Abstract
Acromegaly is usually not a difficult condition to diagnose once the possibility of this disease has been raised. However, a few conditions present with some aspects of acromegaly or gigantism but without growth hormone (GH) excess. Such cases are described as "pseudoacromegaly" or "acromegaloidism". Here we describe a female patient investigated for GH excess at 10 years of age for tall stature since infancy (height and weight > +3 standard deviations) and typical acromegalic features, including large hands/feet, large jaw, tongue, hoarse deep voice, and headache. Results of radiography of the sella turcica and GH response at an oral glucose tolerance test and insulin–arginine– thyrotrophin–luteinizing hormone–releasing hormone test were normal. Ethinylestradiol and medroxyprogesterone were given for 2 years; this successfully stopped further height increase. Although the patient's growth rate plateaued, coarsening of the facial features and acral enlargement also led to investigations for suspicion of acromegaly at 23 and 36 years of age, both with negative results. On referral at the age of 49 years, she had weight gain, sweating, sleep apnea, headaches, joint pain, and enlarged tongue. Endocrine assessment again showing normal GH axis was followed by genetic testing with a macrocephaly/overgrowth syndrome panel. A de novo mutation in the NSD1 gene (c.6605G>C; p.Cys2202Ser) was demonstrated. Mutations affecting the same cysteine residue have been identified in patients with Sotos syndrome. In summary, Sotos syndrome and other overgrowth syndromes can mimic the clinical manifestations of acromegaly or gigantism. Genetic assessment could be helpful in these cases.
Keywords: NSD1 gene, overgrowth, pseudoacromegaly, Sotos syndrome
This case report describes a patient with tall stature and acromegalic features, but no GH excess, at four independent evaluations from ages 10 to 49 years. Genetic studies showed a novel NSD1-mutation causing Sotos syndrome.
Diagnosis of acromegaly is often delayed several years after onset of symptoms [1]. However, once the disease is suspected, the diagnostic process is usually uncomplicated [2]. Patients occasionally present with clinical aspects of acromegaly or gigantism but without growth hormone (GH) excess: pseudoacromegaly or acromegaloidism. Here we present such a case and the diagnostic work-up.
1. Case Report
In 2012, a 49-year-old woman was referred to the endocrine clinic for suspicion of acromegaly. This was her fourth referral and investigation for suspected GH excess since the age of 10 years.
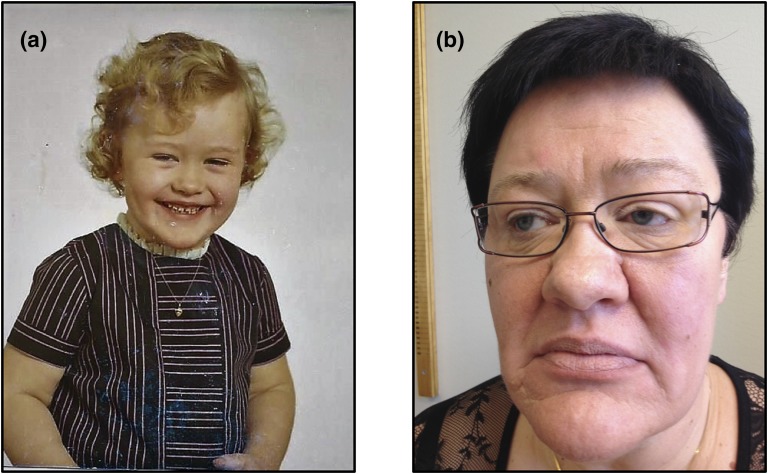
There was no family history of abnormal growth: The patient's mother’s height was 164 cm (−0.2 standard deviation [SD]), her father’s height was 175 cm (−0.5 SD), and both her brother and her sister were of normal height. She was born at 41 weeks' gestation, her length at birth was 52 cm (+1 SD), and her birthweight was 4120 g (+1.5 SDs). At 9 months of age, she weighed 16 kg (> +3 SDs), and her shoe size was 28 in the European (EU) sizing scheme. From age 7 years, her height was above +3 SDs. She had her first teeth at 2 months, and at age 3 years permanent teeth began to replace her deciduous teeth (normal age range, 5 to 7 years); at age 8 years, she had the teeth maturation of a 12-year-old. Her performance in school was below average, with difficulties in concentration and learning. However, no formal cognitive tests were performed. The parents did notice her early accelerated growth rate, but because she was their first child they did not seek medical attention [Fig. 1(a)].
Figure 1.
Patient at ages 5 (a) and 49 (b) years.
At 10 years of age, she was seen by an ophthalmologist because of apparent visual problems. Ophthalmologic status, including Goldmann perimetry, was normal, but as a result of apparent acromegalic features she was referred to the pediatric clinic. She was large, at a height of 160 cm (> +3 SDs) and weight of 60 kg (> +3 SDs), with skeletal age corresponding to 15 years and a full set of permanent teeth except wisdom teeth. However, pubertal development corresponded to her chronologic age: Tanner stages I (breast) and II (pubic hair). Typical acromegalic features were noted: large hands and feet [shoe size, 43 EU], large jaw and tongue, and hoarse and deep voice. Endocrine evaluation (GH, oral glucose tolerance test, insulin–arginine–thyrotrophin-releasing hormone–luteinizing hormone–releasing hormone test, other pituitary hormones, and radiography of the sella turcica) was normal. She was treated with ethinylestradiol and medroxyprogesterone; this treatment successfully reduced and stopped further gain in height but increased her weight gain. Treatment was finally discontinued because of heavy menstrual bleeding and development of a uterus myoma after almost 2 years’ treatment. At this point (age 12 years), her height was 171 cm and her weight was 78 kg.
During the next 40 years, she trained and worked as an assistant nurse. She had an operation for carpal tunnel syndrome (age 32 years), hallux valgus (age 34 years), and osteoarthritis in her wrist (age 45 years). Because of endometriosis and large endometrial cysts, she had a left salpingo-oophorectomy and resection of a rather large cyst in the right ovary at age 31 years. At age 39 years she noted vitiligo; she was also diagnosed with mild hyperthyroidism due to an autonomous thyroid nodule, which was treated with radioiodine followed by stable 125 µg/d l-thyroxine replacement therapy. She is also receiving oral vitamin B12 treatment.
At 23 years of age, she was referred to the endocrine clinic because of clinical suspicion of acromegaly. Endocrine evaluation, including a GH curve, was normal, and no pituitary adenoma was found on pituitary computed tomography.
At 36 years of age, she was referred from a fertility clinic, where acromegaly was again suspected; endocrine evaluation was repeated and again was normal. Efforts to induce pregnancy, including in vitro fertilization, were unsuccessful. The couple adopted a daughter in 2003.
At 49 years of age, she was referred for the fourth time for suspicion of acromegaly with fatigue, increasing swelling of hands and feet, and increasing size of the tongue. She also had numbness; weight gain; pain in the knees, hands, fingers, and lumbar back; headaches; and depression. She was increasingly troubled with sweating and snoring. At the time of this referral, the magnitude of her health problems had led to sick leave. She was generally obese (height, 175 cm; weight, 112 kg; body mass index, 36.6 kg/m2); her blood pressure was 125/75 mm Hg; and she showed acromegalic features, with moderate enlargement of the tongue, nose, and lips, large hands (index finger diameter, 28 mm), and feet (shoe size, 45 EU) [Fig. 1(b)]. Results of routine laboratory tests, including pituitary hormone levels, were unremarkable. Magnetic resonance imaging of the sella showed no pituitary adenoma, and endocrine evaluation showed no GH excess, with an insulin-like growth factor-1 level of 205 to 213 µg/L (reference range, 54 to 307 µg/L), normal GH day curve (mean GH level, 0.167 µg/L; minimum, 0.034 µg/L), and normal GH suppression in response to an oral glucose tolerance test (GH minimum, 0.033 µg/L).
Because of her history of excessive growth in childhood, genetic testing with a panel for macrocephaly/overgrowth syndrome genes was performed.
2. Genetic Studies
DNA was isolated from whole blood by using the QIAamp DNA Blood Maxi kit (Qiagen, Hilden, Germany). Sequencing of the proband’s genomic DNA for the macrocephaly/overgrowth syndromes panel (NSD1, CUL4B, EZH2, GLI3, MED12, NFIX, PHF6, PTCH, PTEN, and UPF3B) was performed at GeneDx (Gaithersburg, MD) using a HiSeq instrument (Illumina, San Diego, CA). Confirmation of the proband’s mutation and her parents' genotyping were performed with Sanger sequencing (primers: ACCAGCAGGGAAATGGGAAT and GGAGGCACATACTCACGGAT).
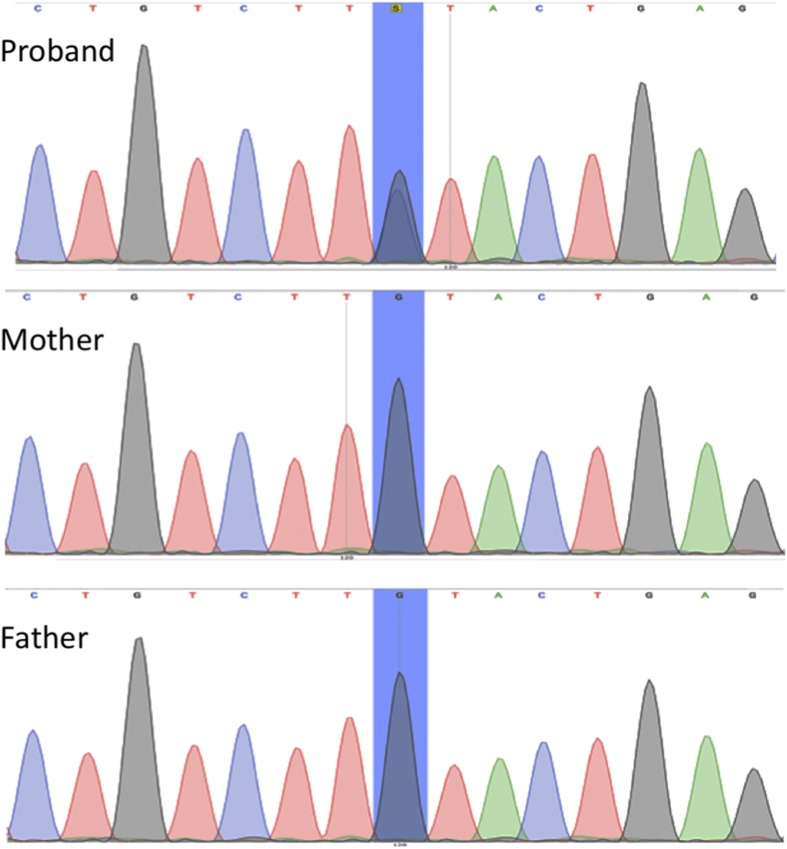
A heterozygous mutation was identified in the NSD1 gene (c.6605G>C; p.Cys2202Ser) (Fig. 2). This variant has not been previously described, but mutations affecting the same cysteine residue (C2202R and C2202Y) have also been reported in the Human Gene Mutation Database in association with Sotos syndrome and gigantism [3]. This mutation is located in the fifth plant homeodomain (PHD-V), a cysteine- and histidine-rich zinc finger–like motif, where other missense mutations have also been described in patients with Sotos syndrome [4]. The mutation has likely arisen de novo because it was not identified in the analysis of the patient's parents’ genome (Fig. 2).
Figure 2.
Sequencing electropherogram of NSD1 exon 23 from the patient (top) and her parents (bottom). The double peak (in blue) confirms the presence of a de novo heterozygous missense mutation at c.6605G>C (p.Cys2202Ser). No mutation was found in the patient's mother and father.
3. Discussion
Sotos syndrome (Mendelian Influence in Man 117550) was first described by Sotos et al. in 1964, as a congenital overgrowth syndrome with clinical features, including excessive overgrowth with advanced bone age, acromegalic features, macrocephaly, characteristic facial appearance, and intellectual disability [5–8]. Additional features that may be present include neonatal hypotonia, neonatal jaundice, seizures, scoliosis, strabismus, congenital heart defects, hypothyroidism, hypopigmentation, and cancer [5–8]. Because macrocephaly is a feature of the syndrome, "cerebral gigantism" was an early term for this condition. Interestingly, a note in the pediatric records of our patient stated that clinical features of cerebral gigantism were absent (age 10 years).
In 2002, the syndrome was associated with haploinsufficiency of the nuclear receptor SET domain protein 1 (NSD1) gene on chromosome 5, first in cases with microdeletions of 5q35 and later also with intragenic mutations of the NSD1 gene, which is the most common cause of Sotos syndrome [7]. Sotos syndrome is an autosomal dominant disorder, and most cases are due to de novo mutations [7, 8]. These data suggest that the underlying NSD1 mutational mechanism may influence reproductive function, and delayed menarche, oligomenorrhea, spontaneous abortions, and stillbirths have been reported in patients with Sotos syndrome [9].
NSD1 encodes a histone methyltransferase involved in chromatin regulation, which is important for multiple aspects of normal embryonic development. NSD1 binds near various promoter elements to regulate transcription via interactions with histone H3 methylation and recruitment of RNA polymerase II and has been suggested to both negatively and positively regulate transcription [7]. In addition to its causal role in Sotos syndrome, NSD1 is a proto-oncogene, and epigenetic alterations induced by NSD1 may also be implicated in malignancies [7, 8].
Although pituitary gigantism and acromegaly were suspected and excluded repeatedly in this patient, the correct diagnosis, Sotos syndrome, was delayed for several decades. Sotos syndrome was first described in 1964, and the pediatric evaluation in our patient took place in 1974. In current pediatric practice, tall stature with advanced bone age and some level of learning difficulties would lead to suspicion of Sotos syndrome [10]. However, the wide variability of the phenotype of Sotos syndrome associated with NSD1 mutations can make the diagnosis very difficult [6–8]. Although most individuals with Sotos syndrome have mild intellectual disability, normal intellectual ability has also been reported [7, 11]. In the current case, no formal cognitive testing was performed, but intellectual and social functions were considered to be within the normal range. Another discrepancy with current pediatric practice is that sex steroids are no longer widely recommended to halt growth because of their short- and long-term consequences [10], and the possibility that this treatment contributed to our patient’s infertility cannot be excluded.
In addition to Sotos syndrome, the differential diagnosis for acromegaly/pituitary gigantism, include several genetic overgrowth syndromes, such as Beckwith–Wiedemann (IGF2), Weaver (EZH2), Malan (NFIX), and Tatton–Brown–Rahman (DNMT3) syndromes, or diseases that feature tall stature, such as Berardinelli–Seip lipodystrophy (AGPAT2) or abnormalities of natriuretic peptide C pathway (NPR2, CNP) [8, 10, 12–14]. In adults with acromegaloid features, pachydermoperiostosis should also be considered [15].
This case illustrates that genetic overgrowth syndromes should be considered as differential diagnoses in patients with acromegaloid features without excess GH secretion, a group of conditions also called pseudoacromegaly. As genetic diagnostic testing is becoming more accessible and affordable, it should be considered in these patients to allow correct diagnosis and relevant monitoring and treatment of concomitant health problems related to the syndrome, such as ophthalmologic and psychiatric disease and possibly increased risk for malignancies. Our patient was grateful and relieved to finally receive a diagnosis because it explained her symptoms and signs and allowed her to be treated symptomatically.
Acknowledgments
Acknowledgments
Disclosure Summary: P.M. is supported by a Barts and the London Charity Clinical Training Fellowship. Otherwise, the authors have nothing to disclose.
Footnotes
- EU
- European Union
- GH
- growth hormone
- SD
- standard deviation
- UK
- United Kingdom.
References and Notes
- 1.Reid TJ, Post KD, Bruce JN, Nabi Kanibir M, Reyes-Vidal CM, Freda PU. Features at diagnosis of 324 patients with acromegaly did not change from 1981 to 2006: acromegaly remains under-recognized and under-diagnosed. Clin Endocrinol (Oxf). 2010;72(2):203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A, Wass JA; Endocrine Society . Acromegaly: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–3951. [DOI] [PubMed] [Google Scholar]
- 3.Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014;133(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Douglas J, Hanks S, Temple IK, Davies S, Murray A, Upadhyaya M, Tomkins S, Hughes HE, Cole TR, Rahman N. NSD1 mutations are the major cause of Sotos syndrome and occur in some cases of Weaver syndrome but are rare in other overgrowth phenotypes. Am J Hum Genet. 2003;72(1):132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sotos JF, Dodge PR, Muirhead D, Crawford JD, Talbot NB. Cerebral gigantism in childhood. N Engl J Med. 1964;271(3):109–116. [DOI] [PubMed] [Google Scholar]
- 6.Tatton-Brown K, Rahman N. Clinical features of NSD1-positive Sotos syndrome. Clin Dysmorphol. 2004;13(4):199–204. [PubMed] [Google Scholar]
- 7.Tatton-Brown K, Rahman N. The NSD1 and EZH2 overgrowth genes, similarities and differences. Am J Med Genet C Semin Med Genet. 2013;163C(2):86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tatton-Brown K, Rahman N. Sotos syndrome. GeneReviews® [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK1479/. Published 19 November 2015. Accessed 24 March 2017.
- 9.Opitz JM, Weaver DW, Reynolds JF Jr. The syndromes of Sotos and Weaver: reports and review. Am J Med Genet. 1998;79(4):294–304. [DOI] [PubMed] [Google Scholar]
- 10.Albuquerque EVA, Scalco RC, Jorge AAL Management of endocrine disease: diagnostic and therapeutic approach of tall stature. Eur J Endocrinol. 2017;176(6):R339–R353. [DOI] [PubMed] [Google Scholar]
- 11.Lane C, Milne E, Freeth M. Cognition and behaviour in Sotos syndrome: a systematic review. PLoS One. 2016;11(2):e0149189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatton-Brown K, Rahman N. EZH2-related overgrowth. GeneReviews® [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK148820/. Published 18 July 2013, updated 6 August 2015. Accessed 30 May 2017.
- 13.Miura K, Kim O-H, Lee HR, Namba N, Michigami T, Yoo WJ, Choi IH, Ozono K, Cho TJ. Overgrowth syndrome associated with a gain-of-function mutation of the natriuretic peptide receptor 2 (NPR2) gene. Am J Med Genet A. 2014;164A(1):156–163. [DOI] [PubMed] [Google Scholar]
- 14.Seip M. Congenital hyperpituitarism of hypothalamic origin: a new diencephalic syndrome with endocrine manifestations. Acta Paediatr Suppl. 1959;48(Suppl 118):154–155. [DOI] [PubMed] [Google Scholar]
- 15.Karimova MM, Halimova ZY, Urmanova YM, Korbonits M, Cranston T, Grossman AB. Pachydermoperiostosis masquerading as acromegaly. J Endocr Soc. 2017;1(2):109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]