Abstract
Context:
The effects of maternal inorganic iodine therapy on infant thyroid function are not well known.
Objective:
This study investigated the effects on infant thyroid function of maternal inorganic iodine therapy when administered to lactating mothers with Graves disease.
Design and Setting:
This study was a prospective case series performed at the Tajiri Thyroid Clinic, Kumamoto, Japan.
Participants:
Subjects were 26 infants of lactating mothers with Graves disease treated with potassium iodide (KI) for postpartum thyrotoxicosis.
Main Outcome Measures:
Infant blood levels of thyroid-stimulating hormone (TSH) and free thyroxine were measured using the dried filter-paper method. Iodine concentrations in breast milk and infant urine were measured on the same day. Subclinical hypothyroidism was defined as a blood TSH level of ≥10 or ≥5 μIU/mL in <6-month-old and 6- to 12-month-old infants, respectively.
Results:
The median age of the infants was 3 months (range, 0 to 10 months). The median KI dose was 50 mg/d (range, 10 to 100 mg/d). High median iodine concentrations were detected in breast milk (15,050 μg/L; range, 831 to 72,000 μg/L) and infant urine (15,650 μg/L; range, 157 to 250,000 μg/L). Twenty-five of 26 infants had normal thyroid function. Although one infant had subclinical hypothyroidism (blood TSH, 12.3 μIU/mL), the TSH level normalized to 2.3 μIU/mL at 2 months after KI discontinuation.
Conclusion:
In Japan, where iodine intake is sufficient, administration of inorganic iodine to lactating mothers with Graves disease did not affect thyroid function in most infants despite high levels of exposure to iodine via breast milk.
Keywords: breast milk iodine, infant urine iodine, potassium iodide, thyrotoxicosis
Thyroid function was assessed in infants of lactating mothers with Graves disease who were treated with inorganic iodine. Despite iodine overload, thyroid function was not affected in most infants.
For patients with Graves disease, the effects of inorganic iodine therapy appear quickly but are often considered transient [1]. Generally, inorganic iodine is used for preparation of surgery, for treatment of thyroid storm, and after radioiodine therapy for Graves disease [2]. Iodine treatment may be associated with thyroid dysfunction and rarely with allergic reaction. In Japan, inorganic iodine is empirically administered to Graves disease patients with mild hyperthyroidism and to those with a history of adverse reactions to antithyroid drugs. It is reported that in some cases, long-term control of thyroid function as well as remission of Graves disease can be achieved with inorganic iodine alone [3, 4]. In daily clinical practice, inorganic iodine may be administered to pregnant and lactating women with Graves disease for whom adverse reactions preclude the use of antithyroid drugs. However, inorganic iodine is transferred to fetuses via the placenta and to infants via breast milk [5]. Therefore, it is important to know the effects of this type of therapy on fetal and infant thyroid function.
There have been case reports of congenital hypothyroidism induced by excess maternal iodine ingestion during pregnancy outside the setting of Graves disease [6]. On the other hand, in Japan, it has been reported that administration of inorganic iodine to pregnant women with Graves disease did not cause hypothyroidism in almost all of their fetuses [7, 8]. Studies of the administration of inorganic iodine during breastfeeding found that when lactating mothers with euthyroidism received overdoses of iodine, preterm newborn infants and neonates ingested excessive levels of iodine through the breast milk and developed hypothyroidism [9–12]. However, no studies have evaluated the effects of inorganic iodine administered to lactating mothers with Graves disease on infant thyroid function. We therefore prospectively investigated thyroid function in infants of lactating mothers with Graves disease who were treated with inorganic iodine for thyrotoxicosis and simultaneously measured iodine concentrations in breast milk and infant urine.
1. Subjects and Methods
A. Subjects
Twenty-six infants for whom iodine concentrations in breast milk and infant urine could be measured simultaneously were selected from among the infants of lactating mothers with Graves disease who were treated with potassium iodide (KI) alone for thyrotoxicosis between September 2012 and August 2015. Some infants were exclusively breastfed, whereas others received mixed feedings (breast milk and formula). These 26 infants included two brothers and two infants from a set of triplets; thus, there were 23 breastfeeding mothers. Informed consent was obtained from all mothers. The study protocol was approved by our clinical Institutional Review Board.
KI was administered to patients with mild hyperthyroidism, those with a history of adverse reactions to antithyroid drugs, and those with postpartum thyrotoxicosis in whom it was difficult to differentiate between Graves disease and painless thyroiditis. Of the 23 mothers, 14 had continuously received oral KI since before delivery. Nine mothers started or resumed oral KI therapy after the development or exacerbation of postpartum thyrotoxicosis. KI dose was adjusted to maintain normal maternal thyroid function every 1 to 3 months.
B. Methods
Blood samples were collected from infants using an easy-to-perform filter paper procedure. Briefly, the outer edge of the footpad was pricked with a sterile needle, and blood was blotted onto filter paper. The levels of free thyroxine (FT4) and thyroid-stimulating hormone (TSH) in the dried blotted blood samples were then measured using an enzyme-linked immunosorbent assay (FT4: ENZAPLATE N-FT4, Bayer Medical Ltd., Osaka, Japan; TSH: Cretin TSH ELISA II, Eiken Chemical Co., Ltd., Tokyo, Japan). TSH concentrations are expressed as serum concentrations, which are calculated by multiplying the whole blood values by 1.6 [13]. These blood levels were measured during every maternal outpatient visit while the mother received oral KI therapy. However, in this study, we used the blood levels of FT4 and TSH that were obtained when the data on iodine concentrations in breast milk and infant urine were obtained. Subclinical hypothyroidism was defined as a blood TSH level of ≥10 μIU/mL or ≥5 μIU/mL in <6-month-old or 6- to 12-month-old infants, respectively, according to the Japanese Guidelines for Mass Screening of Congenital Hypothyroidism (2014 revision) [14].
This study also evaluated breast milk and infant urine iodine concentrations. Breast milk was collected by manual expression, and infant urine was collected in a self-adhesive sterile urine bag on the same day as the infant thyroid function analyses. Supernatants of centrifuged breast milk samples and urine samples were stored at −20°C until further analysis. Iodine concentration measurements were outsourced to the Hitachi Chemical Clinical Laboratory (Tokyo, Japan), which implemented a microplate method based on the Sandell–Kolthoff reaction [15].
C. Statistical Analysis
Data are expressed as medians and ranges. The Mann-Whitney U test was used for statistical comparison, and the Spearman rank correlation coefficient was used to evaluate correlations. A P value of <0.05 was considered statistically significant. All statistical analyses were performed with EZR software (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [16], which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics.
2. Results
According to maternal interviews, all infants underwent mass screening for congenital hypothyroidism at 4 to 7 days after birth and received normal results.
Table 1 shows characteristics of breast-fed infants of mothers treated with KI and iodine levels in breast milk. The raw data and case numbers are shown in Supplemental Table 1 (39.7KB, docx) . The 26 infants included 14 boys and 12 girls, with a median age of 3 months (range, 0 to 10 months) and a median gestational age at birth of 38 weeks (range, 35 to 41 weeks). The median KI dosing periods were 9 months (range, 1 to 78 months) from before through after delivery and 2 months (range, 1 to 9 months) after delivery. The median KI dose was 50 mg/d (range, 10 to 100 mg/d). Nineteen infants were exclusively breastfed, and seven infants received mixed feedings. There was no statistical significance between the two groups regarding blood TSH levels [exclusively breastfed vs mixed feedings, median (range); 2.1 (0.6 to 12.3) μIU/mL vs 1.9 (0.9 to 5.7) μIU/mL; P = 0.71] or iodine concentrations in breast milk [14,200 (831 to 48,000) μg/L vs 26,400 (1170 to 72,000) μg/L; P = 1.0] and infant urine [15,200 (520 to 250,000) μg/L vs 16,100 (157 to 53,000) μg/L; P = 0.73].
Table 1.
Characteristics of Breast-Fed Infants of Mothers Treated With KI and Iodine Levels in Breast Milk
| Characteristics | Median (Range) |
|---|---|
| Age, mo | 3 (0–10) |
| Birth age, wk | 38 (35–41) |
| KI dosing period, mo | 9 (1–78) |
| KI dosing period after birth, mo | 2 (1–9) |
| KI dose, mg/d | 50 (10–100) |
| Infant FT4, ng/dL | 1.01 (0.61–1.55) |
| Infant TSH, μIU/mL | 2.1 (0.6–12.3) |
| Infant urine iodine, μg/L | 15,650 (157–250,000) |
| Breast milk iodine, μg/L | 15,050 (831–72,000) |
The median iodine concentrations of all 26 infants were 15,050 μg/L (range, 831 to 72,000 μg/L) in breast milk and 15,650 μg/L (range, 157 to 250,000 μg/L) in infant urine, and these concentrations correlated significantly (r = 0.699; P < 0.001). The KI doses correlated with the iodine concentrations in both breast milk (r = 0.636; P < 0.001) and infant urine (r = 0.610; P < 0.001) (Table 2).
Table 2.
Spearman Correlation Coefficients (r) Between Each Pair of Variables in all Infants (n = 26)
| KI Dose, mg/d | Breast Milk Iodine, μg /L | Infant Urine Iodine, μg /L | Infant TSH, μIU/mL | |
|---|---|---|---|---|
| KI dose, mg/d | ||||
| r | 1.000 | |||
| P value | ||||
| Breast milk iodine, μg/L | ||||
| r | 0.636 | 1.000 | ||
| P value | <0.001 | |||
| Infant urine iodine, μg/L | ||||
| r | 0.610 | 0.699 | 1.000 | |
| P value | <0.001 | <0.001 | ||
| Infant TSH, μIU/mL | ||||
| r | 0.0478 | −0.246 | −0.146 | 1.000 |
| P value | 0.82 | 0.23 | 0.48 |
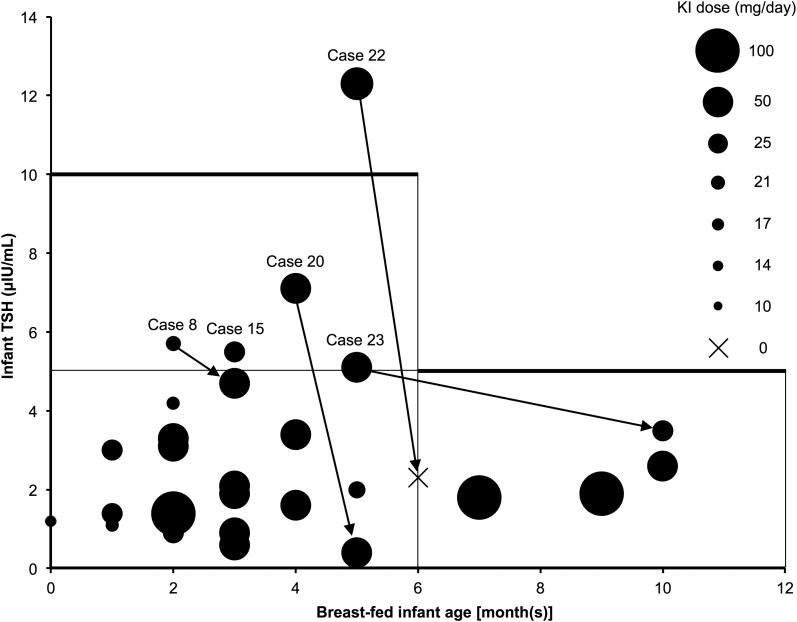
Twenty-five of 26 infants had normal thyroid function. One 5-month-old infant (case 22) presented with subclinical hypothyroidism, indicated by a blood TSH level of 12.3 μIU/mL, after 1 month of maternal KI therapy. Two months after KI discontinuation, the blood TSH level normalized to 2.3 μIU/mL (Fig. 1).
Figure 1.
The relationship between TSH level and age among infants of lactating mothers with Graves disease treated with KI. Each circle indicates the KI dose administered to one mother. Arrows indicate data from the same cases. Case numbers are shown in Supplemental Table 1 (39.7KB, docx) . Only one infant (case 22) showed subclinical hypothyroidism, and the blood TSH level normalized after discontinuing KI. Four infants (cases 8, 15, 20, 23) had blood TSH levels ≥5 μIU/mL within 6 months after birth; these levels decreased to <5 μIU/mL in three infants (cases 8, 20, and 23) during KI administration. We were unable to follow case 15.
Four infants had blood TSH levels ≥5 μIU/mL within 6 months after birth; these levels decreased to <5 μIU/mL in three infants during KI administration. In case 8, the blood TSH level was 5.7 μIU/mL at 2 months after birth. At the same time, because the mother's hyperthyroidism worsened, the maternal KI dose was increased, and thiamazole was initiated at a dose of 15 mg. However, the infant's blood TSH level decreased to 4.7 μIU/mL after 1 month. In case 15, the blood TSH level was 5.5 μIU/mL at 3 months after birth; however, we were unable to follow this case. In case 20, the blood TSH level was 7.1 μIU/mL at 4 months after birth but decreased to 0.4 μIU/mL after 1 month; the maternal KI dose was maintained during this period. In case 23, the blood TSH level was 5.1 μIU/mL at 5 months after birth but decreased to 3.5 μIU/mL at 6 months after the KI dose was halved (Fig. 1). The blood TSH level did not correlate with the KI dose (r = 0.0478; P = 0.82) or with the iodine concentrations in breast milk (r = −0.246; P = 0.23) and infant urine (r = −0.146; P = 0.48) in all the infants (Table 2). When statistical correlations were evaluated only in the exclusively breastfed infants, there were no significant correlations between the blood TSH level and each variable (Supplemental Table 2 (48KB, docx) ).
3. Discussion
This study evaluated the effects of inorganic iodine therapy administered to lactating mothers with Graves disease on the thyroid function of breastfed infants. In this study, maternal inorganic iodine therapy did not affect the thyroid function of most breastfed infants (25 of 26 infants), as determined by TSH levels.
According to studies in the United States, the median iodine concentrations in breast milk from ordinary lactating mothers ranges from 35 to 155 μg/L [5]. In contrast, we observed markedly higher levels of iodine in breast milk from inorganic iodine–treated lactating mothers with Graves disease (median, 15,050 μg/L; range, 831 to 72,000 μg/L). The reported median urinary iodine concentration among 30-day-old infants in Japan is 271 μg/L [17], and the World Health Organization epidemiological criteria for iodine nutritional status defines iodine overload as a median urinary iodine concentration of ≥300 μg/L (based on the median urinary iodine concentration in school-aged children) [18]. In contrast, we observed a high median infant urinary iodine concentration of 15,650 μg/L (range, 157 to 250,000 μg/L). Iodine concentrations in breast milk correlated significantly with those in infant urine, and KI doses correlated with breast milk and infant urine iodine concentrations, which strongly suggests that the KI administration to lactating mothers contributed to iodine overload in the study infants through breast milk.
Despite our finding that oral KI–treated lactating mothers transferred large amounts of iodine to their infants via breast milk, all but one infant maintained normal thyroid function. We have devised two explanations for this outcome. First, this might be an example of the escape from the Wolff–Chaikoff effect. In other words, thyroid function might have normalized after transient hypothyroidism. Excessive iodine intake inhibits thyroid hormone synthesis through the organification defect. Because the Wolff–Chaikoff effect is generally transient in the normal thyroid, thyroid hormone synthesis would normalize within a few days because of the so-called escape phenomenon [19]. This phenomenon is thought to have occurred in case 20, in which the blood TSH level was 7.1 μIU/mL after 1 month of KI administration but decreased to 0.4 μIU/mL after another month, despite no change in the KI dose. The escape phenomenon may occur after 36 to 40 weeks of gestation [20]; our study suggests that it may occur in postneonatal infants. In case 22, which was the only case affected by subclinical hypothyroidism, the high blood TSH level after 1 month of KI administration normalized immediately after KI discontinuation. Therefore, we cannot determine whether the TSH levels would have eventually normalized via the escape phenomenon, even if KI administration had continued.
Regarding the second possibility, the Japanese diet includes high daily levels of iodine ingested via seaweed [21]. The normal thyroid function observed in most infants included in this study might be associated with the sufficient iodine intake in Japan. People with less iodine intake in daily life are presumed more susceptible to excessive iodine. The tolerable upper limit for daily iodine intake varies from region to region (0.3 mg/d in the iodine-deficient region [18], 1.1 mg/d in adults in the United States [22], and 3.0 mg/d in adults in Japan [23]). We speculate that infants in regions characterized by low iodine intake and iodine deficiency might be more strongly affected by iodine overload.
The 2017 American Thyroid Association guidelines strongly recommend against sustained iodine intake exceeding 500 to 1100 μg/d while breastfeeding due to concerns about the potential for inducing hypothyroidism in the infant [24]. Several reports have indicated that the administration of an iodine overdose to lactating mothers causes hypothyroidism in their infants [9–12]. However, those studies differed from our study in two aspects. First, the previously reported studies included both preterm newborn infants and neonates, with the former group being more likely to be affected by iodine overload [12]. In our study, all examined infants had surpassed the newborn stage and were considered less likely to experience iodine overload–related effects on thyroid function. Second, the lactating mothers in the earlier studies did not have Graves disease. However, the infants of lactating mothers with Graves disease might be less likely to have elevated blood TSH levels for 2 to 3 months after birth because of the effects of maternal thyrotropin receptor antibodies.
We observed that infant blood TSH levels did not correlate with oral KI doses or breast milk and urinary iodine concentrations in the exclusively breastfed infants as well as in all the infants, suggesting that sensitivity to iodine varies among individuals. Moreover, our findings might have been affected by intersubject differences in the duration of iodine exposure and the variable timing of oral KI administration, breastfeeding, breast milk expression, and infant urine collection [25]. In this study, some infants were exclusively breastfed, and others received mixed feedings. However, there was no statistical significance between the two groups regarding blood TSH levels or the iodine concentrations in breast milk and infant urine. To analyze the differences between these two groups more accurately, data on frequency of formula feeding and iodine content of formula, which were not available in this study, are needed. The iodine content of the formula manufactured in Japan varies depending on the manufacturer, and its median value in formula from the five major manufacturers is 39 μg/L (range, 13 to 152). Because we did not ask the mothers which manufacturer's formulas were used, the iodine content of the formula that each infant ingested was unknown. However, our data strongly suggest that, regardless of type of feeding (exclusively breastfed, mixed fed), almost all infants were exposed to excess iodine by ingesting breast milk with high iodine content. In addition to these factors, the small sample size might contribute to the lack of correlation and the lack of statistical significance observed in this study.
We note the scarcity of available data regarding inorganic iodine therapy for lactating mothers with Graves disease and suggest that therapeutic indications should be considered carefully. However, inorganic iodine therapy appears to be a potentially useful alternative for mothers with Graves disease with adverse reactions to antithyroid drugs who are contraindicated for iodine-131 therapy because they wish to continue breastfeeding (due to the superiority of breast milk to formula or due to infant rejection of formula) and do not wish to undergo surgery.
As demonstrated in this study, the administration of inorganic iodine to lactating mothers with Graves disease may occasionally cause subclinical hypothyroidism in their infants. Given the importance of thyroid hormones to normal brain development during infancy, the thyroid function of infants should be closely monitored to ensure a rapid recovery from subclinical hypothyroidism while their mothers receive inorganic iodine. Moreover, because iodine sensitivity varies greatly among individuals, a further accumulation of cases may reveal overt hypothyroidism. Further studies are warranted.
In summary, our study found that inorganic iodine therapy, when administered to lactating mothers with Graves disease, did not affect the thyroid function in most infants, despite high levels of exposure to iodine via breast milk. We note that the study setting of Japan, where dietary iodine intake is sufficient, may have buffered the effects of excess iodine exposure.
Acknowledgments
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- FT4
- free thyroxine
- TSH
- thyroid-stimulating hormone.
References and Notes
- 1.Cooper DS. Treatment of thyrotoxicosis In: Braverman LE, and Cooper DS, eds. Werner and Ingbar’s The Thyroid. 10th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2013:492–516. [Google Scholar]
- 2.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–1421. [DOI] [PubMed] [Google Scholar]
- 3.Uchida T, Goto H, Kasai T, Komiya K, Takeno K, Abe H, Shigihara N, Sato J, Honda A, Mita T, Kanazawa A, Fujitani Y, Watada H. Therapeutic effectiveness of potassium iodine in drug-naïve patients with Graves’ disease: a single-center experience. Endocrine. 2014;47(2):506–511. [DOI] [PubMed] [Google Scholar]
- 4.Okamura K, Sato K, Fujikawa M, Bandai S, Ikenoue H, Kitazono T. Remission after potassium iodide therapy in patients with Graves’ hyperthyroidism exhibiting thionamide-associated side effects. J Clin Endocrinol Metab. 2014;99(11):3995–4002. [DOI] [PubMed] [Google Scholar]
- 5.Leung AM, Pearce EN, Braverman LE. Iodine nutrition in pregnancy and lactation. Endocrinol Metab Clin North Am. 2011;40(4):765–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connelly KJ, Boston BA, Pearce EN, Sesser D, Snyder D, Braverman LE, Pino S, LaFranchi SH. Congenital hypothyroidism caused by excess prenatal maternal iodine ingestion. J Pediatr. 2012;161(4):760–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Momotani N, Hisaoka T, Noh J, Ishikawa N, Ito K. Effects of iodine on thyroid status of fetus versus mother in treatment of Graves’ disease complicated by pregnancy. J Clin Endocrinol Metab. 1992;75(3):738–744. [DOI] [PubMed] [Google Scholar]
- 8.Yoshihara A, Noh JY, Watanabe N, Mukasa K, Ohye H, Suzuki M, Matsumoto M, Kunii Y, Suzuki N, Kameda T, Iwaku K, Kobayashi S, Sugino K, Ito K. Substituting potassium iodide for methimazole as the treatment for Graves’ disease during the first trimester may reduce the incidence of congenital anomalies: a retrospective study at a single medical institution in Japan. Thyroid. 2015;25(10):1155–1161. [DOI] [PubMed] [Google Scholar]
- 9.Chanoine JP, Boulvain M, Bourdoux P, Pardou A, Van Thi HV, Ermans AM, Delange F. Increased recall rate at screening for congenital hypothyroidism in breast fed infants born to iodine overloaded mothers. Arch Dis Child. 1988;63(10):1207–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koga Y, Sano H, Kikukawa Y, Ishigouoka T, Kawamura M. Effect on neonatal thyroid function of povidone-iodine used on mothers during perinatal period. J Obstet Gynaecol (Tokyo 1995). 1995;21(6):581–585. [DOI] [PubMed] [Google Scholar]
- 11.Casteels K, Pünt S, Brämswig J. Transient neonatal hypothyroidism during breastfeeding after post-natal maternal topical iodine treatment. Eur J Pediatr. 2000;159(9):716–717. [DOI] [PubMed] [Google Scholar]
- 12.Chung HR, Shin CH, Yang SW, Choi CW, Kim BI. Subclinical hypothyroidism in Korean preterm infants associated with high levels of iodine in breast milk. J Clin Endocrinol Metab. 2009;94(11):4444–4447. [DOI] [PubMed] [Google Scholar]
- 13.Information Center for Specific Pediatric Chronic Diseases. Diagnosis guide. Thyroid-stimulating hormone (TSH) deficiency. Available at: http://www.shouman.jp/instructions/5_11_19/ (in Japanese). Accessed 8 September 2017.
- 14.Nagasaki K, Minamitani K, Anzo M, Adachi M, Ishii T, Onigata K, Kusuda S, Harada S, Horikawa R, Minagawa M, Mizuno H, Yamakami Y, Fukushi M, Tajima T; Mass Screening Committee; Japanese Society for Pediatric Endocrinology; Japanese Society for Mass Screening . Guidelines for mass screening of congenital hypothyroidism (2014 revision). Clin Pediatr Endocrinol. 2015;24(3):107–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizokami T, Fukata S, Hishinuma A, Kogai T, Hamada K, Maruta T, Higashi K, Tajiri J. Iodide transport defect and breast milk iodine. Eur Thyroid J. 2016;5(2):145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuse Y, Ogawa H, Fujita M, Arata N, Harada S, Ohashi T, Shishiba Y, Irie M. Maternal-neonatal relationship of iodine metabolism in perinatal period: changes in urinary iodine excretion in Japanese mothers and newborn infants. Presented at 15th International &14th European Congress of Endocrinology 2012, Florence, Italy. In: Endocrine Abstracts. 2012;29:P1640. [Google Scholar]
- 18.WHO/UNICEF/ICCIDD Assessment of iodine deficiency disorders and monitoring their elimination, 3rd ed. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 19.Wolff J, Chaikoff IL, Goldberg RC, Meier JR. The temporary nature of the inhibitory action of excess iodine on organic iodine synthesis in the normal thyroid. Endocrinology. 1949;45(5):504–513, illust. [DOI] [PubMed] [Google Scholar]
- 20.Fisher DA, Brown RS. The maturation of thyroid function in the perinatal period and during childhood In: Braverman LE, and Cooper DS, eds. Werner and Ingbar’s The Thyroid. 10th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2013:775–786. [Google Scholar]
- 21.Nagataki S. The average of dietary iodine intake due to the ingestion of seaweeds is 1.2 mg/day in Japan. Thyroid. 2008;18(6):667–668. [DOI] [PubMed] [Google Scholar]
- 22.Food and Nutrition Board, Institute of Medicine Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 23.Ministry of Health, Labour and Welfare. Overview of dietary reference intakes for Japanese (2015). Available at: http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/Overview.pdf. Accessed 8 September 2017.
- 24.Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, Grobman WA, Laurberg P, Lazarus JH, Mandel SJ, Peeters RP, Sullivan S. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27(3):315–389. [DOI] [PubMed] [Google Scholar]
- 25.Leung AM, Braverman LE, He X, Heeren T, Pearce EN. Breastmilk iodine concentrations following acute dietary iodine intake. Thyroid. 2012;22(11):1176–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]