Abstract
Objective:
This study aimed to estimate the annual health care burden for patients with adrenal insufficiency [AI; primary (PAI), secondary to pituitary disorder (PIT), and congenital adrenal hyperplasia (CAH)] using real-world data.
Methods:
Using a US-based payer database comprising >108 million members, strict inclusion criteria with diagnostic codes and pharmacy records were used to identify 10,383 patients with AI. This included 1014 patients with PAI, 8818 with PIT, and 551 with CAH, followed for >12 months. Patients were matched 1:1 to controls, based on age (±5 years), sex, insurance, and region. Multivariable expenditure models were estimated for each AI cohort vs controls as well as subsets by glucocorticoid therapy (hydrocortisone, dexamethasone, prednisone, or multiple therapies). A separate multivariable model was estimated to assess the association between adherence and expenditures.
Results:
Total annual health care expenditure estimates were significantly higher (P < 0.0001) in all AI cohorts compared with matched controls (PAI $18,624 vs $4320, PIT $32,218 vs $6956, CAH $7677 vs $4203). Patients with AI have more frequent inpatient hospital stays with up to eight to 10 times more days in the hospital per year than their matched controls. In each AI cohort, patients on multiple steroid therapies had higher expenditures in comparison with patients using hydrocortisone therapy alone. In PAI and PIT cohorts taking hydrocortisone only, fewer expenditures were found in higher adherence subsets.
Conclusion:
Patients with AI demonstrate a substantial annual health care burden. Expenditures vary by underlying cause and treatment and are reduced in patients with higher adherence to glucocorticoid replacement.
Keywords: adrenal insufficiency, hypoadrenalism, congenital adrenal hyperplasia, health care costs, health economics, health care expenditures
Annual health care burden was estimated for insured patients with AI in comparison with matched controls using multivariable models. Models accounted for glucocorticoid therapy and adherence.
Adrenal insufficiency (AI) is a rare, life-threatening endocrine disease characterized by insufficient production of corticosteroid hormones [1]. AI can be classified as primary or secondary. Primary AI (PAI), is a condition affecting the adrenal glands, associated with loss of both glucocorticoid and mineralocorticoid hormones. Congenital adrenal hyperplasia (CAH), an inherited form of PAI, affects one in 10,000 to one in 20,000 newborns per year [2]. Hypopituitarism, or secondary AI, arises due to a hypothalamic or pituitary disorder (PIT) with glucocorticoid deficiency alone when the renin–angiotensin–aldosterone axis remains intact [3]. Conventional treatment of both PAI and secondary AI involves a lifelong regimen of steroid replacement therapy, with once-, twice-, or thrice-daily dosing with a glucocorticoid [4]. The most commonly prescribed glucocorticoid for this regimen is hydrocortisone, with alternatives being prednisone, prednisolone, and dexamethasone [3].
The literature on cost of treatment of AI is very limited. A recent study involving about 20,000 patients in the United Kingdom estimated the cost per patient of £1922 (USD $3,411) giving a total annual cost for the AI patient population of the UK of £39.7 million (USD $70.5 million) [5]. There are several cost drivers of AI that include but are not limited to diagnosis, less efficacious treatment strategies, comorbidities associated with the underlying disease, and possible side effects induced by the glucocorticoid replacement. The symptoms of AI are relatively nonspecific, often leading to delayed diagnosis, which may result in hospital admission with an acute life-threatening adrenal crisis [1]. The chronic nature of AI significantly increases cost because it requires lifelong replacement therapy and physician office visits. During glucocorticoid therapy, adherence may be made even more difficult by problems related to adrenal crisis and hospitalization, as well as patient concerns about the use of glucocorticoid therapy [6–8]. These long-term effects include decreased quality of life, reduced productivity (absenteeism), premature mortality, and long-term morbidity including obesity, osteoporosis, impaired glucose tolerance, cardiovascular disease, and infection [3].
A recent analysis, using the same source dataset, showed that AI patients have significant comorbidities, including increased risk of diabetes mellitus, hypertension, hyperlipidemia, depression, and anxiety, in comparison with matched controls [9]. In addition to increased comorbidity burden, PAI and PIT patients experienced significantly higher rate of hospital admissions than the matched controls, largely due to infection. The purpose of this study is to build upon those clinical findings of increased inpatient hospitalizations and morbidity utilizing a large national payer database in the United States to estimate the annual health care burden for patients with AI.
1. Methods
A. Data Source
This study used the same database and patient acquisition as our recent study on comorbidities of patients with AI [9], which used administrative health claims data from Truven Health MarketScan® Commercial and Medicare databases from January 2006 to June 2011, including a total of 108,271,287 patients. MarketScan contains individual-level, deidentified health care claims information from employers, health plans, hospitals, and Medicare programs in the United States and is fully compliant with the Health Insurance Portability and Accountability Act of 1996 [10].
B. Cohort Definitions and Selection Criteria
Patients were classified into three cohorts (PAI, PIT, or CAH) based on the algorithm from Stewart et al. [9], which applies strict inclusion criteria with diagnostic codes and pharmacy records of steroid prescriptions. To exclude patients who may have been prescribed high doses of steroids for conditions other than AI, in all cohorts, patients were excluded if there was documentation of glucocorticoid and/or mineralocorticoid usage with a pharmacy fill within the last 30 days of >10 mg/d of prednisolone/prednisone, >1 mg/d of dexamethasone, or >50 mg/d of hydrocortisone.
Following selection of subjects with the inclusion/exclusion criteria from Stewart et al. [9], each AI patient cohort was further classified into monotherapy (hydrocortisone only, dexamethasone only, or prednisolone/prednisone only) or multiple therapies based on the pharmacy fills in the 6 to 12 months after the first diagnosis time window. A lag of 6 months from the date of diagnosis was important for the adherence calculation to ensure the stability of a patients’ medication regimen. Furthermore, because glucocorticoid adherence can only be estimated based on pharmacy fill claims, patients with adherence of ≥50% were identified, and sample size permitting, analyzed separately. This subanalysis used ≥50% adherence as a threshold to ensure that cohort definitions were robust to varying degrees of drug adherence.
By setting this threshold, some appropriate patients might be omitted, but this analysis intentionally errs on the side of caution in order not to reach inaccurate conclusions about AI and replacement therapy.
AI patients across the three cohorts utilizing hydrocortisone only with threshold adherence rates of ≥50% (hereby known as the hydro-only subset) were analyzed as a subset to ensure the robustness of the cohorts. Finally, to explore the relationship between adherence and expenditures, the hydro-only subset was further divided by taking the initial threshold adherence criterion of ≥50% and creating the following two categories: patients who were 50% to 75% adherent vs patients who were >75% adherent.
C. Statistical Analysis
Every patient meeting inclusion and exclusion criteria within each AI cohort (PAI, PIT, and CAH) was matched one-to-one to a control group in the same insurance database (matched control). The matched control patients are not necessarily “healthy” controls, as they are in a database related to receiving health care, but rather, patients whose medical condition(s) does not include AI. Patients were matched using the greedy algorithm based on age (within 5 years), sex, insurance type (commercial or Medicare), and region (Northeast, Northcentral, South, West, or unknown) [11, 12].
All study measures were summarized using descriptive statistics. Patient demographics and expenditures were summarized in separate data tables and figures. Continuous variables were presented as the mean and standard deviation. Categorical variables were summarized with the count and percentage in each category. Health care expenditures included all inpatient and outpatient (emergency room, office visits, laboratory, radiology, and other procedures) expenditures in the first year after index diagnosis. Average inpatient admissions, average total hospital days, and average outpatient visits were reported for each AI cohort, as well as the PAI and PIT hydro-only subsets and the PIT 50% adherence subset.
To estimate the incremental annual health care expenditures for each AI cohort overall (PAI, PIT, and CAH) and their two subsets (adherence of ≥50% and hydro-only) compared with their matched control, multivariable generalized linear models were estimated using the gamma log-link function. The log-gamma model was chosen due to the nature of the outcome variable, health care expenditures, which is often right skewed. This model takes into account the distribution of the outcome variable without the need for complex retransformation procedures (e.g., natural logarithmic transformation). Expenditures (2006 to 2011) were converted to 2015 dollars using the Consumer Price Index from the Bureau of Labor Statistics [13]. Model adjustments after matching included year of diagnosis, and important patient demographics including age, sex, insurance plan, and plan type. Multivariable expenditure models were also estimated within each AI cohort by drug treatment (hydrocortisone, dexamethasone, prednisone, or multiple therapies) and, data permitting, their two subsets with the reference group for pairwise comparisons being the hydrocortisone only group.
Additional multivariable expenditure models were estimated for the hydro-only cohort by the two categories of their hydrocortisone adherence (50% to 75% vs >75%). Due to the small sample size of CAH patients taking only hydrocortisone, this analysis was only possible for the PAI and PIT cohorts.
2. Results
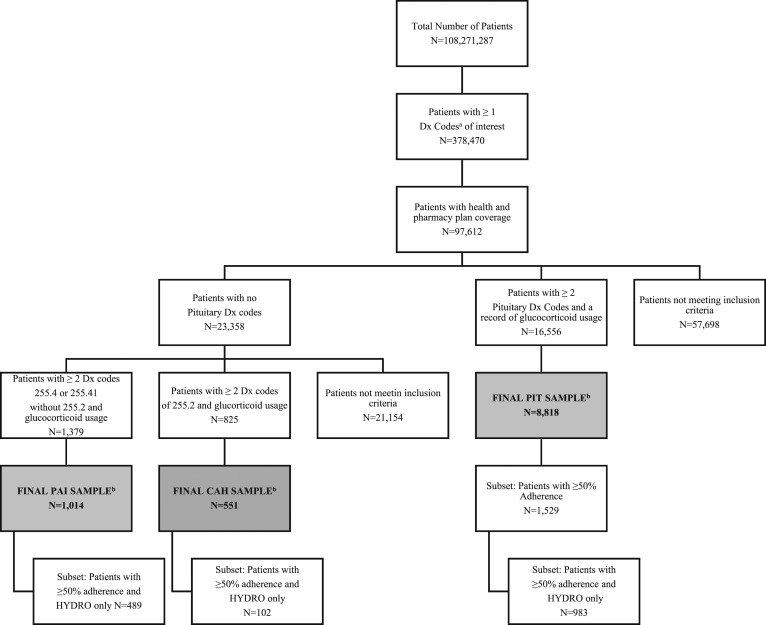
A total of 10,383 AI patients were identified from MarketScan commercial and Medicare databases for inclusion in the PAI (n = 1014), PIT (n = 8818), and CAH (n = 551) cohorts. Full patient attrition is shown in Fig. 1. Baseline demographics were similar between AI and matched control patients as designed (Table 1). Mean age (±standard deviation) was lowest in the CAH group (32.0 ± 18.3 years) compared with PAI (50.8 ± 17.2 years) and PIT (48.0 ± 16.0 years), with almost 30% of CAH patients under 18 years of age. Female sex predominated in all AI cohorts (PAI, 64.4%; PIT, 59.3%; and CAH, 67.9%). The majority of patients had commercial insurance coverage (PAI, 81.0%; PIT, 87.7%; and CAH, 97.3%).
Figure 1.
Attrition diagram. aICD-9 diagnosis (Dx) codes: 227.3–Benign neoplasm of pituitary gland and craniopharyngeal duct, 237.0–Neoplasm of uncertain behavior of pituitary gland and craniopharyngeal duct, 239.7–Neoplasm of unspecified nature of endocrine glands and other parts of nervous system, 253.2–Panhypopituitarism, 253.4–Other anterior pituitary disorders, 253.7–Iatrogenic pituitary disorders, 253.8–Other disorders of the pituitary and other syndromes of diencephalohypophyseal origin, 253.9–Unspecified disorder of the pituitary gland and its hypothalamic control, 255.2–Adrenogenital disorders, 255.4–Corticoadrenal insufficiency, 255.41–Glucocorticoid deficiency. bFinal AI samples matched to a cohort by age, sex, insurance type, and region. Only the PIT 50% adherence subset is displayed due to available sample size for each cohort. Adapted with permission from [9].
Table 1.
Patient Demographics at Baseline
| PAI | PIT | CAH | |
|---|---|---|---|
| Matched pairs | N = 1014 | N = 8818 | N = 551 |
| Age, y | |||
| Mean | 50.8 | 48.0 | 31.9 |
| Standard deviation | 17.2 | 16.0 | 18.3 |
| Sex, % | |||
| Male | 35.6 | 40.8 | 32.1 |
| Female | 64.4 | 59.3 | 67.9 |
| Insurance coverage, % | |||
| Commercial | 81.0 | 87.7 | 97.3 |
| Medicare | 19.0 | 12.3 | 2.7 |
| Region, % | |||
| Northeast | 12.3 | 14.6 | 15.4 |
| Northcentral | 31.0 | 25.7 | 22.5 |
| South | 33.8 | 43.3 | 43.6 |
| West | 22.4 | 15.3 | 17.6 |
| Unknown | 0.5 | 1.0 | 0.9 |
Adapted with permission from [9].
Total annual health care expenditure estimates were significantly higher (P < 0.0001) in all AI cohorts compared with their matched controls (PAI, $18,624 vs $4320; PIT, $32,218 vs $6956; CAH, $7677 vs $4203; Table 2). Health care expenditure estimates were highest in the PIT cohort with an incremental annual health care burden of $25,262, followed by PAI $14,304 and CAH $3474. For the PIT cohort, when examining the subset of patients with at least 50% adherence, their annual estimated costs were confirmatory (PIT, $32,218, vs PIT at least 50% adherence, $32,456). When analyzing patients with both adherence ≥50% and hydrocortisone recorded as the only glucocorticoid replacement (hydro-only subset), their estimated annual health expenditures were lower than the full sample (Table 2; PAI, $18,624, vs PAI hydro-only, $10,714; PIT, $32,218, vs PIT hydro-only, $26,251).
Table 2.
Total Annual Expenditures by AI Cohort and Matched Controls
| Pairs, n | Cohort | Case Estimate | Control Estimate |
|---|---|---|---|
| 1014 | PAI | $18,624 | $4320 |
| 489 | PAI hydro-onlya | $10,714 | $2901 |
| 8818 | PIT | $32,218 | $6956 |
| 983 | PIT hydro-only | $26,251 | $6566 |
| 1529 | PIT 50% onlyb | $32,456 | $6846 |
| 551 | CAH | $7677 | $4203 |
Hydro-only refers to patients in the PAI and PIT cohorts with hydrocortisone use only and a threshold of ≥50% adherence.
PIT 50% only refers to the subset of the PIT cohort with ≥50% adherence. Only PIT 50% adherence subset is displayed due to available sample size for each cohort.
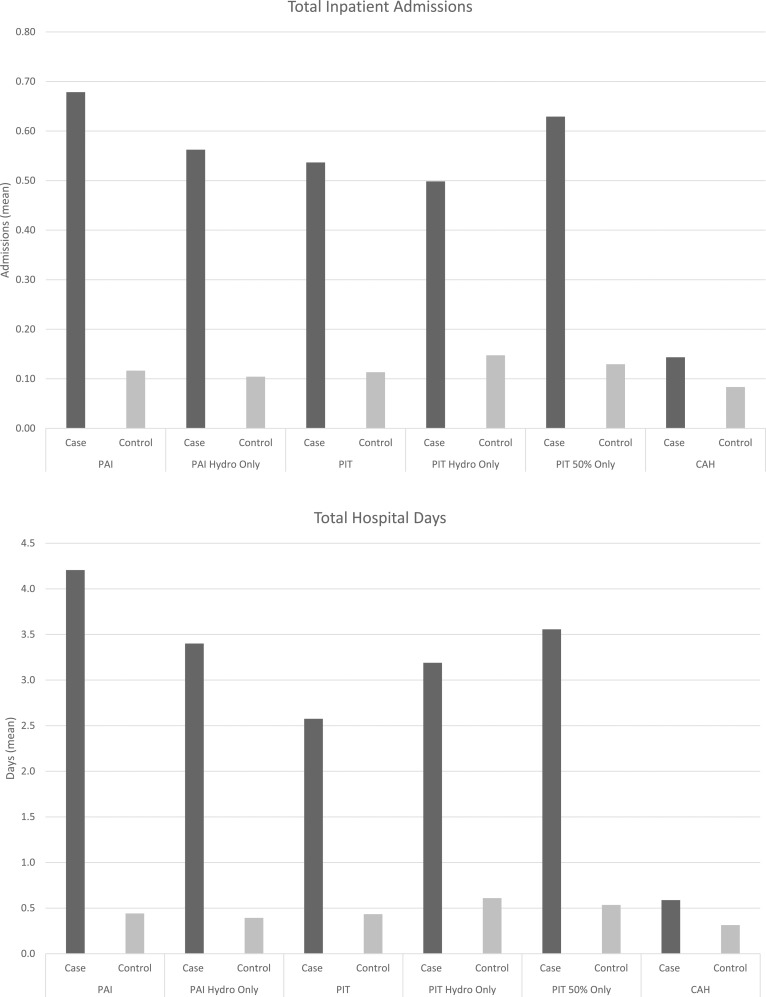
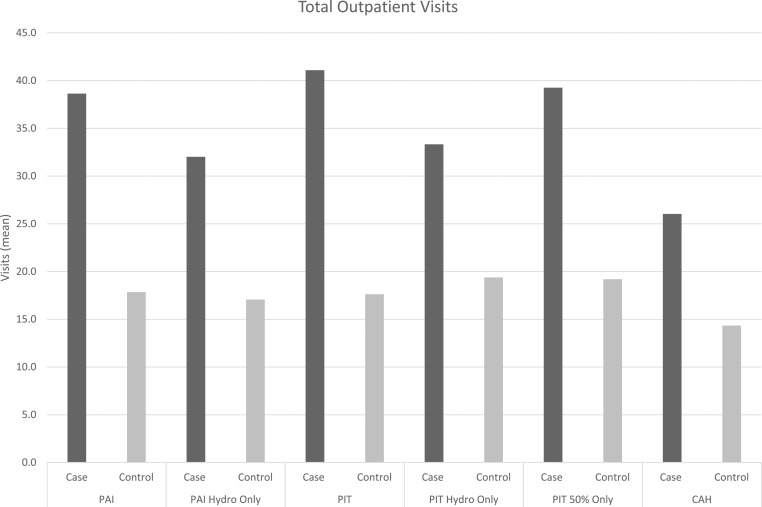
Substantial differences were found in total annual health care expenditure estimates for AI patients in comparison with matched controls (Table 2). The substantial increase in expenditures for the AI cohorts are on the basis of a mix of both inpatient and outpatient health care utilization. When examining this incremental burden for each AI cohort, the distribution of health care utilization by inpatient vs outpatient is evenly split with the exception of the CAH cohort (PAI: inpatient, 59%, vs outpatient, 41%; PIT: inpatient, 54%, vs outpatient, 46%; CAH: inpatient, 3%, vs outpatient, 97%). To understand these differences in greater detail, summary statistics of health care utilization measures including inpatient admissions, average total days spent in the hospital, and average outpatient visits were compared (Fig. 2). PAI patients had average annual number of inpatient admissions of 0.68 compared with 0.12 in the matched control; the PAI hydro-only subset had a similar difference (PAI hydro-only, 0.56 vs 0.10). The PIT cohort had an average of 0.54 admissions vs 0.11 for the matched control and this was similar for the hydro-only subset and ≥50% adherent cohort (0.63 vs 0.13). Among the CAH cohort, the CAH patients had 0.14 inpatient admissions compared with the 0.08 average annual admissions among matched controls. The average total hospitalized days were highest in the PAI cohort with 4.2 days in comparison with 0.4 among controls. AI patients were hospitalized more frequently and spent up to eight to 10 times more days in the hospital than their matched controls. Furthermore AI patients had approximately twice as many outpatient visits per year compared with matched controls (Fig. 2). Outpatient visits included those to the emergency department, laboratory, radiology unit, physician office, and other services. PAI patients had an average of 38.6 total outpatient visits compared with 17.8 among controls. The PIT cohort had an average of 41.1 visits with less in the hydro-only cohort (33.3 visits).
Figure 2.
Average health care utilization (inpatient, hospital days, outpatient) by AI cohort.
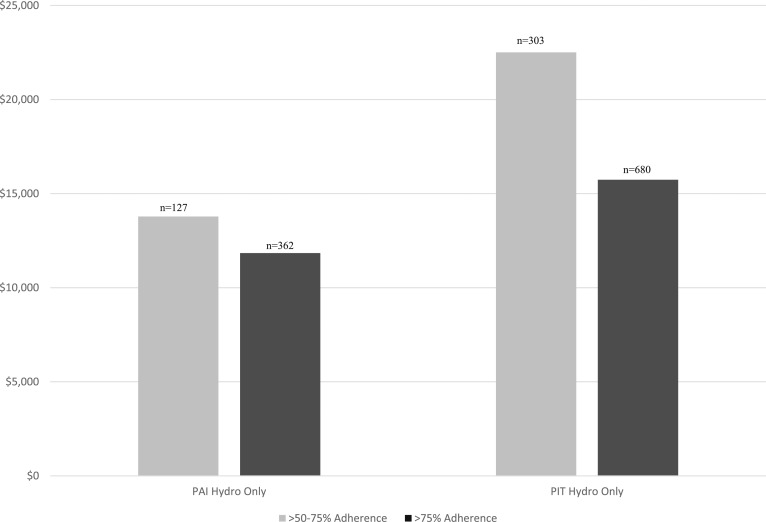
Annual health care expenditures for the AI cohorts were compared with each other based on their record of glucocorticoid replacement therapy. The annual health care expenditures by four types of glucocorticoid replacement therapy were estimated for each of the three AI cohorts: (1) hydrocortisone, (2) dexamethasone, (3) prednisone, and (4) multiple therapies. For the PIT cohort, Table 3 also reports findings for the subset of patients with threshold adherence of at least 50%. Across all three cohorts, estimated annual health care expenditures for patients on dexamethasone therapy compared with those taking hydrocortisone were not statistically significantly different. However, the PAI dexamethasone group was very small with only 15 patients. Patients on prednisone therapy had significantly higher total expenditures in the PAI and CAH cohorts (PAI, P = 0.009; CAH, P = 0.0134) and significantly lower total expenditures in the PIT cohort (P ≤ 0.0001) in comparison with patients in the same cohort on hydrocortisone. However, in the PIT subset of patients with ≥50% adherence, these findings were no longer statistically significant. In all AI cohorts including the PIT ≥50% adherence cohort, patients who used multiple glucocorticoid replacement regimens had higher total annual expenditures compared with patients who received hydrocortisone alone (PAI, P ≤ 0.0001; PIT, P ≤ 0.0001; PIT 50%, P ≤ 0.0001; and CAH, P = 0.0350; Table 3). When examining the two levels of adherence by expenditures for the hydro-only subset of patients with PAI and PIT, both cohorts showed a decrease in expenditures as adherence increased (Fig. 3).
Table 3.
Total Annual Expenditures by AI Drug Cohort
| AI Cohort Drug Cohort | Cohorts |
50% Adherencea |
||||
|---|---|---|---|---|---|---|
| N | Expenditures | P Valueb | N | Expenditures | P Valueb | |
| PAI | ||||||
| Hydrocortisone | 631 | $17,358 | — | |||
| Dexamethasone | 15 | $9431 | 0.0847 | |||
| Prednisone | 166 | $23,701 | 0.009 | |||
| Multiple therapies | 202 | $27,045 | <0.0001 | |||
| PIT | ||||||
| Hydrocortisone | 1802 | $37,281 | — | 983 | $24,479 | — |
| Dexamethasone | 1077 | $40,749 | 0.0511 | 27 | $27,424 | 0.6679 |
| Prednisone | 4881 | $21,955 | <0.0001 | 163 | $23,376 | 0.6939 |
| Multiple therapies | 1058 | $55,862 | <0.0001 | 356 | $46,336 | <0.0001 |
| CAH | ||||||
| Hydrocortisone | 147 | $5048 | — | |||
| Dexamethasone | 148 | $5210 | 0.8296 | |||
| Prednisone | 187 | $7132 | 0.0134 | |||
| Multiple therapies | 69 | $7354 | 0.0350 | |||
Only PIT 50% adherence subset is displayed due to available sample size for each cohort.
P values compare total expenditures of drug cohort vs hydrocortisone (hydrocortisone is the reference group for pairwise comparisons).
Figure 3.
Expenditures by adherence subsets.
3. Discussion
This study examined the real-world health care expenditures associated with AI and further investigated expenditures based on types of glucocorticoid replacement therapy and presumed medication adherence. Substantial health care expenditures occurred in each AI cohort, ranging from two to four times higher than matched controls. Health care expenditure estimates were highest in the PIT cohort ($32,218 vs $6956) followed by PAI ($18,624 vs $4320) and CAH cohorts ($7677 vs $4203). These findings are similar to the results of a study of diabetes treatment cohorts in the same claims database. Bonafede et al. [14] found that all cause medical expenditures for the first year after type 2 diabetes diagnosis ranged from $8591 to $20,350, based on severity cohort. Increased health care utilization across the three cohorts was explained by a mix of both inpatient and outpatient visits. This increased burden was confirmed even in the cohort with ≥50% adherence.
Annual health care expenditures were estimated for the following four categories of glucocorticoid replacement therapy in each cohort: (1) hydrocortisone, (2) dexamethasone, (3) prednisone, and (4) multiple therapies. Across cohorts, the expenditures associated with dexamethasone were not statistically higher than the expenditures associated with hydrocortisone treatment. Significantly higher annual expenditures were estimated for patients on prednisone therapy in both the PAI and CAH cohorts. In the PIT cohort, patients taking prednisone had significantly lower annual expenditures in comparison with PIT patients on hydrocortisone.
In each AI cohort, patients with multiple glucocorticoid replacement therapies had the highest expenditures. This finding highlights the difficulty in treating AI and how challenging it can be to find the most effective therapy for each patient [15]. Titration must be based on clinical judgment due to the lack of an ideal biochemical test. The choice of therapy may be due to attempts to control symptoms and signs related to AI which may reflect the use of multiple therapies in older sicker patients who have higher health care costs.
In the PAI and PIT hydro-only cohorts, higher adherence resulted in lower expenditures. This may be due to patients being less likely to experience adrenal crisis if they are more adherent to glucocorticoid replacement therapy. A case series of three AI patients found that with high adherence due to continuous subcutaneous hydrocortisone infusion, patients reported symptom improvement and two of the three cases had reduced hospital admission rates. The decrease in hospital admissions and resulting reduction in inpatient length of stay, reduced treatment costs in comparison with the year prior to continuous infusion [16]. Adherence is commonly linked to reduced cost of treatment, and alternatively, poor adherence has been cited by the World Health Organization as a driver of health care cost worldwide [17]. The World Health Organization report addressed the effect of adherence in chronic illnesses including hypertension, asthma, and diabetes. In the case of diabetes, the direct and indirect costs of complications attributed to poor control of diabetes were three to four times higher than the cost of complications in patients in good control. A survey on medication adherence and patient experience reported on adherence to glucocorticoid therapy. Only 15% of surveyed AI patients reported being fully adherent to the doses and timing of doses [8].
It is notable that the CAH cohort had the lowest cost as compared with the other cohorts. This may be due to the lower mean age of the CAH cohort; nearly 30% were under the age of 18. Annual expenditures in the PIT group were higher than in the PAI and CAH groups. This finding may be due to several factors, including care for the underlying condition which includes multiple hormonal deficiencies. For example, PIT patients selected into this analysis are likely to have panhypopituitarism as well as diabetes insipidus. Therefore, along with their glucocorticoid therapy they would also need desmopressin, sex steroid, and thyroxine replacement as well as the potential for recombinant human growth hormone. Although this analysis did not investigate magnetic resonance imaging (MRI) use specifically, PIT patients have regular head MRIs to monitor their pituitary-area tumor and typically have other pituitary hormone deficiencies also requiring treatment.
The UK cost of illness study suggested that inpatient admissions are a major cost driver of AI [5]. In a recent analysis, using the same cohorts as the current study, we found hospital inpatient admissions to be significantly higher in AI patients vs controls: for one admission in the matched control group, there were 4.64 admissions in the PAI cohort P < 0.0001. This finding was similar in the PIT cohort with four inpatient admissions to every one in the matched controls (P < 0.0001) [9]. These higher inpatient admissions are consistent with the high expenditures found in this analysis.
A. Data Limitations
We most likely did not capture all AI patients in the database. For the purpose of this analysis, we were striving to avoid including patients where we lacked a high level of certainty that the diagnosis was correct, particularly to ensure we excluded those who might have been treated with therapeutic (vs replacement) glucocorticoids for other conditions that are known to be associated with increased morbidity. We recognize that this strategy omitted some appropriate patients, but preferred to err on the side of caution in order not to reach inaccurate conclusions about AI and its replacement.
The limitations of administrative claims-based data—including lack of generalizability to noninsured populations, clinical outcomes being imputed from data not prepared for research purposes, and the underreporting of certain events and diagnoses—are well known. Administrative claims data are collected for the purpose of billing and reimbursement, not for coordinating medical care or conducting outcomes research. The data are subject to coding errors and underreporting of clinical conditions which do not trigger a billable event. Laboratory results, death outside the hospital setting, and physician notes are absent so specific medical details cannot be determined. Although prescription fills are available, such data do not reveal when or if the patient actually took the prescribed medication; likewise, physician instructions for taking medication are not available in the database and must be imputed using package size, pill dose strength, and number of pills dispensed. Furthermore, in this database, the drug claims are not linked to diagnoses, which mean that for this analysis, multiple steroid therapies may relate to the fact that the patient has other comorbidities that require other steroid treatments. Finally, limitations common to all retrospective research apply: most importantly, the lack of random allocation to treatment and the absence of protocols for follow up of all treatment cohorts, starting at a similar point in their disease course.
Despite these shortcomings, administrative data have been widely used to evaluate the association between treatments and clinical outcomes, particularly when a portrayal of patient experience outside the controlled setting of the clinical trial is useful, such as in the rare disorder AI.
4. Conclusion
This analysis of claims data included more than 10,000 AI patients divided into PAI, PIT, and CAH cohorts. Substantial annual health care expenditures were found for each of the AI cohorts in comparison with matched controls, with the PIT cohort having the highest annual expenditures. Patients with AI have more frequent inpatient hospital stays with up to eight to 10 times more days in the hospital per year than their matched controls. When comparing AI patients within each cohort based on their drug regimen, patients receiving prednisone therapy vs hydrocortisone therapy had significantly higher total annual expenditures in the PAI and CAH and significantly lower total expenditures in the PIT cohort. Patients taking only hydrocortisone and meeting the threshold of ≥50% adherence were found to have lower expenditures when medication adherence was 75% or higher. Future research is needed to further understand the increase in health care utilization (surgery, radiotherapy, MRI, etc.) that was observed in AI pituitary patients. In conclusion, compared with matched controls, patients with AI, irrespective of cause, demonstrate a substantial annual health care burden.
Appendix.
ICD-9 Codes Used to Define Cohorts
| ICD-9 Diagnosis Code | ICD-9 Diagnosis Description | Cohort |
|---|---|---|
| 255.4 | Corticoadrenal insufficiency | PAI |
| 255.41 | Glucocorticoid deficiency | PAI |
| 227.3 | Neoplasm, benign, pituitary gland | PIT |
| 237.0 | Neoplasm, uncertain behavior, pituitary gland | PIT |
| 239.7 | Neoplasm, unspecified nature, endocrine glands | PIT |
| 253.2 | Panhypopituitarism | PIT |
| 253.4 | Other anterior pituitary disorders | PIT |
| 253.7 | Disorder, iatrogenic pituitary | PIT |
| 253.8 | Other disorders of the pituitary and other syndromes of diencephalohypophyseal origin | PIT |
| 253.9 | Unspecified disorders of the pituitary gland and its hypothalamic control | PIT |
| 255.2 | Adrenogenital disorders | CAH |
Acknowledgments
B.M.K.B. and G.J. are members of the Endocrine Society.
Acknowledgments
This study was sponsored by Shire.
Disclosure Summary: C.G., M.P.R., and E.R.B. are employees of CTI Clinical Trial and Consulting Services, which is a paid consultant to Shire. C.M. is an employee of Shire International GmbH. P.M.S. has received speaker fees from Shire and Novartis. G.J. has received speaker’s honoraria and grants from Novartis, Novo Nordisk, Pfizer, and Sandoz; speaker’s honoraria from Merck Serono and Otsuka; and consultancy fees from Astra Zeneca and Shire. B.M.K.B. is the principal investigator on grants to Massachusetts General Hospital from Cortendo, Novartis, NovoNordisk, and Versartis and has received occasional consulting honoraria from Aeterna Zentaris, Chiasma, Cortendo, HRA Pharma, Ipsen, Novartis, NovoNordisk, and Pfizer.
Footnotes
- AI
- adrenal insufficiency
- CAH
- congenital adrenal hyperplasia
- PAI
- primary adrenal insufficiency
- PIT
- pituitary disorder.
References and Notes
- 1.Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart PM, Newell-Price JDC. The adrenal cortex. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology. 13th edPhiladelphia, PA: Elsevier; 2016:490–555. [Google Scholar]
- 3.Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014;383(9935):2152–2167. [DOI] [PubMed] [Google Scholar]
- 4.Johannsson G, Falorni A, Skrtic S, Lennernäs H, Quinkler M, Monson JP, Stewart PM. Adrenal insufficiency: review of clinical outcomes with current glucocorticoid replacement therapy. Clin Endocrinol (Oxf). 2015;82(1):2–11. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan R, Lee D. Adrenal insufficiency: burden of disease and cost of illness. Value Health. 2013;16:A436. [Google Scholar]
- 6.Smans LC, Souverein PC, Leufkens HG, Hoepelman AI, Zelissen PM. Increased use of antimicrobial agents and hospital admission for infections in patients with primary adrenal insufficiency: a cohort study. Eur J Endocrinol. 2013;168(4):609–614. [DOI] [PubMed] [Google Scholar]
- 7.Björnsdottir S, Sundström A, Ludvigsson JF, Blomqvist P, Kämpe O, Bensing S. Drug prescription patterns in patients with Addison’s disease: a Swedish population-based cohort study. J Clin Endocrinol Metab. 2013;98(5):2009–2018. [DOI] [PubMed] [Google Scholar]
- 8.Chapman SC, Llahana S, Carroll P, Horne R. Glucocorticoid therapy for adrenal insufficiency: nonadherence, concerns and dissatisfaction with information. Clin Endocrinol (Oxf). 2016;84(5):664–671. [DOI] [PubMed] [Google Scholar]
- 9.Stewart PM, Biller BM, Marelli C, Gunnarsson C, Ryan MP, Johannsson G. Exploring inpatient hospitalizations and morbidity in patients with adrenal insufficiency. J Clin Endocrinol Metab. 2016;101(12):4843–4850. [DOI] [PubMed] [Google Scholar]
- 10.Truven Health Analytics. MarketScan studies: abbreviated bibliography: 2014. Available at: http://sites.truvenhealth.com/bibliography/2014TruvenHealthMarketScanBibliography.pdf. Accessed 10 March 2016.
- 11.Mayo Clinic. Locally written SAS macros: Gmatch. Available at: http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros. Accessed 23 June 2016.
- 12.Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. In: Proceedings of the 26th Annual SAS Users Group International Conference; April 22–25, 2001; Long Beach, CA. Paper 214-26. Available at: http://www2.sas.com/proceedings/sugi26/p214-26.pdf. Accessed 23 June 2016.
- 13.Bureau of Labor Statistics. U.S. medical care consumer price index. Available at: http://www.bls.gov/news.release/cpi.toc.htm. Accessed 23 June 2016.
- 14.Bonafede M, Chandran A, DiMario S, Saltiel-Berzin R, Saliu D. Medication usage, treatment intensification, and medical cost in patients with type 2 diabetes: a retrospective database study. BMJ Open Diabetes Res Care. 2016;4(1):e000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray RD, Ekman B, Jones BA, Marelli C, Quinkler M, Zelissen PMJ. Clinical management of adrenal insufficiency (AI) shows notable heterogeneity: data from the European AI Registry (EU-AIR). In: Proceedings of the 17th European Congress of Endocrinology; May 16–20, 2015; Dublin, Ireland. Poster EP-39. [Google Scholar]
- 16.Khanna A, Khurana R, Kyriacou A, Davies R, Ray DW. Management of adrenocortical insufficiency with continuous subcutaneous hydrocortisone infusion: long-term experience in three patients. Endocrinol Diabetes Metab Case Rep. 2015;2015:150005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. Available at: http://apps.who.int/iris/bitstream/10665/42682/1/9241545992.pdf. Accessed 23 June 2016.