibition. The induction of PhoBR leads to transcription of yaiC from the promoter of phoA, which leads to the biosynthesis of c-di-GMP, repression of ipuA and ipbA, and then reduced expression of the fim operon by promoting the off orientation of fimS. It is not known (question mark on the right) whether c-di-GMP directly or indirectly inhibits the expression of ipuA and ipbA. Furthermore, c-di-GMP could inhibit the recombinase activity instead of its transcription. As for ipuA and ipbA, it is not known (question mark on the left) whether c-di-GMP directly or indirectly affects FimB or fimB.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The E. coli strains and plasmids used in this study are listed in Table 1. Bacteria were grown in lysogeny broth (LB) at 37°C. Bacteria were also grown in MOPS minimal medium (Teknova) supplemented with 0.4% glucose, 0.2% (NH4)2SO4, 1.32 mM K2HPO4, and 1 μg/ml thiamine (high phosphate). MOPS low-phosphate medium contained 1 μM K2HPO4 (57). 5637 human bladder cells (ATCC HTB-9) were grown in RPMI 1640 medium (Wisent Bioproducts) supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 4.5 g/liter glucose, and 1.5 g/liter sodium bicarbonate. Antibiotics and reagents were added as required at the following concentrations: kanamycin, 40 μg/ml; ampicillin, 100 to 200 μg/ml; chloramphenicol, 30 μg/ml; gentamicin, 15 μg/ml; diaminopimelic acid (DAP), 50 μg/ml; 5-bromo-4-chloro-3 indolylphosphate disodium (BCIP), 40 μg/ml; and isopropyl-β-d-1-thiogalactopyranoside (IPTG), 500 μM.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristicsa | Source or reference(s) |
|---|---|---|
| Strains | ||
| CFT073 | UPEC wild-type pyelonephritis strain | 70, 71 |
| MGN-617 | thi thr leu tonA lacY glnV supE DΔasdA4 recA::RP4 2-Tc::Mu [pir]; Kmr | 72 |
| ORN172 | thr-1 leuB thi-1 Δ(argF-lac)U169 xyl-7 ara-13 mtl-2 gal-6 rpsL tonA2 supE44 Δ(fimBEACDFGH)::kan pilG1 | 73 |
| CFT073 Δlac (QT1081) | CFT073 ΔlacZYA::FRT | 66 |
| Δpst mutant (QT1911) | CFT073 ΔpstSCA::FRT | 10 |
| Δpst compl. mutant (QT2117) | QT1911::Tn7T-Gm::pstSCA; Gmr | 10 |
| CFT073 Δfim (QT2138) | CFT073 ΔfimAICDFGH::km; Kmr | 10 |
| CFT073 fim L-ON (QT2285) | CFT073 fimS phase locked-on; Cmr | 10 |
| ΔphoA mutant (QT2334) | CFT073 ΔphoA::FRT | This study |
| ΔpsiF mutant (QT2335) | CFT073 ΔpsiF::FRT | This study |
| Δpst ΔphoA mutant (QT2336) | QT1911 ΔphoA::FRT | This study |
| Δpst ΔpsiF mutant (QT2337) | QT1911 ΔpsiF::FRT | This study |
| Δpst ΔyaiC mutant (DMY; QT2065) | QT1911 ΔyaiC::FRT | This study |
| ΔyaiC mutant (QT2100) | CFT073 ΔyaiC::FRT | This study |
| DMY compl. yaiC mutant (QT2222) | QT1911::Tn7T-Gm::yaiC; Gmr | This study |
| DMY compl. AC mutant (QT2244) | QT1911::Tn7T-Gm::phoA-psiF-yaiC; Gmr | This study |
| PphoA_PM mutant (QT2729) | QT1911:: PphoA_PM (mutations in promoter of phoA | This study |
| DMC mutant (QT2099) | QT1911 ΔcsgD::FRT | This study |
| ΔcsgD mutant (QT2141) | CFT073 ΔcsgD::FRT | This study |
| QT2209 | MGN-617/pIJ297; Apr Gmr | This study |
| QT2208 | MGN-617/pIJ296; Apr Gmr | This study |
| QT2087 | MGN-617/pLOF/Km; Apr Kmr | This study |
| QT2893 | CFT073/pTRC::ydaM; Apr | This study |
| Δpst mutant/pTRC::ydaM (QT2895) | QT1911/pTRC::ydaM; Apr | This study |
| QT2894 | CFT073/pTRC::yhjH; Apr | This study |
| Δpst mutant/pTRC::yhjH (QT2896) | QT1911/pTRC::yhjH; Apr | This study |
| QT2059 | CFT073/pIJ280; Apr | This study |
| QT2067 | QT1911/pIJ280; Apr | This study |
| DMY ΔipuA mutant (QT3015) | QT2065 ΔipuA::Cm; Cmr | This study |
| DMY ΔipbA mutant (QT3016) | QT2065 ΔipbA::Km; Kmr | This study |
| Δ4 mutant (QT3031) | QT3015 ΔipbA::Km; Cmr Kmr | This study |
| Plasmids | ||
| pCP20 | FLP helper plasmid Ts replicon; Apr Cmr | 60 |
| pSTNSK | pST76-K::tnsABCD; Kmr | 62 |
| pKD13 | Template plasmid for amplification of the km cassette bordered by FRT sites; Apr Kmr | 60 |
| pKD46 | λ-Red recombinase plasmid Ts replicon; Apr | 60 |
| pGP-Tn7-Gm | pGP704::Tn7T-Gm; Apr Gmr | 62 |
| pIJ280 | pBAD24::yaiC; Apr | |
| pIJ297 | pGP-Tn7-Gm::yaiC; Apr Gmr | This study |
| pIJ296 | pGP-Tn7-Gm::phoA-psiF-yaiC (AC); Apr Gmr | This study |
| pGEM-T | TA cloning of PCR product; Apr | Promega |
| pSG76C | oriR6K suicide vector possessing an I-SceI cleavage site; Cmr | 64 |
| pGEM::PphoA | pGEM-T:: PphoA-phoA (phoA with its promoter) | This study |
| pGEM::PphoA_PM | pGEM-T::P-phoAPM-phoA; introduction of mutations (PM) into the promoter of phoA; | This study |
| pSG76C:: PphoA_PM | pSG76C::PphoAPM-phoA | This study |
| pPIRK | Helper plasmid carrying the pir gene and the pSC101ts origin; Kmr | 64 |
| pST76-ASceP | PSC101ts origin suicide vector expression the I-SceI meganuclease; Apr | 63 |
| pLOF-Km | Tn10-based transposon vector delivery plasmid; Apr Kmr | 74 |
| pTRC::ydaM | pTRC99a::ydaM; Apr | This study |
| pTRC::yhjH | pTRC99a::yhjH; Apr | This study |
Kmr, kanamycin resistance; Gmr, gentamicin resistance; Cmr, chloramphenicol resistance; Apr, ampicillin resistance; Ts, temperature sensitive.
Transposon mutagenesis.
Transposon mutagenesis was performed as described by Simms and Mobley (58). Briefly, the MGN-617/pLOF-Km) donor strain and recipient strain CFT073 Δpst were cultured overnight (O/N) at 37°C in LB with appropriate antibiotics and supplements. Cultures were gently mixed at a 1:4 donor-to-recipient ratio, placed onto LB agar plates supplemented with DAP and IPTG, and incubated for 5 h at 37°C. Following incubation, the bacterial lawn was suspended in 1 ml of phosphate-buffered saline (PBS), washed twice in PBS, serially diluted, and spread onto LB agar plates supplemented with kanamycin and incubated O/N at 37°C to select the recovery of kanamycin-resistant transposon mutants of the CFT073 Δpst recipient strain. To confirm the loss of the pLOF-Km vector, transconjugants were screened for their susceptibility to ampicillin (100 μg/ml).
Evaluation of type 1 fimbrial production.
The production of type 1 fimbriae by transposon mutants was quantified by a yeast agglutination assay (10, 37). The transposon mutants were cultured in 96-well microtiter plates to mid-log phase of growth. Following centrifugation, the pellet was suspended in 40 μl of PBS and transferred to other microtiter wells containing equal volumes of a 3% commercial yeast suspension in PBS. After 30 min of incubation on ice, yeast aggregation was monitored visually, and the agglutination titer was recorded as the most diluted bacterial sample giving a positive agglutination reaction. To inhibit type 1 fimbria-dependent agglutination, a final concentration of 1.5% α-d-mannopyranose was added to the samples.
Preparation of fimbrial extracts and Western blotting.
Preparation of fimbrial extracts and Western blotting were performed, as previously described (37), with anti-FimA serum from E. coli strain BAM.
Site-specific integration of Tn10.
Transposon site-specific integrations were identified as described by Nichols et al. (59) and as shown in Fig. 1. Chromosomal DNA isolated from the transposon mutants was digested with RsaI. The fragmented DNA was self-ligated with T4 DNA ligase (Fermentas), and a first round of inverted PCR, with primers Pri1 and Pri2 (see Table S1 in the supplemental material), was performed onto the circularized DNA. The PCR product was diluted 1:10, and 1 μl was used as the template for a second round of inverted PCR using Pri3 and Pri4. PCR products from this second round were gel electrophoresed, and fragments were sequenced (Génome Québec Innovation Centre, McGill University). BLASTN was used to identify the site-specific integration site in the genome of the CFT073 Δpst strain.
Construction of nonpolar mutants and complemented strains.
All mutants were generated by the procedure described by Datsenko and Wanner using plasmid pKD3 as the kanamycin resistance cassette (60). The primers used are listed in Table S1 in the supplemental material. Antibiotic cassettes flanked by FLIP recombination target (FRT) sequences were removed by transforming the mutant strains with pCP20 expressing the FLP recombinase (61).
The ΔpstSCA ΔyaiC (DMY) mutant strain was complemented as described by Crépin et al. (62) by inserting the yaiC gene or phoA-psiF yaiC genes with their respective promoters into the chromosomal attTn7 site, resulting in DMY compl. (attTn7::yaiC) and DMY compl. AC (attTn7::phoA-psiF-yaiC) mutant strains, respectively. Briefly, yaiC and its native promoter were amplified with primers CMD1156 and CMD1157 (Table S1) and cloned into the XmaI and XhoI sites of pGP-Tn7-Gm, creating vector pGP-Tn7-Gm-yaiC. The phoA-psiF-yaiC region, with the promoter of phoA, were amplified with primers CMD1260 and CMD1261 and cloned into the XmaI and XhoI sites of pGP-Tn7-Gm, creating vector pGP-Tn7-Gm-AC. The DMY mutant strain (pSTNSK) was conjugated overnight with strain MGN-617 (pGP-Tn7-Gm-yaiC or pGP-Tn7-Gm-AC) at 30°C on LB agar plates supplemented with DAP. After incubation, the bacterial lawn was suspended in 1 ml of PBS, washed twice in PBS, serially diluted, spread on LB agar supplemented with gentamicin, and incubated at 37°C. Colonies were verified for sensitivity to kanamycin and ampicillin, the expected phenotype for integration at attTn7, and loss of the transposase-containing plasmid pSTNSK. Tn7 insertion into the attTn7 site was verified by PCR using primers CMD1070 and CMD1072 (Table S1).
The introduction of point mutations into the promoter (Pho box) of phoA was performed as described by Pósfai et al. (63, 64). The phoA gene and its upstream promoter (Pho box) were amplified with primers CMD1245 and CMD1336 and were cloned in pGEM-T vector (Promega), creating vector pGEM::PphoA. The introduction of 13 point mutations into the Pho box of phoA was performed according to the QuikChange site-directed mutagenesis kit (Stratagene) with primers CMD1334 and CMD1335. The point mutations were confirmed by sequencing the vector with M13-specific primers. The resulting plasmid (pGEM::PphoA_PM) was digested with SacI and SphI and cloned into the respective sites of pSG76-C, creating vector pSG76C::PphoA_PM. Plasmid pSG76C::PphoA_PM was introduced in strain CFT073 Δpst, containing the p-PIRK vector, and grown for 5 h at 30°C. This step allowed the replication of the pSG76C::PphoA_PM vector as the π protein is provided by p-PIRK. Following growth at 30°C, the bacterial suspension was spread on LB with chloramphenicol (LB-Cm) plates and grown 5 h at 42°C and O/N at 37°C. This step was done to inhibit the replication of pSG76C::PphoA_PM while selecting for integration by Cm selection, as the helper plasmid is unable to replicate at these temperatures. In this manner, pSG76C::PphoA_PM integrates by single-crossover recombination at the homologous site, i.e., the phoA gene and its promoter (Pho box) into the CFT073 Δpst mutant strain. The replacement of the native pPhoA promoter with a modified promoter was achieved by introducing the pST76-ASceP vector in the resulting strain. The expression of SceP from pST76-ASceP can introduce a double strand break at the I-SceI homing endonuclease site on pSG76C::PphoA_PM, which can be avoided through homologous recombination between the mutant and the WT allele (64), generating the markerless exchange of the pPhoA promoter. Loss of pST76-ASceP temperature-sensitive plasmid is then achieved by growing the bacteria at 37°C or 42°C. The resulting strain was named PphoA_PM.
Experimental UTI in CBA/J mice.
Experimental infections were carried out using coinfection models as described by Hagberg et al. (65) and Sabri et al. (66). Prior to inoculation, strains were grown for 16 h at 37°C with shaking (250 rpm) in 55 ml of LB medium. Cultures were then centrifuged, and pellets of the WT and derivative strains were mixed 1:1. Six-week-old CBA/J female mice were transurethrally inoculated with 20 μl of the 1:1 mixture containing 5 × 108 CFU of UPEC CFT073 ΔlacZYA strain and 5 × 108 CFU of either CFT073 ΔpstSCA strain or CFT073 ΔpstSCA ΔyaiC (DMY). The CFT073 Δlac strain is as virulent as the CFT073 wild-type parent and presented no statistical differences from the WT strain (66). Furthermore, the Δlac strain provided a differential Lac-negative phenotype on MacConkey agar plates. At 48 h p.i., the mice were euthanized; bladders and kidneys were aseptically removed, homogenized, diluted, and plated onto MacConkey agar to determine bacterial counts.
RNA extraction and quantification of gene expression.
RNAs from bacterial cultures grown to mid-log phase in LB or MOPS minimal medium to mid-log phase were extracted using TRIzol reagent (Invitrogen), according to the manufacturer's recommendations, with the exception that DNase I treatment was performed twice. The iScript cDNA synthesis kit and the SsoFast Evagreen Supermix kit (Bio-Rad) were used for qRT-PCR, according to the manufacturer's instructions. The tus gene was used as a housekeeping control (37). Each qRT-PCR run was done in quadruplicate, and for each reaction, the calculated threshold cycle (CT) was normalized to the CT of the tus gene amplified from the corresponding sample. The fold change was calculated using the 2−ΔΔCT method (67). Genes with a fold change above or below the defined threshold of 2 were considered to be differentially expressed.
Quantification of the on/off state of the fimS region.
Quantification of the orientation of the fimS switch was performed by quantitative PCR (qPCR) with iQ SYBR Green Supermix (Bio-Rad), according to Crépin et al. (10). The qPCR was performed on 10 ng of genomic DNA (gDNA) extracted from bacteria grown to mid-log phase of growth in LB. Primers CMD1246 and CMD1248 were used to amplify the on orientation, while the CMD1247 and CMD1248 primers amplified the off orientation. The threshold cycle (CT) of the on and off orientations was normalized to the CT of the vat gene (amplified with primers CMD96 and CMD97), an uninvertible element. The fold change was calculated using the 2−ΔΔCT method (67). A fold change above or below the defined threshold of 2 was considered to be differentially oriented.
Adhesion assay.
The human bladder epithelial cell line 5637 (ATCC HTB-9) was grown to confluence in 24-well plates in RPMI 1640. UPEC CFT073 and its derivative strains were grown in LB medium at 37°C to mid-log phase of growth (optical density [OD], 0.6). The bacterial cells were centrifuged, washed twice with PBS, resuspended at a 106 CFU · ml−1 suspension in RPMI 1640 medium (Wisent Bio Products, St-Bruno, Canada), supplemented with 10% fetal bovine serum, and added to each well. Bacterium-host cell contact was enhanced by a 5-min centrifugation at 600 × g. At 2 h postadhesion, cells were washed three times and lysed with PBS–0.1% sodium deoxycholate (DOC), serially diluted, and plated onto LB agar plates. Quantification of cell-associated bacteria was performed as previously described (16, 68). To block adherence mediated by type 1 fimbriae, 1.5% α-d-mannopyranose was added to the culture medium.
Determination of intracellular c-di-GMP levels.
c-di-GMP was quantified as described by Waters et al. (69). Bacteria were grown in LB medium to mid-log phase of growth. After cell lysis, supernatants were collected and analyzed by liquid chromatography-tandem mass spectrometry on a Finnigan TSQ Quantum Discovery MAX on a Quattro-Micro triple quadrupole mass spectrometer (Waters Corporation, Milford, MA), coupled with an Alliance high-performance liquid chromatography (HPLC) system (Waters Corporation). The MassLynx software from Waters was used for instrument control, data acquisition, and data processing. c-di-GMP was detected by using selected reaction monitoring (SRM) in negative ionization mode at m/z 689.0/150.1. SRM was used for simultaneous tracking of m/z 6,893,344 at 32 eV and m/z 6,893,150 at 45 eV, which gave a signal ratio of 1:0.4. The mass spectrometry parameters were as follows: cone energy, 45 eV; collision energy, 42 eV; capillary, 3,300 V; desolvation gas, 500 liters/h; and cone gas, 0 liters/h. The temperature source was 120°C and the temperature desolvation was 450°C. The interscan delay was 0.01 s. Purified c-di-GMP (Biolog, Germany) was used to generate the standard curve. Data are given as the c-di-GMP concentration in nanograms per milliliter per unit of optical density at 600 nm and are the means of the results from three independent experiments. Each sample was quantified in duplicate and showed less than 10% variation between duplicates.
Expression of GGDEF and EAL genes from an inducible promoter.
The yaiC gene was amplified with primers CMD1099 and CMD1100. The amplified product was then cloned into pBAD24 at the XbaI and HindIII sites, creating plasmid pIJ280. The induction of yaiC from the pBAD promoter was induced with 0.05% l-arabinose. The gene ydaM (GGDEF) was amplified with primers CMD1461 and CMD1462 and yhjH (EAL) with primers CMD1463 and CMD1464. The respective genes were cloned into the NcoI and PstI sites of pTRC99a (Pharmacia Biotech). Gene expression was induced by adding 50 μM IPTG to the culture medium.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to France Daigle (Université de Montréal), who kindly provided the pLOF-Km vector. We also thank Christine Martin for assistance and collaboration with the Metabolism Exploration: from genes to metabolites (PFEM) platform (INRA-Clermont-Ferrand) for quantification of the c-di-GMP.
S.C. was supported by scholarships from the Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT), the Fondation Armand-Frappier and the Centre de Recherche en Infectiologie Porcine (CRIP). G.P. was supported by scholarship from the Fondation Armand-Frappier. This work was supported by a Canada Research Chair (2009-2014) and from the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grants (RGPIN 250129-07 and 2014-06622) to C.M.D. and by an NSERC Discovery grant (RGPIN-2015-05373) to J.H.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00168-17.
REFERENCES
- 1.West AH, Stock AM. 2001. Histidine kinases and response regulator proteins in two-component signaling systems. Trends Biochem Sci 26:369–376. doi: 10.1016/S0968-0004(01)01852-7. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh YJ, Wanner BL. 2010. Global regulation by the seven-component Pi signaling system. Curr Opin Microbiol 13:198–203. doi: 10.1016/j.mib.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wanner BL. 1996. Phosphorus assimilation and control of the phosphate regulon, p 1357–1381. In Neidhardt RCI, Ingraham JL, Lin EEC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella: cellular and molecular biology, vol 1 American Society for Microbiology, Washington, DC. [Google Scholar]
- 4.Blanco AG, Sola M, Gomis-Ruth FX, Coll M. 2002. Tandem DNA recognition by PhoB, a two-component signal transduction transcriptional activator. Structure 10:701–713. doi: 10.1016/S0969-2126(02)00761-X. [DOI] [PubMed] [Google Scholar]
- 5.Yuan ZC, Zaheer R, Morton R, Finan TM. 2006. Genome prediction of PhoB regulated promoters in Sinorhizobium meliloti and twelve proteobacteria. Nucleic Acids Res 34:2686–2697. doi: 10.1093/nar/gkl365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chekabab SM, Jubelin G, Dozois CM, Harel J. 2014. PhoB activates Escherichia coli O157:H7 virulence factors in response to inorganic phosphate limitation. PLoS One 9:e94285. doi: 10.1371/journal.pone.0094285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crépin S, Chekabab SM, Le Bihan G, Bertrand N, Dozois CM, Harel J. 2011. The Pho regulon and the pathogenesis of Escherichia coli. Vet Microbiol 153:82–88. doi: 10.1016/j.vetmic.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 8.Lamarche MG, Wanner BL, Crépin S, Harel J. 2008. The phosphate regulon and bacterial virulence: a regulatory network connecting phosphate homeostasis and pathogenesis. FEMS Microbiol Rev 32:461–473. doi: 10.1111/j.1574-6976.2008.00101.x. [DOI] [PubMed] [Google Scholar]
- 9.Santos-Beneit F. 2015. The Pho regulon: a huge regulatory network in bacteria. Front Microbiol 6:402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crépin S, Houle S, Charbonneau ME, Mourez M, Harel J, Dozois CM. 2012. Decreased expression of type 1 fimbriae by a pst mutant of uropathogenic Escherichia coli reduces urinary tract infection. Infect Immun 80:2802–2815. doi: 10.1128/IAI.00162-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hannan TJ, Totsika M, Mansfield KJ, Moore KH, Schembri MA, Hultgren SJ. 2012. Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection. FEMS Microbiol Rev 36:616–648. doi: 10.1111/j.1574-6976.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielubowicz GR, Mobley HL. 2010. Host-pathogen interactions in urinary tract infection. Nat Rev Urol 7:430–441. doi: 10.1038/nrurol.2010.101. [DOI] [PubMed] [Google Scholar]
- 13.Rahdar M, Rashki A, Miri HR, Rashki Ghalehnoo M. 2015. Detection of pap, sfa, afa, foc, and fim adhesin-encoding operons in uropathogenic Escherichia coli isolates collected from patients with urinary tract infection. Jundishapur J Microbiol 8:e22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stærk K, Khandige S, Kolmos HJ, Moller-Jensen J, Andersen TE. 2016. Uropathogenic Escherichia coli express type 1 fimbriae only in surface-adherent populations under physiological growth conditions. J Infect Dis 213:386–394. doi: 10.1093/infdis/jiv422. [DOI] [PubMed] [Google Scholar]
- 15.Conover MS, Hadjifrangiskou M, Palermo JJ, Hibbing ME, Dodson KW, Hultgren SJ. 2016. Metabolic requirements of Escherichia coli in intracellular bacterial communities during urinary tract infection pathogenesis. mBio 7(2):e00104-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J 19:2803–2812. doi: 10.1093/emboj/19.12.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snyder JA, Haugen BJ, Buckles EL, Lockatell CV, Johnson DE, Donnenberg MS, Welch RA, Mobley HL. 2004. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect Immun 72:6373–6381. doi: 10.1128/IAI.72.11.6373-6381.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subashchandrabose S, Hazen TH, Brumbaugh AR, Himpsl SD, Smith SN, Ernst RD, Rasko DA, Mobley HL. 2014. Host-specific induction of Escherichia coli fitness genes during human urinary tract infection. Proc Natl Acad Sci U S A 111:18327–18332. doi: 10.1073/pnas.1415959112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orndorff PE, Falkow S. 1984. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol 159:736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abraham JM, Freitag CS, Clements JR, Eisenstein BI. 1985. An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci U S A 82:5724–5727. doi: 10.1073/pnas.82.17.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klemm P. 1986. Two regulatory fim genes, fimB and fimE, control the phase variation of type 1 fimbriae in Escherichia coli. EMBO J 5:1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gally DL, Leathart J, Blomfield IC. 1996. Interaction of FimB and FimE with the fim switch that controls the phase variation of type 1 fimbriae in Escherichia coli K-12. Mol Microbiol 21:725–738. doi: 10.1046/j.1365-2958.1996.311388.x. [DOI] [PubMed] [Google Scholar]
- 23.Bryan A, Roesch P, Davis L, Moritz R, Pellett S, Welch RA. 2006. Regulation of type 1 fimbriae by unlinked FimB- and FimE-like recombinases in uropathogenic Escherichia coli strain CFT073. Infect Immun 74:1072–1083. doi: 10.1128/IAI.74.2.1072-1083.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannan TJ, Mysorekar IU, Chen SL, Walker JN, Jones JM, Pinkner JS, Hultgren SJ, Seed PC. 2008. LeuX tRNA-dependent and -independent mechanisms of Escherichia coli pathogenesis in acute cystitis. Mol Microbiol 67:116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarkar S, Roberts LW, Phan MD, Tan L, Lo AW, Peters KM, Paterson DL, Upton M, Ulett GC, Beatson SA, Totsika M, Schembri MA. 2016. Comprehensive analysis of type 1 fimbriae regulation in fimB-null strains from the multidrug resistant Escherichia coli ST131 clone. Mol Microbiol 101:1069–1087. doi: 10.1111/mmi.13442. [DOI] [PubMed] [Google Scholar]
- 26.Jenal U, Reinders A, Lori C. 2017. Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol 15:271–284. doi: 10.1038/nrmicro.2016.190. [DOI] [PubMed] [Google Scholar]
- 27.Römling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryjenkov DA, Simm R, Romling U, Gomelsky M. 2006. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J Biol Chem 281:30310–30314. doi: 10.1074/jbc.C600179200. [DOI] [PubMed] [Google Scholar]
- 29.Hengge R, Galperin MY, Ghigo JM, Gomelsky M, Green J, Hughes KT, Jenal U, Landini P. 2015. Systematic nomenclature for GGDEF and EAL domain-containing cyclic di-GMP turnover proteins of Escherichia coli. J Bacteriol 198:7–11. doi: 10.1128/JB.00424-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Römling U, Rohde M, Olsen A, Normark S, Reinkoster J. 2000. AgfD, the checkpoint of multicellular and aggregative behaviour in Salmonella Typhimurium regulates at least two independent pathways. Mol Microbiol 36:10–23. doi: 10.1046/j.1365-2958.2000.01822.x. [DOI] [PubMed] [Google Scholar]
- 31.Römling U, Sierralta WD, Eriksson K, Normark S. 1998. Multicellular and aggregative behaviour of Salmonella Typhimurium strains is controlled by mutations in the agfD promoter. Mol Microbiol 28:249–264. doi: 10.1046/j.1365-2958.1998.00791.x. [DOI] [PubMed] [Google Scholar]
- 32.Simm R, Morr M, Kader A, Nimtz M, Romling U. 2004. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol Microbiol 53:1123–1134. doi: 10.1111/j.1365-2958.2004.04206.x. [DOI] [PubMed] [Google Scholar]
- 33.Zogaj X, Bokranz W, Nimtz M, Romling U. 2003. Production of cellulose and curli fimbriae by members of the family Enterobacteriaceae isolated from the human gastrointestinal tract. Infect Immun 71:4151–4158. doi: 10.1128/IAI.71.7.4151-4158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brombacher E, Baratto A, Dorel C, Landini P. 2006. Gene expression regulation by the Curli activator CsgD protein: modulation of cellulose biosynthesis and control of negative determinants for microbial adhesion. J Bacteriol 188:2027–2037. doi: 10.1128/JB.188.6.2027-2037.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brombacher E, Dorel C, Zehnder AJ, Landini P. 2003. The curli biosynthesis regulator CsgD co-ordinates the expression of both positive and negative determinants for biofilm formation in Escherichia coli. Microbiology 149:2847–2857. doi: 10.1099/mic.0.26306-0. [DOI] [PubMed] [Google Scholar]
- 36.Barnhart MM, Chapman MR. 2006. Curli biogenesis and function. Annu Rev Microbiol 60:131–147. doi: 10.1146/annurev.micro.60.080805.142106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crépin S, Lamarche MG, Garneau P, Seguin J, Proulx J, Dozois CM, Harel J. 2008. Genome-wide transcriptional response of an avian pathogenic Escherichia coli (APEC) pst mutant. BMC Genomics 9:568. doi: 10.1186/1471-2164-9-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertrand N, Houle S, LeBihan G, Poirier E, Dozois CM, Harel J. 2010. Increased Pho regulon activation correlates with decreased virulence of an avian pathogenic Escherichia coli O78 strain. Infect Immun 78:5324–5331. doi: 10.1128/IAI.00452-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simm R, Fetherston JD, Kader A, Romling U, Perry RD. 2005. Phenotypic convergence mediated by GGDEF-domain-containing proteins. J Bacteriol 187:6816–6823. doi: 10.1128/JB.187.19.6816-6823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogasawara H, Yamada K, Kori A, Yamamoto K, Ishihama A. 2010. Regulation of the Escherichia coli csgD promoter: interplay between five transcription factors. Microbiology 156:2470–2483. doi: 10.1099/mic.0.039131-0. [DOI] [PubMed] [Google Scholar]
- 41.Pesavento C, Becker G, Sommerfeldt N, Possling A, Tschowri N, Mehlis A, Hengge R. 2008. Inverse regulatory coordination of motility and curli-mediated adhesion in Escherichia coli. Genes Dev 22:2434–2446. doi: 10.1101/gad.475808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Claret L, Miquel S, Vieille N, Ryjenkov DA, Gomelsky M, Darfeuille-Michaud A. 2007. The flagellar sigma factor FliA regulates adhesion and invasion of Crohn disease-associated Escherichia coli via a cyclic dimeric GMP-dependent pathway. J Biol Chem 282:33275–33283. doi: 10.1074/jbc.M702800200. [DOI] [PubMed] [Google Scholar]
- 43.Lamarche MG, Dozois CM, Daigle F, Caza M, Curtiss R III, Dubreuil JD, Harel J. 2005. Inactivation of the pst system reduces the virulence of an avian pathogenic Escherichia coli O78 strain. Infect Immun 73:4138–4145. doi: 10.1128/IAI.73.7.4138-4145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamarche MG, Harel J. 2010. Membrane homeostasis requires intact pst in extraintestinal pathogenic Escherichia coli. Curr Microbiol 60:356–359. doi: 10.1007/s00284-009-9549-x. [DOI] [PubMed] [Google Scholar]
- 45.Lamarche MG, Kim SH, Crepin S, Mourez M, Bertrand N, Bishop RE, Dubreuil JD, Harel J. 2008. Modulation of hexa-acyl pyrophosphate lipid A population under Escherichia coli phosphate (Pho) regulon activation. J Bacteriol 190:5256–5264. doi: 10.1128/JB.01536-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng C, Tennant SM, Azzopardi KI, Bennett-Wood V, Hartland EL, Robins-Browne RM, Tauschek M. 2009. Contribution of the pst-phoU operon to cell adherence by atypical enteropathogenic Escherichia coli and virulence of Citrobacter rodentium. Infect Immun 77:1936–1944. doi: 10.1128/IAI.01246-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferreira GM, Spira B. 2008. The pst operon of enteropathogenic Escherichia coli enhances bacterial adherence to epithelial cells. Microbiology 154:2025–2036. doi: 10.1099/mic.0.2008/016634-0. [DOI] [PubMed] [Google Scholar]
- 48.Pratt JT, Ismail AM, Camilli A. 2010. PhoB regulates both environmental and virulence gene expression in Vibrio cholerae. Mol Microbiol 77:1595–1605. doi: 10.1111/j.1365-2958.2010.07310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pratt JT, McDonough E, Camilli A. 2009. PhoB regulates motility, biofilms, and cyclic di-GMP in Vibrio cholerae. J Bacteriol 191:6632–6642. doi: 10.1128/JB.00708-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lybecker MC, Samuels DS. 2007. Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol Microbiol 64:1075–1089. doi: 10.1111/j.1365-2958.2007.05716.x. [DOI] [PubMed] [Google Scholar]
- 51.Kovacikova G, Lin W, Skorupski K. 2005. Dual regulation of genes involved in acetoin biosynthesis and motility/biofilm formation by the virulence activator AphA and the acetate-responsive LysR-type regulator AlsR in Vibrio cholerae. Mol Microbiol 57:420–433. doi: 10.1111/j.1365-2958.2005.04700.x. [DOI] [PubMed] [Google Scholar]
- 52.Bobrov AG, Kirillina O, Ryjenkov DA, Waters CM, Price PA, Fetherston JD, Mack D, Goldman WE, Gomelsky M, Perry RD. 2011. Systematic analysis of cyclic di-GMP signalling enzymes and their role in biofilm formation and virulence in Yersinia pestis. Mol Microbiol 79:533–551. doi: 10.1111/j.1365-2958.2010.07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamayo R, Schild S, Pratt JT, Camilli A. 2008. Role of cyclic di-GMP during el tor biotype Vibrio cholerae infection: characterization of the in vivo-induced cyclic Di-GMP phosphodiesterase CdpA. Infect Immun 76:1617–1627. doi: 10.1128/IAI.01337-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tischler AD, Camilli A. 2005. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect Immun 73:5873–5882. doi: 10.1128/IAI.73.9.5873-5882.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kai-Larsen Y, Luthje P, Chromek M, Peters V, Wang X, Holm A, Kadas L, Hedlund KO, Johansson J, Chapman MR, Jacobson SH, Romling U, Agerberth B, Brauner A. 2010. Uropathogenic Escherichia coli modulates immune responses and its curli fimbriae interact with the antimicrobial peptide LL-37. PLoS Pathog 6:e1001010. doi: 10.1371/journal.ppat.1001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raterman EL, Shapiro DD, Stevens DJ, Schwartz KJ, Welch RA. 2013. Genetic analysis of the role of yfiR in the ability of Escherichia coli CFT073 to control cellular cyclic dimeric GMP levels and to persist in the urinary tract. Infect Immun 81:3089–3098. doi: 10.1128/IAI.01396-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bougdour A, Gottesman S. 2007. ppGpp regulation of RpoS degradation via anti-adaptor protein IraP. Proc Natl Acad Sci U S A 104:12896–12901. doi: 10.1073/pnas.0705561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simms AN, Mobley HL. 2008. Multiple genes repress motility in uropathogenic Escherichia coli constitutively expressing type 1 fimbriae. J Bacteriol 190:3747–3756. doi: 10.1128/JB.01870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nichols BP, Shafiq O, Meiners V. 1998. Sequence analysis of Tn10 insertion sites in a collection of Escherichia coli strains used for genetic mapping and strain construction. J Bacteriol 180:6408–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cherepanov PP, Wackernagel W. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9–14. doi: 10.1016/0378-1119(95)00193-A. [DOI] [PubMed] [Google Scholar]
- 62.Crepin S, Harel J, Dozois CM. 2012. Chromosomal complementation using Tn7 transposon vectors in Enterobacteriaceae. Appl Environ Microbiol 78:6001–6008. doi: 10.1128/AEM.00986-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pósfai G, Kolisnychenko V, Bereczki Z, Blattner FR. 1999. Markerless gene replacement in Escherichia coli stimulated by a double-strand break in the chromosome. Nucleic Acids Res 27:4409–4415. doi: 10.1093/nar/27.22.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pósfai G, Koob MD, Kirkpatrick HA, Blattner FR. 1997. Versatile insertion plasmids for targeted genome manipulations in bacteria: isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. J Bacteriol 179:4426–4428. doi: 10.1128/jb.179.13.4426-4428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hagberg L, Engberg I, Freter R, Lam J, Olling S, Svanborg Eden C. 1983. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun 40:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sabri M, Houle S, Dozois CM. 2009. Roles of the extraintestinal pathogenic Escherichia coli ZnuACB and ZupT zinc transporters during urinary tract infection. Infect Immun 77:1155–1164. doi: 10.1128/IAI.01082-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 68.Léveillé S, Caza M, Johnson JR, Clabots C, Sabri M, Dozois CM. 2006. Iha from an Escherichia coli urinary tract infection outbreak clonal group A strain is expressed in vivo in the mouse urinary tract and functions as a catecholate siderophore receptor. Infect Immun 74:3427–3436. doi: 10.1128/IAI.00107-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Waters CM, Lu W, Rabinowitz JD, Bassler BL. 2008. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J Bacteriol 190:2527–2536. doi: 10.1128/JB.01756-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV, Jones BD, Warren JW. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun 58:1281–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Welch RA, Burland V, Plunkett G III, Redford P, Roesch P, Rasko D, Buckles EL, Liou SR, Boutin A, Hackett J, Stroud D, Mayhew GF, Rose DJ, Zhou S, Schwartz DC, Perna NT, Mobley HL, Donnenberg MS, Blattner FR. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A 99:17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dozois CM, Dho-Moulin M, Bree A, Fairbrother JM, Desautels C, Curtiss R III. 2000. Relationship between the Tsh autotransporter and pathogenicity of avian Escherichia coli and localization and analysis of the Tsh genetic region. Infect Immun 68:4145–4154. doi: 10.1128/IAI.68.7.4145-4154.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woodall LD, Russell PW, Harris SL, Orndorff PE. 1993. Rapid, synchronous, and stable induction of type 1 piliation in Escherichia coli by using a chromosomal lacUV5 promoter. J Bacteriol 175:2770–2778. doi: 10.1128/jb.175.9.2770-2778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Herrero M, de Lorenzo V, Timmis KN. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in Gram-negative bacteria. J Bacteriol 172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.