Abstract
Context:
In obese men with lowered testosterone levels, testosterone treatment augments diet-associated loss of body fat.
Objective:
We hypothesized that testosterone treatment modulates circulating concentrations of hormonal mediators of fat mass and energy homeostasis in obese men undergoing a weight loss program.
Design:
Prespecified secondary analysis of a randomized, double-blind, placebo-controlled trial.
Setting:
Tertiary referral center.
Participants:
Obese men (body mass index ≥30 kg/m2) with a repeated total testosterone level ≤12 nmol/L.
Intervention:
One hundred participants mean age 53 years (interquartile range 47 to 60 years) receiving 10 weeks of a very low–energy diet followed by 46 weeks of weight maintenance were randomly assigned at baseline to 56 weeks of intramuscular testosterone undecanoate (cases, n = 49) or matching placebo (controls, n = 51). Eighty-two men completed the study.
Main outcomes:
Between-group differences in leptin, adiponectin, ghrelin, glucagon like peptide-1, gastric inhibitory polypeptide, peptide YY, pancreatic polypeptide, and amylin levels.
Results:
At study end, compared with controls, cases had greater reductions in leptin [mean adjusted difference (MAD), −3.6 ng/mL (95% CI, −5.3 to −1.9); P < 0.001]. The change in leptin levels between cases and controls was dependent on baseline fat mass, as the between-group difference progressively increased with increasing fat mass [MAD, −0.26 ng/mL (95% CI, −0.31 to −0.26); P = 0.001 per 1 kg of baseline fat mass]. Weight loss–associated changes in other hormones persisted during the weight maintenance phase but were not modified by testosterone treatment.
Conclusions:
Testosterone treatment led to reductions in leptin beyond those achieved by diet-associated weight loss. Testosterone treatment may reduce leptin resistance in obese men.
Keywords: testosterone, obesity, leptin, energy homeostasis
We conducted an RCT of testosterone treatment in obese men subjected to weight loss and found that testosterone decreased leptin levels over and above the decrease achieved with weight loss alone.
In men, obesity is commonly associated with lowered circulating testosterone levels [1]. In modest obesity, decreases in total testosterone predominate because of insulin resistance–associated reductions in sex hormone–binding globulin levels. More severe obesity, however, is also associated with reductions in free testosterone levels. This is due to central suppression of the hypothalamic-pituitary-testicular (HPT) axis in obese men because luteinizing hormone levels are usually low or inappropriately normal [1]. This obesity-associated HPT axis suppression is thought to be functional and mediated by excess adiposity, given that weight loss is associated with increases in luteinizing hormone and testosterone levels [2, 3]. Although the exact underlying mechanisms are unknown, the reversal of HPT axis suppression with weight loss suggests a pathogenic role for adipose tissue. Indeed, adipose tissue–derived adipokines such as leptin [4] and adiponectin [5] have been implicated in contributing to this obesity-associated HPT axis suppression. In addition, there is evidence that gut-derived hormones such as glucagonlike peptide-1 (GLP-1), gastric inhibitory polypeptide (GIP), peptide YY (PYY), pancreatic polypeptide (PP), ghrelin, and amylin have regulatory actions on the HPT axis [6]. Common to these adipose and gut-derived hormones are their roles in the modulation of body weight, fat mass, and appetite. A close interaction between nutritional status and reproduction in men is well documented, and there is compelling evidence that changes in circulating concentrations of these hormones play important roles in linking the regulation of reproduction to the control of energy homeostasis [6]. In contrast, potential reciprocal effects of gonadal hormones, such as testosterone, on circulating levels of adipokines and gut hormones are less well studied.
Testosterone is not only important in the regulation of reproductive function but is also an important determinant of body composition in men. In randomized controlled clinical trials, testosterone treatment consistently reduced fat mass and increased lean mass [7, 8]. In observational studies, long-term testosterone treatment has also been associated with a reduction in body weight [9]. The mechanisms by which testosterone promotes fat loss may include effects on adipocyte differentiation and function [10] and improvements in mood and energy levels [11], and in rodents, increased exercise [12]. Whether testosterone treatment affects hormonal regulators of adiposity, body weight, and energy balance is less well-known.
We recently showed that among obese men with lowered testosterone levels undergoing a rigorous weight loss program with a very low–energy diet (VLED), the addition of testosterone treatment led to greater loss of fat mass than that achieved by diet alone [13]. This led us to hypothesize that testosterone may modulate the circulatory concentrations of adipokines and gut hormones as a potential contributing mechanism to this effect. In a prespecified secondary analysis of this double-blind, randomized, placebo-controlled trial (RCT) in obese men with lowered testosterone levels, we comprehensively examined the effects of testosterone treatment on the key circulating hormonal adipokines and gut hormones implicated in biologically important roles in the regulation of fat mass and body weight, in addition to a rigorous weight loss program.
1. Methods
A. Design Overview
The study design was reported previously in detail (ClinicalTrials.gov no. NCT01616732) [13]. Briefly, this study was an RCT of testosterone treatment or matching placebo, administered for 56 weeks in men undergoing a strict weight loss program. The trial was conducted from April 2013 through November 2015 at a tertiary referral center (Austin Health, Melbourne, Australia). The trial protocol was approved by the local ethics committee (HREC 2012/04495). Each participant provided written informed consent.
B. Setting and Participants
Adult men recruited from the local community were eligible to participate in the trial if they were obese [body mass index (BMI) >30 kg/m2] and had two morning (8 am to 10 am) fasting total testosterone levels ≤12 nmol/L drawn at least 1 week apart. Eligibility was based on electrochemiluminescence immunoassay used at the study hospital for routine clinical care. Total testosterone was remeasured by liquid chromatography–tandem mass spectroscopy from frozen samples stored at −80°C at study end [14]. Key exclusion criteria [13] included classic hypogonadism due to pituitary or testicular disease, contraindications to testosterone treatment, use of weight-altering medications including insulin, previous VLED failure, and bariatric surgery. Participants were randomized to receive either 1000 mg of testosterone undecanoate (the standard ampoule strength in Australia) or visually identical placebo by deep intramuscular buttock injection at weeks 0, 6 (manufacturer-recommended loading dose), 16, 26, 36, and 46 to ensure therapeutic trough levels of 10 to 15 nmol/L [13]. In addition, all subjects received a VLED providing 640 kcal per day for 10 weeks, followed by a 46-week weight maintenance phase based on the Australian Commonwealth Scientific and Industrial Research Organization Total Wellbeing Diet [13].
C. Main Outcome Measures
The primary outcome of the RCT was change in fat mass [13]. The main prespecified exploratory outcome measures of this secondary analysis were changes in adipokine and gut hormone levels. Adipokines and gut hormones were measured as previously described [15]. All blood tests were drawn in the fasting morning state (8 am to 10 am), including into a BD P800 tube (BD, Franklin Lakes, NJ) for preservation of gut hormones, and were assayed in a single run. Total PP, PYY, and amylin levels were measured using the Millipore Human Metabolic Panel Milliplex kit (Merck, Darmstadt, Germany), with intra-assay coefficient of variation (CVs) of 5.86%, 6.73%, and 2.90% and interassay CVs of 5.25%, 9.60%, and 8.71%, respectively. Leptin, active GLP-1, GIP, and total ghrelin levels were measured using the Bio-plex diabetes panel (Bio-Rad, Hercules, CA) with intra-assay CVs of 5.51%, 7.37%, 3.57%, and 2.47% and interassay CVs of 13.44%, 6.90%, 3.15%, and 5.25%, respectively. Adiponectin was measured using a duo enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN) with an intra-assay CV of 5.37% and an interassay CV of 7.64%.
D. Statistical Methods
Comparison of baseline characteristics was based on the Welch t test for normally distributed parameters, as assessed by the Kolmogorov-Smirnov test with Lilliefors correction, or the Wilcoxon rank-sum test in cases of nonnormal distribution. Data shown are mean (standard deviation) or median [interquartile range]. Correlations are based on the Kendall Tau rank test.
Changes in the levels of gut hormones between cases and controls over the follow-up visits were analyzed using a linear mixed model with restricted maximum likelihood estimator. The model included random intercepts per subject and, as fixed variables, the baseline levels of the respective outcome variable, three time points (i.e., at 0, 10, and 56 weeks), the group (i.e., placebo and testosterone), and the interaction of time point × group. The latter represents the measure of interest (i.e., between-group change over time), which was quantitatively expressed as mean adjusted difference (MAD) surrounded by the profiled 95% confidence interval (CI). P values for the change from 0 to 10, 10 to 56, and 0 to 56 weeks were conventionally adjusted for multiple testing by the Holm-Bonferroni method. In addition, the influence of baseline fat mass on leptin change was assessed in a three-way interaction. All tests were two-tailed, with P < 0.05 denoting statistical significance. Analyses were conducted using R version 3.3.2 for Mac, effects 3.1-2, and lme4 package 1.1-12 [16, 17] and SPSS version 22 (SPSS Inc., Chicago, IL).
2. Results
A. Study Subjects
As described previously, of the 100 trial participants, 49 men were randomized to testosterone (i.e., cases) and 51 to placebo (i.e., controls) [13]. Eighty-two men completed the trial, 44 of 49 cases (90%) and 38 of 51 controls (75%; P = 0.099). The most common reason for noncompletion was failure to attend visits (testosterone = 3, placebo = 12).
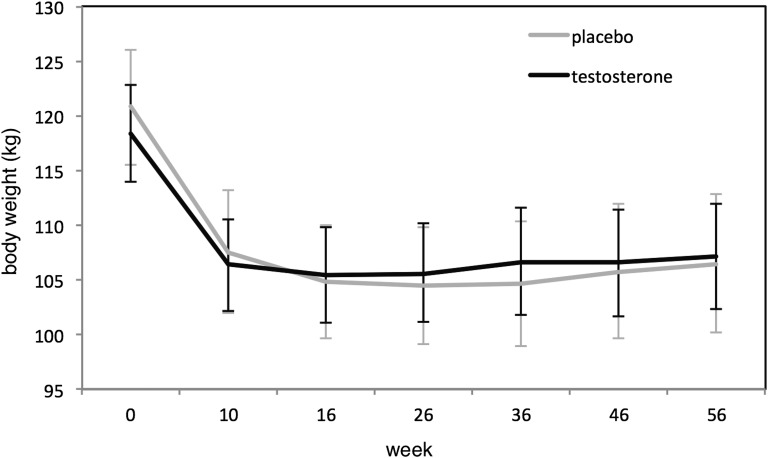
Baseline characteristics, including circulating concentrations of adipokines and gut hormones, were comparable between the groups except for ghrelin, which was higher in the cases (Table 1), likely a chance finding. Following 56 weeks of testosterone treatment, trough total testosterone level increased to 14.1 nmol/L in cases (recommended trough range, 10 to 15 nmol/L), whereas trough total testosterone level at study end increased to 10.0 nmol/L in controls, both P < 0.05 compared with baseline and significantly different between groups (P < 0.001) [13]. Weight loss after the 10-week VLED phase was −12.0 kg in cases and −13.5 kg in controls and was maintained at study end, −11.4 kg in cases and −10.9 kg in controls (Fig. 1). The weight lost by cases comprised a greater loss of fat mass (MAD, 2.9 kg [−5.7, −0.2]; P = 0.04), whereas controls lost more lean mass (MAD, 3.4 kg [1.3, 5.5]; P = 0.002), such that the total body weight lost at study end was no different between groups (MAD, −0.5 kg [−4.3, 3.3]; P = 0.80) [13].
Table 1.
Baseline Characteristics of Randomly Assigned Study Participants
| Testosterone Group (n = 49) | Placebo Group (n = 51) | P Value | |
|---|---|---|---|
| Age, y | 54 [47.3, 59.8] | 52.8 [47.6, 60.1] | 0.93 |
| Weight, kg | 118.3 (15.7) | 120.7 (19.6) | 0.51 |
| BMI, kg/m2 | 37.5 [34.9, 40.5] | 37.3 [34.7, 41.6] | 0.60 |
| Waist circumference, cm | 124 [118, 131] | 123 [117, 136] | 0.62 |
| Fat mass, kg | 44.3 (10.0) | 46.4 (10.6) | 0.30 |
| Lean mass, kg | 68.1 (7.3) | 67.4 (9.1) | 0.67 |
| TT, nmol/L | 6.8 (2.0) | 7.0 (1.6) | 0.55 |
| cFT, pmol/L | 159 (46) | 172 (44) | 0.15 |
| Estradiol, pmol/L | 123 (73) | 128 (58) | 0.56 |
| Leptin, ng/mL | 7.58 [4.60, 11.3] | 6.60 [5.29, 10.2] | 0.99 |
| Adiponectin, μg/mL | 2.67 [2.32, 3.00] | 2.71 [2.20, 3.15] | 0.87 |
| GLP-1, pg/mL | 54.5 [1.10, 96.4] | 58.5 [1.10, 105] | 0.99 |
| GIP, pg/mL | 124 [87.7, 168] | 114 [88.5, 160] | 0.78 |
| PYY, pg/mL | 193 [120, 262] | 150 [86.1, 221] | 0.44 |
| PP, pg/mL | 29.6 [16.9, 56.1] | 30.4 [17.3, 56.3] | 0.90 |
| Ghrelin, pg/mL | 252 [172, 327] | 182 [140, 225] | 0.002 |
| Amylin, pg/mL | 48.2 [38.8, 64.0] | 49.8 [37.9, 66.9] | 0.69 |
Data are mean (standard deviation) or median [interquartile range].
Abbreviations: cFT, calculated free testosterone; TT, total testosterone.
Figure 1.
Body weights in placebo- and testosterone-treated men during the 56-week study.
B. Change in Circulating Levels of Adipokines and Gut Hormones
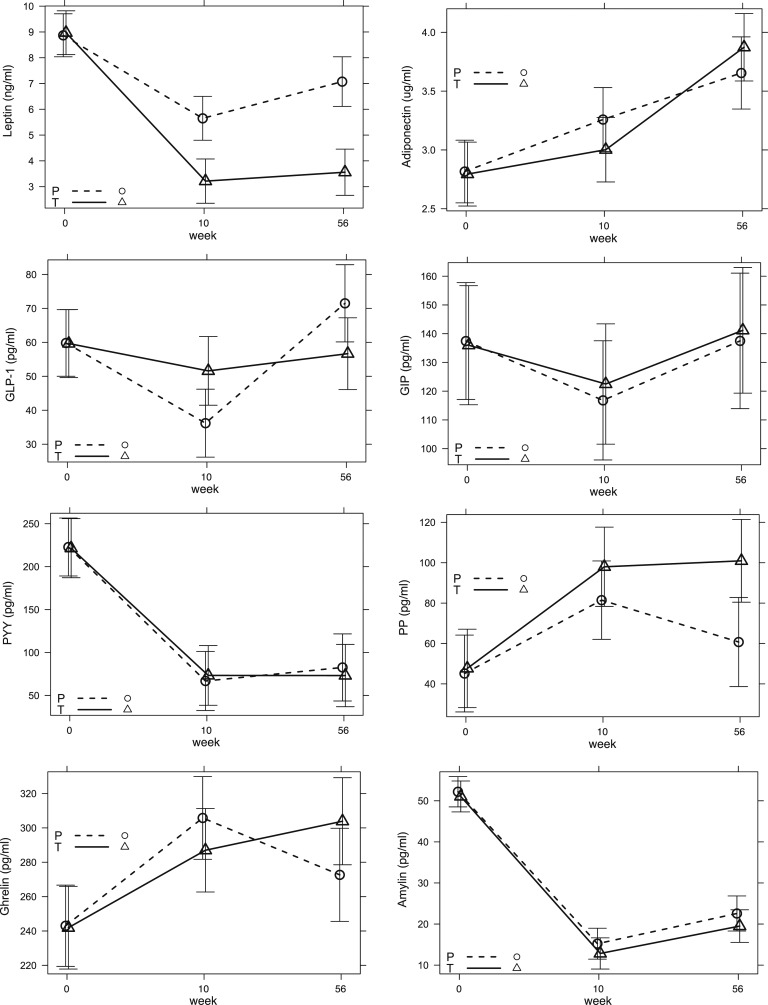
At the end of the rapid weight loss VLED phase of the trial (week 10), both cases and controls showed changes in adipokine levels, with decreases in leptin and increases in adiponectin, and changes in gut-derived hormones, including decreases in PYY and amylin and increases in PP and ghrelin. These changes generally persisted at study end (week 56) (Fig. 2). During the week 10 to week 56 weight maintenance phase, only GLP-1 levels changed significantly (MAD, −30.2 pg/mL [−50.6, −9.9]; P = 0.011), whereas there were no significant between-group changes for other adipokines or gut hormones (P > 0.05).
Figure 2.
Change in hormone levels between testosterone and placebo groups. Shown are MADs (surrounded by 95% CIs) in circulating concentrations of leptin, adiponectin, GLP-1, GIP, PYY, PP, ghrelin, and amylin at weeks 10 and 56. For statistical comparison between groups, refer to Table 2. P, placebo (dashed lines); T, testosterone (solid lines).
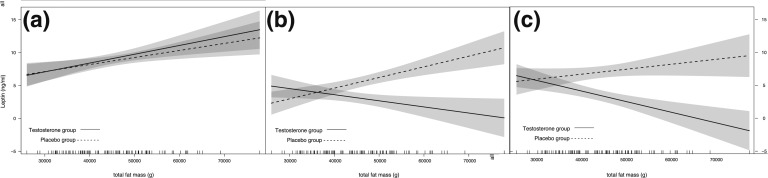
With respect to group differences at study end (week 56) (i.e., the primary outcome measure of this current analysis), cases had reduced leptin levels (MAD, −3.6 ng/mL, [−5.3, −1.9]; P < 0.001) compared with controls (Table 2). The leptin change between testosterone- and placebo-treated men was dependent on baseline fat mass, as the between-group difference progressively increased with increasing fat mass (Fig. 3); the MADs for the three-way interaction per 1 kg of fat mass were −0.28 ng/mL [−0.35, −0.13], P = 0.0004, at 10 weeks, and −0.26 ng/mL [−0.31, −0.26], P = 0.001, at 56 weeks. There were no between-group differences in other hormones (Table 2).
Table 2.
Change in Main Outcomes
| Testosterone Groupa | Placebo Groupa | Mean Adjustedb Difference |
P Value |
|
|---|---|---|---|---|
| (n = 49) |
(n = 51) |
|||
| Leptin, ng/mL | ||||
| Week 0 | 7.58 [4.60, 11.3] | 6.60 [5.29, 10.2] | — | — |
| Week 10 | 2.27 [1.39, 4.61] | 3.74 [2.07, 6.79] | −2.54 (−4.13 to −0.94) | 0.004 |
| Week 56 | 3.11 [1.66, 4.53] | 4.93 [2.62, 8.60] | −3.61 (−5.28 to −1.93) | <0.001 |
| Adiponectin, μg/mL | ||||
| Week 0 | 2.67 [2.32, 3.00] | 2.71 [2.20, 3.15] | — | — |
| Week 10 | 2.95 [2.24, 3.51] | 3.06 [2.50, 3.92] | −0.23 (−0.73 to 0.26) | 0.71 |
| Week 56 | 3.33 [2.62, 4.25] | 3.33 [2.74, 4.18] | 0.24 (−0.28 to 0.76) | 0.71 |
| GLP-1, pg/mL | ||||
| Week 0 | 54.5 [1.10, 96.4] | 58.5 [1.10, 105] | — | — |
| Week 10 | 37.6 [1.10, 69.5] | 37.1 [1.10, 58.3] | 15.58 (−3.68 to 34.84) | 0.23 |
| Week 56 | 53.3 [1.10, 94.9] | 65.2 [1.10, 120] | −14.66 (−34.88 to 5.54) | 0.23 |
| GIP, pg/mL | ||||
| Week 0 | 124 [87.7, 168] | 114 [88.5, 160] | — | — |
| Week 10 | 94.5 [62.9, 158] | 113 [61.8, 138] | 7.17 (−32.68 to 46.98) | 1.00 |
| Week 56 | 107 [66.6, 150] | 97.3 [69.3, 152] | 5.13 (−36.64 to 47.02) | 1.00 |
| PYY, pg/mL | ||||
| Week 0 | 193 [120, 262] | 150 [86.1, 221] | — | — |
| Week 10 | 61.5 [48.9, 87.6] | 58.5 [39.5, 79.7] | 7.83 (−59.88 to 75.54) | 1.00 |
| Week 56 | 61.5 [44.0, 87.3] | 65.5 [41.8, 87.6] | −8.06 (−78.96 to 62.83) | 1.00 |
| PP, pg/mL | ||||
| Week 0 | 29.6 [16.9, 56.1] | 30.4 [17.3, 56.3] | — | — |
| Week 10 | 60.5 [43.6, 140] | 51.6 [30.2, 104] | 0.50 (−24.22 to 52.19) | 0.50 |
| Week 56 | 70.9 [44.6, 112] | 55.1 [17.9, 85.8] | 3.35 (−2.28 to 77.71) | 0.20 |
| Ghrelin, pg/mL | ||||
| Week 0 | 252 [172, 327] | 182 [140, 225] | — | — |
| Week 10 | 312 [204, 373] | 231 [183, 343] | −17.67 (−60.09 to 24.75) | 0.42 |
| Week 56 | 284 [209, 379] | 216 [184, 266] | 32.32 (−12.37 to 77.03) | 0.32 |
| Amylin, pg/mL | ||||
| Week 0 | 48.2 [38.8, 64.0] | 49.8 [37.9, 66.9] | — | — |
| Week 10 | 10.4 [8.13, 16.8] | 12.9 [8.11, 18.7] | −1.23 (−8.63 to 6.17) | 1.00 |
| Week 56 | 17.3 [13.3, 22.5] | 20.6 [14.0, 27.6] | −1.92 (−9.67 to 5.82) | 1.00 |
Data are reported as median [interquartile range] and mean adjusted difference (95% CIs) between groups (see Methods).
Median interquartile range.
Mean adjusted difference (95% CIs).
Figure 3.
Differences in leptin levels between testosterone and placebo groups. Changes in leptin levels are shown according to the baseline amount of total body fat mass (continuous and dashed lines represent leptin levels in testosterone- and placebo-treated men, respectively). (a) Leptin level at baseline. (b and c) Divergence in leptin levels between testosterone- and placebo-treated men at 10 and 56 weeks. The shaded areas represent the 95% CIs. Depending on baseline fat mass, leptin levels at weeks 10 and 56 diverged and progressively decreased significantly in the testosterone group compared with the placebo group. The vertical lines immediately above the x-axis correspond to the baseline total fat mass of an individual study subject. The three-way interaction was statistically significant (see Results).
At the end of the weight loss phase, the change in leptin level in the testosterone-treated men correlated with the change in circulating calculated free testosterone level (τ = 0.24; P = 0.02) and with the change in circulating estradiol level (τ = 0.24; P = 0.01) but not with the change in circulating total testosterone level (τ = 0.13; P = 0.20).
C. Associations of Adipokines and Gut Hormones With Body Composition and Sex Steroid Levels at Baseline
In the entire group, leptin correlated with body weight (τ = 0.27; P < 0001), BMI (τ = 0.36; P < 0.0001), and total fat mass (τ = 0.44; P < 0.0001) but not with visceral fat mass—quantified from single axial CT images at the L4-L5 intervertebral disk space as described [13]—or lean mass. Leptin level correlated with circulating estradiol level (τ = 0.28; P < 0.0001) but not with total or free testosterone level. Ghrelin correlated inversely with BMI (τ = −0.157; P = 0.020). GLP-1 (τ = 0.16; P = 0.025), PP (τ = 0.16; P = 0.025) and amylin (τ = 0.14; P = 0.036) correlated with visceral fat mass. Other correlations of adipokines and gut hormones with body composition parameters or sex steroid levels were not significant.
In the entire group, baseline concentrations of adipokines or gut hormones were not predictive of the amount of body weight or fat mass lost during the study.
3. Discussion
In this 56-week RCT of obese men subjected to a rigorous weight loss program, the addition of testosterone treatment led to reductions in circulating leptin concentrations beyond what was achieved by caloric restriction alone. Remarkably, the leptin change between testosterone- and placebo-treated men was dependent on their baseline fat mass, as the between-group difference progressively increased with increasing fat mass: the higher the baseline adiposity, the greater the leptin-lowering effect of testosterone treatment compared with placebo treatment. Changes in the concentrations of adiponectin and gut hormone levels were similar between testosterone- and placebo-treated men during the study. In both groups, weight loss-associated–changes in these hormones were evident after the 10-week VLED phase. These changes persisted at week 56 despite 46 weeks of weight maintenance, confirming and extending previous findings [15]. Thus, although testosterone treatment had an independent effect on leptin levels, changes in body weight and fat mass during diet exerted a more dominant effect on circulating concentrations of other key modulators of energy homeostasis than did testosterone treatment.
To date, only a few smaller RCTs have assessed the effects of testosterone treatment on adipokines and gut hormones to compare our findings. No previous RCT focused exclusively on obese men or included a rigorous weight loss program. In addition, previous RCTs did not adjust testosterone treatment effects for changes in fat mass, itself closely correlated with circulating concentrations of adipokines and gut hormones. Although testosterone treatment consistently decreased leptin levels in these previous RCTs [18–21], effects on adiponectin levels were conflicting, with some [19, 22] but not all [21, 23] reporting a decrease in adiponectin with testosterone treatment. RCTs reporting the effects of testosterone treatment on gut hormones are not available.
A. Testosterone Treatment and Adipokines
Physiologically, leptin reduces fat mass by promoting satiety and increasing energy expenditure. However, obese men are commonly leptin resistant, consistent with the positive correlation of circulating leptin levels with body weight and total adiposity in our cohort. Although previous RCTs did not adjust for changes in fat mass [18–21], our findings indicate that the testosterone effect on reducing circulating leptin level is not simply an indirect effect mediated by testosterone-associated reductions in body fat. Instead, they suggest that testosterone may directly inhibit leptin production in adipose tissue. This is consistent with reports that testosterone treatment suppresses leptin messenger RNA and leptin secretion from human adipose tissue in vitro [24] and that male mice with an adipose tissue–specific deletion of the androgen receptor have hyperleptinemia in the absence of increased body weight or adiposity [25]. Whether such testosterone-associated alterations in leptin levels, evident over and above those achieved by caloric restriction alone, are mechanistically involved in the regulation of body fat mass by testosterone requires further study. However, as suggested previously [26], low testosterone levels may contribute to leptin resistance in obese men. Therefore, it is tempting to speculate that testosterone treatment may restore HPT axis responsiveness to leptin, which may in turn promote further reduction in fat mass.
Increasing data have pointed to the importance of estradiol in regulating adipose tissue in men [27]. Although our study was not designed to examine this issue, the changes in leptin levels correlated with on-treatment changes in circulating levels of both free testosterone and estradiol.
As expected, weight loss was associated with progressive increases in circulating adiponectin levels in our cohort, but testosterone treatment had no independent effect on adiponectin levels. This is consistent with prospective studies in men with prostate cancer initiating androgen-deprivation therapy that reported an initial increase in adiponectin levels due to profound testosterone deficiency, which was subsequently offset by increased fat mass [28]. Thus, previous RCTs of testosterone treatment reporting a decrease in adiponectin levels [19, 22] may have been confounded by concomitant reductions in fat mass.
B. Testosterone Treatment and Gut Hormones
Testosterone treatment had no effect on circulating concentrations of gut hormones in dieting men. Although previous studies have implicated testosterone treatment in modulating circulating concentrations of gut hormones such as ghrelin [29], our data suggest that any potential actions of testosterone on circulating gut hormones are overridden by mechanisms associated with changes in body weight and fat mass. In our cohort, initial weight loss was associated with changes in circulating concentrations of adipokines and gut hormones, and these changes generally persisted at study end following a 46-week period of successful weight maintenance. Similar persistent changes have been reported previously and have been hypothesized to facilitate weight regain after diet-induced weight loss [15]. This previous study was smaller (n = 50), had a higher dropout rate, and enrolled predominantly (68%) female participants, and participants regained weight during the study [15]. Hence, the current study confirmed these findings in a larger, exclusively male cohort with successful weight maintenance after the VLED phase.
C. Strengths and Limitations
Strengths of the study include the exclusive focus on obese men with testosterone levels, measured by validated mass spectrometry [14], that were substantially lower than levels reported in unselected community-dwelling obese men [30, 31]; the administration of effective intramuscular testosterone, eliminating compliance issues; and the successful implementation of a rigorous weight loss program with a relatively low dropout rate.
Limitations include the fact that this study was a secondary, albeit prespecified, analysis of an RCT designed to assess the effects of testosterone treatment on body composition [13]. In addition, we did not assess changes in appetite or dynamic food intake–dependent changes in adipokine or gut hormone levels.
4. Conclusions
In this comprehensive evaluation of testosterone effects on circulating levels of key adipokines and gut hormones in an RCT among obese men subjected to a rigorous weight loss program, we found that testosterone treatment reduced leptin levels above and beyond the effects of diet alone. The lower leptin levels in testosterone-treated men may reflect a lessening of leptin resistance by mechanisms other than a reduction in adiposity, as leptin levels were lower in testosterone-treated men even with adjustments for the loss of fat mass. By contrast, changes in weight and fat mass during diet exerted a more dominant effect on circulating concentrations of other key modulators of energy homeostasis compared with testosterone treatment. Whether this potential reduction in leptin resistance is clinically important in mediating testosterone-related changes in body composition requires further study. In conjunction with the metabolically favorable effects of testosterone on body composition and modest improvements in androgen deficiency–like symptoms added to diet-induced weight loss reported elsewhere [13, 32], we posit that obese middle-aged men with lowered testosterone levels constitute a population that should be targeted in larger clinical trials to further assess the long-term benefits and risks of testosterone treatment in combination with lifestyle measures.
Acknowledgments
Acknowledgments
M.G. was supported by a career development fellowship (1024139), and M.N.T.F. was supported by a postgraduate scholarship (1055305), both from the National Health and Medical Research Council,Australia. Bayer Pharma AG (Berlin, Germany) provided testosterone, placebo, and financial support to conduct investigations but had no role in trial design, data analysis, or writing of the manuscript.
Clinical trial registry: Clinicaltrials.gov no. NCT01616732 (registered 8 June 2012).
Disclosure Summary: M.G. has received research funding from Bayer Pharma, Novartis, Weight Watchers, Lilly, and speaker’s honoraria from Besins Healthcare. M.N.T.F. has received research funding from Bayer Pharma. R.H. has nothing to disclose.
Footnotes
- BMI
- body mass index
- CI
- confidence interval
- CV
- coefficient of variation
- GIP
- gastric inhibitory polypeptide
- GLP-1
- glucagonlike peptide-1
- HPT
- hypothalamic-pituitary-testicular
- MAD
- mean adjusted difference
- PP
- pancreatic polypeptide
- PYY
- peptide YY
- RCT
- randomized controlled trial
- VLED
- very low–energy diet.
References and Notes
- 1.Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, Finn JD, Bartfai G, Boonen S, Casanueva FF, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Vanderschueren D, Huhtaniemi IT, Wu FC; EMAS Group . Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95(4):1810–1818. [DOI] [PubMed] [Google Scholar]
- 2.Camacho EM, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, Lee DM, Tajar A, Bartfai G, Boonen S, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Keevil B, Lean ME, Pendleton N, Punab M, Vanderschueren D, Wu FC; EMAS Group . Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168(3):445–455. [DOI] [PubMed] [Google Scholar]
- 3.Corona G, Rastrelli G, Monami M, Saad F, Luconi M, Lucchese M, Facchiano E, Sforza A, Forti G, Mannucci E, Maggi M. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168(6):829–843. [DOI] [PubMed] [Google Scholar]
- 4.Isidori AM, Caprio M, Strollo F, Moretti C, Frajese G, Isidori A, Fabbri A. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J Clin Endocrinol Metab. 1999;84(10):3673–3680. [DOI] [PubMed] [Google Scholar]
- 5.Caminos JE, Nogueiras R, Gaytán F, Pineda R, González CR, Barreiro ML, Castaño JP, Malagón MM, Pinilla L, Toppari J, Diéguez C, Tena-Sempere M. Novel expression and direct effects of adiponectin in the rat testis. Endocrinology. 2008;149(7):3390–3402. [DOI] [PubMed] [Google Scholar]
- 6.Comninos AN, Jayasena CN, Dhillo WS. The relationship between gut and adipose hormones, and reproduction. Hum Reprod Update. 2014;20(2):153–174. [DOI] [PubMed] [Google Scholar]
- 7.Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63(3):280–293. [DOI] [PubMed] [Google Scholar]
- 8.Bhasin S. The brave new world of function-promoting anabolic therapies: testosterone and frailty. J Clin Endocrinol Metab. 2010;95(2):509–511. [DOI] [PubMed] [Google Scholar]
- 9.Saad F, Yassin A, Doros G, Haider A. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int J Obes. 2016;40(1):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144(11):5081–5088. [DOI] [PubMed] [Google Scholar]
- 11.Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A, Santanna J, Loh L, Lenrow DA, Holmes JH, Kapoor SC, Atkinson LE, Strom BL. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85(8):2670–2677. [DOI] [PubMed] [Google Scholar]
- 12.Wood RI. Oral testosterone self-administration in male hamsters: dose-response, voluntary exercise, and individual differences. Horm Behav. 2002;41(3):247–258. [DOI] [PubMed] [Google Scholar]
- 13.Ng Tang Fui M, Prendergast LA, Dupuis P, Raval M, Strauss BJ, Zajac JD, Grossmann M. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med. 2016;14(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harwood DT, Handelsman DJ. Development and validation of a sensitive liquid chromatography-tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clin Chim Acta. 2009;409(1-2):78–84. [DOI] [PubMed] [Google Scholar]
- 15.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604. [DOI] [PubMed] [Google Scholar]
- 16. R Core Team. 2016 R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: https://cran.r-project.org. Accessed 11 February 2016.
- 17.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- 18.Sih R, Morley JE, Kaiser FE, Perry HM III, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661–1667. [DOI] [PubMed] [Google Scholar]
- 19.Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156(5):595–602. [DOI] [PubMed] [Google Scholar]
- 20.Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf). 2010;73(5):602–612. [DOI] [PubMed] [Google Scholar]
- 21.Dhindsa S, Ghanim H, Batra M, Kuhadiya ND, Abuaysheh S, Sandhu S, Green K, Makdissi A, Hejna J, Chaudhuri A, Punyanitya M, Dandona P. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care. 2016;39(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frederiksen L, Højlund K, Hougaard DM, Mosbech TH, Larsen R, Flyvbjerg A, Frystyk J, Brixen K, Andersen M. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur J Endocrinol. 2012;166(3):469–476. [DOI] [PubMed] [Google Scholar]
- 23.Gianatti EJ, Dupuis P, Hoermann R, Strauss BJ, Wentworth JM, Zajac JD, Grossmann M. Effect of testosterone treatment on glucose metabolism in men with type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37(8):2098–2107. [DOI] [PubMed] [Google Scholar]
- 24.Wabitsch M, Blum WF, Muche R, Braun M, Hube F, Rascher W, Heinze E, Teller W, Hauner H. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J Clin Invest. 1997;100(4):808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu IC, Lin HY, Liu NC, Wang RS, Sparks JD, Yeh S, Chang C. Hyperleptinemia without obesity in male mice lacking androgen receptor in adipose tissue. Endocrinology. 2008;149(5):2361–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jockenhövel F, Blum WF, Vogel E, Englaro P, Müller-Wieland D, Reinwein D, Rascher W, Krone W. Testosterone substitution normalizes elevated serum leptin levels in hypogonadal men. J Clin Endocrinol Metab. 1997;82(8):2510–2513. [DOI] [PubMed] [Google Scholar]
- 27.Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton EJ, Gianatti E, Strauss BJ, Wentworth J, Lim-Joon D, Bolton D, Zajac JD, Grossmann M. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol (Oxf). 2011;74(3):377–383. [DOI] [PubMed] [Google Scholar]
- 29.Pagotto U, Gambineri A, Pelusi C, Genghini S, Cacciari M, Otto B, Castañeda T, Tschöp M, Pasquali R. Testosterone replacement therapy restores normal ghrelin in hypogonadal men. J Clin Endocrinol Metab. 2003;88(9):4139–4143. [DOI] [PubMed] [Google Scholar]
- 30.Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A, Huhtaniemi IT, Kula K, Punab M, Boonen S, Vanderschueren D; European Male Aging Study Group . Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737–2745. [DOI] [PubMed] [Google Scholar]
- 31.Dhindsa S, Miller MG, McWhirter CL, Mager DE, Ghanim H, Chaudhuri A, Dandona P. Testosterone concentrations in diabetic and nondiabetic obese men [published correction appears in Diabetes Care. 2010;33(8):1911]. Diabetes Care. 2010;33(6):1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng Tang Fui M, Hoermann R, Prendergast LA, Zajac JD, Grossmann M. Symptomatic response to testosterone treatment in dieting obese men with low testosterone levels in a randomized, placebo-controlled clinical trial [published online ahead of print January 17, 2017]. Int J Obes (Lond). [DOI] [PubMed] [Google Scholar]