Abstract
Context:
Testosterone (T) is a central androgenic hormone, and sex hormone-binding globulin (SHBG) is the major determinant of its bioactivity. There are no acknowledged genetic variants with clear-cut clinical implications, modulating T levels in men.
Objective:
To confirm genetic associations of top loci (SHBG, GCKR, SLCO1B1, and JMJD1C) from genome-wide association (GWA) studies for serum SHBG and T.
Design, Patients:
Groups differing in general and reproductive parameters: young men (n = 540; 19.3 ± 1.8 years), severe idiopathic male infertility patients (n = 641; 31.6 ± 6.0 years), and male partners of pregnant women (n = 324; 31.9 ± 6.6 years). All patients were recruited at the Andrology Centre, Tartu University Hospital, Estonia.
Main Outcome Measure(s):
Genetic associations with reproductive hormones, testicular and sperm parameters (linear regression, additive model); intergroup allele/genotype distribution comparisons.
Results:
Associations with serum SHBG levels were robust for SHBG −68 G>A [rs1799941; meta-analysis: P = 3.7 × 10−14; allelic effect (standard error) = 4.67 (0.62) nmol/L], SHBG +1091 C>T [rs727428; P = 7.3 × 10−11; −3.74 (0.57)], SHBG Pro185Leu [rs6258; P = 1.2 × 10−4, −12.2 (3.17)], and GCKR Pro446Leu [rs1260326; P = 1.5 × 10−4; −2.2 (0.59)]. Measured T concentrations correlated with genetically modulated levels of SHBG (r = 0.48 to 0.74, P < 0.0001), guaranteeing stable availability of free T. Among infertile men, SHBG Pro185Leu substitution showed additional downstream effect on luteinizing hormone [P = 5.1 × 10−5; −1.66 (0.57) IU/L] and follicle-stimulating hormone [P = 3.4 × 10−3; −2.48 (1.23) IU/L]. No associations with male reproductive parameters were detected for SHBG Asp327Asn (rs6259), SLCO1B1 Val174Ala (rs4149056), and JMJD1C intronic variant rs7910927.
Conclusions:
Claims were replicated and additional associations were detected for four of seven tested GWAS top loci. Perspective clinical investigations of these variants are hypotestosteronemia among aging men and pharmacogenetics of hormone replacement therapy.
Only four of seven top-associated SNPs from GWAS meta-analysis for serum SHBG and T were robustly replicated in fertile and infertile men of reproductive age, representing SHBG and GCKR gene variants.
Despite the rising importance in personalized patient management in the clinical practice, the current understanding of common genetic variation contributing to male reproductive hormone levels is moderate. Perspective clinical implications of genetic testing include more accurate diagnostics of clinical conditions with impaired reproductive physiology, and development of stratified treatment schemes taking into account patients’ genetic profile. There are few examples of polymorphisms with generally acknowledged robust genetic effects on male hormonal profile and so far, the focus has been vastly targeted to gonadotropin action. The variant with strongest and widest effect on male reproductive physiology is FSHB −211 G>T (rs10835638) located in the promoter of the follicle-stimulating hormone (FSH) β-subunit encoding gene [1]. T-allele carriers have substantially lower serum FSH, and TT-homozygosity is associated with decreased testes volume and higher risk to idiopathic male infertility [2]. Pharmacogenetic potential of FSHB −211 G>T in male FSH treatment has been suggested to detect men with genetically inherited low FSH [3]. Other examples of extensively analyzed variants in andrology settings have smaller genetic effects on male reproductive parameters, e.g. FSH receptor (p.Asn680Ser/p.Thr307Ala) [4, 5], FSHR −29 G>A (rs1394205) [6, 7], and luteinizing hormone (LH) isoforms (V-LH, Trp8Arg/ Ile15Thr) [8, 9].
A central androgenic hormone in men is testosterone (T). There is growing evidence that low T level is related to a wide variety of general health problems from metabolic syndrome [10] to increased general mortality risk [11, 12]. Sex hormone-binding globulin (SHBG) is the high-affinity binding protein for androgens and estrogens and the major modulator of their bioactivity by limiting diffusion into target tissues [13]. Only a small fraction (2% to 5%) of T represents free T (FT). So far, there are no acknowledged genetic variants with clear-cut clinical implications that modulate male T levels. However, recent genome-wide association (GWA) studies have extensively targeted the genetic determinants of both, circulating total T and the key modulator of FT, sex-hormone binding globulin (SHBG) [14–16]. These studies have highlighted previously known loci from candidate gene studies and/or associated with other metabolic phenotypes (SHBG, GCKR, and SLCO1B1), and novel genes potentially contributing to SHBG and T levels (JMJD1C, FAM9B, PRMT6, and ZBTB10).
The current study aimed to analyze the association of single-nucleotide polymorphisms (SNPs) identified in the GWA studies as top-associated loci as top loci for circulating T and SHBG. Seven variants were selected for the analysis, including four in the SHBG gene. The 5′UTR regulatory variant SHBG −68 G>A (rs1799941; reference transcript: NM_001040.4) was shown to modulate SHBG and T levels in candidate-gene [17] and GWA reports [14–16]. Variant +1091 C>T (rs727428) at the 3′ downstream region has been associated with female serum SHBG [18, 19] and male T and DHT [16]. Missense variants SHBG Pro185Leu (rs6258) and Asp327Asn (rs6259) were shown to affect serum SHBG level in both sexes [14, 15, 20]. We also analyzed three novel genes (GCKR, JMJD1C, and SLCO1B1) highlighted among the top 12 findings in the largest conducted GWAS meta-analysis for SHBG that incorporated data from 16 epidemiologic cohorts (n = 28,837; 14,938 men and 10,899 women) [15]. Among these, the Pro446Leu (rs1260326) in the GCKR gene encoding hepatic glucokinase regulator protein has been previously shown to modulate several metabolic traits and disorders [21]. A variant in the SLCO1B1 gene, Val174Ala (rs4149056) with a functional effect on the encoded OATP1B1 protein activity has been extensively explored in the context of statin pharmacogenetics [22]. SNPs in JMJD1C, a gene involved in spermatogenesis [23], have been associated with SHBG (rs7910927, intron 1) [15] and total T (rs10822184) [16] in two independent GWA studies.
The main objective of our study was to robustly confirm or reject the claimed associations with male serum SHBG and/or T levels, as well as to uncover additional genetic effects in an independent and clinically well characterized sample set with available broad range of hormonal and fertility-related parameters. The study was designed using three groups of young and middle-age men recruited at the Andrology Centre, Tartu University Hospital, Estonia (total, n = 1505): young men (n = 540), patients with severe idiopathic male factor infertility (n = 641), and fertile men recruited among partners of pregnant women (n = 324). This choice of alternative patient groups also enabled us to assess age and fertility status related effects. For four of the seven analyzed variants, original claims were replicated and additional genetic associations were detected. No associations with male reproductive parameters were detected for three SNPs, previously highlighted as top findings in a large GWA studies meta-analysis [15].
1. Methods
A. Ethics Statement
The study has been approved by the Ethics Review Committee on Human Research of the University of Tartu, Estonia. The study was conducted according to the Declaration of Helsinki principles. Written informed consent for evaluation and use of their clinical data for scientific purposes was obtained from each patient prior to recruitment.
B. Study Groups for the Genetic Analysis
Genetic analysis was carried out for three study groups of men, differing in their general and reproductive parameters, as well as fertility status (Table 1). All men were recruited at the Andrology Centre, Tartu University Hospital (AC-TUH). All study participants were born and living in Estonia.
Table 1.
Characteristics of the Study Groups
| Parameter | Young Men Cohort | Partners of Pregnant Women | Idiopathic Infertility Patients | Kruskal–Wallis Test P Value |
|---|---|---|---|---|
| n | 540 | 324 | 641 | |
| Age, y | 19.3 ± 1.8 | 31.9 ± 6.6 | 31.6 ± 6.0 | <0.0001 |
| 18.7 (17.2‒22.9) | 31.0 (22.8‒45.0) | 30.9 (23.5‒42.0) | ||
| BMIa | 22.3 ± 2.7 | 25.5 ± 3.8 | 26.6 ± 4.4 | <0.0001 |
| 22.1 (18.7‒27.5) | 24.8 (20.0‒32.3) | 25.8 (20.9‒34.7) | ||
| Abstinence period, h | 114.9 ± 55.2 | 108.3 ± 109.0 | 92.3 ± 51.0 | <0.0001 |
| 98.0 (58.0‒227.0) | 72.0 (48.0‒240.0) | 72.0 (48.0‒168.0) | ||
| FSH, IU/L | 3.2 ± 1.8 | 4.1 ± 2.3 | 7.3 ± 6.0 | <0.0001 |
| 2.9 (1.2‒6.7) | 3.6 (1.5‒8.3) | 5.5 (1.9‒19.8) | ||
| LH, IU/L | 4.0 ± 1.7 | 3.8 ± 1.7 | 4.4 ± 2.1 | 0.0003 |
| 3.8 (1.8‒7.2) | 3.6 (1.5‒6.7) | 4.0 (1.7‒8.1) | ||
| Total T, nmol/L | 29.2 ± 9.2 | 17.0 ± 5.9 | 18.6 ± 6.4 | <0.0001 |
| 27.8 (15.5‒46.3) | 16.5 (8.8‒27.2) | 17.9 (10.1‒30.0) | ||
| SHBG, nmol/L | 34.4 ± 14.1 | 34.6 ± 14.6 | n/d | 0.6394b |
| 32.0 (18.0‒57.0) | 30.9 (16.4‒63.6) | |||
| Semen volume, mL | 3.4 ± 1.6 | 4.1 ± 1.8 | 4.2 ± 1.8 | <0.0001 |
| 3.2 (1.2‒6.4) | 3.7 (1.7‒8.0) | 4.0 (1.7‒7.8) | ||
| Sperm concentration, mln/mL | 87.7 ± 79.4 | 98.1 ± 80.1 | 7.8 ± 5.9 | <0.0001 |
| 67.3 (8.2‒224.5) | 76.0 (16.7‒236.0) | 7.0 (0.1‒18.0) | ||
| Total sperm count, mln/ejaculate | 287.5 ± 291.4 | 383.0 ± 330.1 | 33.9 ± 31.2 | <0.0001 |
| 222.8 (18.4‒782.1) | 295.2 (60.0‒980.1) | 25.2 (0.4‒94.5) | ||
| Total testes volume, mL | 50.7 ± 10.6 | 46.9 ± 10.0 | 40.3 ± 10.3 | <0.0001 |
| 50.0 (35.0‒70.0) | 46.0 (34.0‒63.0) | 40.0 (24.0‒56.0) |
Data are presented as mean ± standard deviation and median (5th to 95th percentiles).
Abbreviations: BMI, body mass index; mln, million; n/d, not determined.
Data for BMI available for 327 patients of the idiopathic infertility group.
P value for Mann-Whitney U test comparing distribution of SHBG levels among young men cohort versus partners of pregnant women.
B-1. Estonian young men cohort
Young men (n = 578) were recruited by the AC-TUH between May 2003 and June 2004 in the framework of a prospective study Environment and Reproductive Health (EU sixth FP project QLRT-2001-02911). The study group has been used previously in a number of genetic association studies of male reproductive parameters [1, 9]. The current study excluded subjects with severe genital pathologies (cryptorchidism, n = 9) or missing data (no DNA, n = 27; incomplete clinical records, n = 3). The final number of genotyped young men was 540 (aged 19.3 ± 1.8 years).
B-2. Estonian idiopathic infertility patients
Idiopathic oligozoospermia cases (n = 750) were recruited at the AC-TUH between June 2003 and August 2008 among the male partners of couples failing to conceive a child for a period of ≥12 months [24]. Oligozoospermia was diagnosed according to the World Health Organization criteria valid at the time of recruitment (sperm concentration <20 mln/mL) [25]. Patients with causal factors for male infertility were excluded from the genetic analysis (Supplemental data (268.9KB, pdf) ) [2, 7, 9]. The final number of genotyped patients was 641 (aged 31.6 ± 6.0 years).
B-3. Partners of pregnant women
The group was formed from the partners of pregnant women, who presented for prenatal care at Tartu University Women’s Clinic and West-Tallinn Central Hospital Women’s Clinic, Estonia in 2010 to 2014. Male partners of the informed pregnant women were invited to participate in the study; final recruitment and clinical assessment of men (n = 364) were conducted at the AC-TUH. The details of the group formation are described by Punab et al. [26]. The current genetic analysis excluded cases with pregnancies achieved by in vitro fertilization (n = 3), borderline oligozoospermia (n = 10), and extended time (>12 months) taken to achieve pregnancy (n = 27). The number of genotyped subjects in this study was 324 (aged 31.9 ± 6.6 years).
C. Clinical Examination and Laboratory Procedures
Patients were examined by specialist clinicians, who had received respective training in clinical assessment and standardized andrological workup, locally and in collaboration with other EAA accredited centers [27]. The applied routine andrological pipeline to collect and document epidemiological, laboratory, and clinical examination data at the AC-TUH is described in detail by Punab et al. [26]. Semen samples were obtained by patient masturbation, and all semen values were determined in accordance to the World Health Organization recommendations at the time of recruitment [25, 28]. The protocol of semen analysis is detailed in the Supplemental data (268.9KB, pdf) . Physical examination for the assessment of genital pathology and testicular size (orchidometer, made of birch wood; Pharmacia & Upjohn, Denmark) was performed with the man in standing position. The total testes volume is the sum of right and left testicles. Venous blood was obtained from the cubital vein in the morning (08.00 to 13.00) and serum was separated immediately. Analyses of reproductive hormone levels (serum FSH, LH, total T, and SHBG) are detailed in the Supplemental data (268.9KB, pdf) . Calculated FT levels (unbound T, cFT) were derived from measured total T and SHBG, using the Vermeulen equation [29]. FT percentage (%FT) was estimated as cFT/total T × 100.
D. Genetic Analysis
In total, seven genetic variants were analyzed in the genomic DNA extracted from the patients’ blood samples. The SHBG regulatory variants −68 G>A in 5′UTR (rs1799941) [30] and +1091 C>T (rs727428) [16] at the 3′ downstream regions, SHBG Pro185Leu (rs6258) [14, 15] and GCKR Pro446Leu substitutions (rs1260326) [15] were genotyped in all study subjects (n = 1505). Three SNPs were genotyped only in young men and infertile patients study groups (n = 1181): SLCO1B1 Val174Ala (rs4149056) [15], SHBG Asp327Asn (rs6259) [15], and the G>T SNP in JMJD1C intron 1 (rs7910927) [15, 16]. As genetic association testing with all parameters in these two samples resulted in no statistically significant outcomes, these three SNPs were excluded from further genotyping among the partners of pregnant women. All polymorphisms were genotyped by polymerase chain reaction and allelic discrimination assay on the ABI PRISM 7900HT detection system (Applied Biosystems, Foster City, CA) and details are provided in the Supplemental data (268.9KB, pdf) . Median genotyping efficiency (call rate) across SNPs and study samples was 99.8%. Genotype frequencies for all the SNPs in all studied samples were in concordance with Hardy-Weinberg equilibrium (P ≥ 0.2; Supplemental Table 1 (268.9KB, pdf) ).
E. Statistical Analysis
Mean, standard deviation, median, and 5th to 95th percentiles were calculated for general characteristics and outcome variables using Stata/SE version 13.1. Statistical tests were performed using PLINK software, version 1.07 [31], unless stated otherwise. Tests for Hardy-Weinberg equilibrium of the genotyped SNPs and tests for population differentiation (χ2 test, allelic and genotypic) for all pairs of studied samples were performed. Marker-trait association testing was performed using multiple linear regression applying additive genetic model. If required, the natural log-transformation was used to obtain an approximate normal distribution of values. Regression testing was performed with adjustment for appropriate cofactors (Supplemental Methods (268.9KB, pdf) ). Results of individual study groups were combined in meta-analysis using the meta package [32] for the statistical package R using inverse variance method under fixed-effects model. Statistical significance threshold after correction for multiple testing was estimated α = 0.05/119 = 4.2 × 10−4 (Supplemental Methods (268.9KB, pdf) ). Correlations between total T and SHBG levels were evaluated using Pearson product-moment correlation coefficients. Subjects with outlier values for T and SHBG (<1st and >99th percentiles) have been excluded from the correlation analysis.
2. Results
A. Clinical Comparison of the Three Study Groups
To assess the robustness of genetic associations, the current study was designed to include three groups of men with different reproductive histories and health: young men (n = 540; aged 19.3 ± 1.8 years), and middle-aged patients with either severe idiopathic male factor infertility (n = 641; 31.6 ± 6.0 years) or proven fertility (partners of pregnant women; n = 324; 31.9 ± 6.6 years; Table 1). Both middle-aged study groups, men with idiopathic infertility and partners of pregnant women, had significantly higher body mass index than young men (median 22.1 versus 24.8 and 25.8, respectively). As expected from the study group formation criteria, fertile men exhibited 10-fold higher sperm counts (median 295.2; 5th to 95th percentiles, 60.0 to 980.1 million) compared with infertility patients (25.2; 0.4 to 94.5 million). Reflecting their impaired testicular function, infertility patients were measured statistically higher serum FSH (5.5; 1.9 to 19.8 IU/L) compared with young male cohort (2.9; 1.2 to 6.7 IU/L) and fertile middle-aged men (3.6; 1.5 to 8.3 IU/L; P < 0.05). Young men exhibited higher total T and larger total testes volume, but lower semen volume compared with middle-aged men.
B. Common SHBG Variants Coregulate Circulating SHBG and Total T
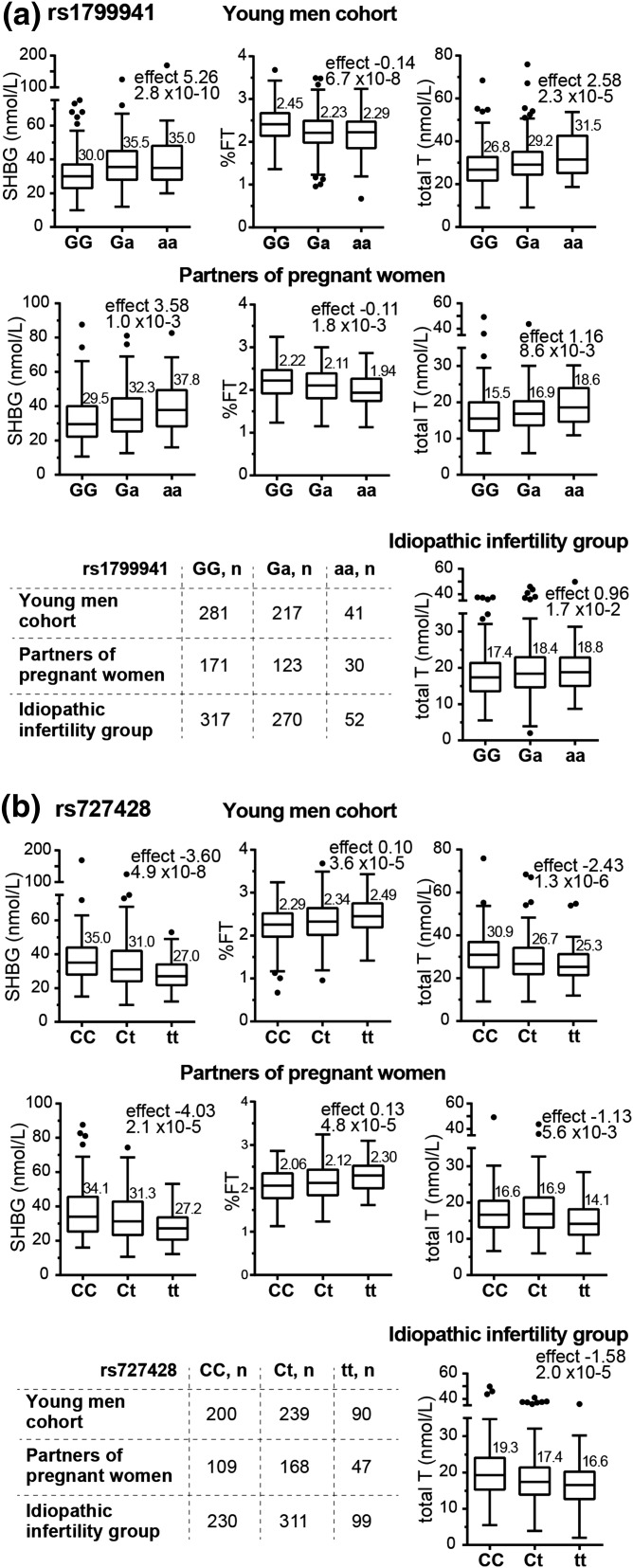
Regulatory variant −68 G>A [rs1799941; minor allele frequency (MAF), 27.7% to 29.3%] at the SHBG 5′UTR exhibited a significant primary effect on increased SHBG in both study groups with available SHBG measurements: young men [YM; P = 2.8 × 10−10; A-allele effect (SE) = 5.26 (0.77) nmol/L] and partners of pregnant women [PP; P = 1.0 × 10−3; 3.58 (1.04) nmol/L] [Fig. 1(a); Table 2]. Meta-analysis enhanced the statistical significance of the association [P = 3.7 × 10−14; 4.67 (0.62) nmol/L]. High levels of SHBG in A-allele carriers were accompanied by increased total T [meta-analysis, all groups: P = 2.1 × 10−7; 1.36 (0.26) nmol/L], unchanged cFT level [young men + partners of pregnant women: P = 2.5 × 10−1; 0.01 (0.01) nmol/L], and decreased %FT [young men + partners of pregnant women: P = 3.9 × 10−10; −0.13% (0.02%)]. The regulatory variant +1091 C>T (rs727428; MAF 39.6% to 40.4%) downstream of the SHBG gene exhibited the same magnitude, but opposite genetic effect compared with the −68 G>A [Fig. 1(b); Table 2]. T-allele carriers had decreased SHBG [meta-analysis, young men + partners of pregnant women: P = 7.3 × 10−11; allelic effect (SE) = −3.74 (0.57) nmol/L] and total T [meta-analysis, all groups: P = 6.3 × 10−7; −1.64 (0.33) nmol/L), unaffected cFT level [meta-analysis, young men + partners of pregnant women: P = 7.6 × 10−2, −0.01 (0.01) nmol/L], but higher %FT [meta-analysis, young men + partners of pregnant women: P = 6.5 × 10−9; 0.11% (0.02%)].
Figure 1.
Tukey boxplots for the distribution of the SHBG, percentage of free testosterone (%FT), and total testosterone values in the young men cohort (n = 540), partners of pregnant women (n = 324), and idiopathic infertility patient study groups (n = 641) stratified according to the genotypes of the SHBG gene variants (a) rs1799941 (−68 G>A) and (b) rs727428 (+1091 C>T). Free testosterone (cFT) percentage (%FT) expresses the fraction of free testosterone of total measured testosterone. cFT was calculated based on total testosterone and SHBG measurements, using the Vermeulen’s equation [29]. The numbers above the boxes represent median values. For idiopathic infertility patients, measurements of serum SHBG were not available. Minor allele effect (statistic β from linear regression under additive model) and statistical significance (P value) are shown on each graph.
Table 2.
Marker–Trait Association Analysis for the SHBG Variants rs1799941, rs727428, and rs6258, and GCKR Variant rs1260326
| Parameter | Linear Regression (Additive Model) | Young Men Cohort | Partners of Pregnant Women | Idiopathic Infertility Group (II) | Meta-Analysisa Young Men + Partners of Pregnant Women | Meta-Analysisa Young Men + Partners of Pregnant Women + II |
|---|---|---|---|---|---|---|
| SHBG rs1799941 (G>A),b −68 G/A | ||||||
| MAF, % | 27.7 | 28.2 | 29.3 | n/a | n/a | |
| Total T, nmol/L | Effect (SE) | 2.58 (0.58) | 1.16 (0.43) | 0.96 (0.40) | 1.66 (0.35) | 1.36 (0.26) |
| P value | 2.3 × 10−5 | 8.6 × 10−3 | 1.7 × 10−2 | 1.6 × 10−6 | 2.1 × 10−7 | |
| SHBG, nmol/L | Effect (SE) | 5.26 (0.77) | 3.58 (1.04) | n/d | 4.67 (0.62) | n/d |
| P value | 2.8 × 10−10 | 1.0 × 10−3 | 3.7 × 10−14 | |||
| cFT, nmol/L | Effect (SE) | 0.02 (0.02) | 0.00 (0.01) | n/d | 0.01 (0.01) | n/d |
| P value | 1.9 × 10−1 | 5.5 × 10−1 | 2.5 × 10−1 | |||
| %FT, % | Effect (SE) | −0.14 (0.03) | −0.11 (0.03) | n/d | −0.13 (0.02) | n/d |
| P value | 6.7 × 10−8 | 1.8 × 10−3 | 3.9 × 10−10 | |||
| FSH, IU/L | Effect (SE) | −0.02 (0.10) | 0.14 (0.16) | 0.04 (0.25) | 0.03 (0.08) | 0.03 (0.08) |
| P value | 8.4 × 10−1 | 3.7 × 10−1 | 8.6 × 10−1 | 7.7 × 10−1 | 7.4 × 10−1 | |
| LH, IU/L | Effect (SE) | 0.06 (0.10) | −0.04 (0.14) | 0.05 (0.12) | 0.03 (0.08) | 0.03 (0.07) |
| P value | 5.7 × 10−1 | 8.0 × 10−1 | 6.9 × 10−1 | 7.6 × 10−1 | 6.3 × 10−1 | |
| Sperm count, mln | Effect (SE) | 7.76 (14.2) | −31.8 (20.8) | 3.27 (1.67) | −4.82 (11.7) | 3.11 (1.65) |
| P value | 5.8 × 10−1 | 9.2 × 10−2 | 5.9 × 10−2 | 6.8 × 10−1 | 6.0 × 10−2 | |
| Sperm concentration, mln/mL | Effect (SE) | 0.25 (4.21) | −8.36 (5.17) | 1.03 (0.38) | −3.18 (3.27) | 0.97 (0.38) |
| P value | 9.5 × 10−1 | 7.6 × 10−2 | 1.0 × 10−2 | 3.3 × 10−1 | 1.0 x 10−2 | |
| Total testes volume, mL | Effect (SE) | 0.59 (0.71) | 1.06 (0.80) | −0.51 (0.68) | 0.79 (0.53) | 0.31 (0.42) |
| P value | 4.1 × 10−1 | 1.9 × 10−1 | 4.5 × 10−1 | 1.3 × 10−1 | 4.7 × 10−1 | |
| SHBG rs727428 (C>T), +1091 C/T | ||||||
| MAF | 39.6 | 40.4 | 39.8 | n/a | n/a | |
| Total T, nmol/L | Effect (SE) | −2.43 (0.52) | −1.13 (0.42) | −1.58 (0.37) | −1.64 (0.33) | −1.61 (0.25) |
| P value | 1.3 × 10−6 | 5.6 × 10−3 | 2.0 × 10−5 | 6.3 × 10−7 | 4.8 × 10−11 | |
| SHBG, nmol/L | Effect (SE) | −3.60 (0.70) | −4.03 (1.01) | n/d | −3.74 (0.57) | n/d |
| P value | 4.9 × 10−8 | 2.1 × 10−5 | 7.3 × 10−11 | |||
| cFT, nmol/L | Effect (SE) | −0.04 (0.01) | 0.00 (0.01) | n/d | −0.01 (0.01) | n/d |
| P value | 1.1 × 10−2 | 5.6 × 10−1 | 7.6 × 10−2 | |||
| %FT, % | Effect (SE) | 0.10 (0.02) | 0.13 (0.03) | n/d | 0.11 (0.02) | n/d |
| P value | 3.6 × 10−5 | 4.8 × 10−5 | 6.5 × 10−9 | |||
| FSH, IU/L | Effect (SE) | −0.04 (0.09) | 0.06 (0.16) | −0.35 (0.23) | −0.01 (0.08) | −0.05 (0.07) |
| P value | 6.7 × 10−1 | 6.9 × 10−1 | 9.9 × 10−2 | 8.6 × 10−1 | 5.0 × 10−1 | |
| LH, IU/L | Effect (SE) | −0.16 (0.09) | −0.02 (0.14) | −0.11 (0.11) | −0.12 (0.08) | −0.12 (0.06) |
| P value | 8.2 × 10−2 | 8.6 × 10−1 | 2.8 × 10−1 | 1.3 × 10−1 | 6.8 × 10−2 | |
| Sperm count, mln | Effect (SE) | −6.65 (12.7) | 24.8 (20.6) | −1.80 (1.53) | 2.01 (10.8) | −1.73 (1.51) |
| P value | 5.8 × 10−1 | 2.3 × 10−1 | 2.0 × 10−1 | 8.5 × 10−1 | 2.5 × 10−1 | |
| Sperm concentration, mln/mL | Effect (SE) | −0.39 (3.78) | 6.22 (5.12) | −0.55 (0.35) | 1.94 (3.04) | −0.51 (0.35) |
| P value | 9.1 × 10−1 | 2.3 × 10−1 | 9.5 × 10−2 | 5.2 × 10−1 | 1.4 × 10−1 | |
| Total testes volume, mL | Effect (SE) | −1.45 (0.64) | −1.48 (0.79) | 1.49 (0.63) | −1.46 (0.50) | −0.32 (0.39) |
| P value | 2.4 × 10−2 | 5.6 × 10−2 | 1.9 × 10−2 | 3.3 × 10−3 | 4.0 × 10−1 | |
| SHBG rs6258 (C>T), Pro185Leu | ||||||
| MAF | 1.12 | 0.62 | 0.94 | n/a | n/a | |
| Total T, nmol/L | Effect (SE) | −5.89 (2.73) | −4.16 (2.67) | 4.59 (1.94) | −5.00 (1.91) | −4.80 (1.36) |
| P value | 1.1 × 10−2 | 5.2 × 10−2 | 1.8 × 10−2 | 8.8 × 10−3 | 4.1 × 10−4 | |
| SHBG, nmol/L | Effect (SE) | −12.3 (3.65) | −12.09 (6.39) | n/d | −12.2 (3.17) | n/d |
| P value | 9.9 × 10−6 | 9.4 × 10−3 | 1.2 × 10−4 | |||
| cFT, nmol/L | Effect (SE) | 0.06 (0.07) | −0.03 (0.05) | n/d | 0.00 (0.04) | n/d |
| P value | 4.2 × 10−1 | 5.7 × 10−1 | 9.7 × 10−1 | |||
| %FT, % | Effect (SE) | 0.48 (0.12) | 0.47 (0.19) | n/d | 0.48 (0.10) | n/d |
| P value | 6.3 × 10−5 | 1.4 × 10−2 | 2.2 × 10−6 | |||
| FSH, IU/L | Effect (SE) | −0.15 (0.47) | −1.48 (1.04) | −2.48 (1.23) | −0.38 (0.43) | −0.61 (0.40) |
| P value | 7.2 × 10−1 | 3.6 × 10−2 | 3.4 × 10−3 | 3.8 × 10−1 | 1.3 × 10−1 | |
| LH, IU/L | Effect (SE) | −0.08 (0.49) | 0.18 (0.90) | −1.66 (0.57) | −0.02 (0.43) | −0.62 (0.34) |
| P value | 8.6 × 10−1 | 8.3 × 10−1 | 5.1 × 10−5 | 9.6 × 10−1 | 7.1 × 10−2 | |
| Sperm count, mln | Effect (SE) | −47.9 (72.4) | 130.1 (145.0) | 7.81 (8.74) | −12.4 (64.8) | 7.45 (8.66) |
| P value | 3.6 × 10−1 | 3.6 × 10−1 | 3.6 × 10−1 | 8.5 × 10−1 | 3.9 × 10−1 | |
| Sperm concentration, mln/mL | Effect (SE) | −16.8 (21.2) | 39.70 (35.59) | 2.97 (1.92) | −2.0 (18.18) | 2.91 (1.91) |
| P value | 2.8 × 10−1 | 2.7 × 10−1 | 1.5 × 10−1 | 9.1 × 10−1 | 1.3 × 10−1 | |
| Total testes volume, mL | Effect (SE) | −5.71 (3.14) | 0.68 (4.85) | 4.46 (3.28) | −3.82 (2.64) | −0.57 (2.05) |
| P value | 7.0 × 10−2 | 8.8 × 10−1 | 1.8 × 10−1 | 1.5 × 10−1 | 7.8 × 10−1 | |
| GCKR rs1260326 (C>T), Pro446Leu | ||||||
| MAF | 41.1 | 37.4 | 36.8 | n/a | n/a | |
| Total T, nmol/L | Effect (SE) | −0.03 (0.55) | −0.83 (0.41) | −0.42 (0.38) | −0.54 (0.33) | −0.49 (0.25) |
| P value | 9.5 × 10−1 | 3.7 × 10−2 | 2.7 × 10−1 | 1.0 × 10−1 | 5.0 × 10−2 | |
| SHBG, nmol/L | Effect (SE) | −1.61 (0.73) | −3.38 (1.00) | n/d | −2.2 (0.59) | n/d |
| P value | 2.3 × 10−2 | 3.1 × 10−4 | 1.5 × 10−4 | |||
| cFT, nmol/L | Effect (SE) | 0.02 (0.01) | −0.01 (0.01) | n/d | 0.00 (0.01) | n/d |
| P value | 1.7 × 10−1 | 4.8 × 10−1 | 9.7 × 10−1 | |||
| %FT, % | Effect (SE) | 0.06 (0.02) | 0.11 (0.03) | n/d | 0.08 (0.02) | n/d |
| P value | 1.2 × 10−2 | 5.1 × 10−4 | 3.5 × 10−5 | |||
| FSH, IU/L | Effect (SE) | −0.04 (0.09) | −0.07 (0.15) | 0.06 (0.23) | −0.05 (0.08) | −0.04 (0.07) |
| P value | 6.8 × 10−1 | 6.2 × 10−1 | 7.9 × 10−1 | 5.5 × 10−1 | 6.3 × 10−1 | |
| LH, IU/L | Effect (SE) | 0.02 (0.10) | −0.27 (0.13) | 0.13 (0.11) | −0.08 (0.08) | −0.01 (0.06) |
| P value | 8.8 × 10−1 | 3.3 × 10−2 | 2.4 × 10−1 | 2.9 × 10−1 | 8.6 × 10−1 | |
| Sperm count, mln | Effect (SE) | 3.41 (13.0) | −15.9 (20.1) | 0.57 (1.57) | −2.29 (10.9) | 0.51 (1.56) |
| P value | 7.9 × 10−1 | 4.0 × 10−1 | 7.1 × 10−1 | 8.34 × 10−1 | 7.4 × 10−1 | |
| Sperm concentration, mln/mL | Effect (SE) | 3.05 (3.88) | −0.54 (5.00) | 0.19 (0.36) | 1.70 (3.06) | 1.00 (1.23) |
| P value | 4.3 × 10−1 | 9.1 × 10−1 | 5.9 × 10−1 | 5.8 × 10−1 | 4.1 × 10−1 | |
| Total testes volume, mL | Effect (SE) | −0.92 (0.65) | 0.98 (0.77) | 0.47 (0.65) | −0.14 (0.50) | 0.09 (0.40) |
| P value | 1.6 × 10−1 | 2.1 × 10−1 | 4.7 × 10−1 | 7.9 × 10−1 | 8.3 × 10−1 | |
Minor allele effect is shown as the estimated linear regression (additive model) statistic β, and SE of the regression is shown in parentheses. P values resistant to Bonferroni correction for multiple testing are indicated in bold.
Abbreviations: mln, million; n/a, not applicable; n/d, not determined.
Results in individual study groups were combined into meta-analysis using inverse variance method under fixed-effects model.
Major allele > minor allele.
The genetic associations of rs1799941 and rs727428 with SHBG, total T, and %FT were robust across study groups and remained statistically significant after correction for multiple testing. Neither of the variants affected the level of serum FSH and LH levels (Table 2).
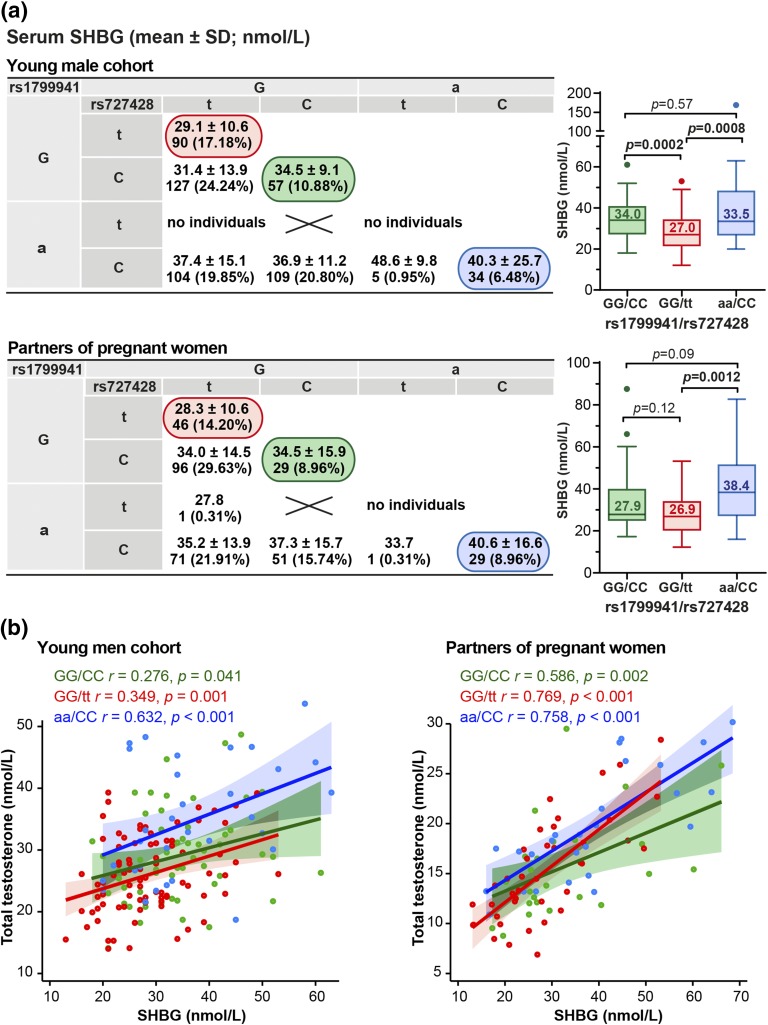
The two common SHBG variants −68 G>A (rs1799941) and +1091 C>T (rs727428) are located 4369 base pairs apart, and the distribution of their alleles exhibits low correlation (linkage disequilibrium estimate across all study subjects, r2 = 0.241). When the carriers of the alternative genotype combinations were compared, the lowest circulating SHBG was detected for the GG-homozygotes at −68 G>A position combined with TT-homozygosity at the +1091 C>T position [young men: 29.1 ± 10.6, partners of pregnant women: 28.3 ± 10.6 nmol/L; Fig. 2(a)]. This level of SHBG was 27.5% lower compared with the opposite genotype combination AA/CC [young men: 40.3 ± 25.7, partners of pregnant women: 40.6 ± 16.6 nmol/L; P ≤ 0.0012; Fig. 2(a)]. The measured serum SHBG and total T exhibited more prominent positive correlation among middle-aged (n = 292, r = 0.738, P < 0.0001) compared with young men (n = 473, r = 0.484, P < 0.0001). The strength of interrelation between SHBG and T was also dependent on the genotype combination of SHBG variant [Fig. 2(b)]. Interestingly, in both groups the lowest correlation between circulating SHBG and T levels was detected among the carriers of the major alleles of both variants [GG/CC genotype combination: young men, n = 55, r = 0.276, P = 0.041; partners of pregnant women, n = 26, r = 0.586; P = 0.002; Fig. 2(b)]. This indicates in these men the weakest compensatory mechanism to keep their T in normal level when the SHBG concentration is either increased or decreased.
Figure 2.
Effect of the carrier status of SHBG rs1799941 (−68G/A; 5′UTR) and rs727428 (+1091 C>T; 3′downstream) genotype combinations on SHBG and total T levels in the study groups of young men (n = 540) and partners of pregnant women (n = 324). (a) SHBG levels in the nine possible SHBG genotype combinations formed by the −68G/A and +1091 C/T variants. Respective number of carriers is shown under the SHBG data presented as mean ± SD. The prevalence of each genotype combination (%) is shown in parentheses. Box plots show the genetic effect on double homozygotes of the two studied variants, rs1799941/rs727428 genotypes (GG/CC, green; GG/tt, red; aa/CC, blue). The numbers inside the boxes represent median values. Statistical significance between the groups was assessed by Mann-Whitney U test. (b) Scatter plots assessing correlations between SHBG and total testosterone levels in rs1799941/rs727428 genotype combinations GG/CC (green), GG/tt (red), and aa/CC (blue) from young men cohort and partners of pregnant women. Best-fit regression line, 5th to 95th confidence intervals, Pearson product-moment correlation coefficients (r), and significance of correlation (P) are shown.
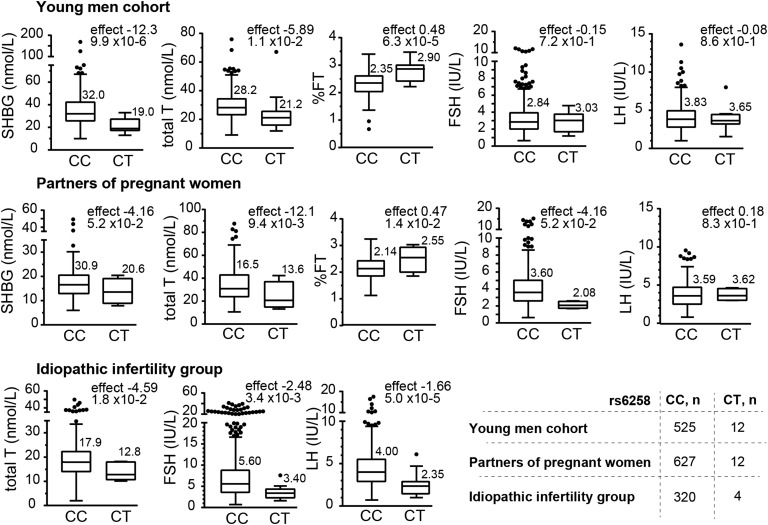
C. Rare Variant SHBG Pro185Leu Exhibits Large Effects on Circulating Reproductive Hormones
A rare missense variant Pro185Leu in the SHBG gene (rs6258, C>T; MAF 0.62% to 1.12%) showed a strong functional effect that exceeded manifold the respective effects of the regulatory SNPs and was consistent across study groups. Carriers of the Pro>Leu substitution had only 60% to 66% of the circulating SHBG level compared with Pro-Pro homozygotes [meta-analysis, young men + partners of pregnant women: P = 1.2 × 10−4, Leu-allele effect (SE) −12.2 (3.17) nmol/L; Fig. 3; Table 2]. Total T levels in heterozygote mutation carriers compared with wild-type variant were also substantially decreased, ranging from 71.5% to 82% [meta-analysis, all groups: P = 4.1 × 10−4; −4.80 (1.36) nmol/L]. As a consequence of low SHBG and T, the Leu-variant carriers have ~20% higher %FT [young men + partners of pregnant women: P = 2.2 × 10−6; 0.48% (0.10%); Fig. 3; Table 2]. No effect was detected on the level of cFT.
Figure 3.
Tukey boxplots for the distribution of the total testosterone, SHBG, and %FT values in the young men cohort (n = 540), partners of pregnant women (n = 324), and idiopathic infertility patient study groups (n = 641) stratified according to the genotypes of the SHBG gene variant rs6258 (C>T, Pro185Leu). The numbers above the boxes represent median values. Minor allele effect (statistic β from linear regression under additive model) and statistical significance (P value) are shown on each graph.
Only among infertile men, the SHBG Pro185Leu substitution showed additional downstream effect on reduced serum gonadotropin levels, LH [P = 5.1 × 10−5; −1.66 (0.57) IU/L] and FSH [P = 3.4 × 10−3; −2.48 (1.23) IU/L]. Heterozygotes for this missense variant may represent a subgroup of idiopathic infertility patients with low T, but no consequent increase in FSH and LH. Notably, a trend for reduced FSH was also detected among partners of pregnant women [P = 3.6 × 10−2; −1.48 (1.04) IU/L], potentially indicative to a progressive effect with increasing age.
D. Main Effect of GCKR Variant Pro446Leu Is on Decreased SHBG
GCKR Pro446Leu missense variant (rs1260326; MAF 36.8% to 41.1%) exhibited functional effect only on serum SHBG [P = 1.5 × 10−4; allele effect −2.2 (0.59) nmol/L] and %FT [P = 3.5 × 10−5; 0.08% (0.02%); Table 2]. This genetic effect was weaker than was identified for the analyzed SHBG variants. Consequently, no significant association was detected with total T.
Interestingly, we observed a trend for an increasing genetic effect of GCKR Pro446Leu with age, as the effect on both, SHBG and %FT was approximately doubled among the middle-aged partners of pregnant women (aged 31.9 ± 6.6 years) compared with young men cohort (19.3 ± 1.8 years; Table 2). As this missense variant has a vast cascade of functional effects on shaping the serum metabolomics profile and susceptibility cardio-metabolic diseases [21], the age-related progressive effect may indicate decreasing actions of compensatory mechanisms.
E. No Confirmed Effect of Three GWAS Top-SNPs on Male Reproductive Hormones
Three SNPs, highlighted in recent GWA studies [15, 16], showed no statistically significant associations with any of the analyzed male reproductive parameters in young men and infertility patients (Supplemental Table 2 (268.9KB, pdf) ). These SNPs were missense variants in the SHBG Asp327Asn (rs6259, G>A, MAF = 7.7% to 8.0%), SLCO1B1 Val174Ala (rs4149056, T>C, MAF = 19.6% to 23.9%), and an intronic variant in JMJD1C (rs7910927 G>T, MAF = 48.3% to 50.8%).
F. Analyzed Genetic Variants Were Not Significantly Associated With Infertility Status, and Seminal And Testicular Parameters
None of the tests comparing allele and genotype distributions between the three study groups, including comparison between infertility patients and men with proved fertility, reached statistical significance (Supplemental Table 1 (268.9KB, pdf) ). Thus, the current data exclude the major role of the studied seven SNPs in predisposition to severe male factor infertility.
Consistently, none of the tested seven SNPs was significantly associated with semen and total testicular volume, and total sperm count and concentration (Table 2; Supplemental Table 2 (268.9KB, pdf) ).
3. Discussion
The major aim of the current study was to identify genetic variants with robust and reproducible effects on male reproductive parameters. Such variants may have potential applicability in andrology practices in diagnostic purposes, as well as in the development of optimal treatment options taking into consideration an individual’s genetic profile. The strength and advantage of our study compared with the previous reports was the analysis of genetic associations across a broad range of hormonal and testicular parameters available for the three independent study groups of men with different reproductive histories (young and middle-aged, fertile and infertile). All the patients involved in the current study had been clinically phenotyped and passed the standardized andrology workup conducted by trained andrology specialists at a dedicated clinical center [26]. This represents an additional strength of the study. This study tests the targeted variants for the link to male infertility status. Furthermore, no studies have addressed the association of polymorphisms in the GCKR, SLCO1B1, and JMJD1C with sperm parameters and impaired fertility, despite highlighting them in GWA studies for male reproductive hormones.
In our clinical study groups, we replicated four of seven top genetic associations previously highlighted in GWA studies for serum SHBG and/or T levels (Table 2; Figs. 1 and 3). The corroborated genetic associations represented regulatory and coding variants, common and rare variants. Three of the confirmed associations had been originally demonstrated in candidate gene studies. A common polymorphism, SHBG −68 G>A (rs1799941), was shown to contribute to circulating SHBG and T levels in men already a decade ago [17]. The SHBG downstream variant +1091 C>T (rs727428) was initially highlighted as the SHBG-level modulating SNP predisposing to breast cancer [19], and the GCKR Pro446Leu substitution (rs1260326) has been extensively studied in the context of metabolic disorders [21]. Only the missense rare variant SHBG Pro185Leu (rs6258), exhibiting large effect on both serum SHBG and T, had been discovered using the GWA approach [14]. Ohlsson et al. [14] demonstrated that leucine at the SHBG position 185 resulted in its altered steroid-binding capacity affecting T bioavailability and action at the target tissue level.
One previous study on a mixed sample including men with normal and abnormal semen quality (n = 677) has demonstrated a potential association of two variants in the SHBG gene, −68 G>A (rs1799941) and rs6259 (Asp327Asn) with sperm concentration and motility [33]. So far, no studies have replicated these claims. In our study, none of the tested SNPs showed a clear effect on fertility status and seminal and testicular parameters in the three homogenously formed study groups. What could be the clinical implications and impact of these genetic effects? Among our young and middle-aged men study groups, genetically determined level of serum SHBG was tightly positively correlated with the level of total T. Increased levels of circulating SHBG were accompanied by increased production of T [Table 2; Fig. 2(b)], guaranteeing optimal availability of T to organs and tissues in the male body. This refers to fine-tuned feedback mechanisms to maintain the amount of FT at a constant level (Table 2). Although this compensatory mechanism is expected to work well for the young and middle-aged men, it may gradually decline along the age and in the context of overall impaired health. In elderly men, even in the case of normal serum levels of T, low cFT levels are associated with androgen-deficiency related symptoms [34]. In addition to cFT, there is rising acknowledgment of the importance of %FT (FT fraction of total T) in clinical practice. Recently, higher %FT has been associated with poor-prognosis prostate cancer [35]. We have shown that in the SHBG variants −68 G>A (rs1799941) and +1091 C>T (rs727428), the SHBG Pro185Leu (rs6258) and GCKR Pro446Leu (rs1260326) substitutions are significantly associated with %FT levels. Regarding the SHBG Pro185Leu variant, our results are consistent with the study by Ohlsson et al. [14] reporting that the this substitution is associated with higher %FT due to lower steroid-binding capacity of serum SHBG. Thus, in the assessment of male reproductive physiology and health, introduction of routine estimation of cFT and %FT appear to be equally important to the measurement of serum total T [34]. There is an increased global interest in T deficiency in men, as respective sexual and nonsexual symptoms can negatively affect the quality of life and cause general health concerns such as depression, fatigue, and decreased concentration and memory [36]. The genetic variants shown in the current report with a robust effect on serum SHBG and T represent prime candidates for the future studies of hypogonadism, i.e. T deficiency among aging men. Our study suggests that the efficiency of the compensatory mechanism for the altered level of SHBG is dependent on the genetic variation in the SHBG gene [Fig. 2(b)].
Further impact in andrology clinic may arise from the analysis of the highlighted rare missense variants. Men carrying the SHBG Pro185Leu (rs6258) substitution represent a subgroup of idiopathic infertility patients with low T, but no consequent increase in FSH and LH. This genetic stratification may assist in choosing the optimal workup scheme for these patients. A substitution Pro446Leu (rs1260326) in the hepatic GCKR gene had a weaker effect on the studied parameters and thus, it was only statistically significant on serum SHBG. Overall, this variant exhibits a pleiotropic effect on a number of metabolic parameters (e.g. on triglycerides, serum urate levels) [37, 38] and thus may additionally contribute to the profile of clinical symptoms in hypogonadism among aging men.
The current study among young and middle-aged men did not confirm three genetic associations with SHBG, initially reported by a large meta-analysis of GWA reports. These represent the SHBG Asp327Asn substitution variant (rs6259) and two SNPs in the genes involved in either hepatic function (SLCO1B1, rs4149056) or spermatogenesis (JMJD1C, rs7910927). The GWA studies meta-analysis included 16 epidemiological cohorts (n = 28,837; 14,938 men and 10,899 women) with various clinical profiles [15]. One possible explanation is that these variants are mainly associated with SHBG among women or in certain clinical groups. Indeed, the seminal study on the genetic effect of the SHBG Asp327Asn was performed among women referred to the clinic for hirsutism, including >50% diagnosed with polycystic ovary syndrome [20]. There is an abundance of literature that the SLCO1B1 missense variant Val174Ala (rs4149056) mainly affects pharmacokinetics and various treatment responses [39]. JMJD1C represents a novel gene linked to male fertility. Although two independent GWA studies have reported association with SHBG [15] and T [16], there is emerging evidence that its role as a candidate histone demethylase is rather in epigenetic regulation in several clinical conditions [23, 40].
In summary, we have robustly replicated the association with serum SHBG for four of seven analyzed genetic variants. Our study alerts to investigations on the role of genetically determined level of T (as well as cFT and %FT) in reproductive and general health of aging men, in predisposition to hypotestosteronemia and pharmacogenetics of hormone replacement therapy in male infertility and hypogonadism.
Acknowledgments
We thank the participating patients. Džonathan Šapoval is acknowledged for technical assistance in genotyping. Kristel Ehala-Aleksejev is thanked for the discussions on the pilot outcome of this study.
Acknowledgments
The study was supported by the European Union through the European Regional Development Fund (project HAPPY PREGNANCY, 3.2.0701.12-0047; for M.L. and M.P.) and Estonian Research Council (Grants IUT34-12 and ETF9030 for M.L., PUT181 to M.P.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- %FT
- free testosterone percentage
- AC-TUH
- Andrology Centre, Tartu University Hospital
- cFT
- calculated free testosterone
- FSH
- follicle-stimulating hormone
- FT
- free testosterone
- GWA
- genome-wide association
- LH
- luteinizing hormone
- MAF
- minor allele frequency
- SE
- standard error
- SHBG
- sex hormone-binding globulin
- SNP
- single-nucleotide polymorphism
- T
- testosterone.
References and Notes
- 1.Grigorova M, Punab M, Ausmees K, Laan M. FSHB promoter polymorphism within evolutionary conserved element is associated with serum FSH level in men. Hum Reprod. 2008;23(9):2160–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grigorova M, Punab M, Poolamets O, Kelgo P, Ausmees K, Korrovits P, Vihljajev V, Laan M. Increased prevalence of the -211 T allele of follicle stimulating hormone (FSH) beta subunit promoter polymorphism and lower serum FSH in infertile men. J Clin Endocrinol Metab. 2010;95:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlin A, Vinanzi C, Selice R, Garolla A, Frigo AC, Foresta C. Toward a pharmacogenetic approach to male infertility: polymorphism of follicle-stimulating hormone beta-subunit promoter. Fertil Steril. 2011;96(6):1344–1349. [DOI] [PubMed] [Google Scholar]
- 4.Simoni M, Gromoll J, Hoppner W, Kamischke A, Krafft T, Stahle D, Nieschlag E. Mutational analysis of the follicle-stimulating hormone (FSH) receptor in normal and infertile men: identification and characterization of two discrete FSH receptor isoforms. J Clin Endocrinol Metab. 1999;84(2):751–755. [DOI] [PubMed] [Google Scholar]
- 5.Grigorova M, Punab M, Poolamets O, Sõber S, Vihljajev V, Žilaitiene B, Erenpreiss J, Matulevičius V, Tsarev I, Laan M. Study in 1790 Baltic men: FSHR Asn680Ser polymorphism affects total testes volume. Andrology. 2013;1(2):293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wunsch A, Ahda Y, Banaz-Yasar F, Sonntag B, Nieschlag E, Simoni M, Gromoll J. Single-nucleotide polymorphisms in the promoter region influence the expression of the human follicle-stimulating hormone receptor. Fertil Steril. 2005;84(2):446–453. [DOI] [PubMed] [Google Scholar]
- 7.Grigorova M, Punab M, Punab AM, Poolamets O, Vihljajev V, Zilaitiene B, Erenpreiss J, Matulevicius V, Laan M. Reproductive physiology in young men is cumulatively affected by FSH-action modulating genetic variants: FSHR -29G/A and c.2039 A/G, FSHB -211G/T. PLoS One. 2014;9(4):e94244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pettersson K, Ding YQ, Huhtaniemi I. An immunologically anomalous luteinizing hormone variant in a healthy woman. J Clin Endocrinol Metab. 1992;74(1):164–171. [DOI] [PubMed] [Google Scholar]
- 9.Punab AM, Grigorova M, Punab M, Adler M, Kuura T, Poolamets O, Vihljajev V, Zilaitiene B, Erenpreiss J, Matulevicius V, Laan M. Carriers of V-LH among 1593 Baltic men have significantly higher serum LH. Andrology. 2015;3(3):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haring R, Volzke H, Felix SB, Schipf S, Dorr M, Rosskopf D, Nauck M, Schofl C, Wallaschofski H. Prediction of metabolic syndrome by low serum testosterone levels in men: results from the study of health in Pomerania. Diabetes. 2009;58(9):2027–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pye SR, Huhtaniemi IT, Finn JD, Lee DM, O’Neill TW, Tajar A, Bartfai G, Boonen S, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Rutter MK, Vanderschueren D, Wu FC, Group ES. Late-onset hypogonadism and mortality in aging men. J Clin Endocrinol Metab. 2014;99(4):1357–1366. [DOI] [PubMed] [Google Scholar]
- 12.Haring R, Volzke H, Steveling A, Krebs A, Felix SB, Schofl C, Dorr M, Nauck M, Wallaschofski H. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. Eur Heart J. 2010;31(12):1494–1501. [DOI] [PubMed] [Google Scholar]
- 13.Laurent MR, Hammond GL, Blokland M, Jardi F, Antonio L, Dubois V, Khalil R, Sterk SS, Gielen E, Decallonne B, Carmeliet G, Kaufman JM, Fiers T, Huhtaniemi IT, Vanderschueren D, Claessens F. Sex hormone-binding globulin regulation of androgen bioactivity in vivo: validation of the free hormone hypothesis. Sci Rep. 2016;6:35539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohlsson C, Wallaschofski H, Lunetta KL, Stolk L, Perry JR, Koster A, Petersen AK, Eriksson J, Lehtimaki T, Huhtaniemi IT, Hammond GL, Maggio M, Coviello AD, Group ES, Ferrucci L, Heier M, Hofman A, Holliday KL, Jansson JO, Kahonen M, Karasik D, Karlsson MK, Kiel DP, Liu Y, Ljunggren O, Lorentzon M, Lyytikainen LP, Meitinger T, Mellstrom D, Melzer D, Miljkovic I, Nauck M, Nilsson M, Penninx B, Pye SR, Vasan RS, Reincke M, Rivadeneira F, Tajar A, Teumer A, Uitterlinden AG, Ulloor J, Viikari J, Volker U, Volzke H, Wichmann HE, Wu TS, Zhuang WV, Ziv E, Wu FC, Raitakari O, Eriksson A, Bidlingmaier M, Harris TB, Murray A, de Jong FH, Murabito JM, Bhasin S, Vandenput L, Haring R. Genetic determinants of serum testosterone concentrations in men. PLoS Genet. 2011;7:e1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coviello AD, Haring R, Wellons M, Vaidya D, Lehtimaki T, Keildson S, Lunetta KL, He C, Fornage M, Lagou V, Mangino M, Onland-Moret NC, Chen B, Eriksson J, Garcia M, Liu YM, Koster A, Lohman K, Lyytikainen LP, Petersen AK, Prescott J, Stolk L, Vandenput L, Wood AR, Zhuang WV, Ruokonen A, Hartikainen AL, Pouta A, Bandinelli S, Biffar R, Brabant G, Cox DG, Chen Y, Cummings S, Ferrucci L, Gunter MJ, Hankinson SE, Martikainen H, Hofman A, Homuth G, Illig T, Jansson JO, Johnson AD, Karasik D, Karlsson M, Kettunen J, Kiel DP, Kraft P, Liu J, Ljunggren O, Lorentzon M, Maggio M, Markus MR, Mellstrom D, Miljkovic I, Mirel D, Nelson S, Morin Papunen L, Peeters PH, Prokopenko I, Raffel L, Reincke M, Reiner AP, Rexrode K, Rivadeneira F, Schwartz SM, Siscovick D, Soranzo N, Stockl D, Tworoger S, Uitterlinden AG, van Gils CH, Vasan RS, Wichmann HE, Zhai G, Bhasin S, Bidlingmaier M, Chanock SJ, De Vivo I, Harris TB, Hunter DJ, Kahonen M, Liu S, Ouyang P, Spector TD, van der Schouw YT, Viikari J, Wallaschofski H, McCarthy MI, Frayling TM, Murray A, Franks S, Jarvelin MR, de Jong FH, Raitakari O, Teumer A, Ohlsson C, Murabito JM, Perry JR. A genome-wide association meta-analysis of circulating sex hormone-binding globulin reveals multiple Loci implicated in sex steroid hormone regulation. PLoS Genet. 2012;8(7):e1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin G, Sun J, Kim ST, Feng J, Wang Z, Tao S, Chen Z, Purcell L, Smith S, Isaacs WB, Rittmaster RS, Zheng SL, Condreay LD, Xu J. Genome-wide association study identifies a new locus JMJD1C at 10q21 that may influence serum androgen levels in men. Hum Mol Genet. 2012;21(23):5222–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eriksson AL, Lorentzon M, Mellstrom D, Vandenput L, Swanson C, Andersson N, Hammond GL, Jakobsson J, Rane A, Orwoll ES, Ljunggren O, Johnell O, Labrie F, Windahl SH, Ohlsson C. SHBG gene promoter polymorphisms in men are associated with serum sex hormone-binding globulin, androgen and androgen metabolite levels, and hip bone mineral density. J Clin Endocrinol Metab. 2006;91(12):5029–5037. [DOI] [PubMed] [Google Scholar]
- 18.Wickham EP III, Ewens KG, Legro RS, Dunaif A, Nestler JE, Strauss JF III. Polymorphisms in the SHBG gene influence serum SHBG levels in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2011;96(4):E719–E727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson DJ, Healey CS, Baynes C, Kalmyrzaev B, Ahmed S, Dowsett M, Folkerd E, Luben RN, Cox D, Ballinger D, Pharoah PD, Ponder BA, Dunning AM, Easton DF; Studies in Epidemiology and Risks of Cancer Heredity Team . Identification of common variants in the SHBG gene affecting sex hormone-binding globulin levels and breast cancer risk in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3490–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cousin P, Calemard-Michel L, Lejeune H, Raverot G, Yessaad N, Emptoz-Bonneton A, Morel Y, Pugeat M. Influence of SHBG gene pentanucleotide TAAAA repeat and D327N polymorphism on serum sex hormone-binding globulin concentration in hirsute women. J Clin Endocrinol Metab. 2004;89(2):917–924. [DOI] [PubMed] [Google Scholar]
- 21.Brouwers MC, Jacobs C, Bast A, Stehouwer CD, Schaper NC. Modulation of glucokinase regulatory protein: a double-edged sword? Trends Mol Med. 2015;21(10):583–594. [DOI] [PubMed] [Google Scholar]
- 22.Kitzmiller JP, Mikulik EB, Dauki AM, Murkherjee C, Luzum JA. Pharmacogenomics of statins: understanding susceptibility to adverse effects. Pharm Genomics Pers Med. 2016;9:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakajima R, Okano H, Noce T. JMJD1C exhibits multiple functions in epigenetic regulation during spermatogenesis. PLoS One. 2016;11:e0163466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Punab M. Male Fertility and Its Risk Factors in Estonia [PhD thesis]. Tartu, Estonia: University of Tartu Press; 2007:25–28. [Google Scholar]
- 25.World Health Organization Laboratory Manual for the Examination of the Human Semen and Sperm—Cervical Mucus Interaction. 4th ed. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- 26.Punab M, Poolamets O, Paju P, Vihljajev V, Pomm K, Ladva R, Korrovits P, Laan M. Causes of male infertility: a 9-year prospective monocentre study on 1737 patients with reduced total sperm counts. Hum Reprod. 2017;32(1):18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlsen E, Andersen AG, Buchreitz L, Jorgensen N, Magnus O, Matulevicuus V, Nermoen I, Petersen JH, Punab M, Suominen J, Zilaitiene B, Giwercman A. Inter-observer variation in the results of the clinical andrological examination including estimation of testicular size. Int J Androl. 2000;23(4):248–253. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization Laboratory Manual for the Examination and Processing of Human Semen. 5th edGeneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 29.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. [DOI] [PubMed] [Google Scholar]
- 30.Low YL, Taylor JI, Grace PB, Dowsett M, Folkerd E, Doody D, Dunning AM, Scollen S, Mulligan AA, Welch AA, Luben RN, Khaw KT, Day NE, Wareham NJ, Bingham SA. Polymorphisms in the CYP19 gene may affect the positive correlations between serum and urine phytoestrogen metabolites and plasma androgen concentrations in men. J Nutr. 2005;135(11):2680–2686. [DOI] [PubMed] [Google Scholar]
- 31.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(13):559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarzer G. Meta: an R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 33.Lee IW, Kuo PH, Su MT, Kuan LC, Hsu CC, Kuo PL. Quantitative trait analysis suggests polymorphisms of estrogen-related genes regulate human sperm concentrations and motility. Hum Reprod. 2011;26(6):1585–1596. [DOI] [PubMed] [Google Scholar]
- 34.Antonio L, Wu FC, O’Neill TW, Pye SR, Ahern TB, Laurent MR, Huhtaniemi IT, Lean ME, Keevil BG, Rastrelli G, Forti G, Bartfai G, Casanueva FF, Kula K, Punab M, Giwercman A, Claessens F, Decallonne B, Vanderschueren D. European Male Ageing Study Group . Low free testosterone is associated with hypogonadal signs and symptoms in men with normal total testosterone. J Clin Endocrinol Metab. 2016;101(7):2647–2657. [DOI] [PubMed] [Google Scholar]
- 35.Albisinni S, De Nunzio C, Tubaro A, Barry WT, Banez LL, Freedland SJ. Greater percent-free testosterone is associated with high-grade prostate cancer in men undergoing prostate biopsy. Urology. 2012;80(1):162–167. [DOI] [PubMed] [Google Scholar]
- 36.Aversa A, Morgentaler A. The practical management of testosterone deficiency in men. Nat Rev Urol. 2015;12(11):641–650. [DOI] [PubMed] [Google Scholar]
- 37.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, Twee-Hee Ong R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PK, Lucas G, Luben R, Loos RJ, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, Konig IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens AC, Ingelsson E, Igl W, Kees Hovingh G, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Doring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJ, de Faire U, Crawford G, Collins FS, Chen YD, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kottgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C, Pistis G, Ruggiero D, O’Seaghdha CM, Haller T, Yang Q, Tanaka T, Johnson AD, Kutalik Z, Smith AV, Shi J, Struchalin M, Middelberg RP, Brown MJ, Gaffo AL, Pirastu N, Li G, Hayward C, Zemunik T, Huffman J, Yengo L, Zhao JH, Demirkan A, Feitosa MF, Liu X, Malerba G, Lopez LM, van der Harst P, Li X, Kleber ME, Hicks AA, Nolte IM, Johansson A, Murgia F, Wild SH, Bakker SJ, Peden JF, Dehghan A, Steri M, Tenesa A, Lagou V, Salo P, Mangino M, Rose LM, Lehtimaki T, Woodward OM, Okada Y, Tin A, Muller C, Oldmeadow C, Putku M, Czamara D, Kraft P, Frogheri L, Thun GA, Grotevendt A, Gislason GK, Harris TB, Launer LJ, McArdle P, Shuldiner AR, Boerwinkle E, Coresh J, Schmidt H, Schallert M, Martin NG, Montgomery GW, Kubo M, Nakamura Y, Tanaka T, Munroe PB, Samani NJ, Jacobs DR Jr, Liu K, D’Adamo P, Ulivi S, Rotter JI, Psaty BM, Vollenweider P, Waeber G, Campbell S, Devuyst O, Navarro P, Kolcic I, Hastie N, Balkau B, Froguel P, Esko T, Salumets A, Khaw KT, Langenberg C, Wareham NJ, Isaacs A, Kraja A, Zhang Q, Wild PS, Scott RJ, Holliday EG, Org E, Viigimaa M, Bandinelli S, Metter JE, Lupo A, Trabetti E, Sorice R, Doring A, Lattka E, Strauch K, Theis F, Waldenberger M, Wichmann HE, Davies G, Gow AJ, Bruinenberg M; LifeLines Cohort Study, Stolk RP, Kooner JS, Zhang W, Winkelmann BR, Boehm BO, Lucae S, Penninx BW, Smit JH, Curhan G, Mudgal P, Plenge RM, Portas L, Persico I, Kirin M, Wilson JF, Mateo Leach I, van Gilst WH, Goel A, Ongen H, Hofman A, Rivadeneira F, Uitterlinden AG, Imboden M, von Eckardstein A, Cucca F, Nagaraja R, Piras MG, Nauck M, Schurmann C, Budde K, Ernst F, Farrington SM, Theodoratou E, Prokopenko I, Stumvoll M, Jula A, Perola M, Salomaa V, Shin SY, Spector TD, Sala C, Ridker PM, Kahonen M, Viikari J, Hengstenberg C, Nelson CP; CARDIoGRAM Consortium; DIAGRAM Consortium; ICBP Consortium; MAGIC Consortium, Meschia JF, Nalls MA, Sharma P, Singleton AB, Kamatani N, Zeller T, Burnier M, Attia J, Laan M, Klopp N, Hillege HL, Kloiber S, Choi H, Pirastu M, Tore S, Probst-Hensch NM, Volzke H, Gudnason V, Parsa A, Schmidt R, Whitfield JB, Fornage M, Gasparini P, Siscovick DS, Polasek O, Campbell H, Rudan I, Bouatia-Naji N, Metspalu A, Loos RJ, van Duijn CM, Borecki IB, Ferrucci L, Gambaro G, Deary IJ, Wolffenbuttel BH, Chambers JC, Marz W, Pramstaller PP, Snieder H, Gyllensten U, Wright AF, Navis G, Watkins H, Witteman JC, Sanna S, Schipf S, Dunlop MG, Tonjes A, Ripatti S, Soranzo N, Toniolo D, Chasman DI, Raitakari O, Kao WH, Ciullo M, Fox CS, Caulfield M, Bochud M, Gieger C. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45(2):145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reimer T, Kempert S, Gerber B, Thiesen HJ, Hartmann S, Koczan D. SLCO1B1*5 polymorphism (rs4149056) is associated with chemotherapy-induced amenorrhea in premenopausal women with breast cancer: a prospective cohort study. BMC Cancer. 2016;16:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe S, Watanabe K, Akimov V, Bartkova J, Blagoev B, Lukas J, Bartek J. JMJD1C demethylates MDC1 to regulate the RNF8 and BRCA1-mediated chromatin response to DNA breaks. Nat Struct Mol Biol. 2013;20(12):1425–1433. [DOI] [PubMed] [Google Scholar]