Abstract
Objectives:
Posttraumatic stress disorder (PTSD) is associated with hypothalamus-pituitary-adrenal (HPA) axis response to stressors, but links to neurophysiological and neuroanatomical changes are unclear. The purpose of this study was to determine whether stress-induced cortisol alters negative feedback on pituitary corticotroph function and pituitary volume.
Design:
Prospective controlled study in an outpatient clinic.
Methods:
Subjects with PTSD and matched control subjects underwent pituitary volume measurement on magnetic resonance imaging, with pituitary function assessed by 24-hour urine free cortisol (UFC), 8:00 am cortisol, and adrenocorticotropic hormone (ACTH) levels, and ACTH levels after 2-day dexamethasone/corticotropin-releasing hormone test. Primary outcome was pituitary volume; secondary outcomes were ACTH area under the curve (AUC) and 24-hour UFC.
Results:
Thirty-nine subjects were screened and 10 subjects with PTSD were matched with 10 healthy control subjects by sex and age. Mean pituitary volume was 729.7 mm3 [standard deviation (SD), 227.3 mm3] in PTSD subjects vs 835.2 mm3 (SD, 302.8 mm3) in control subjects. ACTH AUC was 262.5 pg/mL (SD, 133.3 pg/mL) L in PTSD vs 244.0 pg/mL (SD, 158.3 pg/mL) in control subjects (P = 0.80). In PTSD subjects, UFC levels and pituitary volume inversely correlated with PTSD duration; pituitary volume correlated with ACTH AUC in control subjects (Pearson correlation coefficient, 0.88, P = 0.0009) but not in PTSD subjects.
Conclusions:
The HPA axis may be downregulated and dysregulated in people with PTSD, as demonstrated by discordant pituitary corticotroph function and pituitary volume vs intact HPA feedback and correlation of pituitary volume with ACTH levels in healthy control subjects. The results suggest a link between pituitary structure and function in PTSD, which may point to endocrine targeted therapeutic approaches.
Keywords: pituitary gland, pituitary volume, cortisol, PTSD, hypothalamic-pituitary-adrenal axis
In a pilot study, the HPA axis was dysfunctional and downregulated in PTSD subjects vs matched healthy control subjects, suggesting the potential of endocrine targeting approaches in PTSD patients.
Posttraumatic stress disorder (PTSD) occurs in patients exposed to or who witnessed an extreme traumatic event. Lifetime prevalence is 8%, with a substantial burden on mental and physical health [1, 2]. The complex PTSD phenotype likely reflects interactions of multiple genetic and environmental factors.
Disruption of the hypothalamic-pituitary-adrenal (HPA) axis has been implicated in the pathogenesis of PTSD. Hormonal changes in PTSD are subtle but appear to represent a failure of the stress-response system to regain homeostasis, as would be expected in healthy subjects. The sympathetic nervous system controls immediate responses to stressors, whereas subsequent HPA axis responses reinstate homeostasis and long-lasting adaptive changes. The HPA axis stress response is essentially terminated through glucocorticoid negative feedback inhibition of the hypothalamus and pituitary, and basal HPA axis activity is restored. However, when stress is chronic or severe, as in PTSD, the HPA axis stress response may be prolonged or the system may fail, and mediators that promote termination of the response are no longer protective. This failure is manifested by lower cortisol levels but elevated catecholamine levels, which lead PTSD patients to show a persistent response to an external challenge that is no longer actively present. Patients remain hypervigilant, with intrusive memories of traumatic events leading to heightened autonomic response [1].
Studies in PTSD have shown some hormonal changes consistent with altered HPA axis activity, but results are not consistent. Cortisol levels may be lower in PTSD [3], with nonpharmacological stress challenges in PTSD patients evoking cortisol levels [4], but these results have not been confirmed [5], and a meta-analysis showed no difference in basal cortisol, urine free cortisol (UFC), or salivary cortisol levels [6]. Others showed that patients with PTSD had lower adrenocorticotropic hormone (ACTH) responses to corticotropin-releasing hormone (CRH) [7] and that onset of stress-induced cortisol response is delayed in PTSD, with increased [8] or unchanged [9] CRH-induced cortisol.
Because the enhanced HPA axis suppression observed in PTSD suggests alterations in glucocorticoid sensitivity at the pituitary level, it is postulated that neuroendocrine alterations in PTSD could be linked to changes in pituitary structure. Pituitary gland volume changes during the lifespan and these anatomic alterations may reflect functional pituitary gland status. However, studies of HPA functional changes in PTSD, using standard HPA testing, have not been conducted, and it remains unknown whether the pituitary structural changes observed in some psychiatric disorders are similarly seen in PTSD. In pediatric PTSD patients, pituitary volumes were higher in pubertal/postpubertal subjects but similar in prepubertal subjects compared with control subjects [10]. Pituitary volume was smaller in adult PTSD subjects than in healthy control subjects, although some PTSD subjects were receiving antidepressants and anxiolytics and control subjects were not matched for age, sex, or body mass index [11]. Data are limited on pituitary size in PTSD and, importantly, there are no reports of pituitary structure and function associations in PTSD. We conducted a study to address these gaps in understanding the HPA axis in PTSD. We hypothesized that patients with PTSD would exhibit enhanced negative feedback on pituitary corticotrophs, which, in turn, would lead to smaller pituitary volumes. The objective of our pilot, prospective study in patients with PTSD and healthy control subjects was to identify a unique neuroendocrine signature that could be targeted therapeutically to alter HPA axis activity and modify disease.
1. Materials and Methods
A. Study Participants
Subjects with PTSD were recruited at Cedars-Sinai Medical Center, Los Angeles, CA, in response to advertisements and referrals from treating psychiatrists. Healthy control subjects were recruited from the community in response to advertisements. PTSD subjects were ages 18 to 80 years, and all met Diagnostic and Statistical Manual-IV criteria for PTSD. Subjects were excluded if they had uncontrolled medical or endocrine disease or a pituitary adenoma, or if they were currently taking antidepressant medications, psychotropic medications, glucocorticoids, estrogen therapy, or ketoconazole. Both men and women were recruited.
Subjects came for three study visits. The first visit was the initial screening, during which a blood sample was collected for complete blood count, liver function tests, and basic metabolic profile, and urine toxicology was performed. Washout of antidepressants and anxiolytics to allow for study enrollment was permitted if approved by the treating physician. The washout period was 5 weeks for fluoxetine and 2 weeks for other antidepressants and anxiolytics. At visit 2, baseline 8:00 am endocrine tests were conducted and dexamethasone (Dex) tablets dispensed. At visit 3, subjects underwent CRH stimulation after having taken Dex for the prior 48 hours (described in 2D. Endocrine tests), and then underwent a magnetic resonance imaging (MRI). All subjects provided informed consent, and the study was approved by the Institutional Review Board.
B. Questionnaires
The Mini International Neuropsychiatric Interview [12] was administered to confirm that the subject met the criteria for PTSD or, alternatively, was a healthy control subject. Trauma exposure was confirmed on the Early Trauma Inventory Self Report, which is a 28-item self-administered questionnaire evaluating childhood or adolescent abuse and neglect [13]. Severity of PTSD was assessed in PTSD subjects using the Clinician Administered PTSD Scale, a 30-item structured interview assessing PTSD symptoms, impact of symptoms on social and occupational performance, and severity [14]. Severity scores were calculated by summing the frequency and intensity ratings for each symptom. Active depression was screened through self-administration of the Quick Inventory of Depressive Symptoms questionnaire [15].
C. Imaging
Subjects underwent pituitary MRI without gadolinium enhancement. Pituitary volume was calculated using the formula for volume (i.e., length × height × width). Three-dimensional volumetric analysis was performed via rendering software from T1 postcontrast images. After identification of the pituitary gland, rather than relying on automation, manual contour segmentation was used to determine volume of the area of interest to provide the most accurate assessment of the pituitary gland. After determining the volume of the pituitary after contour cuts, the remaining cranial anatomy segments were separated and set as an overlay with transparency settings of 50% to showcase the pituitary within the cranium.
D. Endocrine Tests
At visit 2, 24-hour UFC was measured and 8:00 am cortisol and ACTH levels assessed. Thyroid-stimulating hormone (TSH), free T4, insulin-like growth factor-1, and prolactin (PRL) levels, as well as testosterone level in males and luteinizing hormone and follicle-stimulating hormone levels and menstrual history in women were also assessed. Subjects then underwent the 2-day Dex/CRH test. Starting at noon on day 1, subjects took 0.5 mg of Dex every 6 hours, with the final dose taken at 6:00 am on day 3. At visit 3, at 8:00 am, serum levels of cortisol and ACTH were determined and then 100 μg of ovine CRH was injected intravenously. Plasma Dex levels were measured before CRH injection and blood samples were collected for cortisol and ACTH measurements every 15 minutes for 60 minutes. Urine toxicology screen was performed at each study visit.
ACTH was measured by immunoassay, and free T4 was measured by direct equilibrium dialysis at Quest Diagnostics (San Juan Capistrano, CA). UFC, insulin-like growth factor-1, total and free testosterone, and Dex levels were measured by liquid chromatography mass spectrometry at Quest Diagnostics. Serum cortisol and TSH were measured by chemiluminescence and PRL was measured by microparticle enzyme immunoassay at Cedars-Sinai.
E. Outcomes
The primary outcome of the study was the difference in pituitary volume on MRI in PTSD subjects vs matched, healthy control subjects. Secondary outcomes were differences in ACTH area under the curve (AUC) and UFC values, as well as correlation of pituitary volumes with measures of HPA function, including ACTH AUC, UFC, and serum cortisol values. These outcomes were also correlated with age, duration of PTSD, and severity of PTSD.
F. Statistical Analysis
Numerical results were summarized by mean [standard deviation (SD)] for normally distributed variables and median (range) for nonnormally distributed variables. Pearson correlation coefficients were calculated and paired t test was used to determine differences in matched pairs for variables that were normally distributed. Spearman correlation coefficients and Wilcoxon signed rank were used for nonnormally distributed variables. Statistical significance was defined as P < 0.05. All statistical tests were two-sided. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
We determined that 10 subjects in each group, for a total of 20 subjects overall, would be sufficient for this pilot study and would be the minimum number of subjects needed to provide feasibility and logistics for a larger study in the future. To allow for dropouts, we planned to screen a maximum of 30 PTSD subjects and 15 healthy control subjects, with a goal of enrolling a maximum of 20 subjects in the pilot study.
2. Results
A. Baseline Clinical Characteristics
Thirty-nine subjects were screened for the study. Six PTSD subjects were excluded because they were unable to undergo a washout of psychiatric medications. Six subjects (two PTSD and four control subjects) withdrew after the first visit. One PTSD subject had a pituitary microadenoma identified on MRI and, therefore, was excluded. Two subjects (one PTSD and one control subject) were withdrawn on the third visit because of adverse events; one experienced a rash and flushing from the Dex, and the other had syncope after the CRH injection. Three subjects (one control subject and two PTSD subjects) were excluded because of unstable medical conditions, including transaminitis, untreated hypothyroidism, and anemia. One control subject became pregnant before the second visit and was withdrawn.
Ten subjects with PTSD (nine women, one man) and 10 sex-matched, healthy control subjects completed the study. Mean age and body mass index in the PTSD group were 43.7 years (SD, 13.5 years) and 29.9 kg/m2 (SD, 7.3 kg/m2) vs 42.4 years (SD, 13.6 years) and 27.2 kg/m2 (SD, 6.1 kg/m2) in the control group, respectively (Table 1). Mean duration of PTSD was 19.1 years (SD, 17.9 years). In all subjects, onset of PTSD was >6 months from the start of the study. Severity of PTSD was measured on the Clinician Administered PTSD Scale, with mean score of 61. Dex levels measured a mean of 393.7 ng/dL and 382.6 ng/dL in PTSD and control subjects, respectively, and all subjects had cortisol levels <1.8 μg/dL after Dex administration, confirming appropriate completion of the Dex/CRH test.
Table 1.
HPA Function Test Results in Patients With PTSD
| Variable | Cohort | Mean (SD) | P Value |
|---|---|---|---|
| Age, y | PTSD | 43.7 (13.5) | NA |
| HC | 42.4 (13.6) | ||
| BMI, kg/m2 | PTSD | 29.9 (7.3) | NA |
| HC | 27.2 (6.1) | ||
| Pituitary volume, mm3 | PTSD | 665.3 (202.6) | 0.30 |
| HC | 835.2 (302.8) | ||
| Volumetrics, mm3 | PTSD | 854 (203) | 0.87 |
| HC | 834 (275) | ||
| ACTH AUC, pg/mL | PTSD | 262.5 (133.3) | 0.80 |
| HC | 244.0 (158.3) | ||
| UFC, μg | PTSD | 24.8 (12.0) | 0.59 |
| HC | 21.4 (10) | ||
| Basal ACTH, pg/mL | PTSD | 18.6 (11.0) | 0.15 |
| HC | 12.8 (2.8) | ||
| Basal cortisol, μg/dL | PTSD | 15.3 (8.0) | 0.95 |
| HC | 15.6 (5.8) | ||
| PRL, ng/mL | PTSD | 12.3 (4.0) | 0.67 |
| HC | 11.3 (5.6) |
Abbreviations: HC, healthy control subjects; NA, not applicable.
B. Pituitary Size and HPA Function Testing
Mean pituitary volume was 729.7 mm3 (SD, 227.3 mm3) in PTSD subjects vs 835.2 mm3 (SD, 302.8 mm3) in healthy control subjects (P = 0.49; Fig. 1). Volumetric analysis similarly showed no significant differences, with a mean of 854 mm3 (SD, 203 mm3) in PTSD subjects compared with 834 mm3 (SD, 275 mm3) in control subjects. Volumetrics correlated with calculated mean pituitary volume [Spearman correlation coefficient, −0.82 (P = 0.0038) in the PTSD group and 0.85 (P = 0.0016) in the control group].
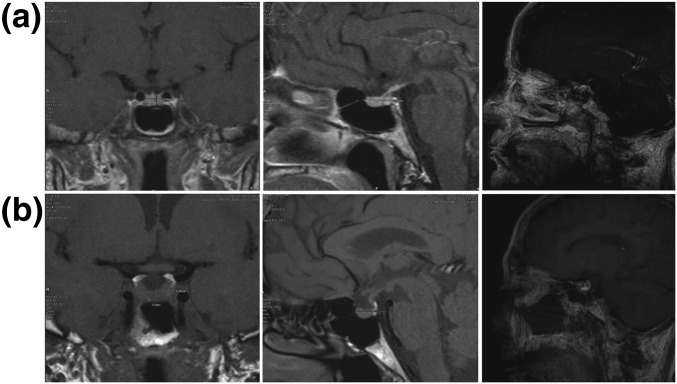
Figure 1.
MRI images (coronal and sagittal) and volumetric analysis of (a) a 60-year-old woman with PTSD (geometric volume, 403 mm3, volumetrics, 566 mm3) and (b) a matched healthy control subject (volume, 1379 mm3, volumetrics, 1080 mm3).
ACTH AUC was 262.5 pg/mL (SD, 133.3 pg/mL) in PTSD subjects vs 244.0 pg/mL (158.3 pg/mL) in control subjects, and 24-hour UFC was 24.8 μg (SD, 12.0 μg) in PTSD subjects vs 21.4 μg (9.9 μg) in control subjects. Basal 8:00 am cortisol and basal ACTH levels were 15.3 μg/dL (SD, 8.0 μg/dL) and 18.6 pg/mL (SD, 11.0 pg/mL) in PTSD subjects vs 15.6 μg/dL (SD, 5.8 μg/dL) and 12.8 pg/mL (SD, 2.8 pg/mL) in healthy control subjects, respectively. None of these differences were statistically significant. Furthermore, mean ACTH levels at each time point upon CRH stimulation were similar in both groups.
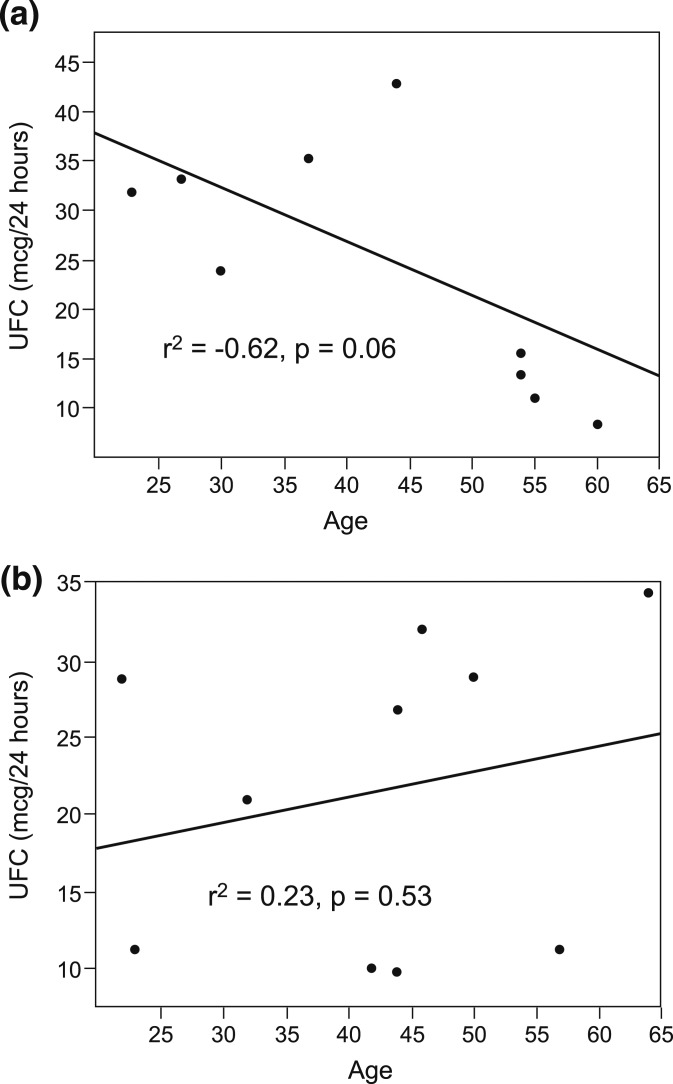
In the PTSD group, UFC levels inversely correlated with both duration of PTSD (Pearson correlation coefficient, −0.59; P = 0.07) and age (Pearson correlation coefficient, −0.62; P = 0.056), but these correlations were not observed in control subjects (Fig. 2). Remaining endocrine axes were intact, and PRL levels were normal.
Figure 2.
Correlation of 24-hour UFC levels with age in (a) PTSD subjects and (b) control subjects. In PTSD subjects, UFC levels decreased with age, whereas there was no similar correlation in control subjects.
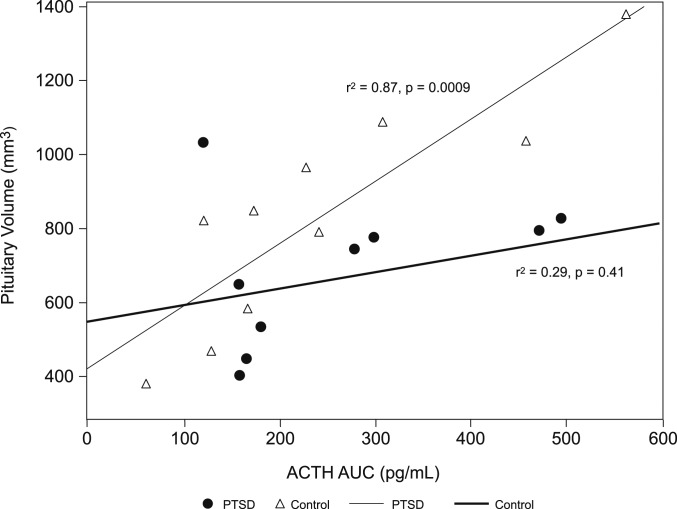
Although pituitary volume did not correlate with ACTH AUC and other measures of HPA function in PTSD subjects, pituitary volume significantly correlated with ACTH AUC in healthy control subjects [Pearson correlation coefficient, 0.88 (P = 0.0009); Fig. 3]. Pituitary volume correlated with duration of PTSD (Pearson correlation coefficient, −0.67; P = 0.03). Volumetrics in control subjects did not correlate with ACTH AUC (Spearman correlation coefficient, 0.58; P = 0.08).
Figure 3.
Correlation of pituitary volume and ACTH AUC. Mean pituitary volume was 729.7 mm3 (SD, 227.3 mm3) in PTSD subjects (circles) compared with 835.2 mm3 (SD, 302.8 mm3) in healthy control subjects (triangles). ACTH AUC was 262.5 pg/mL (SD, 133.3 pg/mL) in PTSD subjects vs 244.0 pg/mL (SD, 158.3 pg/mL) in control subjects. In healthy control subjects (gray line), pituitary volume significantly correlated with ACTH AUC (Pearson correlation coefficient, 0.88; P = 0.0009) but not in PTSD subjects (dark line) (Pearson correlation coefficient, 0.29; P = 0.41).
3. Discussion
This study attempts to link pituitary structure and HPA axis function in patients with PTSD. We posit that the HPA axis may be downregulated and dysregulated in this disorder, as evidenced by decreasing UFC with increased age and duration of PTSD, and a lack of correlation between ACTH levels and pituitary size. In contrast, matched, healthy control subjects maintained an intact HPA axis, exhibiting a robust pituitary size, stable UFC levels regardless of age, and significant correlation of pituitary size with ACTH levels.
Patterns of basal HPA activity typically change over the lifespan, with a positive correlation seen between age and diurnal cortisol secretion. Levels rise gradually during childhood, more rapidly in adolescence, and remain elevated in the elderly [16]. In contrast, we found that PTSD patients have lower cortisol production, and others have found both lower morning UFC levels as well as a decreasing cortisol response on Dex suppression test (DST) with advanced age [17].
Proposed mechanisms for the observed altered HPA axis activity in PTSD include enhanced feedback sensitivity, reduced pituitary sensitivity to glucocorticoids, reduced adrenal sensitivity to ACTH, and enhanced dexamethasone bioavailability [18]. Biologic effects of glucocorticoids are determined by HPA output as well as by glucocorticoid tissue sensitivity. Sensitivity is influenced by genetics, local glucocorticoid availability, abundance and affinity of glucocorticoid receptors (GRs), and disrupted glucocorticoid signaling [19].
In particular, the observed low cortisol and low glucocorticoid signaling in PTSD may reflect enhanced GR responsiveness [1]. Suppressive effects of Dex on ACTH would be expected to be at least as robust as effects on cortisol, owing to pituitary GR sensitivity. The DST in PTSD leads to enhanced ACTH suppression [18] and blunted ACTH response to CRH relative to healthy control subjects [20]. Changes in GR may also mediate these changes; decreased cytosolic GR lymphocytes derived from patients with PTSD correlated with enhanced Dex suppression [21]. However, higher GR abundance has also been reported in lymphocytes of those with PTSD [22].
Combined Dex/CRH testing detects subtle changes in pituitary GR sensitivity [18]. In healthy subjects, administration of Dex for 2 days suppresses corticotrophs, and CRH-elicited responses of ACTH and cortisol are attenuated [23]. However, downregulation of the HPA axis, as in PTSD, seems to alter these responses: In patients with borderline personality disorder and comorbid PTSD, ACTH increase is blunted with the Dex/CRH test [24]. Although a pilot study of 14 subjects with PTSD showed no differences in ACTH levels, using a modified Dex/CRH test [25], use of the standard 2-day Dex/CRH test has not been evaluated.
Further evidence of a dysfunctional, downregulated HPA axis in PTSD is suggested by our finding that pituitary size correlated with ACTH levels in healthy control subjects but not in PTSD subjects. The pituitary is plastic and dynamically adjusts to stimuli that affect hormone secretion and homeostasis [26]. Animal studies have shown that corticotrophs enlarge after acute CRH infusion [27] and pituitary size has been shown to correlate in patients with asthenia independent of age [28]. In patients with major depression, HPA hyperactivity may be associated with increased pituitary gland volume and circulating cortisol levels, especially in elderly patients [29], although not all studies confirm this observation [30]. Acute psychosis is associated with HPA hyperactivity, and larger pituitary volumes were observed in prodromal psychosis [31]. Pituitary volumes increase in schizophrenia spectrum disorders at a rate of 2.7% to 3.6% per year [32], whereas patients with chronic schizophrenia have smaller pituitary volumes [33]. Furthermore, psychiatric inpatients exhibit elevated post-DST cortisol concentrations that correlate with larger pituitary volume [34].
In our study, healthy control subjects with presumably intact glucocorticoid sensitivity had appropriate correlation of pituitary gland size with ACTH response to Dex/CRH stimulation, whereas subjects with PTSD exhibited enhanced negative feedback that led to discordance between pituitary size and ACTH secretion. Of note, ACTH levels after the Dex/CRH test were not different in PTSD subjects in our study, unlike prior work showing suppressed ACTH responses to CRH or Dex/CRH in this population [20]. Discrepancy in these findings may be due to differences in testing standards. The overnight DST using 0.5 mg of dexamethasone led to greater suppression of cortisol and ACTH levels in PTSD subjects [35], and the use of the 1-mg Dex overnight test similarly resulted in significantly lower ACTH levels [36]. We used the standard Dex/CRH test of 0.5 mg of dexamethasone every 6 hours for 48 hours, which may have led to more consistent ACTH suppression. Timing of trauma may also influence study outcomes. Patients with different traumatic histories have distinct peripheral-blood gene expression and DNA methylation profiles [37], suggesting epigenetic changes may be a factor. For example, expression of FKBP5, but not NR3C1, is significantly lower in PTSD patients who have a blunted HPA-axis response, suggesting a need to account for clinically and biologically distinct HPA-axis reactivity endophenotypes in PTSD [38].
We demonstrated in patients with PTSD that UFC decreased with age, and serum cortisol and pituitary volumes were lower with duration of PTSD. ACTH levels, however, did not correlate with pituitary size. Our results, therefore, suggest there may be a unique neuroendocrine signature associated with PTSD that could help identify treatment options, such as glucocorticoid supplementation. Several studies have assessed effects of hydrocortisone administration for a short time, or to augment psychotherapy. In a study randomly assigning military veterans to 30 mg of oral hydrocortisone or placebo before prolonged exposure therapy for PTSD, those receiving hydrocortisone were more likely to remain in treatment longer, with a greater reduction in PTSD symptoms [39].
An alternative approach may be to prevent onset of PTSD by administering a single, high glucocorticoid dose in the acute aftermath of trauma. In a randomized study, no subjects given intravenous hydrocortisone,100 to 140 mg, within 6 hours of a traumatic event developed PTSD at 3 months’ follow-up [40]. A systematic review pooling data from four studies similarly showed a relative risk reduction of 83% in development of PTSD among adults exposed to traumatic events who were given hydrocortisone [41]. A single dose of glucocorticoid may acutely reduce GR number, lower tonic glucocorticoid signaling by transiently activating available GRs, and recalibrate GR responsiveness. It may also change expression of genes that will lead to restored basal cortisol, allowing induced cortisol adaptive changes [1].
Although our results suggest potential areas to explore in larger studies, this was a pilot study with a small sample size; therefore, the results need to be interpreted with caution. Furthermore, another limitation is the sex imbalance. Lifetime prevalence of PTSD is twice as high in women as in men [42], which likely contributed to the higher recruitment of women in this study (nine of 10 in each cohort). HPA axis activity differs between men and women, with lower mean cortisol levels in premenopausal women compared with men of the same age [43] and lower HPA responses to psychological stress [44]. In a meta-analysis of HPA function in PTSD, male sex was associated with lower daily cortisol output [17], whereas premenopausal women have higher ACTH response to metyrapone than men [45], particularly in times of high estrogen secretion [46]. Fluctuations in salivary cortisol have been noted during the menstrual cycle [47], but no changes in CRH-expressing neurons were seen in postmortem tissue after menopause [48]. In our study, although our statistical model comparing all PTSD patients with all normal control subjects did not account for menopausal status or timing of menstruation or pregnancy history, outcomes were similar even after excluding the one male PTSD subject and his matched healthy control. Robust studies of HPA axis activity in PTSD with larger cohorts of men, premenopausal women with menstrual-cycle timing, and postmenopausal women would be needed to rigorously assess the impact of estrogen.
Timing and type of trauma may have played a role in response to HPA axis testing and pituitary size, as shown with those exposed to childhood trauma [24], but this was not addressed in our statistical models and should be explored further. Also, multiple factors could impact cellular glucocorticoid sensitivity and glucocorticoid receptor signaling and affect the clinical findings of this study. Finally, our observations could be secondary to the overall stress response. However, the persistent symptoms experienced by patients with PTSD would suggest that stress adaptation is not sufficient to explain these phenomena.
In conclusion, this pilot study presents preliminary evidence for a dysfunctional HPA axis that translates to structural pituitary changes, and suggests that enhanced negative feedback may play a role in the biology of PTSD. These findings necessitate confirmation in larger studies and open the door for therapeutic modulation of the HPA axis in patients with PTSD.
Acknowledgments
We thank Grace Labrado and Shira Berman for help with manuscript preparation.
Acknowledgments
This project described was supported by the National Center for Advancing Translational Sciences (Grant UL1TR000124 (7/1/11 - 2/29/16) and UL1TR001881 (7/1/16 - 5/31/17) and the National Institutes of Health (Grants K23DK085148 to O.C., T32DK007770 to S.M., and DK103198 to S.M.).
Clinical trials registry: ClinicalTrials.gov no. NCT00815204 (registered 24 December 2008).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- CRH
- corticotropin-releasing hormone
- GR
- glucocorticoid receptor
- HPA
- hypothalamus-pituitary-adrenal
- PRL
- prolactin
- PTSD
- posttraumatic stress disorder
- TSH
- thyroid-stimulating hormone
- UFC
- urine free cortisol.
References and Notes
- 1.Daskalakis NP, Lehrner A, Yehuda R. Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol Metab Clin North Am. 2013;42(3):503–513. [DOI] [PubMed] [Google Scholar]
- 2.Chriguer RS, Elias LL, da Silva IM Jr, Vieira JG, Moreira AC, de Castro M. Glucocorticoid sensitivity in young healthy individuals: in vitro and in vivo studies. J Clin Endocrinol Metab. 2005;90(11):5978–5984. [DOI] [PubMed] [Google Scholar]
- 3.Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL Jr, Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J Nerv Ment Dis. 1990;178(6):366–369. [DOI] [PubMed] [Google Scholar]
- 4.Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Afzal N, McGlashan T, Elzinga B, Anderson GM, Heninger G, Southwick SM, Charney DS. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28(6):733–750. [DOI] [PubMed] [Google Scholar]
- 5.Liberzon I, Abelson JL, Flagel SB, Raz J, Young EA. Neuroendocrine and psychophysiologic responses in PTSD: a symptom provocation study. Neuropsychopharmacology. 1999;21(1):40–50. [DOI] [PubMed] [Google Scholar]
- 6.Klaassens ER, Giltay EJ, Cuijpers P, van Veen T, Zitman FG. Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: a meta-analysis. Psychoneuroendocrinology. 2012;37(3):317–331. [DOI] [PubMed] [Google Scholar]
- 7.Smith MA, Davidson J, Ritchie JC, Kudler H, Lipper S, Chappell P, Nemeroff CB. The corticotropin-releasing hormone test in patients with posttraumatic stress disorder. Biol Psychiatry. 1989;26(4):349–355. [DOI] [PubMed] [Google Scholar]
- 8.Rasmusson AM, Lipschitz DS, Wang S, Hu S, Vojvoda D, Bremner JD, Southwick SM, Charney DS. Increased pituitary and adrenal reactivity in premenopausal women with posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):965–977. [DOI] [PubMed] [Google Scholar]
- 9.Kellner M, Yassouridis A, Hübner R, Baker DG, Wiedemann K. Endocrine and cardiovascular responses to corticotropin-releasing hormone in patients with posttraumatic stress disorder: a role for atrial natriuretic peptide? Neuropsychobiology. 2003;47(2):102–108. [DOI] [PubMed] [Google Scholar]
- 10.Thomas LA, De Bellis MD. Pituitary volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry. 2004;55(7):752–758. [DOI] [PubMed] [Google Scholar]
- 11.Atmaca M, Ozer O, Korkmaz S, Taskent I, Yildirim H. Evidence for the changes of pituitary volumes in patients with post-traumatic stress disorder. Psychiatry Res. 2017;260:49–52. [DOI] [PubMed] [Google Scholar]
- 12.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 13.Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis. 2007;195(3):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8(1):75–90. [DOI] [PubMed] [Google Scholar]
- 15.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. [DOI] [PubMed] [Google Scholar]
- 16.Deuschle M, Gotthardt U, Schweiger U, Weber B, Körner A, Schmider J, Standhardt H, Lammers CH, Heuser I. With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sci. 1997;61(22):2239–2246. [DOI] [PubMed] [Google Scholar]
- 17.Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. 2012;32(4):301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yehuda R, Golier JA, Halligan SL, Meaney M, Bierer LM. The ACTH response to dexamethasone in PTSD. Am J Psychiatry. 2004;161(8):1397–1403. [DOI] [PubMed] [Google Scholar]
- 19.Quax RA, Manenschijn L, Koper JW, Hazes JM, Lamberts SW, van Rossum EF, Feelders RA. Glucocorticoid sensitivity in health and disease. Nat Rev Endocrinol. 2013;9(11):670–686. [DOI] [PubMed] [Google Scholar]
- 20.Ströhle A, Scheel M, Modell S, Holsboer F. Blunted ACTH response to dexamethasone suppression-CRH stimulation in posttraumatic stress disorder. J Psychiatr Res. 2008;42(14):1185–1188. [DOI] [PubMed] [Google Scholar]
- 21.Yehuda R, Boisoneau D, Lowy MT, Giller EL Jr. Dose-response changes in plasma cortisol and lymphocyte glucocorticoid receptors following dexamethasone administration in combat veterans with and without posttraumatic stress disorder. Arch Gen Psychiatry. 1995;52(7):583–593. [DOI] [PubMed] [Google Scholar]
- 22.Gotovac K, Sabioncello A, Rabatic S, Berki T, Dekaris D. Flow cytometric determination of glucocorticoid receptor (GCR) expression in lymphocyte subpopulations: lower quantity of GCR in patients with post-traumatic stress disorder (PTSD). Clin Exp Immunol. 2003;131(2):335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanovski JA, Cutler GB Jr, Chrousos GP, Nieman LK. The dexamethasone-suppressed corticotropin-releasing hormone stimulation test differentiates mild Cushing's disease from normal physiology. J Clin Endocrinol Metab. 1998;83(2):348–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinne T, de Kloet ER, Wouters L, Goekoop JG, DeRijk RH, van den Brink W. Hyperresponsiveness of hypothalamic-pituitary-adrenal axis to combined dexamethasone/corticotropin-releasing hormone challenge in female borderline personality disorder subjects with a history of sustained childhood abuse. Biol Psychiatry. 2002;52(11):1102–1112. [DOI] [PubMed] [Google Scholar]
- 25.Muhtz C, Wester M, Yassouridis A, Wiedemann K, Kellner M. A combined dexamethasone/corticotropin-releasing hormone test in patients with chronic PTSD--first preliminary results. J Psychiatr Res. 2008;42(8):689–693. [DOI] [PubMed] [Google Scholar]
- 26.Hodson DJ, Mollard P. Navigating pituitary structure and function - defining a roadmap for hormone secretion. J Neuroendocrinol. 2013;25(7):674–675. [DOI] [PubMed] [Google Scholar]
- 27.Westlund KN, Aguilera G, Childs GV. Quantification of morphological changes in pituitary corticotropes produced by in vivo corticotropin-releasing factor stimulation and adrenalectomy. Endocrinology. 1985;116(1):439–445. [DOI] [PubMed] [Google Scholar]
- 28.Yamada T, Nojiri K, Sasazawa H, Tsukui T, Miyahara Y, Nakayama K, Komatsu M, Aizawa T, Komiya I. Correlation between the pituitary size and function in patients with asthenia. Endocr J. 2005;52(4):441–444. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan KR, Doraiswamy PM, Lurie SN, Figiel GS, Husain MM, Boyko OB, Ellinwood EH Jr, Nemeroff CB. Pituitary size in depression. J Clin Endocrinol Metab. 1991;72(2):256–259. [DOI] [PubMed] [Google Scholar]
- 30.Eker C, Ovali GY, Ozan E, Eker OD, Kitis O, Coburn K, Gonul AS. No pituitary gland volume change in medication-free depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(7):1628–1632. [DOI] [PubMed] [Google Scholar]
- 31.Garner B, Pariante CM, Wood SJ, Velakoulis D, Phillips L, Soulsby B, Brewer WJ, Smith DJ, Dazzan P, Berger GE, Yung AR, van den Buuse M, Murray R, McGorry PD, Pantelis C. Pituitary volume predicts future transition to psychosis in individuals at ultra-high risk of developing psychosis. Biol Psychiatry. 2005;58(5):417–423. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi T, Zhou SY, Nakamura K, Tanino R, Furuichi A, Kido M, Kawasaki Y, Noguchi K, Seto H, Kurachi M, Suzuki M. Longitudinal volume changes of the pituitary gland in patients with schizotypal disorder and first-episode schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):177–183. [DOI] [PubMed] [Google Scholar]
- 33.Pariante CM, Dazzan P, Danese A, Morgan KD, Brudaglio F, Morgan C, Fearon P, Orr K, Hutchinson G, Pantelis C, Velakoulis D, Jones PB, Leff J, Murray RM. Increased pituitary volume in antipsychotic-free and antipsychotic-treated patients of the AEsop first-onset psychosis study. Neuropsychopharmacology. 2005;30(10):1923–1931. [DOI] [PubMed] [Google Scholar]
- 34.Axelson DA, Doraiswamy PM, Boyko OB, Rodrigo Escalona P, McDonald WM, Ritchie JC, Patterson LJ, Ellinwood EH Jr, Nemeroff CB, Krishnan KR. In vivo assessment of pituitary volume with magnetic resonance imaging and systematic stereology: relationship to dexamethasone suppression test results in patients. Psychiatry Res. 1992;44(1):63–70. [DOI] [PubMed] [Google Scholar]
- 35.Golier JA, Schmeidler J, Legge J, Yehuda R. Enhanced cortisol suppression to dexamethasone associated with Gulf War deployment. Psychoneuroendocrinology. 2006;31(10):1181–1189. [DOI] [PubMed] [Google Scholar]
- 36.Duval F, Crocq MA, Guillon MS, Mokrani MC, Monreal J, Bailey P, Macher JP. Increased adrenocorticotropin suppression after dexamethasone administration in sexually abused adolescents with posttraumatic stress disorder. Ann N Y Acad Sci. 2004;1032:273–275. [DOI] [PubMed] [Google Scholar]
- 37.Zannas AS, Provençal N, Binder EB. Epigenetics of posttraumatic stress disorder: current evidence, challenges, and future directions. Biol Psychiatry. 2015;78(5):327–335. [DOI] [PubMed] [Google Scholar]
- 38.Zaba M, Kirmeier T, Ionescu IA, Wollweber B, Buell DR, Gall-Kleebach DJ, Schubert CF, Novak B, Huber C, Köhler K, Holsboer F, Pütz B, Müller-Myhsok B, Höhne N, Uhr M, Ising M, Herrmann L, Schmidt U. Identification and characterization of HPA-axis reactivity endophenotypes in a cohort of female PTSD patients. Psychoneuroendocrinology. 2015;55:102–115. [DOI] [PubMed] [Google Scholar]
- 39.Yehuda R, Bierer LM, Pratchett LC, Lehrner A, Koch EC, Van Manen JA, Flory JD, Makotkine I, Hildebrandt T. Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology. 2015;51:589–597. [DOI] [PubMed] [Google Scholar]
- 40.Zohar J, Yahalom H, Kozlovsky N, Cwikel-Hamzany S, Matar MA, Kaplan Z, Yehuda R, Cohen H. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur Neuropsychopharmacol. 2011;21(11):796–809. [DOI] [PubMed] [Google Scholar]
- 41.Amos T, Stein DJ, Ipser JC. Pharmacological interventions for preventing post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2014;7(7):CD006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry. 1998;55(7):626–632. [DOI] [PubMed] [Google Scholar]
- 43.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81(7):2468–2473. [DOI] [PubMed] [Google Scholar]
- 44.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61(2):154–162. [DOI] [PubMed] [Google Scholar]
- 45.Inslicht SS, Richards A, Madden E, Rao MN, O’Donovan A, Talbot LS, Rucker E, Metzler TJ, Hauger RL, Neylan TC. Sex differences in neurosteroid and hormonal responses to metyrapone in posttraumatic stress disorder. Psychopharmacology (Berl). 2014;231(17):3581–3595. [DOI] [PubMed] [Google Scholar]
- 46.Lund TD, Munson DJ, Haldy ME, Handa RJ. Dihydrotestosterone may inhibit hypothalamo-pituitary-adrenal activity by acting through estrogen receptor in the male mouse. Neurosci Lett. 2004;365(1):43–47. [DOI] [PubMed] [Google Scholar]
- 47.McCormick CM, Teillon SM. Menstrual cycle variation in spatial ability: relation to salivary cortisol levels. Horm Behav. 2001;39(1):29–38. [DOI] [PubMed] [Google Scholar]
- 48.Bao AM, Swaab DF. Gender difference in age-related number of corticotropin-releasing hormone-expressing neurons in the human hypothalamic paraventricular nucleus and the role of sex hormones. Neuroendocrinology. 2007;85(1):27–36. [DOI] [PubMed] [Google Scholar]