Abstract
Hypophosphatasia is an inherited disease characterized by reduced alkaline phosphatase activity, extracellular accumulation of inorganic pyrophosphate, and impaired bone mineralization. Asfotase alfa (AA) is a recombinant human alkaline phosphatase therapy approved for treatment of pediatric-onset hypophosphatasia. Studies show promising outcome in AA-treated children with hypophosphatasia; however, data on adults with pediatric-onset hypophosphatasia are scarce. We report on a 59-year-old woman with childhood-onset hypophosphatasia and a history of multiple fractures and orthopedic procedures. Owing to renal failure (histological diagnosis: focal segmental glomerulosclerosis), hemodialysis was started in 2013. By the end of 2015, the patient was unable to walk, could only stand for 30 seconds, and was completely dependent on help for activities of daily living. After 13 months of AA therapy, the patient showed a dramatic increase in quality of life (increased mobility), reduction in pain medication, and a significant improvement in bone mineralization throughout the skeleton, including consolidation of existing fractures and no occurrence of new fractures. This case report demonstrates a relevant therapeutic success of AA treatment in an adult hemodialysis patient with childhood onset of hypophosphatasia.
Keywords: alkaline phosphatase, asfotase alfa, bone mineralization, hypophosphatasia
We report a 59-year-old woman with childhood onset hypophosphatasia and multiple fractures. After 13 months of asfotase alfa therapy she showed dramatic improvement in bone mineralization.
Hypophosphatasia (HPP) is an inherited disease characterized by reduced alkaline phosphatase (AP) activity leading to extracellular accumulation of inorganic pyrophosphate, which inhibits bone and tooth mineralization. It is caused by recessively or dominantly inherited mutations within the tissue nonspecific isoenzyme of AP (TNSALP) gene on chromosome 1p36-34 [1]. The prevalence of severe and moderate HPP in Europe has been estimated at 1 per 300,000 and 1 per 6370, respectively [1].
As >330 mutations of the TNSALP gene have been described, patients may present with a wide spectrum of clinical characteristics ranging from antenatal death to severe bone deformities in early childhood, to dental complications without bone involvement in adults. In decreasing order of severity and according to age of onset, clinicians categorize the perinatal, infantile, childhood, adult, and odontologic forms of HPP [1].
No approved therapy for HPP has been available until recently [2]. Asfotase alfa (AA) is a recombinant human TNSALP combined with the constant region of human immunoglobulin G1–Fc domain and deca-aspartate for bone targeting because it has high affinity for hydroxyapatite [2]. Since late 2015, treatment has been approved in Europe, Canada, and the United States for pediatric-onset HPP. So far, studies show excellent outcomes in AA-treated children [2]. The necessity of a treatment option for adult HPP patients has been underlined by a recent study revealing a substantial burden of disease in adult HPP patients [3]. Even though approved for all childhood-onset HPP patients independent of their current age, data about the effects of the treatment in adults are scarce.
In this report we document a 59-year-old woman with childhood-onset HPP, treated with AA for 13 months, who responded with significant clinical and radiographic improvement.
1. Case
In summer 2009, a 51-year-old woman presented to our clinic. The diagnosis of HPP had been made in her first year of life. She reported that early childhood development was accompanied by feeding problems, delay in the start of walking to age 4 years after several leg surgeries, failure to grow resulting in short stature (130 cm) (father, 173 cm; mother, 155 cm), and loss of teeth during adolescence. Crutches for walking had been necessary continuously after left lower leg fracture at age 26 years, followed by pseudoarthrosis of the right tibia and the left femur in the following years. Table 1 provides an overview of the main fractures and orthopedic procedures during her adulthood.
Table 1.
Main Orthopedic Events and Procedures During Adulthood in a 59-Year-Old Female With the Childhood-Onset Form of HPP
| Events and Procedures |
|---|
| • Fracture left lower leg 1984 (age 26 years), intramedullary nailing 1988 |
| • Pseudoarthrosis right lower leg and intramedullary nailing 1989 (age 31 years), removal 1990 |
| • External fixation right lower leg 1993 to 1994, orthosis and intramedullary nailing 2003 |
| • Pseudoarthrosis left femur and intramedullary nailing 2002 |
| • Pseudoarthrosis right humerus 2004 and intramedullary nailing July 2008 |
| • Pseudoarthrosis and fracture left humerus, plate and screw August 2009 |
| • Fracture glenoid bone left September 2010 |
| • Implant loosening left distal humerus October 2010 and open reposition and internal refixation with double plate osteosynthesis plus autologous stem cells |
| • Implant removal left distal humerus and neurolysis nervus ulnaris left February 2012 |
| • Orthosis left arm |
| • Periprosthetic fracture right humerus April 2013 |
Direct sequencing of exons 1 to 12 revealed homozygous TNSALP mutations in exon 5 (p.Ala116Ser) and heterozygous mutation in exon 11 (p.Asn417Ser). The mutation p.Ala116Ser is not functionally characterized; however, a p.Ala116Thr mutation results in negligible activity [4]. The mutation p.Asn417Ser abolishes AP activity, and as a heterozygous genotype reduces activity to ~25% [5].
At referral, she reported pain in all bones, difficulty walking, and poor quality of life. She had received bisphosphonate therapy for a year in the past. For analgesia she was taking 4 × 30 drops (=3 g)/d of a nonopiod analgesia (metamizol). In addition to her HPP, she had hypertension, hypercholesterolemia, chronic kidney disease stage 4 with renal anemia, and nephrolithiasis, with the latter possibly related to HPP.
Owing to implant loosening in her left distal humerus and continuous pseudoarthrosis of the right humerus, therapy with teriparatide (parathyroid hormone 1-34) was started in 2011 but withdrawn after 4 months due to proteinuria (2.6 g/L) and decreased kidney function. Renal biopsy revealed focal segmental glomerulosclerosis (no calcium deposits). Owing to the lack of alternative treatment options, she was given transdermal hormone replacement therapy (0.025 mg if 17β-estradiol and 0.125 mg of norethisterone acetate) in 2013. In the same year, hemodialysis was started.
Owing to new fractures (Table 1) and failure of prevalent fractures to heal, the patient’s health and quality of life further deteriorated accompanied by severe bone pain. By the end of 2015, the patient could no longer walk, could only stand for 30 seconds, and was completely dependent on help for activities of daily living and personal care.
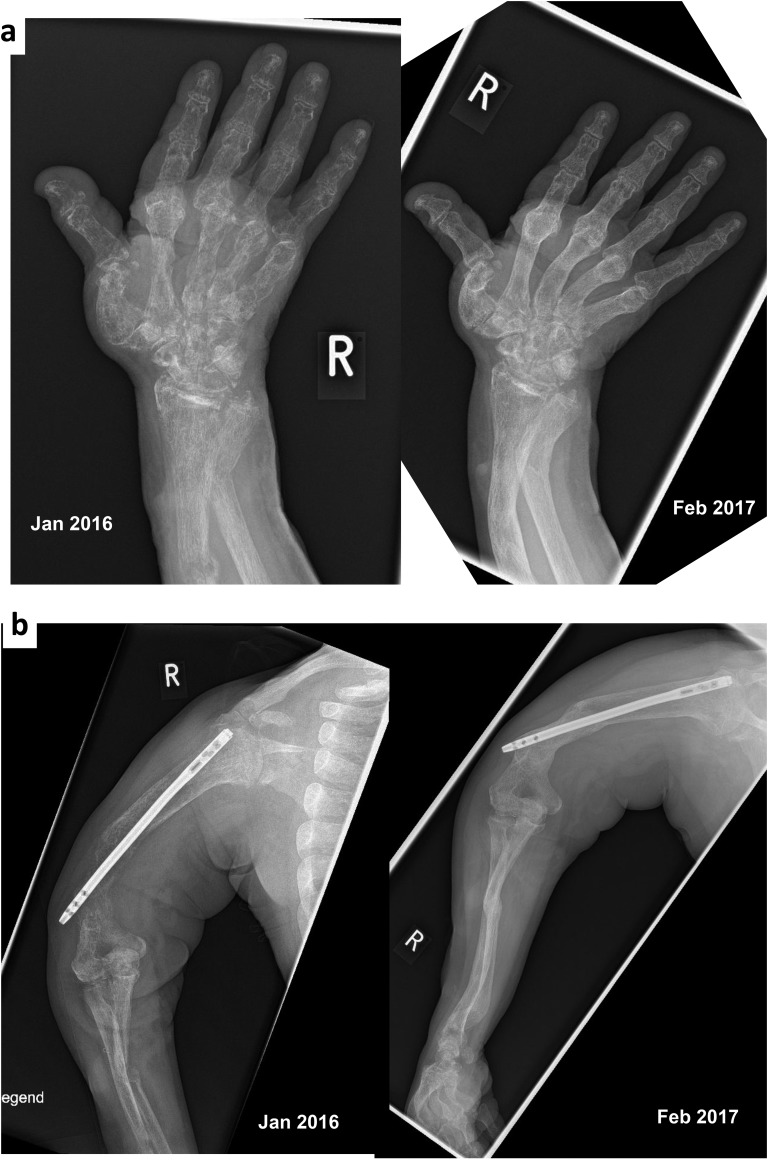
At the end of 2015, AA was approved as therapy for pediatric-onset HPP in Germany. Radiographs of arms, hands, legs, and hips were taken in January 2016 showing widespread progressive demineralization (compared with 2013), distinct osseous deformities, and ongoing destruction of bone structure, especially of the hands (Fig. 1a and 1b, left side). Compared with previous examinations, new radial and bilateral ulnar fractures, as well as a new fracture around a femoral nail, were apparent. At that time, medical therapy comprised bisoprolol (1.25 mg/d), ramipril (5 mg/d), torsemide (200 mg/d), sodium polystyrene sulfonate (450 mg twice each week), lanthanum carbonate (750 mg/d), darbepoetin (15 μg every 2 weeks), calcium (500 mg), colecalciferol (20,000 IU every 2 weeks), and transdermal estrogen.
Figure 1.
X-ray images of the right hand (a) and right arm (b) in January 2016 (left side) before asfotase therapy and in February 2017 (right side) after 13 months of asfotase therapy in a 59-year-old female patient with the childhood-onset form of HPP.
Given the patient’s progressive skeletal deformity and functional impairment, treatment was started with subcutaneous injections of 80 mg of AA three times per week (given directly after hemodialysis) after discussion between specialists within our clinic in January 2016. During the first 6 weeks of treatment she reported slight burning of the hands and knees and fatigue directly after injections. Laboratory data before and during treatment are presented in Table 2.
Table 2.
Laboratory Results Before (January 2016) and During Therapy (July 2016 and January 2017) With AA in a 59-Year-Old Female With the Childhood-Onset Form of HPP
| January 2016 | July 2016 | January 2017 | |
|---|---|---|---|
| Calcium, mmol/L (n: 2.15–2.5) | 2.32 | 2.30 | 2.21 |
| Phosphate, mmol/L (n: 0.87–1.45) | 1.96 | 1.68 | 1.35 |
| AP, μkat/L (n: <2.15) | 0.33 | >110 | >110 |
| Bone AP, ostase, μg/L (n: 6–22.7) | 8.0 | 3300 | 3340 |
| Parathyroid hormone, ng/L (n: 11–43) | 116 | 331 | 293 |
| 25-Hydroxy vitamin D3, μg/L (n: 20–70) | 51 | 68 | 43 |
| Hemoglobin, g/dL (n: 12–16) | 12.6 | 11.6 | 11.9 |
| GFR, mL/min (n: >90) | 6.6 | 6.8 | 6.7 |
| Osteocalcin, μg/L (n: 14–42) | nd | nd | 319 |
Abbreviations: GFR, glomerular filtration rate [calculated with Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula]; n, normal range; nd, not done.
After 13 months of AA treatment the patient could stand and walk independently from the wheelchair to bed (3 m). Her ability to elevate her arms was still significantly impaired, but was clearly improved compared with January 2016. Bone pain had reduced and less pain medication was necessary. Radiographs in February 2017 showed significantly improved mineralization at all skeletal sites with greater improvement in the arms and hands than in the legs, full consolidation of the radial, ulnar, and femoral fractures, and no occurrence of new fractures (Fig. 1a and 1b, right side). Medical therapy was otherwise unchanged.
2. Discussion
No clinical trial data for adults with childhood-onset HPP treated with AA have been published except in abstract form [6]. Data from neonates and infants with severe HPP are available showing significantly improved survival and skeletal mineralization after AA treatment [2, 7, 8].
In our patient with the childhood form of HPP, therapy with AA resulted in marked improvement of mobility and pain, and only mild side effects. In addition to a significant positive impact on her reported quality of life, bone mineralization and fracture healing improved significantly and no new fractures occurred.
Her chronic renal failure (CRF) was attributed to hypertension, nonsteroidal anti-inflammatory medication taken for bone pain, and chronic nephrolithiasis. Unfortunately, we obtained no bone biopsies of our patient. However, it is likely that she had renal osteodystrophy with secondary hyperparathyroidism due to CRF. This was previously shown in an adult HPP patient with CRF requiring hemodialysis [9]. In contrast to the reported case [9], our patient never experienced hypercalcemia after fracture. Recently, a 55-year-old man with odontohypophosphatasia was reported who was asymptomatic until he developed CRF, resulting in numerous disabling fractures [10]. After successful renal transplantation, the patient improved significantly, suggesting that renal failure might further deteriorate bone changes in HPP. Additionally, the administration of bisphosphonate could potentially be an additional factor in worsening of the bone disease.
In our case hemodialysis was an additional challenging point in treatment, and no experience existed regarding hemodialysis and AA treatment. Increased phosphate levels previously reported during AA treatment were not seen in our case, probably due to hemodialysis. Under AA treatment we provided calcium and colecalciferol treatment.
3. Conclusion
This case report shows a significant therapeutic success of AA treatment in an adult hemodialysis patient with the childhood form of HPP.
Acknowledgments
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- asfotase alfa
- AP
- alkaline phosphatase
- CRF
- chronic renal failure
- HPP
- hypophosphatasia
- TNSALP
- tissue-nonspecific isoenzyme of alkaline phosphatase.
References and Notes
- 1.Whyte MP. Hypophosphatasia—aetiology, nosology, pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2016;12(4):233–246. [DOI] [PubMed] [Google Scholar]
- 2.Whyte MP, Greenberg CR, Salman NJ, Bober MB, McAlister WH, Wenkert D, Van Sickle BJ, Simmons JH, Edgar TS, Bauer ML, Hamdan MA, Bishop N, Lutz RE, McGinn M, Craig S, Moore JN, Taylor JW, Cleveland RH, Cranley WR, Lim R, Thacher TD, Mayhew JE, Downs M, Millán JL, Skrinar AM, Crine P, Landy H. Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med. 2012;366(10):904–913. [DOI] [PubMed] [Google Scholar]
- 3.Weber TJ, Sawyer EK, Moseley S, Odrljin T, Kishnani PS. Burden of disease in adult patients with hypophosphatasia: results from two patient-reported surveys. Metabolism. 2016;65(10):1522–1530. [DOI] [PubMed] [Google Scholar]
- 4.Ishida Y, Komaru K, Oda K.. Molecular characterization of tissue-nonspecific alkaline phosphatase with an Ala to Thr substitution at position 116 associated with dominantly inherited hypophosphatasia. Biochim Biophys Acta. 2011;1812(3):326–332. [DOI] [PubMed] [Google Scholar]
- 5.Sultana S, Al-Shawafi HA, Makita S, Sohda M, Amizuka N, Takagi R, Oda K. An asparagine at position 417 of tissue-nonspecific alkaline phosphatase is essential for its structure and function as revealed by analysis of the N417S mutation associated with severe hypophosphatasia. Mol Genet Metab. 2013;109(3):282–288. [DOI] [PubMed] [Google Scholar]
- 6.Kishnani P, Rockman-Greenberg C, Whyte MP, Weber T, Mhanni A, Madson K, Reeves A, Mack K, Plotkin H, Kreher N, Landy H. Hypophosphatasia: enzyme replacement therapy (ENB-0040) decreases TNSALP substrate accumulation and improves functional outcome in affected adolescents and adults. In: 15th International and 14th European Congress of Endocrinology; 5–9 May 2012; Florence, Italy. Abstract 29 OC8.1. Available at: http://www.endocrine-abstracts.org/ea/0029/ea0029OC8.1.htm. [Google Scholar]
- 7.Whyte MP, Rockman-Greenberg C, Ozono K, Riese R, Moseley S, Melian A, Thompson DD, Bishop N, Hofmann C. Asfotase alfa treatment improves survival for perinatal and infantile hypophosphatasia. J Clin Endocrinol Metab. 2016;101(1):334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whyte MP, Madson KL, Phillips D, Reeves AL, McAlister WH, Yakimoski A, Mack KE, Hamilton K, Kagan K, Fujita KP, Thompson DD, Moseley S, Odrljin T, Rockman-Greenberg C. Asfotase alfa therapy for children with hypophosphatasia. JCI Insight. 2016;1(9):e85971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whyte MP, Leelawattana R, Reinus WR, Yang C, Mumm S, Novack DV. Acute severe hypercalcemia after traumatic fractures and immobilization in hypophosphatasia complicated by chronic renal failure. J Clin Endocrinol Metab. 2013;98(12):4606–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cundy T, Michigami T, Tachikawa K, Dray M, Collins JF, Paschalis EP, Gamsjaeger S, Roschger A, Fratzl-Zelman N, Roschger P, Klaushofer K. Reversible deterioration in hypophosphatasia caused by renal failure with bisphosphonate treatment. J Bone Miner Res. 2015;30(9):1726–1737. [DOI] [PubMed] [Google Scholar]