Abstract
Management of adult patients with classic congenital adrenal hyperplasia (CAH) is challenging and often complicated by obesity, metabolic syndrome, and adverse cardiovascular risk. Alterations in weight can influence cortisol kinetics. A 19-year-old woman with classic CAH and morbid obesity experienced persistent elevations of androgen levels while receiving oral glucocorticoid therapy. Control of adrenal androgens was improved with continuous subcutaneous hydrocortisone infusion therapy, but obesity-related comorbidities persisted. After undergoing sleeve gastrectomy, the patient experienced dramatic weight loss, with improvement in insulin sensitivity and fatty liver in the postbariatric period. Cortisol clearance studies performed to evaluate changes in hydrocortisone dose requirements showed marked alternations in cortisol pharmacokinetics with decreases in volume of distribution and cortisol clearance, along with an increase in area under the curve for cortisol. Hydrocortisone dose was subsequently decreased 34% by 15 months after surgery. Effective control of androgen excess on this lower hydrocortisone dose was achieved and continues 27 months after surgery. This case highlights obesity-related complications of glucocorticoid replacement therapy in the management of CAH. Individual patient factors, such as fatty liver disease and insulin resistance, can have a clinically important effect on cortisol metabolism. Bariatric surgery was a safe and effective treatment of obesity in this patient with CAH and should be considered for patients with CAH and multiple obesity-related comorbidities.
Keywords: cortisol, pharmacokinetics, congenital adrenal hyperplasia, bariatric, pump, obesity
A patient with CAH and morbid obesity experienced substantial weight loss and improvement in comorbidities after bariatric surgery, resulting in altered cortisol kinetics and a hydrocortisone dose decrease.
Classic congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency is characterized by deficiency of cortisol and aldosterone along with androgen excess. CAH, a state of adrenal insufficiency, requires life-long glucocorticoid therapy [1]. However, treatment with glucocorticoids at doses used to prevent adrenal crisis often fails to control virilization. For many patients, the lowest effective glucocorticoid dose used to control adrenal androgen production results in cushingoid symptoms. Thus, alternative treatment approaches are being developed, including novel pharmacologic strategies that aim to minimize daily glucocorticoid exposure while achieving physiologic cortisol replacement. Circadian cortisol replacement with a modified-release form of oral hydrocortisone (Chronocort; Diurnal, Cardiff, United Kingdom) and continuous subcutaneous hydrocortisone infusion (CSHI) improve control of adrenal androgens using similar or lower glucocorticoid doses than conventional glucocorticoid formulations [2, 3]. However, pre-existing chronic comorbidities may not be reversible and may interfere with cortisol metabolism and clearance. Patients with CAH have excess cardiovascular and metabolic morbidity, including obesity, hypertension, and insulin resistance [4]. The interplay between glucocorticoid dose and these common comorbidities is mostly unknown.
Here we report a case of bariatric surgery in a patient with CAH. We describe a young woman with difficult-to-manage classic CAH who experienced improved control of adrenal androgens while receiving diurnal CSHI therapy, but morbid obesity remained and the glucocorticoid dose needed to adequately control adrenal androgens remained supraphysiologic. She subsequently underwent successful sleeve gastrectomy. We characterize and describe substantial alterations in hydrocortisone pharmacokinetics associated with extreme weight loss and resolution of weight-related comorbidities after bariatric surgery, allowing for lower doses of glucocorticoid therapy.
1. Case Report
Our patient was born with ambiguous genitalia and presented in adrenal crisis at 2 weeks of life. Classic 21-hydroxylase deficiency was diagnosed on the basis of a 17-hydroxyprogesterone (17-OHP) of 42,000 ng/dL (1273 nmol/L). Genetic testing confirmed the diagnosis of classic salt-wasting CAH (homozygous intron 2 IVS2-13A/C>G splice site mutation). She was managed on oral hydrocortisone and fludrocortisone during childhood. She was overweight from age 6 years and continued to gain weight throughout childhood. During her pubertal years, her CAH management became challenging, with persistent elevations of adrenal androgen levels despite glucocorticoid dose adjustments. 17-OHP levels were consistently >2500 ng/dL [>75.8 nmol/L (reference range < 1200 ng/dL; 36.4 nmol/L)] and androstenedione levels were >350 ng/dL [12.2 nmol/L (reference range, 80 to 240 ng/dL; 2.8 to 8.4 nmol/L)]. She had menarche at age 14 years 5 months but experienced irregular menses. Following completion of linear growth at age 16 years, her glucocorticoid regimen was changed to long-acting formulations.
While receiving dexamethasone (375 μg daily), she experienced excessive weight gain and sleep problems that necessitated a change to her glucocorticoid regimen. She was switched to prednisone 3 mg twice daily but continued to have symptoms of androgen excess including a moderate degree of hirsutism (Ferriman–Gallwey score of 20) and menstrual irregularities with eventual secondary amenorrhea. An oral combined contraceptive pill was added to her regimen but was discontinued after 3 months because of migraines. The antiandrogen bicalutamide, 25 mg once daily, was also initiated but was discontinued because of elevation of liver enzymes. Her CAH management was complicated by concomitant comorbidities, including morbid obesity [body mass index (BMI), 50 kg/m2] and fatty liver observed on liver magnetic resonance spectroscopy (MRS) imaging.
At age 19 years, she was treated with CSHI therapy (NCT01859312) [3]. Hormone analysis performed early in the morning (0900 hours) before CSHI initiation showed an 17-OHP level of 3852 ng/dL (116.7 nmol/L) and an androstenedione level of 356 ng/dL [12.4 nmol/L (reference range, 17 to 175 ng/dL and 0.6 to 6.1 nmol/L)]. After 2 months of CSHI therapy, she was noted to have improved adrenal steroid control, with a 17-OHP level of 699 ng/dL (21.2 nmol/L) and an androstenedione level of 136 ng/dL (4.7 nmol/L); by 4 months, her menstrual periods resumed. Additionally, fatigue scores and quality-of-life estimates improved substantially after 2 months of CSHI therapy [3]. Adrenal steroid secretion remained well controlled with a hydrocortisone dose of 35 mg/d on CSHI therapy. After 9 months of CSHI therapy, she underwent sleeve gastrectomy for management of her obesity and tolerated the procedure well.
2. Materials and Methods
Cortisol clearance studies were performed 9 months before surgery and at 9 and 15 months after surgery. At each clearance study, an intravenous bolus of hydrocortisone 100 mg, was administered, followed by serial blood sampling every 10 minutes for a total of 150 minutes, as described by Bryan et al. [5]. Cortisol pharmacokinetics were calculated via noncompartmental analysis using Phoenix WinNonlin software, version 6.4 (Certara, St. Louis, MO). Clearance was calculated following estimation of the cortisol area under the concentration vs. time curve (AUC) from time 0 minutes (0) to infinity (inf) via the “linear up-log down” trapezoidal rule as follows: clearance = dose/AUC0-inf. The elimination rate constant (ke) was calculated from the slope of the regression line of the log-transformed cortisol data vs. time. Volume of distribution (Vd) and half-life (t1/2) were estimated by using the respective formulas: Vd = clearance/ke and t1/2 = 0.693/ke.
Abdominal magnetic resonance imaging with liver MRS was performed to quantify liver fat. MRS imaging was performed before initiation of CSHI therapy (9 months before surgery, on prednisone), 3 months before surgery (on CSHI therapy), and 9 months after surgery.
3. Results
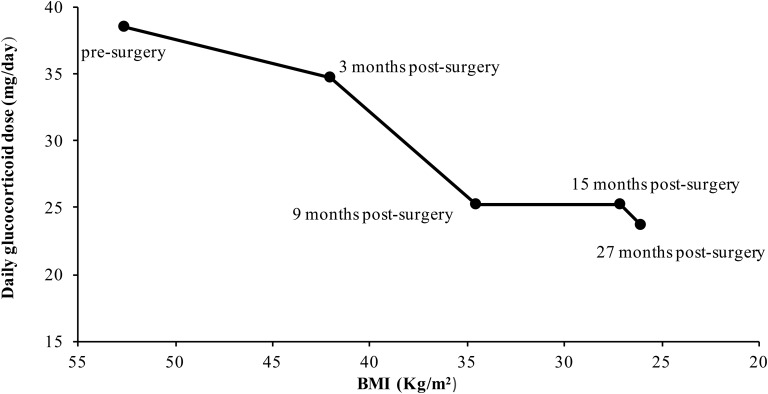
Following sleeve gastrectomy, weight decreased dramatically (Fig. 1). A cortisol clearance study performed 15 months after surgery showed a 43% decrease in clearance, a 74% increase in AUC, and 27% decrease in Vd as compared with presurgery measures (Table 1). As the patient's weight decreased, CSHI rate adjustments were necessary, with a 34% reduction in hydrocortisone dose by 15 months after surgery and a 39% reduction in hydrocortisone dose by 27 months after surgery (Fig. 2). The hydrocortisone dose required to adequately control adrenal androgen production decreased from 38.5 mg/d before surgery to 25 mg/d after bariatric surgery and weight loss. Insulin resistance also improved, and liver fat decreased from 32% before surgery to 5.3% at 9 months after surgery (Fig. 3). Reductions were noted in both subcutaneous and visceral fat estimates. Visceral fat decreased from 900 mL before surgery to 224 mL 9 months after surgery, and subcutaneous fat decreased from 2850 mL before surgery to 1070 mL 9 months after surgery.
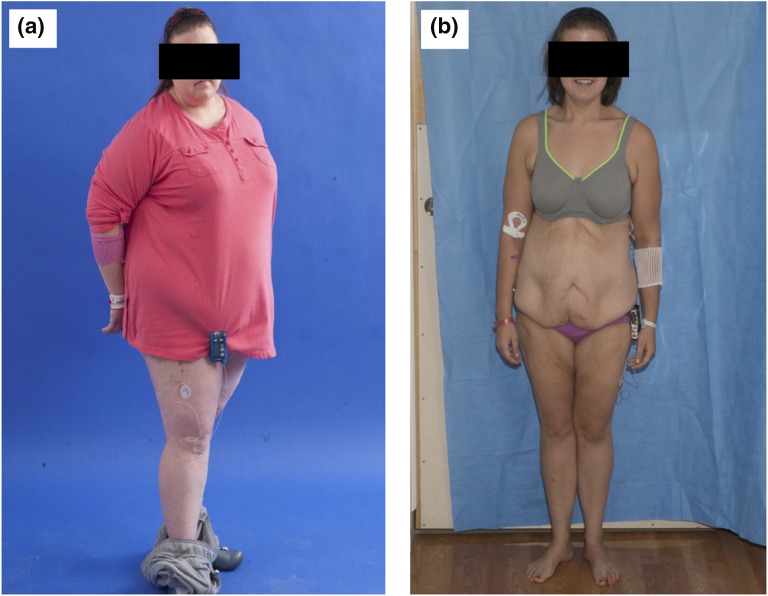
Figure 1.
Drastic weight change noted after bariatric surgery (sleeve gastrectomy). (a) Before surgery. (b) Fifteen months after surgery.
Table 1.
Anthropometrics Measures, Insulin Sensitivity, and Hydrocortisone Pharmacokinetics Before and After Bariatric Surgery
| Variable | Presurgery | 9 Months After Surgery | 15 Months After Surgery |
|---|---|---|---|
| Weight, kg | 126.9 | 83.9 (−34) | 66.3 (− 48) |
| BMI, kg/m2 | 52.6 | 34.5 (−34) | 27.1 (− 48) |
| Waist circumference, cm | 135.9 | 101.5 (− 25) | 100 (−26) |
| Waist/hip ratioa | 0.94 | 1 (6) | 0.8 (−15) |
| HOMA-IR | 5.7 | 1.2 (−79) | 1.2 (−79) |
| Cortisol half-life, min | 94.9 | 94.9 (0) | 120.2 (27) |
| Cortisol AUC0-inf, min × µg/dL | 15653 | 19402 (24) | 27261 (74) |
| Cortisol volume of distribution, dL | 875 | 706 (−19) | 636 (−27) |
| Cortisol clearance, mL/min | 639 | 515 (−19) | 367 (−43) |
Percentage change from presurgery is indicated in parentheses. Conversion factors for cortisol pharmacokinetic parameters: half-life, min × 6 = h; AUC 0-inf: min × µg/dL × 6 = h × ng/mL; volume of distribution, dL × 10 = L; clearance, mL/min × 0.06 = L/hr. AUC0-inf = cortisol area under the curve concentration vs. time curve from time 0 minutes to infinity; HOMA-IR, homeostasis model assessment insulin resistance.
Normal waist/hip ratio for women < 0.85.
Figure 2.
BMI and daily glucocorticoid dose before and after bariatric surgery.
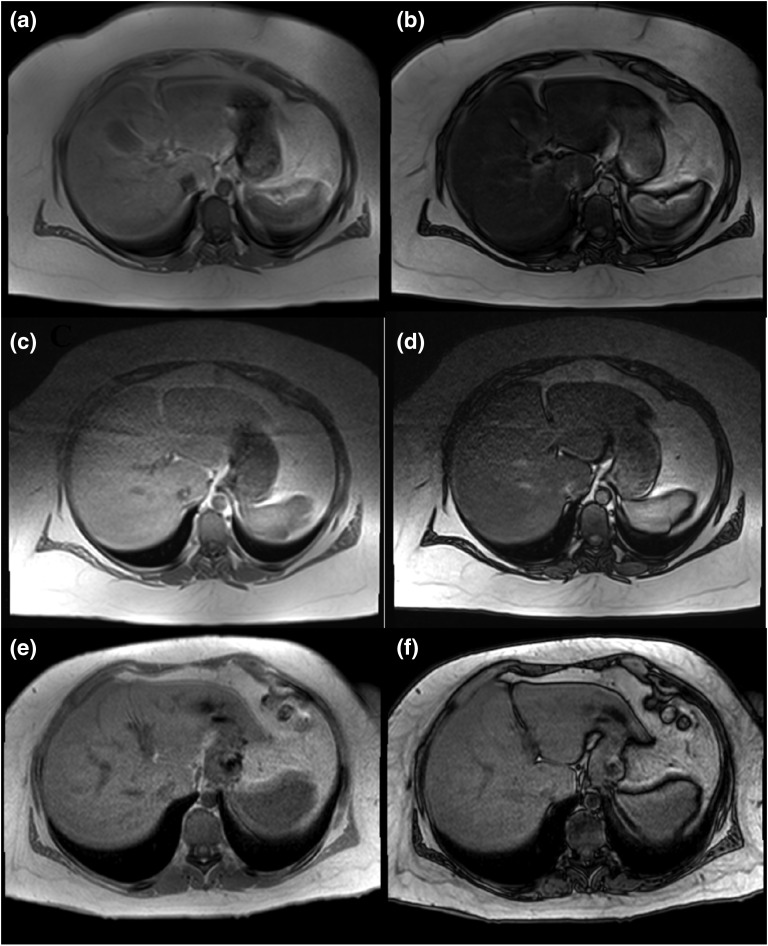
Figure 3.
MRS images before and after bariatric surgery. In-phase (a, c, e) and out-of-phase (b, d, f) images are shown at 9 months before surgery (a and b), 3 months before surgery (c and d), and 9 months after surgery (e and f). Substantial signal drop in the liver in out-of-phase images (b and d) indicates severe liver steatosis before surgery. Liver fat was 32.5% 9 months before surgery (b) and 31.6% 3 months before surgery (d) and decreased to 5.3% 9 months after surgery (f).
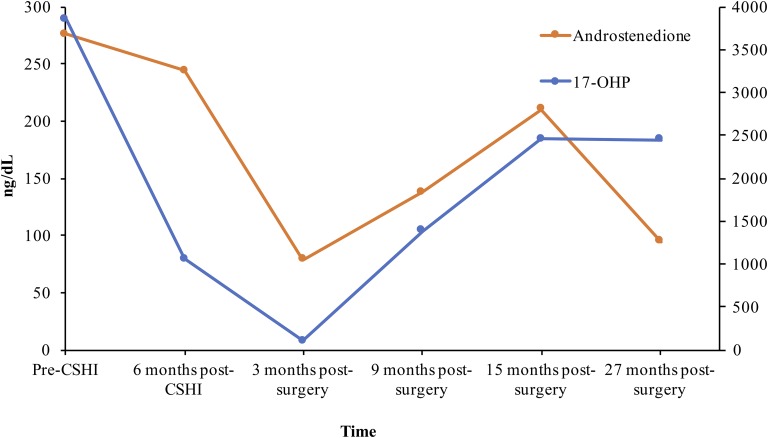
Her weight has remained stable 27 months after surgery (recent BMI, 26.1 kg/m2). She continues on CSHI therapy for her CAH management, with acceptable adrenal steroid control achieved with 17-OHP level of 2448 ng/dL (74.2 nmol/L) and normal androstenedione level of 98 ng/dL (3.4 nmol/L [reference range, 17 to 175 ng/dL and 0.6 to 6.1 nmol/L]) (Fig. 4).
Figure 4.
Early-morning adrenal steroids on CSHI before and after bariatric surgery. Conversion factors: 17-OHP, ng/dL × 0.0303 = nmol/L; androstenedione, ng/dL× 0.0349 = nmol/L. Reference range: androstenedione, 17 to 175 ng/dL (0.6 to 6.1 nmol/L).
4. Discussion
Our patient demonstrates the effect of obesity-related comorbidities on glucocorticoid pharmacokinetics, which, in turn, affects the clinical management of CAH. Her substantial weight loss in the postbariatric period was associated with alterations in hydrocortisone pharmacokinetics, including dramatic decreases in volume of distribution and cortisol clearance and an increase in cortisol AUC. These pharmacokinetic changes explain the ability to maintain optimal adrenal androgen control with concurrent reductions in hydrocortisone dose.
Metabolic syndrome can alter cortisol metabolism. Cortisol clearance inversely correlates with insulin sensitivity, and the presence of fatty liver has also been associated with increased cortisol clearance [6, 7]. Although obesity, insulin resistance, and fatty liver often occur together, increased cortisol clearance is associated with decreased insulin sensitivity, independent of body fat and fatty liver [6]. Changes in cortisol-cortisone metabolism in the liver, adipose tissue, and skeletal muscle have been implicated in the pathogenesis of nonalcoholic fatty liver disease, and tissue-specific dysregulation of glucocorticoid metabolism in the liver and fat may link insulin resistance and fatty liver with obesity [7, 8]. Differential expression of enzymes involved in glucocorticoid metabolism and action, the A-ring reductases (5α and 5β-reductase) in the liver, and 11β-hydroxysteroid dehydrogenase type 1 activity in the liver and adipose tissue contributes to the development of metabolic syndrome and may have played a role in altering hydrocortisone pharmacokinetics following this patient’s drastic weight loss [7, 8]. Our patient had substantial improvement in both insulin sensitivity and liver fat content. Thus, increased insulin sensitivity and decreased liver fat likely contributed to the alterations seen in the hydrocortisone pharmacokinetics.
Data regarding hydrocortisone pharmacokinetics and cortisol clearance in healthy persons is limited, with studies limited by small sample size and mostly restricted to normal-weight males [6, 9]. Pharmacokinetic parameters in our patient showed that cortisol half-life was similar to these published norms before surgery and 9 and 15 months after surgery. However, compared with published norms, our patient’s cortisol clearance was approximately 1.7 times and 1.4 times higher before surgery and 9 months after surgery, respectively, corresponding to her obese state and associated high Vd. With further weight loss at 15 months after surgery and a decrease in Vd, the cortisol clearance decreased and was similar to published norms for healthy persons [9].
Although patients with obesity have normal circulating cortisol, alteration in the hypothalamic–pituitary–adrenal (HPA) axis responsiveness has been described, with increased adrenal drive [10]. There is a close relationship between cortisol clearance and activation of the HPA axis. Increased clearance activates the HPA axis, and, in patients with CAH, this would result in increased adrenal androgen secretion. Thus, obese patients with CAH and metabolic syndrome may require higher doses of glucocorticoid to effectively suppress adrenal androgen secretion. These higher glucocorticoid doses may in turn worsen metabolic syndrome. Although diurnal glucocorticoid replacement may allow for improved ACTH-mediated adrenal androgen production, this improvement may be limited by other factors, especially in obese patients with metabolic syndrome. Our patient is an example of this management dilemma.
Individuals with classic CAH require life-long glucocorticoid therapy. Current available glucocorticoid preparations used in CAH management fall short of replicating diurnal cortisol secretion. Although novel therapeutic approaches aiming to provide physiologic cortisol replacement promise to improve disease- and treatment-related comorbidities, they are unlikely to mitigate long-standing established comorbidities.
Overall, our case highlights several challenges in the management of patients with classic CAH. Non-CAH patient factors, including obesity-related comorbidities, may greatly influence glucocorticoid pharmacokinetics and hence the hormonal management. Early intervention and obesity prevention are needed. However, in the setting of long-standing obesity, insulin resistance, fatty liver, and difficult-to-manage CAH, bariatric surgery is a safe and effective treatment that should be considered.
Acknowledgments
We thank the patient for participating in our National Institutes of Health study.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health (National Institutes of Health), Bethesda, Maryland. Deborah P. Merke received unrelated funding from Diurnal Limited and Millendo Therapeutics through the National Institutes of Health Cooperative Research and Development Agreement and is a Commissioned Officer in the U.S. Public Health Service.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 17-OHP
- 17-hydroxyprogesterone
- AUC
- area under the concentration vs. time curve
- BMI
- body mass index
- CAH
- congenital adrenal hyperplasia
- CSHI
- continuous subcutaneous hydrocortisone infusion
- HPA
- hypothalamic–pituitary–adrenal
- MRS
- magnetic resonance spectroscopy
- Vd
- volume of distribution.
References and Notes
- 1.Speiser PW, Azziz R, Baskin LS, Ghizzoni L, Hensle TW, Merke DP, Meyer-Bahlburg HF, Miller WL, Montori VM, Oberfield SE, Ritzen M, White PC; Endocrine Society . Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(9):4133–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallappa A, Sinaii N, Kumar P, Whitaker MJ, Daley LA, Digweed D, Eckland DJ, Van Ryzin C, Nieman LK, Arlt W, Ross RJ, Merke DP. A phase 2 study of Chronocort, a modified-release formulation of hydrocortisone, in the treatment of adults with classic congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100(3):1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nella AA, Mallappa A, Perritt AF, Gounden V, Kumar P, Sinaii N, Daley LA, Ling A, Liu CY, Soldin SJ, Merke DP. A phase 2 study of continuous subcutaneous hydrocortisone infusion in adults with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2016;101(12):4690–4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falhammar H, Frisén L, Hirschberg AL, Norrby C, Almqvist C, Nordenskjöld A, Nordenström A. Increased cardiovascular and metabolic morbidity in patients with 21-hydroxylase deficiency: a Swedish population-based national cohort study. J Clin Endocrinol Metab. 2015;100(9):3520–3528. [DOI] [PubMed] [Google Scholar]
- 5.Bryan SM, Honour JW, Hindmarsh PC. Management of altered hydrocortisone pharmacokinetics in a boy with congenital adrenal hyperplasia using a continuous subcutaneous hydrocortisone infusion. J Clin Endocrinol Metab. 2009;94(9):3477–3480. [DOI] [PubMed] [Google Scholar]
- 6.Holt HB, Wild SH, Postle AD, Zhang J, Koster G, Umpleby M, Shojaee-Moradie F, Dewbury K, Wood PJ, Phillips DI, Byrne CD. Cortisol clearance and associations with insulin sensitivity, body fat and fatty liver in middle-aged men. Diabetologia. 2007;50(5):1024–1032. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed A, Rabbitt E, Brady T, Brown C, Guest P, Bujalska IJ, Doig C, Newsome PN, Hubscher S, Elias E, Adams DH, Tomlinson JW, Stewart PM. A switch in hepatic cortisol metabolism across the spectrum of non alcoholic fatty liver disease. PLoS One. 2012;7(2):e29531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrew R, Phillips DI, Walker BR. Obesity and gender influence cortisol secretion and metabolism in man. J Clin Endocrinol Metab. 1998;83(5):1806–1809. [DOI] [PubMed] [Google Scholar]
- 9.Tromm A, Möllmann H, Barth J, Hochhaus G, Krieg M, Bigalke C, Möllmann A, Derendorf H. Pharmacokinetics and rectal bioavailability of hydrocortisone acetate after single and multiple administration in healthy subjects and patients. J Clin Pharmacol. 2001;41(5):536–541. [DOI] [PubMed] [Google Scholar]
- 10.Pasquali R. The hypothalamic-pituitary-adrenal axis and sex hormones in chronic stress and obesity: pathophysiological and clinical aspects. Ann N Y Acad Sci. 2012;1264:20–35. [DOI] [PMC free article] [PubMed] [Google Scholar]