Abstract
Context:
F2-isoprostanes (F2-isoPs) are biomarkers for oxidative stress in humans and have been shown to be elevated in obesity, cardiovascular disease, and diabetes. Therefore, F2-isoPs are often implicated in oxidative stress contributing to insulin resistance, although this has not been rigorously examined.
Objective:
To determine whether urinary F2-isoPs are predictive of insulin sensitivity and other clinical metabolic parameters.
Participants:
Sedentary, weight-stable, nondiabetic adults equilibrated on a standard isocaloric diet.
Main Outcome Measures:
Insulin sensitivity via hyperinsulinemic-euglycemic clamp, urinary F2-isoPs by gas chromatography-mass spectrometry, and body composition by dual-energy x-ray absorptiometry.
Results:
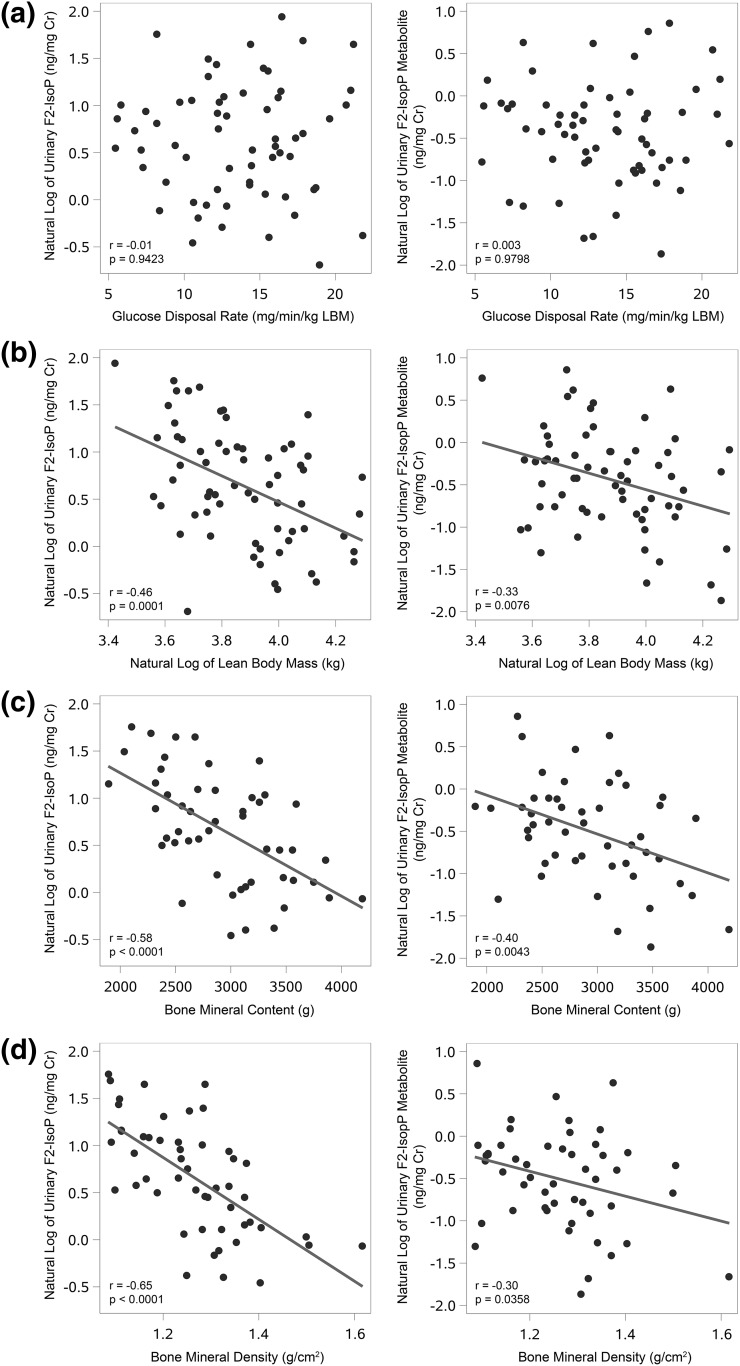
No correlation was found between 15-F2t-IsoP nor its major metabolite, 2,3-dinor-5,6-dihydro-15-F2t-IsoP, with insulin sensitivity, even after adjusting for age, race, sex, BMI, and smoking status. 15-F2t-IsoP was also not associated with body fat. However, there was a strong negative correlation between 15-F2t-IsoP and lean body mass (LBM; r = −0.46, P = 0.0001), bone mineral content (BMC; r = −0.58, P < 0.0001), bone mineral density (BMD; r = −0.65, P < 0.0001), and skeletal muscle protein 4-hydroxynonenal (4-HNE; r = −0.54, P = 0.0239), another marker of oxidative stress. 15-F2t-IsoP was also positively associated with circulating triglycerides and total cholesterol, and increased as a function of age.
Conclusions:
Urinary 15-F2t-IsoP and its major metabolite are not associated with insulin sensitivity, suggesting the lipid peroxidation process that produces F2-isoPs does not reflect oxidative stress reactions operative in insulin resistance. However, urinary F2-isoPs were negatively correlated with LBM, BMC, BMD, and muscle 4-HNE. Because lean and bone mass decline as a function of biological aging, F2-isoPs may reflect the oxidative stress operative in the aging process.
Keywords: F2-isoprostanes, oxidative stress, insulin resistance, lean body mass, bone density, aging
Urinary F2-isoPs were not shown to be predictive of insulin sensitivity (as measured by the clamp technique), but rather with LBM and bone density, which may reflect the aging process.
Oxidative stress has been implicated in the development of insulin resistance [1] and related disease states, such as type 2 diabetes (T2DM), obesity, and metabolic syndrome [2]. An important consequence of oxidative stress and overproduction of reactive oxygen species is lipid peroxidation, which, in particular, has been linked with insulin resistance and diabetes [3, 4]. F2-isoprostanes (F2-isoPs) are prostaglandin-like compounds formed by nonenzymatic, free radical–induced peroxidation of arachidonic acid [5] and thus represent predominant lipid biomarkers for oxidative stress in humans in vivo.
F2-isoPs have been associated with many disease states relating to insulin resistance, including T2DM and cardiometabolic disease [3, 6–9], and are associated with increased mortality from coronary heart disease or stroke in postmenopausal women [10]. Laight et al. showed that obese mice had elevated plasma F2-isoPs in addition to hyperglycemia and hyperinsulinemia, all of which decreased after animals were given supplemental dietary vitamin E as an antioxidant [11]. In humans, obese, insulin-resistant patients had higher levels of urinary F2-isoPs and circulating oxidized low-density lipoprotein (LDL) after a high-fat meal compared with normal weight controls [12]. Other studies have shown that elevated F2-isoPs in T2DM [13] are reduced as a result of antioxidant supplementation [14]. Among nondiabetic individuals, F2-isoPs have been observed to be elevated as a function of high body mass index (BMI), waist-to-hip ratio, and fasting insulin [15]. Based on these findings, oxidative stress contributing to the production of F2-isoPs is widely interpreted to be involved in the pathogenesis of insulin resistance in cardiometabolic disease. However, most studies have examined this relationship using surrogate measures of insulin sensitivity, rather than the gold standard, hyperinsulinemic-euglycemic clamp technique. Thus, we evaluated levels of urinary F2-isoPs in nondiabetic patients across a wide range of BMI and insulin sensitivity, as quantified through use of this clamp technique.
Both urinary and plasma F2-isoPs can be used as biomarkers for in vivo oxidative stress; however, urinary isoprostanes are stable both at room temperature and during multiple freeze-thaw cycles, do not undergo auto-oxidation [6], and are indicative of systemic “whole-body” oxidant stress over time. Urinary F2-isoPs were chosen for use in this study, with a focus on the most abundant and bioactive form, 15-F2t-IsoP (8-iso PGF2α), along with its major urinary metabolite, 2,3-dinor-5,6-dihydro-15-F2t-IsoP. Additionally, we evaluated the association of urinary F2-isoPs with other clinical variables related to insulin resistance and cardiometabolic risk factors, including body composition, substrate oxidation rates, and circulating lipids.
1. Materials and Methods
A. Patient Characteristics
Sixty-five subjects were sequentially recruited for metabolic characterization at the University of Alabama at Birmingham Clinical Research Unit through advertisements and word of mouth. Patients with a BMI >21 kg/m2 were included, with an effort toward equal recruitment of white and black participants. Patients were required to have been sedentary (no regular exercise) and weight-stable for at least 3 months before the study (±3% of body weight). Exclusion criteria included BMI >50 kg/m2; a diagnosis of plasma glucose data indicative of T2DM; the presence of cardiovascular, renal, thyroid, or hepatic disease; and use of pharmacological agents that could affect body composition, lipid, or carbohydrate metabolism. Demographics, such as age, race, and sex were self-reported.
Subjects were equilibrated on a weight maintenance diet (28 to 32 kcal/kg/d; 50% carbohydrates, 30% fat, and 20% protein) for a 3-night stay in the metabolic ward. The study was approved by the institutional review board and written informed consent was obtained from every subject.
B. Body Composition
Anthropometric data (weight, height, waist circumference) were collected and BMI was calculated from weight (kg)/height (m2). Dual-energy x-ray absorptiometry (Lunar Radiation, Madison, WI) was used to assess lean body mass (LBM), body fat, bone mineral content (BMC), and bone mineral density (BMD).
C. Insulin Sensitivity
Insulin sensitivity was assessed as glucose disposal rate (GDR) in vivo using the hyperinsulinemic-euglycemic clamp technique, as previously described [16, 17]. Briefly, after a 12-hour fast, subjects were administered a primed-continuous infusion of regular insulin (Humulin; Eli Lilly, Indianapolis, IN) at a rate of 200 mU/m−2/min−1, producing steady-state insulin concentrations >3000 pmol/L, which are maximally effective for stimulating glucose uptake into skeletal muscle [17]. Serum glucose was clamped at 5.0 mmol/L for at least 3 hours, and maximal glucose uptake for each individual was determined as the mean glucose infusion rate over the final three 20-minute intervals. Whole-body glucose uptake was calculated based on the glucose infusion rate corrected for changes in the glucose pool size, assuming a distribution volume of 19% body weight and pool fraction of 0.65. The GDR was then normalized per kilogram of LBM assessed by dual-energy x-ray absorptiometry.
D. Substrate Oxidation Rates
Basal lipid and carbohydrate oxidation rates were determined through indirect calorimetry after an overnight fast, as previously described [18]. Resting energy expenditure was measured using a Deltratrac metabolic monitor (Deltatrac II; SensorMedics, Yorba Linda, CA) after subjects rested supine on a bed for 30 minutes. The instrument was calibrated with ethanol combustion tests every month against standard gases before each test. Expired air was collected with an adult-size, ventilated canopy system for 20 minutes after a 10-minute equilibration. Whole body oxygen consumption and carbon dioxide were calculated by measuring gradients across the face with known flow rates of air using the Haldane transformation. Substrate oxidation rates were then determined from the respiratory quotient value and the tables of Lusk [19], normalized per kilogram of metabolically active body mass, according to Ravussin et al. [20].
E. Assays
Blood samples were collected after patients fasted overnight for at least 12 hours. Plasma glucose was measured by the glucose oxidase method using a glucose analyzer (YSI 2300; Yellow Springs Instruments, Yellow Springs, OH). Serum insulin levels were measured using immunofluorescence on a TOSOH A1A-II analyzer (TOSOH Corp., South San Francisco, CA); 50 µL serum, sensitivity, 1.0 uU/mL; interassay coefficient of variation (CV), 4.0%; and intra-assay CV, 1.5%. Total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were determined by the colorimetric method on a SIRRUS analyzer (Stanbio Laboratory, Boerne, TX); 3 µL for each; sensitivity, 2 to 5 mg/dL; all assay CVs <6.6%. LDL cholesterol was calculated using the Friedewald equation.
F. Urinary F2-IsoP/Metabolite
Spot urine samples were collected in the morning from each subject, stored at −70°C, and submitted to Dr. G. Milne at Vanderbilt University for analysis. A total of 250 µL of urine was used to analyze the principal urinary F2-isoP, 15-F2t-IsoP (aka 8-iso PGF2α), along with its major urinary metabolite, 2,3-dinor-5,6-dihydro-15-F2t-IsoP, via gas chromatography/negative-ion chemical ionization mass spectrometry [21, 22]. Both the F2-isoP and its metabolite were normalized to urine creatinine, as determined through a modified Jaffe reaction using a commercial chemistry analyzer (Roche COBAS Integra 800; F. Hoffmann-La Roche AG, Basel, Switzerland). The lower limit of detection for the assay was 0.0001 ng/mL, with a CV of 8%. Normal concentrations of F2-isoPs in human urine are 1.6 ± 0.6 ng/mL [23].
G. Statistical Analyses
Descriptive data are presented as means ± standard deviation and range. In a few instances, statistical outliers were identified and removed; however, the conclusions were not affected by the inclusion or exclusion of these data points. Pearson and Spearman correlation coefficients were used to assess the linear relationship between 15-F2t-IsoP and its major metabolite with insulin sensitivity, age, body composition measurements, and metabolic parameters, as appropriate. Multiple linear regression analyses were conducted to ascertain the relationship between the F2-isoP values and body composition measures, with additional adjustments made for age, sex, race, and smoking status, because these all have previously been associated with differences in F2-isoP levels in other studies. Body composition measures were checked for collinearity before entering them into the model. Marginal means were calculated for F2-isoP levels for race, obesity, and smoking status, adjusted for sex. All statistical analyses were conducted using the SAS 9.4 statistical software package (SAS Institute Inc., Cary, NC).
2. Results
A. General Subject Characteristics
The sample population had more females (70%) than males, but was racially balanced with 33 blacks (73% female) and 32 whites (69% female). The average age of participants was 38.6 years. Study subjects were recruited over a wide range of BMI (21.2 to 46.9 kg/m2) and insulin sensitivity as reflected by GDR values (5.46 to 21.82 mg/min/kg LBM). Clinical characteristics of subjects are presented in Table 1.
Table 1.
Descriptive Characteristics
| Age (y) | 38.6 ± 11.1 (20–60) |
| Race | 33 black, 32 white |
| Sex | 19 male, 46 female |
| Weight (kg) | 87.9 ± 19.0 (61.2–139.8) |
| BMI (kg/m2) | 31.0 ± 6.3 (21.2–46.9) |
| Body composition | |
| LBM (kg) | 49.2 ± 10.2 (30.7–73.3) |
| LBM (%) | 56.9 ± 10.6 (40.9–87.9) |
| Total fat mass (kg) (n = 52) | 33.9 ± 14.6 (4.1–71.5) |
| Total body fat (%) | 40.6 ± 11.2 (7.2–58.0) |
| Trunk fat mass (kg) (n = 63) | 18.6 ± 7.7 (4.283–40.7) |
| Waist circumference (cm) | 98.0 ± 14.9 (74.0–141.5) |
| Hip circumference (cm) (n = 64) | 111.5 ± 13.0 (87.5–148.5) |
| Waist-to-hip ratio (n = 64) | 0.88 ± 0.08 (0.72–1.04) |
| BMC (g) (n = 50) | 2928.2 ± 525.5 (1892–4192) |
| BMD (g/cm2) (n = 51) | 1.26 ± 0.12 (1.09–1.62) |
| Fasting glucose (mg/dL) (n = 64) | 95.1 ± 11.54 (72.4–133) |
| GDR (mg/min/kg LBM) (n = 63) | 13.51 ± 4.16 (5.46–21.82) |
| Carbohydrate oxidation rate (kcal/day/kg LBM) (n = 63) | 133.8 ± 58.5 (0–245.7) |
| Fat oxidation rate (kcal/day/kg LBM) (n = 64) | 75.1 ± 35.4 (10.4–197.1) |
| Lipid markers | |
| Triglycerides (mg/dL) (n = 64) | 109.6 ± 55.0 (36–247) |
| Total cholesterol (mg/dL) (n = 63) | 181.2 ± 43.3 (111–340) |
| LDL-cholesterol (mg/dL) (n = 64) | 112.7 ± 38.0 (45.4–211.8) |
| HDL-cholesterol (mg/dL) | 47.6 ± 14.0 (26–81) |
| Systolic blood pressure (mm Hg) | 117.1 ± 15.3 (87–158) |
| Diastolic blood pressure (mm Hg) | 67.5 ± 10.0 (48–91) |
| Urinary F2-isoP (ng/mg creatinine) | 2.28 ± 1.42 (0.5–7.0) |
| Urinary F2-isoP metabolite (ng/mg creatinine) (n = 64) | 0.77 ± 0.48 (0.16–2.36) |
| Smoker | 7 yes, 10 former, 42 no |
Data are presented as mean ± standard deviation (n = 65 unless otherwise noted), unless variables are categorical, in which case the n for each category is reported.
B. Insulin Sensitivity/Substrate Metabolism
No correlation was found between insulin sensitivity as measured by GDR and either 15-F2t-IsoP or its major metabolite, 2,3-dinor-5,6-dihydro-15-F2t-IsoP (Fig. 1). This lack of association persisted even after adjusting for age, race, sex, BMI, and smoking status. Similarly, there were no significant correlations between 15-F2t-IsoP or its major metabolite with either basal carbohydrate oxidation rate or basal fat oxidation rate, although there was a trend for a positive correlation between fasting glucose and 15-F2t-IsoP (P = 0.071) (Table 2).
Figure 1.
Relationships of urinary F2-isoP and its metabolite with clinical parameters. (a) No correlation was observed between either the urinary F2-isoP or its metabolite with insulin sensitivity, as measured by glucose disposal rate via a hyperinsulinemic-euglycemic clamp. Both the urinary F2-isoprostane and its metabolite are negatively correlated with (b) LBM, (c) BMC, (d) and BMD. Cr, creatinine.
Table 2.
Correlation of Metabolic Risk Factors With Urinary F2-IsoP and its Major Metabolite
| Metabolic Risk Factor | Urinary F2-IsoP (ng/mg Cr) (n = 65) | P Value | Urinary F2-IsoP Metabolite (ng/mg Cr) (n = 64) | P Value |
|---|---|---|---|---|
| Age (y) | 0.28 | 0.0241a | 0.10 | 0.4220 |
| Weight (kg) | −0.28 | 0.0261a | 0.02 | 0.9009 |
| BMIb (kg/m2) | −0.09 | 0.4787 | 0.21 | 0.1035 |
| Body composition | ||||
| LBM (kg) | −0.46 | 0.0001c | −0.33 | 0.0076c |
| LBM (%) | −0.20 | 0.1126 | −0.41 | 0.0008c |
| Total fat mass (kg) (n = 52; 51) | −0.04 | 0.7844 | 0.23 | 0.1013 |
| Total body fatb (%) | 0.18 | 0.1492 | 0.40 | 0.0011c |
| Trunk fat massb (g) (n = 63; 62) | 0.01 | 0.9219 | 0.25 | 0.0471a |
| Waist circumference (cm) | −0.06 | 0.6319 | 0.11 | 0.4054 |
| Hip circumference (cm) (n = 64; 63) | −0.07 | 0.5637 | 0.23 | 0.0712 |
| Waist:hip ratio (n = 64; 63) | −0.01 | 0.9234 | −0.13 | 0.3014 |
| BMC (g) (n = 50; 49) | −0.58 | <0.0001c | −0.40 | 0.0043c |
| BMD (g/cm2) (n = 51; 50) | −0.65 | <0.0001c | −0.30 | 0.0358a |
| Fasting glucoseb (mg/dL) | 0.23 | 0.0706 | 0.03 | 0.8180 |
| GDR (mg/min/kg LBM) (n = 63; 62) | −0.01 | 0.9423 | 0.003 | 0.9798 |
| Carbohydrate oxidation rateb (kcal/day/kg LBM) | −0.001 | 0.9928 | −0.03 | 0.8379 |
| Fat oxidation rate (kcal/day/kg LBM) (n = 64) | −0.07 | 0.5922 | −0.01 | 0.9193 |
| Lipid markers | ||||
| Triglycerides (mg/dL) | 0.36 | 0.0031a | 0.23 | 0.0711 |
| Total cholesterolb (md/dL) | 0.32 | 0.0096c | 0.11 | 0.4079 |
| LDL-cholesterol (mg/dL) (n = 64; 63) | 0.23 | 0.0670 | 0.08 | 0.5478 |
| HDL-cholesterolb (mg/dL) | 0.04 | 0.7331 | −0.18 | 0.1580 |
| Systolic blood pressure (mm Hg) | 0.13 | 0.2924 | 0.07 | 0.5868 |
| Diastolic blood pressure (mm Hg) | 0.13 | 0.3228 | −0.13 | 0.3027 |
All correlations are Pearson’s correlation coefficients, unless indicated as Spearman correlation coefficients. Metabolic parameters were otherwise normalized with natural log transformations as appropriate. All values for the urinary isoprostane and its metabolite are natural log-transformed, unless otherwise indicated for Spearman correlation.
P < 0.05.
Spearman correlation coefficient.
P < 0.01.
C. Body Composition
Urinary 15-F2t-IsoP was negatively correlated with body weight and LBM (Table 2, Fig. 1), but not with BMI. 15-F2t-IsoP was not associated with measures of adiposity, including total fat mass, body fat percentage, trunk fat mass, waist circumference, or waist-to-hip ratio (all P = NS).
The F2-isoP metabolite was strongly and negatively correlated with LBM and positively correlated with body fat percentage and trunk fat (Table 2). It was not correlated with body weight.
D. BMC/BMD
Strong inverse correlations were observed between both 15-F2t-IsoP and its major metabolite with both BMC and BMD (Table 2, Fig. 1).
E. Age
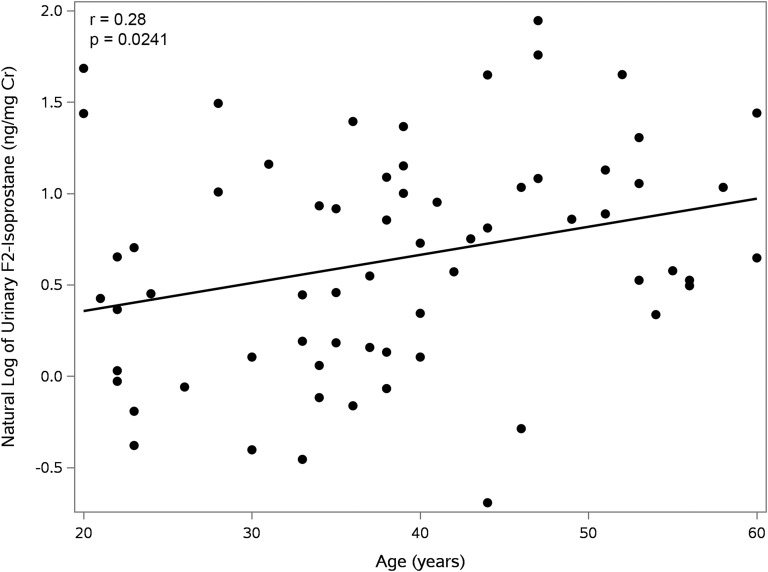
Because decrements in LBM, BMC, and BMD (Fig. 1) all occur as a function of progressive biological aging, we also examined whether 15-F2t-IsoP and its metabolite were correlated with age. Levels of urinary 15-F2t-IsoP were found to rise progressively with advancing age (Fig. 2).
Figure 2.
Correlation of urinary F2-isoprostane with age. 15-F2t-IsoP is positively correlated with age.
F. Circulating Lipids and Lipoproteins
As shown in Table 2, 15-F2t-IsoP was found to have a significant positive correlation with circulating triglycerides and total cholesterol, whereas the trend for an association with LDL-cholesterol did not achieve statistical significance. There were no significant correlations found between these circulating lipids and the metabolite 2,3-dinor-5,6-dihydro-15-F2t-IsoP. No correlation was found between either the F2-isoP or its metabolite with HDL-cholesterol (Table 2). In addition, neither systolic nor diastolic blood pressure was correlated with either of them.
G. Effects of Sex, Race, and Smoking Status and Multiple Linear Regression Analyses
Urinary levels of 15-F2t-IsoP and its metabolite were higher in females than males (2.53 ± 0.22 vs 88 ± 0.07, P = 0.0074; and 1.67 ± 0.22 vs 0.50 ± 0.06, P = 0.0003, respectively). In addition, after adjusting for sex, urinary 15-F2t-IsoP values were higher in whites than blacks (0.68 ± 0.11 and 0.44 ± 0.11, respectively; P = 0.0169), and higher among former and current cigarette smokers compared with those who have never smoked (0.72 ± 0.18, 0.66 ± 0.22, and 0.49 ± 0.09, respectively; P = 0.0375). Additionally, although F2-isoPs were not significantly correlated with BMI, those who were overweight had the highest mean levels of urinary 15-F2t-IsoP (0.77 ± 0.13) compared with either normal weight (0.51 ± 0.15) or obese patients (0.40 ± 0.12) (P = 0.0151) after adjusting for sex.
After ensuring no collinearity between body composition variables (variance inflation factor for LBM = 1.64, BMC = 3.18, BMD = 2.52), a regression model for urinary 15-F2t-IsoP and LBM with BMC and BMD showed that BMD was an independent predictor, whereas BMC was not (Table 3). Subsequent multiple regression analyses revealed that the relationship between 15-F2t-IsoP and either LBM or BMD was still significant after adjusting for age, race, sex, and smoking status (Table 4), although age was a significant covariate in the regression model for lean mass.
Table 3.
Multiple Linear Regression Model of 15-F2t-IsoP With LBM (n = 46)
| Standardized β | P Value | |
|---|---|---|
| LBM | −0.27 | 0.0646 |
| BMC | −0.06 | 0.7853 |
| BMD | −0.45 | 0.0166 |
Full model: adjusted R2 = 0.42, P < 0.0001.
Table 4.
Multiple Linear Regression Analysis of 15-F2t-IsoP With Body Composition and Covariates
|
LBM
(n = 59) |
BMD (n = 48) |
Total Body Fat (%) (n = 59) |
||||
|---|---|---|---|---|---|---|
| Standardized β | P Value | Standardized β | P Value | Standardized β | P Value | |
| Variable of interest | −0.62 | 0.0004 | −0.62 | <0.0001 | 0.045 | 0.7997 |
| Age | 0.29 | 0.0182 | 0.11 | 0.4251 | 0.29 | 0.0360 |
| Race | ||||||
| Black | −0.10 | 0.3984 | 0.11 | 0.4231 | −0.14 | 0.3040 |
| White | 0 | . | 0 | . | 0 | . |
| Sex | ||||||
| Female | −0.15 | 0.3759 | 0.14 | 0.2955 | 0.28 | 0.1195 |
| Male | 0 | . | 0 | . | 0 | . |
| Smoking status | ||||||
| Former | −0.18 | 0.2722 | −0.16 | 0.3652 | −0.10 | 0.5868 |
| No | −0.27 | 0.0912 | −0.16 | 0.3261 | −0.14 | 0.4413 |
| Yes | 0 | . | 0 | . | 0 | . |
| Full model | R2 = 0.33 | 0.0001 | R2 = 0.38 | 0.0002 | R2 = 0.15 | 0.0222 |
Relationships between 15-F2t-IsoP and each body mass composition variable of interest (LBM, BMD, or body fat, respectively). Adjusted R2 values are reported.
H. Additional Markers of Oxidative Stress
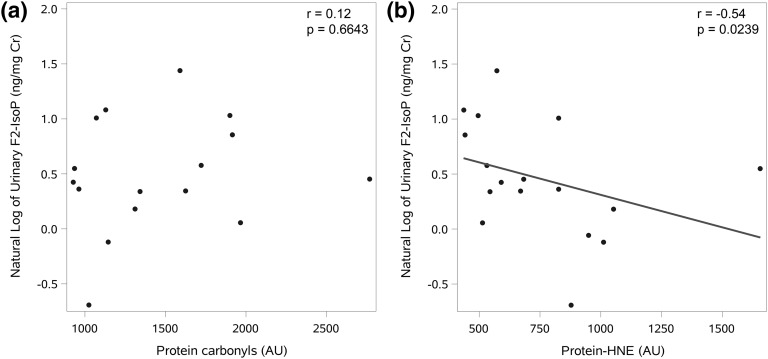
In a subgroup of subjects (58.8% black and 70.6% female, which is very similar to our total sample population), we had available measures of protein 4-hydroxynonenal (protein-HNE; 4-HNE) and carbonyl content in skeletal muscle, which indicate local levels of oxidative stress in the tissue [4]. In these patients (n = 17), urinary 15-F2t-IsoP was correlated with protein-HNE (r = −0.54, P = 0.0239), but not with protein carbonyl content (r = 0.12, P = 0.6643) (Fig. 3).
Figure 3.
Skeletal muscle markers of oxidative stress. The relationship between 15-F2t-IsoP and (a) skeletal muscle protein carbonyl content (n = 16) and (b) skeletal muscle protein-HNE content (n = 17). AU, arbitrary units.
3. Discussion
Oxidative stress participates in the pathophysiology of cardiometabolic disease, and markers of oxidative stress are elevated in those with diabetes, obesity, atherosclerosis, and increased cardiovascular disease risk [23–25]. F2-isoPs have been used widely as biomarkers of oxidative stress in vivo. Plasma and urinary F2-isoP levels have been shown to be elevated in both obesity [12] and diabetes [13], and are reduced following improved glycemic control [14] and weight loss [26]. Furthermore, plasma F2-isoP levels are acutely increased with induced hyperglycemia [27].
Given the central role of insulin resistance in diabetes, obesity, and cardiovascular disease, one might expect that F2-isoP levels would directly reflect oxidative stress that is contributing to the pathogenesis of insulin resistance. However, in the current study, we found no association of either urinary 15-F2t-IsoP or its major metabolite 2,3-dinor-5,6-dihydro-15-F2t-IsoP with insulin sensitivity, as measured by a hyperinsulinemic-euglycemic clamp. Neither did we observe any association of either 15-F2t-IsoP or its metabolite with basal carbohydrate or lipid oxidation rates.
Our observation that F2-isoPs are not quantitatively related to insulin sensitivity in humans suggests that the lipid peroxidation process that produces urinary F2-isoPs may not reflect the oxidative stress processes operative in insulin resistance. F2-isoPs are prostaglandin-like compounds formed by peroxidation of arachidonic acid and constitute a minor component of lipid peroxidation products [24]. F2-isoPs can mediate biological effects such as vaso-reactivity and platelet aggregation; however, these effects are believed to be mediated by receptor binding and not via inherent chemical reactivity and protein modification [6]. This is in contradistinction to 4-HNE, another marker of lipid peroxidation, which is elevated in the skeletal muscle of patients with T2DM and is negatively correlated with GDR in nondiabetic subjects [4].
4-HNE protein adducts are formed in response to mitochondrial generation of reactive oxygen species that interact with n-6 polyunsaturated fatty acids, including arachidonic acid, and other fatty acids such as linoleic acid [28]. It is a highly reactive lipid electrophile, with a prolonged half-life that can diffuse from sites of formation because of its amphiphilic properties [29]. The 4-HNE protein can bind to glutathione and can form covalent bonds with functional proteins in a manner that can alter their function. The 4-HNE adducts form preferentially with specific proteins and can therefore alter function in a cell-specific manner depending on the pattern of protein expression [28, 29]. These different properties may partially explain the observations that 4-HNE adducts in skeletal muscle are correlated with insulin sensitivity [4], whereas F2-isoPs are not. Additionally, the negative correlation between urinary F2-isoPs and tissue 4-HNE noted in our subgroup of patients with skeletal muscle biopsies suggests that local oxidative stress intrinsic to muscle itself can be related to insulin sensitivity in ways that are not reflected by whole-body F2-isoPs. This is likely the result of biochemical differences in the formation of these markers and further demonstrates that the oxidative process that produces F2-isoPs may not be relevant to insulin sensitivity. Collectively, these findings underscore the complex relationship between various oxy-lipid species and insulin sensitivity.
Our results regarding the lack of relationship between F2-isoPs and insulin sensitivity as measured by GDR are consistent with a recent study in healthy youths across the weight spectrum that found no relationship between F2-isoPs and insulin sensitivity as measured by the Homeostatic Model Assessment of Insulin Resistance [30]. Moreover, recent analyses of the Insulin Resistance Atherosclerosis Study discovered an inverse relationship between urinary F2-isoPs and the risk of developing T2DM [31, 32]. These findings, however, are discrepant with those of a smaller study (n = 31) reported by Urakawa et al., who observed a negative correlation between plasma 8-iso-prostaglandin-F2α and clamp measures of insulin sensitivity in lean and obese white men [33].
In terms of body composition, no linear association was found between F2-isoPs and either BMI or measures of body fat (total fat mass, body fat percentage, trunk fat, waist and hip circumference). This finding seems contrary to studies demonstrating that F2-isoP levels are correlated with BMI [15, 34] and with studies that show oxidative stress tend to decrease with weight loss [35]. One possibility for this incongruence is that we provided all participants with a standardized, isocaloric diet for 3 days before study measurements. Because meal intake can affect levels of oxidative stress [36], normalizing the diet for our subjects may have blunted the differences that might otherwise have been observed in our measure of whole-body oxidative stress. Another consideration is that approximately half of our subjects were African American, as Il’yasova et al. reported in the Insulin Resistance Atherosclerosis Study that F2-isoPs rose with BMI in whites but not African Americans. Even so, we failed to find a relationship between F2-isoPs with BMI and measures of fat mass even after controlling for race and in a stratified analysis of whites only (data not shown). However, we observed a higher level of F2-isoPs in overweight subjects compared with lean or obese subjects, whereas the F2-isoPs were similar in obese and lean subjects.
An alternative explanation for this discrepancy in our findings and the literature may be the relationship of F2-isoPs with LBM. We observed that F2-isoP values have a strong negative relationship with LBM, which was independent of BMI or measures of fat mass. Because BMI is a poor predictor of obesity in those with higher LBM, it is possible that the lack of association between F2-isoPs and BMI in our study could be explained by the influence of LBM.
The findings of a relationship between urinary F2-isoPs and LBM may be an important consideration for future studies utilizing F2-isoPs. We also observed that both urinary 15-F2t-IsoP and its major metabolite, 2,3-dinor-5,6-dihydro-15-F2t-IsoP, exhibited strong, significant negative correlations with BMC and BMD. The strength of these correlations was not diminished by adjusting for additional covariates such as age, race, sex, and smoking status. Because decrements in LBM, BMC, and BMD occur as a function of biological aging, it is possible that levels of F2-isoPs reflect oxidative stress contributing to aging. Our data support this hypothesis, as we found a positive correlation between urinary 15-F2t-IsoP levels and age, at least over the delimited age range of our study subjects (i.e., 20 to 60 years).
Regarding sex differences, we found that F2-isoPs were higher in females than males, which is consistent with other studies. However, sex was not a significant factor in the multiple regression equations relating F2-isoP and body composition measures, suggesting that the differences in F2-isoP levels by sex in this study are explained by the effects of LBM and BMD. This may also explain the differences in F2-isoP levels by sex observed in other studies. After controlling for sex, we also observed that whites had higher levels of F2-isoPs than blacks, which is similar to findings by Il’yasova et al. [37], and that smokers and former smokers both had higher F2-isoPs levels than in nonsmokers, as expected [38].
No significant correlation was found between F2-isoP with total body fat (%), even after adjusting for age, race, sex, and smoking status (Table 4). There was, however, a significant correlation between both total body fat (%) and trunk fat with the F2-isoP metabolite. It has been suggested that this metabolite might be a more accurate measure of whole-body oxidative stress over time (because it reduces the likelihood of potential local contribution of free F2-isoP from the kidney) [22]. Thus, future studies are warranted to investigate this compound and its relationship with measures of fat mass and metabolism in more detail.
The strength of this study lies in the use of gold standard measures of in vivo insulin sensitivity in humans and the state-of-the-art methodology for quantification of F2-isoPs. Our subjects represented wide ranges of BMI and insulin sensitivity and excluded the potentially confounding disease state of diabetes. Subjects were weight-stable upon entering the study and were fed an isocaloric diet with a standard macronutrient composition [14] to account for potential dietary effects on study measurements [39, 40].
The main limitation of this study was the use of a single spot morning urine sample instead of a 24-hour collection for F2-isoP analysis, which may have affected outcomes due to the potential of intrasubject daily variability [41]. Even so, the equilibration of research volunteers on a standardized, isocaloric diet with studies conducted in a metabolic ward would minimize daily variation. Another limitation is that our study sample was characterized by an uneven distribution of sex, although we used marginal means to adjust for this unbalanced design in regression analyses. Most importantly, our correlation studies in a cross-section sample population cannot be used to indicate causality; therefore, mechanistically, the results should be regarded as hypothesis-generating. However, the focus of our study was to assess the utility of F2-isoP as a biomarker of oxidative stress in insulin resistance. Despite wide use of F2-isoPs in this context, we found that there is no quantitative relationship with individual differences in insulin sensitivity in a nondiabetic population.
4. Conclusion
In conclusion, oxidative stress, as measured by urinary F2-isoPs, was not associated with insulin sensitivity, substrate oxidation, BMI, or fat mass. However, a significant negative correlation was found between both 15-F2t-IsoP and its major metabolite with LBM, as well as BMC and density. Because LBM, BMC, and BMD decline as a function of biological aging, F2-isoPs may reflect the oxidative stress operative in the aging process. Future studies are needed to identify how aspects of oxidative stress differentially affect pathways involved in the pathogenesis of insulin resistance, bone loss, and aging.
Acknowledgments
We thank Dana Golson, RN, CDE, Robert Petri, Armando Enriquez, MT, and Tracie Thomas, RN, for their help with acquisition of the data for this study.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH; Grants R01DK038765, R01DK083562), the Merit Review program of the Department of Veterans Affairs, the core facilities of the University of Alabama Birmingham (UAB) Diabetes Research Center (including the Human Physiology Core, P30DK079626), and the UAB Medical Scientist Training Program (T32GM008361), Pre-Doctoral Training in Obesity-Related Research (T32HL105349), and Nutrition Obesity Research Center (P30DK056336). F2-isoprostane assays were conducted in core laboratories at the Vanderbilt Diabetes Research and Training Center, supported by the NIH grant P30DK20593.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMC
- bone mineral content
- BMD
- bone mineral density
- BMI
- body mass index
- CV
- coefficient of variation
- F2-isoP
- F2-isoprostane
- GDR
- glucose disposal rate
- HDL
- high-density lipoprotein
- 4-HNE
- protein 4-hydroxynonenal
- LBM
- lean body mass
- LDL
- low-density lipoprotein
- T2DM
- type 2 diabetes mellitus.
References and Notes
- 1.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. [DOI] [PubMed] [Google Scholar]
- 2.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52(4):601–623. [DOI] [PubMed] [Google Scholar]
- 3.Davì G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal. 2005;7(1-2):256–268. [DOI] [PubMed] [Google Scholar]
- 4.Ingram KH, Hill H, Moellering DR, Hill BG, Lara-Castro C, Newcomer B, Brandon LJ, Ingalls CP, Penumetcha M, Rupp JC, Garvey WT. Skeletal muscle lipid peroxidation and insulin resistance in humans. J Clin Endocrinol Metab. 2012;97(7):E1182–E1186. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milne GL, Yin H, Hardy KD, Davies SS, Roberts LJ II. Isoprostane generation and function. Chem Rev. 2011;111(10):5973–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montuschi P, Barnes PJ, Roberts LJ II. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18(15):1791–1800. [DOI] [PubMed] [Google Scholar]
- 7.Basu S. Bioactive eicosanoids: role of prostaglandin F(2α) and F2-isoprostanes in inflammation and oxidative stress related pathology. Mol Cells. 2010;30(5):383–391. [DOI] [PubMed] [Google Scholar]
- 8.Helmersson J, Vessby B, Larsson A, Basu S. Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation. 2004;109(14):1729–1734. [DOI] [PubMed] [Google Scholar]
- 9.Davì G, Falco A, Patrono C. Determinants of F2-isoprostane biosynthesis and inhibition in man. Chem Phys Lipids. 2004;128(1-2):149–163. [DOI] [PubMed] [Google Scholar]
- 10.Roest M, Voorbij HA, Van der Schouw YT, Peeters PH, Teerlink T, Scheffer PG. High levels of urinary F2-isoprostanes predict cardiovascular mortality in postmenopausal women. J Clin Lipidol. 2008;2(4):298–303. [DOI] [PubMed] [Google Scholar]
- 11.Laight DW, Desai KM, Gopaul NK, Anggård EE, Carrier MJ. F2-isoprostane evidence of oxidant stress in the insulin resistant, obese Zucker rat: effects of vitamin E. Eur J Pharmacol. 1999;377(1):89–92. [DOI] [PubMed] [Google Scholar]
- 12.D’Archivio M, Annuzzi G, Varì R, Filesi C, Giacco R, Scazzocchio B, Santangelo C, Giovannini C, Rivellese AA, Masella R. Predominant role of obesity/insulin resistance in oxidative stress development. Eur J Clin Invest. 2012;42(1):70–78. [DOI] [PubMed] [Google Scholar]
- 13.Gopaul NK, Anggård EE, Mallet AI, Betteridge DJ, Wolff SP, Nourooz-Zadeh J. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett. 1995;368(2):225–229. [DOI] [PubMed] [Google Scholar]
- 14.Davì G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T, Costantini F, Capani F, Patrono C. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99(2):224–229. [DOI] [PubMed] [Google Scholar]
- 15.Davì G, Guagnano MT, Ciabattoni G, Basili S, Falco A, Marinopiccoli M, Nutini M, Sensi S, Patrono C. Platelet activation in obese women: role of inflammation and oxidant stress. JAMA. 2002;288(16):2008–2014. [DOI] [PubMed] [Google Scholar]
- 16.Garvey WT, Kwon S, Zheng D, Shaughnessy S, Wallace P, Hutto A, Pugh K, Jenkins AJ, Klein RL, Liao Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes. 2003;52(2):453–462. [DOI] [PubMed] [Google Scholar]
- 17.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34(3):222–234. [DOI] [PubMed] [Google Scholar]
- 18.Wu X, Patki A, Lara-Castro C, Cui X, Zhang K, Walton RG, Osier MV, Gadbury GL, Allison DB, Martin M, Garvey WT. Genes and biochemical pathways in human skeletal muscle affecting resting energy expenditure and fuel partitioning. J Appl Physiol. 1985;2011;110(3):746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lusk G, Du Bois EF. On the constancy of the basal metabolism. J Physiol. 1924;59(2-3):213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, Boyce V, Howard BV, Bogardus C. Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med. 1988;318(8):467–472. [DOI] [PubMed] [Google Scholar]
- 21.Morrow JD, Roberts LJ II. Mass spectrometric quantification of F2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Methods Enzymol. 1999;300:3–12. [DOI] [PubMed] [Google Scholar]
- 22.Morrow JD, Zackert WE, Yang JP, Kurhts EH, Callewaert D, Dworski R, Kanai K, Taber D, Moore K, Oates JA, Roberts LJ. Quantification of the major urinary metabolite of 15-F2t-isoprostane (8-iso-PGF2alpha) by a stable isotope dilution mass spectrometric assay. Anal Biochem. 1999;269(2):326–331. [DOI] [PubMed] [Google Scholar]
- 23.Milne GL, Yin H, Brooks JD, Sanchez S, Jackson Roberts L II, Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol. 2007;433:113–126. [DOI] [PubMed] [Google Scholar]
- 24.Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005;10(Suppl 1):S10–S23. [DOI] [PubMed] [Google Scholar]
- 25.Norris AL, Steinberger J, Steffen LM, Metzig AM, Schwarzenberg SJ, Kelly AS. Circulating oxidized LDL and inflammation in extreme pediatric obesity. Obesity (Silver Spring). 2011;19(7):1415–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magkos F, Fraterrigo G, Yoshino J, Luecking C, Kirbach K, Kelly SC, de Las Fuentes L, He S, Okunade AL, Patterson BW, Klein S. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23(4):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampson MJ, Gopaul N, Davies IR, Hughes DA, Carrier MJ. Plasma F2 isoprostanes: direct evidence of increased free radical damage during acute hyperglycemia in type 2 diabetes. Diabetes Care. 2002;25(3):537–541. [DOI] [PubMed] [Google Scholar]
- 28.Roede JR, Jones DP. Reactive species and mitochondrial dysfunction: mechanistic significance of 4-hydroxynonenal. Environ Mol Mutagen. 2010;51(5):380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrera G, Pizzimenti S, Ciamporcero ES, Daga M, Ullio C, Arcaro A, Cetrangolo GP, Ferretti C, Dianzani C, Lepore A, Gentile F. Role of 4-hydroxynonenal-protein adducts in human diseases. Antioxid Redox Signal. 2015;22(18):1681–1702. [DOI] [PubMed] [Google Scholar]
- 30.Warolin J, Coenen KR, Kantor JL, Whitaker LE, Wang L, Acra SA, Roberts LJ II, Buchowski MS. The relationship of oxidative stress, adiposity and metabolic risk factors in healthy black and white American youth. Pediatr Obes. 2014;9(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Il’yasova D, Morrow JD, Wagenknecht LE. Urinary F2-isoprostanes are not associated with increased risk of type 2 diabetes. Obes Res. 2005;13(9):1638–1644. [DOI] [PubMed] [Google Scholar]
- 32.Il’yasova D, Spasojevic I, Base K, Zhang H, Wang F, Young SP, Millington DS, D’Agostino RB Jr, Wagenknecht LE. Urinary F2-isoprostanes as a biomarker of reduced risk of type 2 diabetes. Diabetes Care. 2012;35(1):173–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urakawa H, Katsuki A, Sumida Y, Gabazza EC, Murashima S, Morioka K, Maruyama N, Kitagawa N, Tanaka T, Hori Y, Nakatani K, Yano Y, Adachi Y. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88(10):4673–4676. [DOI] [PubMed] [Google Scholar]
- 34.Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ; Framingham Study . Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23(3):434–439. [DOI] [PubMed] [Google Scholar]
- 35.Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, Hamouda W, Prabhala A, Afzal A, Garg R. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001;86(1):355–362. [DOI] [PubMed] [Google Scholar]
- 36.Aljada A, Mohanty P, Ghanim H, Abdo T, Tripathy D, Chaudhuri A, Dandona P. Increase in intranuclear nuclear factor kappaB and decrease in inhibitor kappaB in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. Am J Clin Nutr. 2004;79(4):682–690. [DOI] [PubMed] [Google Scholar]
- 37.Il’yasova D, Wang F, Spasojevic I, Base K, D’Agostino RB Jr, Wagenknecht LE. Racial differences in urinary F2-isoprostane levels and the cross-sectional association with BMI. Obesity (Silver Spring). 2012;20(10):2147–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ II. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332(18):1198–1203. [DOI] [PubMed] [Google Scholar]
- 39.Turpeinen AM, Basu S, Mutanen M. A high linoleic acid diet increases oxidative stress in vivo and affects nitric oxide metabolism in humans. Lipids. 1999;34(Suppl):S291–S292. [DOI] [PubMed] [Google Scholar]
- 40.Turpeinen AM, Mutanen M, Aro A, Salminen I, Basu S, Palmquist DL, Griinari JM. Bioconversion of vaccenic acid to conjugated linoleic acid in humans. Am J Clin Nutr. 2002;76(3):504–510. [DOI] [PubMed] [Google Scholar]
- 41.Basu S, Helmersson J. Factors regulating isoprostane formation in vivo. Antioxid Redox Signal. 2005;7(1-2):221–235. [DOI] [PubMed] [Google Scholar]