Abstract
Context:
Genetic defects affecting the expression and function of factors involved in pituitary development have been found to be associated with congenital hypopituitarism (CH). However, for most cases of CH, the etiology remains unknown.
Case Description:
We present an unusual case of an infant with CH, associated with septo-optic dysplasia with an absent anterior pituitary and an ectopic posterior pituitary gland, resulting from a de novo 8.04-Mb interstitial deletion of chromosome 1p31.1-1p31.3. The deleted region includes several genes that might be involved in pituitary development, including LEPR and JAK1.
Conclusions:
Haploinsufficiency of LEPR and/or JAK1 might be associated with CH. This finding suggests a role for LEPR-mediated glycoprotein 130 signaling in human pituitary development.
Keywords: chromosome 1p31, congenital hypopituitarism, microdeletion, LEPR gene, JAK1 gene, leptin
We present a case of congenital hypopituitarism associated with an 8.04-Mb interstitial deletion of chromosome 1p31.1-1p31.3 that suggests a role for LEPR and JAK1 in pituitary development.
Genetic defects affecting the expression and function of factors involved in pituitary development have been found to be associated with congenital hypopituitarism (CH) [1]. However, for most cases of CH, the etiology remains unknown. The identification of other causative genes for CH could provide insight into the interplay of signaling molecules, transcription factors, and other molecular networks involved in pituitary development. We present a case of CH associated with a chromosome 1p31.3 microdeletion involving LEPR and JAK1 that suggests an unusual mechanism for this condition in humans.
1. Methods
Genomic DNA was isolated from whole blood using the Puregene kit (Gentra Systems, Minneapolis, MN). Array comparative genomic hybridization using an oligonucleotide plus single nucleotide polymorphism-based microarray containing 180,000 features (SurePrint G3 GGXChip plus SNP, version 1.0 4x180k; Agilent Technologies, Santa Clara, CA) was performed according to manufacturer’s protocol by Detroit Medical Center University Laboratories (Detroit, MI). The array design and genomic coordinates were based on the National Center for Biotechnology Information Build 37 (hg19) for the Agilent array. Variants were categorized using the American College of Medical Genetics standards and guidelines for interpretation and reporting [2].
The exonic regions of all known genes in the 1p31.3-p31.1 region and 14 additional genes involved in pituitary development (GLI2, HESX1, LHX3, LHX4, OTX2, PITX1, PITX2, POU1F1, PROP1, SHH, SIX3, SOX2, SOX3, and TGIF1) were sequenced using XomeDxSlice testing by GeneDx (Gaithersburg, MD) using the Illumina HiSEquation 2000 sequencing system (Illumina, San Diego, CA).
2. Case Report
The patient was the full-term male of a nonconsanguineous relationship, born to 28-year-old mother with a history of gestational diabetes treated with glyburide. His birth weight was 3.42 kg (50th percentile), his birth length was 48 cm (12th percentile), and his head circumference was 36.1 cm (70th percentile). He was initially vigorous but developed respiratory distress and hypoglycemia soon after birth and required admission to the neonatal intensive care unit.
At the time of birth, he was noted to have multiple dysmorphic features, including trigonocephaly with a prominent brow and metopic ridge; hypotelorism with almond-shaped eyes and infraorbital creases; a flat nasal bridge with anteverted nostrils; small, low-set, posteriorly rotated ears with overfolded helices; a long philtrum, micrognathia, a high arched palate; widely spaced, hypoplastic nipples; a small scrotum and phallus; unilateral cryptorchidism; clenched hands with deep palmar creases and long-appearing fingers; fifth digit clinodactyly of both feet; and a sacral dimple. He also had hyperreflexia in the lower extremities.
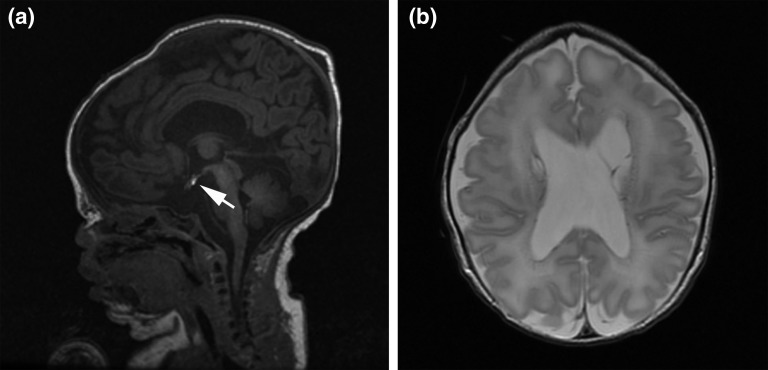
Extensive neonatal imaging studies were performed. A head ultrasound scan on his first day of life revealed midline abnormalities, including the absence of the septum pellucidum and mild lobularity of the medial cortical surfaces, along with ventricular enlargement and several periventricular cystic areas. These findings were subsequently corroborated by magnetic resonance imaging of the brain, which also noted a diminutive sella, ectopic posterior pituitary, and nonvisualization of the anterior pituitary gland (Fig. 1). The corpus callosum appeared hypoplastic. Computed tomography of the head showed evidence of metopic synostosis, and a spinal magnetic resonance imaging study revealed sacralization of L5. At 1 week of age, an electroencephalogram showed a discontinuous electroencephalographic pattern, suggesting diffuse neuronal dysfunction; however, no seizure activity was present. The findings of an ophthalmologic evaluation were normal. The results of his newborn hearing screening test were abnormal, and auditory brainstem response testing performed at 8 months of age found moderate to severe bilateral sensorineural hearing loss. The findings of a newborn echocardiogram were normal, except for a patent foramen ovale, and a skeletal survey showed no abnormal findings and a normal bone mineral density.
Figure 1.
Brain magnetic resonance images showing brain and craniofacial anomalies. (a) Sagittal T1-weighted image showing ectopic location of the posterior pituitary bright spot (arrow), consistent with an ectopic posterior pituitary with a flattened and empty sella turcica. Micrognathia is also apparent. (b) Axial T2-weighted image showing absent septum pellucidum with mildly enlarged lateral ventricles.
Neonatal measurement of the anterior pituitary hormones revealed a low free thyroxine level (0.7 ng/dL) with inappropriately low thyrotropin (13.4 µIU/mL), low adrenocorticotropic hormone (<5 pg/mL), low cortisol (0.8 µg/dL), and low growth hormone levels during two episodes of hypoglycemia (<0.05 ng/mL). His testosterone level was low (<20 ng/dL), the luteinizing hormone level was 0.3 mIU/mL, and the follicle-stimulating hormone level was 2.0 mIU/mL. These findings were consistent with hypopituitarism. Levothyroxine, hydrocortisone, and growth hormone replacement were initiated. His clinical course was notable for substantial global developmental delays. At 1 year of age, he was hypotonic. He was not sitting or crawling, nor did he have any words or meaningful articulations.
The array comparative genomic hybridization revealed a 46XY male chromosomal complement with a de novo 8.04-Mb interstitial deletion of chromosome 1p31.1-1p31.3 involving 30 Online Mendelian Inheritance in Man genes, including LEPR, JAK1, and NFIA (Supplemental Table 1 (21.1KB, docx) ). Sequencing all 30 genes in the 1p31.3p31.1 region did not identify any mutations or variants in the alternate allele. In addition, we did not identify a pathogenic sequence variation in any of the 14 genes outside this locus known to be involved in pituitary development (GLI2, HESX1, LHX3, LHX4, OTX2, PITX1, PITX2, POU1F1, PROP1, SHH, SIX3, SOX2, SOX3, and TGIF1).
3. Discussion
We present the case of an infant with a de novo 8.04-Mb interstitial deletion of chromosome 1p31.1-1p31.3 associated with CH, multiple congenital anomalies, and global developmental delays. Multiple signaling molecules and transcription factors are known to orchestrate the various stages of pituitary development [1]. Mutations in these genes (HESX1, POU1F1, PROP1, LHX3, LHX4, PITX1, PITX2, OTX2, SOX2, and SOX3) result in pituitary dysfunction, including CH [1, 3]. Our patient did not harbor a pathogenic sequence variation in any of these genes, suggesting that cause of his hypopituitarism was the result of an unusual genetic change. Therefore, we postulate that haploinsufficiency of one or more genes in the deleted segment might have been responsible for his CH. Among these genes, several could be involved in pituitary development, including LEPR, NFIA, and JAK1.
Few patients with interstitial deletions of the chromosome 1p31.3 region have been described [4–11] (Table 1). Deletions in this region, in particular, those involving NFIA but not LEPR and JAK1 (Table 1), are typically associated with structural brain abnormalities, including hypoplasia of the corpus callosum and ventriculomegaly, and craniofacial anomalies, metopic synostosis, urinary tract abnormalities, hypotonia, and developmental delays [7]. However, CH has not been described in these cases, suggesting that haploinsufficiency of NFIA is not solely involved in pituitary development.
Table 1.
Summary of Cases With Interstitial Deletions of the Chromosome 1p31.3 Region
| Variable | Barton et al. [10], 1995 | Campbell et al. [4], 2002; Lu et al. [7], 2007 | Koehler et al. [5], 2010 | Petti et al. [6], 2011 | Chen et al. [8], 2011 | Vauthier et al. [9], 2012 | Rao et al. [11], 2013 | Present Study | |
|---|---|---|---|---|---|---|---|---|---|
| Time of diagnosis | Postnatal | Postnatal | Postnatal | Postnatal | Postnatal | Prenatal | Postnatal | Postnatal | Postnatal |
| Method of diagnosis | Karyotyping, FISH | Karyotyping, FISH | Karyotyping, FISH | aCGH | Karyotyping, aCGH | aCGH, FISH | aCGH, MPLC | Karyotyping, FISH, aCGH | aCGH |
| Deletion size | NR | 12 Mb | 12 Mb | 4.93 Mb | 3.2 Mb | 22.2 Mb | 80 Kb | 0.12 Mb | 8 Mb |
| Genes involved | NR | 48 | 48 | 17 | 17 | 91 | 2 | 1 | 30 |
| LEPR | NR | + | + | − | + | NR | + (exon 1,2) | − | + |
| NFIA1 | NR | + | + | + | − | + | − | + (exon 4–9) | + |
| JAK1 | NR | + | + | − | + | NR | − | − | + |
| Age, gender | 18 mo, F | 3 y, F | 11 mo, M | 0.5 y, F | 15 y, M | 30 wk GA, F | 7 y, M | 8 y, F | 2 y, M |
| Phenotype | |||||||||
| Abnormal corpus callosum | ? | + | + | + | ? | + | ? | + | + |
| Hydrocephalus or ventriculomegaly | ? | + | + | + | ? | + | ? | + | + |
| Pituitary abnormality | ? | − | − | − | ? | − | ? | − | + |
| Dysmorphic features | + | + | + | + | + | + | + | + | + |
| Macrocephaly | − | + | + | + | − | + | − | + | − |
| Metopic synostosis | − | − | − | − | − | − | − | + | + |
| Developmental delay | + | + | + | ? | + | NA | + | + | + |
| Urinary tract abnormality | ? | + | + | − | ? | ? | ? | + | − |
| Tethered spinal cord | ? | + | + | − | ? | − | ? | − | + |
| Seizures | − | + | + | − | − | NA | + | − | + |
| Obesity | ? | − | − | − | + | NA | + | + | − |
Abbreviations: +, present; −, absent; ?, no investigations performed to rule it out; aCGH, array comparative genomic hybridization; F, female; FISH, fluorescence in situ hybridization; GA, gestational age; M, male; MPLC, multiplex polymerase chain reaction/liquid chromatography; NA, not applicable; NR, not reported.
Several other genes within the deleted 1p31.1-1p31.3 region are interesting candidates for the phenotype, including LEPR and JAK1. Individuals with homozygous and compound heterozygous mutations in LEPR resulting in leptin receptor deficiency show evidence of pituitary dysfunction with low growth hormone and thyrotropin levels and impaired pubertal development resulting from central hypogonadism [4, 5]. Patients with large interstitial deletions of this region have brain malformations; however, hypopituitarism has not been clearly described [4, 7]. One patient with a homozygous 80-kb deletion of chromosomal 1p31.3 involving the proximal promoter and exons 1 and 2 of LEPR (Table 1) presented with early-onset obesity, mental retardation, and epilepsy, but not CH [5]. Only one other patient with a 3.2-Mb deletion of the chromosome 1p31.3 region that included deletion of LEPR and JAK1 has been reported [6]. The patient had facial dysmorphism, obesity, behavioral problems, and mild intellectual impairment [6]. However, the findings from neuroimaging and endocrinological studies were not reported. The case we have presented is unique in its comprehensive characterization of CH.
The LEPR gene encodes the leptin receptor. Its longest isoform, LEPRb, is a single-transmembrane-domain receptor that belongs to the glycoprotein 130 (gp130) family of cytokine receptors that use the JAK-STAT signal transduction pathway. LEPRb is uniquely expressed in the hypothalamus and pituitary in both rodents and humans [12, 13]. Based on studies in model organisms, it has been suggested that gp130 cytokine signaling mediated by the JAK/STAT signal transduction pathway might have a critical role in hypothalamic and pituitary development [13–16]. In this context, JAK1, a widely expressed membrane-associated phosphoprotein, plays a critical role in neuronal development and survival, mediated by gp130 cytokine signaling [17]. While Jak1−/− neurons cultured in the absence of growth factor are not viable, their viability is maintained in the presence of gp130 receptor family ligands, suggesting an obligate and synergistic relationship between gp130 receptor family members and Jak1 is needed for neuronal development [17]. Extrapolating from these data, we speculate that the combined effect of both LEPR and JAK1 haploinsufficiency could have affected normal pituitary development, resulting in our patient’s phenotype. Future studies are needed to confirm the role gp130 cytokine signaling might have in human pituitary development.
Acknowledgments
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CH
- congenital hypopituitarism
- gp130
- glycoprotein 130.
References and Notes
- 1.Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev. 2009;30(7):790–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kearney HM, Thorland EC, Brown KK, Quintero-Rivera F, South ST. American College of Medical Genetics standards and guidelines for interpretation and reporting of postnatal constitutional copy number variants. Genet Med 2011;13(7):680–685. [DOI] [PubMed] [Google Scholar]
- 3.Reynaud R, Gueydan M, Saveanu A, Vallette-Kasic S, Enjalbert A, Brue T, Barlier A. Genetic screening of combined pituitary hormone deficiency: experience in 195 patients. J Clin Endocrinol Metab. 2006;91(9):3329–3336. [DOI] [PubMed] [Google Scholar]
- 4.Campbell CG, Wang H, Hunter GW. Interstitial microdeletion of chromosome 1p in two siblings. Am J Med Genet. 2002;111(3):289–294. [DOI] [PubMed] [Google Scholar]
- 5.Koehler U, Holinski-Feder E, Ertl-Wagner B, Kunz J, von Moers A, von Voss H, Schell-Apacik C. A novel 1p31.3p32.2 deletion involving the NFIA gene detected by array CGH in a patient with macrocephaly and hypoplasia of the corpus callosum. Eur J Pediatr. 2010;169(4):463–468. [DOI] [PubMed] [Google Scholar]
- 6.Petti M, Samanich J, Pan Q, Huang CK, Reinmund J, Farooqi S, Morrow B, Babcock M. Molecular characterization of an interstitial deletion of 1p31.3 in a patient with obesity and psychiatric illness and a review of the literature. Am J Med Genet A. 2011;155A(4):825–832. [DOI] [PubMed] [Google Scholar]
- 7.Lu W, Quintero-Rivera F, Fan Y, Alkuraya FS, Donovan DJ, Xi Q, Turbe-Doan A, Li QG, Campbell CG, Shanske AL, Sherr EH, Ahmad A, Peters R, Rilliet B, Parvex P, Bassuk AG, Harris DJ, Ferguson H, Kelly C, Walsh CA, Gronostajski RM, Devriendt K, Higgins A, Ligon AH, Quade BJ, Morton CC, Gusella JF, Maas RL. NFIA haploinsufficiency is associated with a CNS malformation syndrome and urinary tract defects. PLoS Genet. 2007;3(5):e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CP, Su YN, Chen YY, Chern SR, Liu YP, Wu PC, Lee CC, Chen YT, Wang W. Chromosome 1p32-p31 deletion syndrome: prenatal diagnosis by array comparative genomic hybridization using uncultured amniocytes and association with NFIA haploinsufficiency, ventriculomegaly, corpus callosum hypogenesis, abnormal external genitalia, and intrauterine growth restriction. Taiwan J Obstet Gynecol. 2011;50(3):345–352. [DOI] [PubMed] [Google Scholar]
- 9.Vauthier V, Jaillard S, Journel H, Dubourg C, Jockers R, Dam J. Homozygous deletion of an 80 kb region comprising part of DNAJC6 and LEPR genes on chromosome 1P31.3 is associated with early onset obesity, mental retardation and epilepsy. Mol Genet Metab. 2012;106(3):345–350. [DOI] [PubMed] [Google Scholar]
- 10.Barton JS, O’Loughlin J, Howell RT, L’e Orme R. Developmental delay and dysmorphic features associated with a previously undescribed deletion on chromosome 1. J Med Genet. 1995;32(8):636–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao A, O’Donnell S, Bain N, Meldrum C, Shorter D, Goel H. An intragenic deletion of the NFIA gene in a patient with a hypoplastic corpus callosum, craniofacial abnormalities and urinary tract defects. Euro J Med Genet. 2014;57(2-3):65–70. [DOI] [PubMed] [Google Scholar]
- 12.Jin L, Zhang S, Burguera BG, Couce ME, Osamura RY, Kulig E, Lloyd RV. Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology. 2000;141(1):333–339. [DOI] [PubMed] [Google Scholar]
- 13.Burguera B, Couce ME, Long J, Lamsam J, Laakso K, Jensen MD, Parisi JE, Lloyd RV. The long form of the leptin receptor (OB-Rb) is widely expressed in the human brain. Neuroendocrinology. 2000;71(3):187–195. [DOI] [PubMed] [Google Scholar]
- 14.Arzt E. gp130 cytokine signaling in the pituitary gland: a paradigm for cytokine-neuro-endocrine pathways. J Clin Invest. 2001;108(12):1729–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaisse C, Halaas JL, Horvath CM, Darnell JE Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14(1):95–97. [DOI] [PubMed] [Google Scholar]
- 16.Popovic V, Damjanovic S, Dieguez C, Casanueva FF. Leptin and the pituitary. Pituitary. 2001;4(1-2):7–14. [DOI] [PubMed] [Google Scholar]
- 17.Rodig SJ, Meraz MA, White JM, Lampe PA, Riley JK, Arthur CD, King KL, Sheehan KC, Yin L, Pennica D, Johnson EM Jr, Schreiber RD. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93(3):373–383. [DOI] [PubMed] [Google Scholar]