Abstract
Context:
Hypothalamic proopiomelanocortin (POMC) is processed to α-melanocyte-stimulating hormone, which interacts with the melanocortin antagonist agouti-related protein (AgRP), to regulate energy balance. The POMC-derived opioid peptide β-endorphin (β-EP) also affects feeding behavior via interactions with brain µ-opioid receptors (MORs), including autoinhibitory interactions with MOR expressed by POMC neurons. The opioid antagonist naltrexone (NTX) stimulates POMC neurons in rodents and decreases food intake.
Objective and Design:
The effect of NTX on brain POMC in humans was assessed by measuring POMC peptide concentrations in lumbar cerebrospinal fluid (CSF). AgRP and cortisol levels were also measured because both are inhibited by opioids. In a double-blinded crossover study, 14 healthy subjects were given NTX (50 mg daily) or placebo for either 2 or 7 days.
Results:
CSF β-EP levels increased after 2 and 7 days of NTX treatment; CSF POMC levels did not change, but the β-EP-to-POMC ratio increased. CSF AgRP levels did not change, but plasma AgRP levels tended to increase after NTX (P = 0.06). Cortisol increased in plasma and CSF after NTX treatment; these changes correlated positively with changes in AgRP levels.
Conclusion:
Opioid antagonism stimulates POMC peptide release into CSF in humans. The increase in the CSF β-EP-to-POMC ratio could indicate selective release of processed peptides or an effect on POMC processing. Furthermore, AgRP and cortisol stimulation by NTX may mitigate POMC-induced decrease in food intake. It remains to be determined if biomarkers in CSF and plasma could be used to predict responses to pharmacotherapy targeting the melanocortin system.
Keywords: agouti-related protein, β-endorphin, cerebrospinal fluid, cortisol, proopiomelanocortin, naltrexone
CSF β-endorphin level increased after 2 and 7 days of naltrexone. CSF and plasma levels of cortisol increased and correlated positively with increases in AgRP, which may mitigate effects of naltrexone on food intake.
Endogenous opioid peptides, including β-endorphin (β-EP), dynorphins, and enkephalins, exert multiple effects on feeding behavior that are mediated by brain μ-, κ-, δ-opioid receptors, respectively [1–3]. These three classes of endogenous opioid peptides have distinct effects on food intake, nutrient selection, and reward. The role of β-EP, which interacts with µ-opioid receptors (MORs), in the regulation of energy balance has been extensively studied but is still not entirely understood.
When injected into the brain, β-EP has short-term stimulatory effects on food intake [4, 5]. β-EP can also interact with MORs expressed by hypothalamic proopiomelanocortin (POMC) and agouti-related protein (AgRP) neurons [6, 7] that play a key role in regulating energy balance [8–10]. POMC is a prohormone that is processed into several active peptides, including the melanocortin receptor (MC4-R) agonist α-melanocyte-stimulating hormone (α-MSH), which inhibits food intake and increases energy expenditure. AgRP is a MC4-R antagonist that increases food intake and inhibits energy expenditure. Deficiencies in POMC synthesis, peptide processing, or MC4-R signaling cause obesity in rodents and humans [11–13]. POMC is also processed to β-EP, which can antagonize the effects of α-MSH on food intake [4]. Electrophysiological studies show that MORs on POMC neurons function as autoinhibitory receptors in response to the release of β-EP [14]. β–EP has also been shown to inhibit AgRP neurons [15].
The opioid receptor antagonist naltrexone (NTX) has high affinity for MORs and can inhibit food intake. NTX has well-established stimulatory effects on POMC neurons, and it has been postulated that NTX decreases food intake in part by stimulating POMC-derived α-MSH release. In rodents, NTX acutely stimulates POMC peptide release and POMC mRNA levels subsequently increase [16–18]. This is accompanied by an increase in POMC prohormone levels in the hypothalamus and a decrease in hypothalamic α-MSH and β-EP content, resulting in a marked decline in the ratio of α-MSH and β-EP to POMC [18].
NTX has also been shown to decrease food intake in humans on a short-term basis, but when it is used alone, it has not been highly effective in producing weight loss [19]. However, effectiveness increases when NTX is used in combination with bupropion, a dopamine and norepinephrine reuptake inhibitor, and this is the basis for the new US Food and Drug Administration-approved weight loss combination drug Contrave (NTX/bupropion; Orexigen Therapeutics, La Jolla, CA) [20]. It has been postulated that stimulation of POMC by bupropion can be enhanced by combination therapy with NTX. This has been confirmed by electrophysiological studies showing that bupropion stimulates POMC neurons and that NTX potentiates this stimulation by blocking β-EP–mediated POMC autoinhibition [21].
Little is known about the effects of NTX on brain POMC in humans or why NTX alone does not have more robust effects on energy balance. In this study, we tested the hypothesis that the effects of NTX on brain POMC in human subjects could be assessed by measuring changes in cerebrospinal fluid (CSF) neuropeptide concentrations. Prior rodent studies have shown that CSF levels of POMC correlate with hypothalamic POMC mRNA levels, and we have shown that high levels of POMC are present in human CSF and may be a biomarker for hypothalamic POMC activity [22, 23]. We also hypothesized that NTX could induce changes in other neuropeptide and hormone systems that could attenuate the effects of POMC stimulation on energy balance, thus providing an explanation for the relatively mild effect of NTX on energy balance. Therefore, we measured AgRP levels in CSF and plasma, because NTX can stimulate AgRP mRNA levels in the rodent hypothalamus [18] and there is evidence that plasma AgRP may be a biomarker of brain AgRP activity [24]. Effects of NTX on cortisol were also studied, because endogenous opioids inhibit the hypothalamic-pituitary-adrenal (HPA) axis and stimulation of the HPA axis by NTX could attenuate effects of POMC stimulation on food intake [25, 26]. Accordingly, we have examined the effects of 2 and 7 days of NTX vs placebo treatment on POMC, β-EP, AgRP, and cortisol levels in CSF and on AgRP and cortisol levels in plasma in 14 healthy subjects. Effects of NTX on leptin, soluble leptin receptor (sOB-R), and insulin concentrations and on measures of hunger or satiety as assessed by the visual analog scale (VAS) were also studied.
1. Materials and Methods
A. Study Participants and Protocol
Fourteen healthy subjects, 10 men and four women, ranging from 19 to 47 years of age, completed the study. Women were studied in the early follicular phase of their menstrual cycle. There were eight lean, three overweight, and three obese participants (body mass index range, 18 to 36 kg/m2). An additional subject developed a mild headache after the first lumbar puncture (LP) and, per study protocol, did not undergo a second LP. All participants were nonsmokers, had no history of substance abuse, and were not taking medications. Subjects were excluded if they had any clinically significant medical condition or history of eating disorder or recent weight change ±5% over the previous 6 months. All participants had a screening visit to confirm their eligibility, which included a physical examination, screening blood work (i.e., complete blood cell count and metabolic panel), electrocardiogram, and urine toxicology to screen for opiate use. Pregnancy was ruled out by β-human chorionic gonadotropin test.
The study was a double-blinded, crossover study with seven subjects per group. The first group received 2 days of NTX or placebo in random order and the second group received 7 days of NTX or placebo in random order. The dosage of NTX was 50 mg daily at 9:00 pm. An LP was performed in the morning between 8:00 am and 10:00 am after a 12-hour overnight fast on day 3 in the first group and on day 8 in the second group. Then, 4 to 12 weeks later, subjects were crossed over from their original random assignment to receive either NTX or placebo for 2 or 7 days and had a second LP on day 3 or 8.
A blood sample was obtained and participants completed a VAS questionnaire at the time of the two LPs. All LPs were performed by one of the study authors (R.S.) using a 25-G Whitcare needle; the first 0.5 mL of CSF was discarded before collection of the 10-mL study sample. The procedure was well tolerated, as we have previously reported [27]. This study was approved by the Columbia University Institutional Review Board and written informed consent was obtained from all subjects before their participation.
B. Assays
POMC was measured by two-site enzyme-linked immunosorbent assay (ELISA) [22, 28]; there is no cross-reactivity with adrenocorticotropic hormone, α-MSH, or β-EP. Affinity-purified human 31K POMC was used for standards; it has a sensitivity of 8 fmol/mL. β-EP was measured with a newly developed two-site ELISA that is specific for β-EP and does not cross-react with β-lipotropin or POMC. This assay uses the same antibody used in the radioimmunoassay (RIA) for capture and a monoclonal antibody [MAB5276; Research Resource Identifier (RRID): AB_95197; Millipore, Temecula, CA] to met-enkephalin (N-terminal of β-EP) that was biotinylated for detection; it has a sensitivity of 2 pg/mL [24]. For comparison, β-EP was also measured by RIA as previously described, and has 3% cross-reactivity with POMC [29].
AgRP was measured by ELISA and RIA with relative specificities for full-length AgRP and AgRP83-132, respectively [22, 30]. The ELISA (R&D Systems, Minneapolis, MN) uses full-length human AgRP standard, which has 17% cross-reactivity on a weight basis with AgRP83-132. The RIA uses an antibody (RRID: AB_2686900) provided by Dr. G. Barsh (Hudson Alpha Institute for Biotechnology, Huntsville, AL) and human AgRP83-132 as the standard (Phoenix Pharmaceuticals, Burlingame, CA); it has 20% cross-reactivity with full-length AgRP on a weight basis.
Cortisol was measured in CSF by sensitive ELISA (Salimetrics, State College, PA) and in serum by chemiluminescent immunometric assay (Siemens Healthcare Diagnostics, Tarrytown, NY). The sensitivity of the Salimetrics ELISA is to 0.007 μg/dL. Leptin and sOB-R were measured in plasma and CSF by ELISA (R&D Systems) [22]. Insulin was measured by Immulite1000 (Siemens Healthcare Diagnostics).
C. Statistical Analysis
Data are expressed as mean ± standard error of the mean (SEM). CSF hormone and neuropeptide levels after placebo and NTX treatment were analyzed by paired t test or paired Wilcoxon signed-rank test. Correlations were determined by linear regression analysis using the Pearson correlation unless indicated differently for nonparametric analysis. Percent changes in hormone and neuropeptide levels after NTX vs placebo were compared in lean and overweight or obese (OW/OB) subjects and analyzed by unpaired t test. Analyses were performed using Prism 6.0 (GraphPad Software, La Jolla, CA).
2. Results
A. Effects of NTX on Leptin and Insulin Levels, Body Weight, and VAS
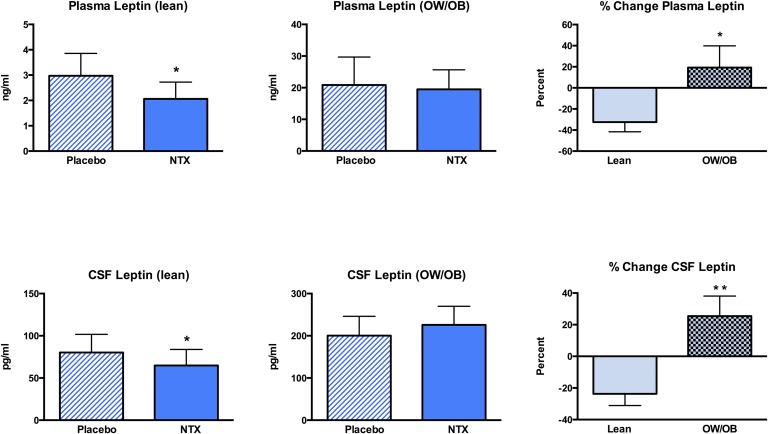
Naltrexone was well tolerated. There were no significant changes in body weight, serum insulin levels, plasma sOB-R levels, or in measures of hunger or satiety as assessed by the VAS (Table 1). Concentrations of leptin in plasma and CSF did not change significantly in the group as a whole (Table 1). However, plasma and CSF leptin levels decreased significantly in lean subjects after NTX treatment (P = 0.04), but there was no significant change in the OW/OB subjects (Fig. 1). When the percent changes in plasma and CSF leptin levels were compared in the lean and OW/OB groups, there were significant differences in the response of both plasma (P = 0.03) and CSF (P = 0.004) leptin to NTX in the lean vs OW/OB groups (Fig. 1).
Table 1.
Effects of NTX vs Placebo on Body Mass Index, Hormone Levels, and Measures of Hunger and Satiety
| Placebo (n = 14) | NTX (n = 14) | P Value | |
|---|---|---|---|
| Body mass index, kg/m2 | 25.8 ± 1.3 | 25.9 ± 1.3 | 0.99 |
| Serum insulin, μIU/mL | 10.6 ± 2.3 | 12.1 ± 2.5 | 0.68 |
| Plasma leptin, ng/mL | 10.6 ± 4.4 | 9.5 ± 3.5 | 0.66 |
| Plasma sOB-R, ng/mL | 23.0 ± 1.5 | 24.6 ± 1.7 | 0.06 |
| CSF leptin, pg/mL | 132 ± 27 | 134 ± 30 | 0.86 |
| VAS | |||
| Hunger | 5.51 ± 0.78 | 4.65 ± 0.79 | 0.44 |
| Satiety | 4.91 ± 0.66 | 5.59 ± 0.60 | 0.46 |
Data given as mean ± standard error of the mean.
Figure 1.
Mean ± SEM changes in (upper panels) plasma and (lower panels) CSF leptin levels after placebo or NTX treatment in lean and OW/OB subjects. (right-side panels) The percent changes in plasma and CSF leptin levels after NTX treatment vs placebo were significantly different in the lean vs the OW/OB groups. *P < 0.05; **P < 0.01.
B. Effects of NTX on POMC and β-EP Levels in CSF
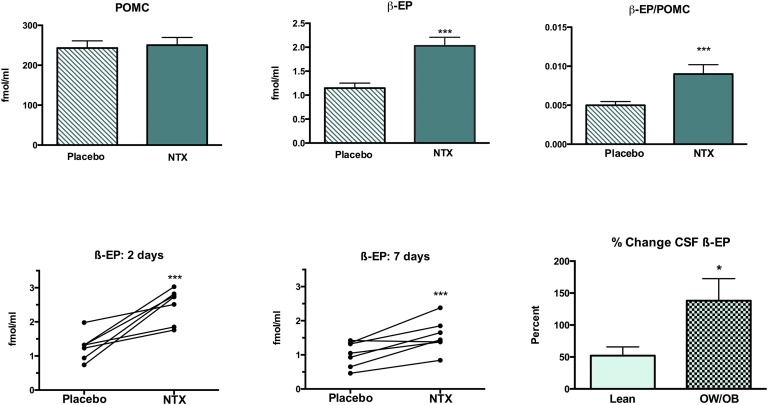
The mean concentration of POMC in CSF did not change significantly in the group as a whole (n=14) after receiving NTX (250 ± 19.3 fmol/mL) compared with placebo (243 ± 18 fmol/mL; Fig. 2). No differences in CSF POMC were noted at either the 2- or 7-day points or when responses to NTX by lean vs OW/OB subjects were compared. In contrast, the concentration of β-EP in CSF increased almost twofold, from 1.15 ± 0.10 fmol/mL to 2.03 ± 0.18 fmol/mL, after NTX, and the β-EP-to-POMC molar ratio increased by 80% (P < 0.001; Fig. 2). Consistent increases in CSF β-EP levels were seen after both 2 and 7 days of NTX treatment (Fig. 2). CSF β-EP levels increased after NTX treatment in both the lean and OW/OB subjects, but the percent increase was significantly greater in the OW/OB group (138% ± 35%) compared with the lean subjects (52.1% ±14%; P = 0.02). A significant increase in CSF β-EP levels after NTX treatment was also noted when β-EP level was measured by the less-specific RIA; CSF levels increased from 22.6 ± 2.1 fmol/mL to 27.6 ± 2.8 fmol/mL (P = 0.005).
Figure 2.
Mean ± SEM CSF POMC and β-EP levels and β-EP-to-POMC ratio after placebo or NTX treatment (upper panels). Individual changes in CSF β-EP levels after placebo vs NTX treatment for (lower left panel) 2 days or (lower middle panel) 7 days. (lower right panel) The percent change in CSF β-EP levels after NTX treatment was greater in the OW/OB than the lean subjects. *P < 0.05; ***P < 0.001.
C. Effects of NTX on AgRP Levels in CSF and Plasma
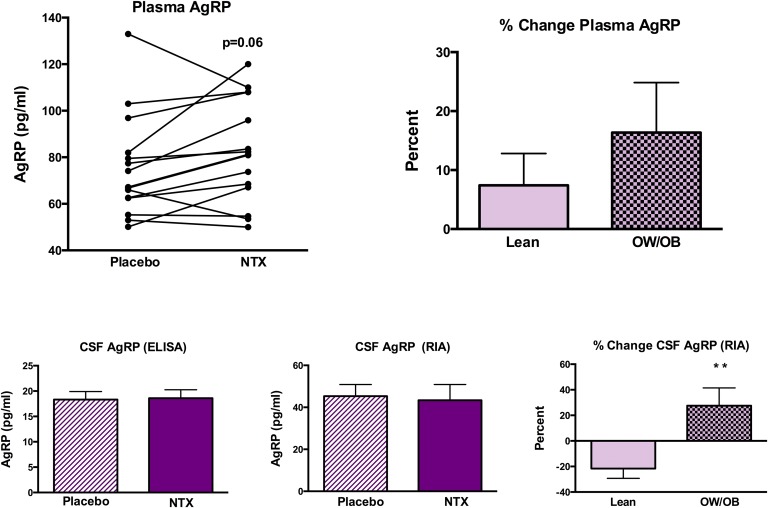
Plasma AgRP levels (measured by ELISA, which is more specific for full-length AgRP) tended to increase after NTX treatment but the change was not statistically significant (P = 0.06; Fig. 3). The percent change in plasma AgRP was not different in lean (7.4% ± 5.4%) vs OW/OB (16.4% ± 8.5%) subjects. There was no change in CSF AgRP levels measured by ELISA after NTX treatment, and percent change was not different between lean and OW/OB subjects. There was no change in CSF AgRP levels measured by RIA (which is more specific for AgRP83-132) in the group as a whole. However, a distinct difference was noted when the percent change in CSF AgRP level was compared between lean and OW/OB subjects (Fig. 3). CSF AgRP levels decreased by 21.6% ± 14% after NTX treatment in the lean subjects, whereas it increased by 27.5% ± 14% in the OW/OB subjects (P = 0.006). There was a significant correlation between the percent change in CSF AgRP level (measured by RIA) and the percent changes in CSF β-EP level (r = 0.601; P = 0.02) and leptin (r = 0.582; P = 0.03).
Figure 3.
Individual changes in plasma AgRP levels after placebo and NTX treatments (upper left panel). (upper right panel) Percent change in plasma AgRP levels after NTX vs placebo treatment in lean vs OW/OB subjects. Mean ± SEM CSF AgRP level after placebo or NTX treatment measured by (lower left panel) ELISA or (lower middle panel) RIA. (lower right panel) The percent change in CSF AgRP level measured by RIA was significantly greater in the OW/OB than the lean subjects. **P < 0.01.
D. Effects of NTX on Plasma and CSF Cortisol Levels
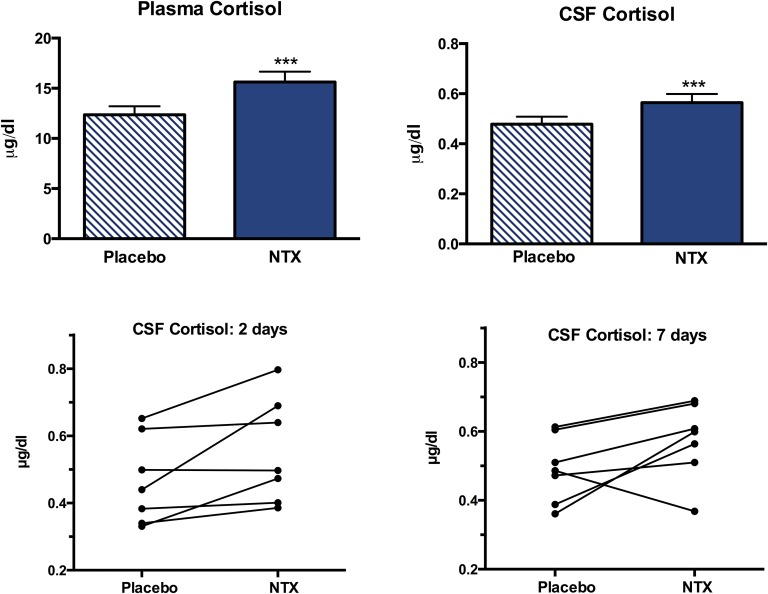
Plasma cortisol levels increased from 12.4 ± 0.85 µg/dL to 15.6 ± 1.0 µg/dL after NTX treatment (P = 0.006) and CSF cortisol increased from 0.479 ± 0.03 µg/dL to 0.564 ± 0.03 µg/dL (P = 0.006; Fig. 4). Overall plasma cortisol and CSF cortisol concentrations increased by 28% and 18%, respectively, after NTX treatment. Similar increases were detected at the 2- and 7-day points, but, when analyzed separately, the increase in plasma cortisol level was only significant at 7 days and the increase in CSF cortisol level was only significant at 2 days. Individual changes in CSF cortisol after 2 and 7 days of NTX treatment are depicted in Figure 4.
Figure 4.
Mean ± SEM (upper left panel) plasma cortisol level and (upper right panel) CSF cortisol level after placebo or NTX treatment. Individual changes in CSF cortisol levels after placebo vs NTX treatment for (lower left panel) 2 days or (lower right panel) 7 days. ***P = 0.006.
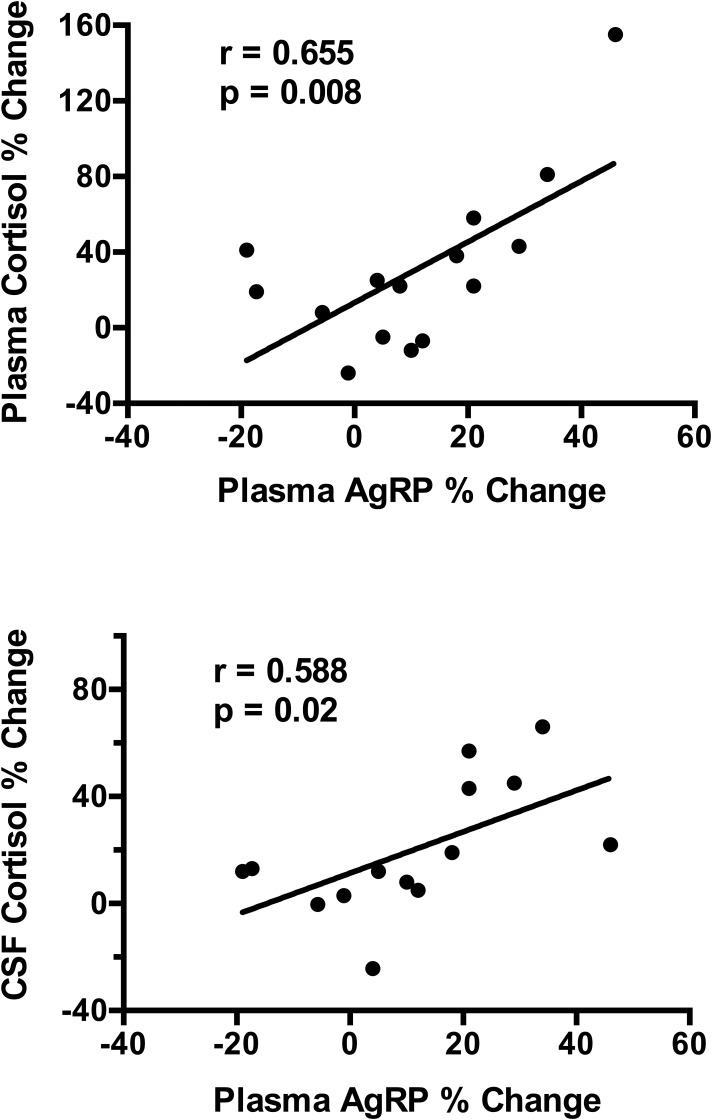
The increases in plasma and CSF cortisol levels were similar when lean and OW/OB subjects were compared. The correlation between the changes in plasma and CSF cortisol levels was r = 0. 715 (P = 0.005 for Spearman correlation). There was a significant positive correlation between the changes in plasma AgRP levels and changes in plasma cortisol levels (r = 0.655; P = 0.008) and CSF cortisol levels (r = 0.588; P = 0.02) after NTX treatment (Fig. 5). In contrast, there were no significant correlations between changes in CSF AgRP levels measured by either ELISA (r = 0.310; P = 0.28) or RIA (r = 0.200; P = 0.49), and changes in plasma or CSF cortisol levels.
Figure 5.
Correlations of the percent change in plasma AgRP levels with the percent change in plasma and CSF cortisol levels.
3. Discussion
Using CSF neuropeptide measurements as a surrogate for brain POMC activity, this study examined the effects of NTX treatment in humans. Significant changes in POMC-derived peptide levels were demonstrated that parallel some of the effects of NTX on brain POMC in rodents. Furthermore, changes in AgRP and cortisol levels were also demonstrated after NTX treatment that could mitigate the effects of POMC stimulation on energy balance. In addition, there is some evidence that the neuropeptide and hormone responses to opioid antagonism differs in lean vs OW/OB individuals.
CSF β-EP levels increased after both 2 and 7 days of NTX treatment, but CSF POMC levels did not change. This is consistent with rodent studies that showed acute stimulatory effects of NTX on β-EP release, whereas stimulation of POMC mRNA occurred after 7 days [16, 17]. Thus, it may be that longer duration of treatment with NTX could lead to an increase in CSF POMC levels. The ratio of β-EP to POMC in CSF increased after NTX treatment by 80% compared with placebo. This is the inverse of the changes noted in the rodent hypothalamus, where levels of β-EP and the ratio of β-EP to POMC fall after NTX treatment [18]. This could indicate selective release of the processed peptide or an effect on POMC processing. CSF β-EP levels increased in both lean and obese subjects, but the percent increase was higher in the obese vs the lean group, consistent with increased endogenous opioid inhibition of POMC neurons in obese subjects. Of note, there is evidence that brain MOR availability is altered in obese subjects [31]. Although it is α-MSH that interacts with brain MC4-Rs, we were unable to reliably detect α-MSH in CSF with a highly specific assay, possibly due to α-MSH degradation or inactivation by the enzyme prolylcarboxypeptidase [32]. Our α-MSH assay is specific for the 13 amino-acid, amidated peptide and does not detect the 12 amino-acid, inactivated peptide. However, prior rodent studies have shown that hypothalamic β-EP and α-MSH levels change in parallel in response to NTX [18]. Thus, it is likely that the NTX-induced changes in CSF β-EP levels reflect changes in both hypothalamic β-EP and α-MSH levels. However, the inability to directly assess α-MSH levels is a weakness of the study.
Despite the stimulatory effects of NTX on POMC in animals and on POMC peptide release in humans, as documented by the current study, effects on feeding and body weight are modest. Therefore, we examined other potential NTX-induced changes that could attenuate the POMC-mediated effects on energy balance. Effects of NTX on AgRP levels in CSF and plasma were examined because there is evidence that β–EP can also inhibit AgRP neurons [15]. Plasma AgRP levels (measured by ELISA) tended to increase after NTX treatment, but the change was not significant (P = 0.06). Changes were similar in lean and obese subjects. There was no change in CSF AgRP levels measured by ELISA or RIA in the group as a whole. However, a distinct difference was noted when the percent change in CSF AgRP levels measured by RIA was compared in the lean vs OW/OB subjects. CSF AgRP levels decreased by 21.6% after NTX treatment in the lean subjects compared with an increase of 27.5% in the OW/OB subjects. Of note, AgRP83-132 possesses more biological activity than the full-length peptide [33]. We have confirmed by high-performance liquid chromatography that both forms of AgRP are present in CSF [22, 30]. Thus, in the obese subjects in the current study, there was a more robust increase in AgRP as well as in β-EP after NTX treatment.
Effects of NTX treatment on plasma and CSF cortisol levels were also studied because endogenous opioids inhibit the HPA axis and stimulation of the HPA axis by NTX could also limit the effects of NTX on food intake [25, 26]. Plasma and CSF cortisol levels increased after 2 and 7 days of NTX treatment. Endogenous opioids inhibit the HPA axis in rodents via inhibition of hypothalamic corticotropin-releasing hormone [25], and opioid antagonism results in stimulation of the HPA axis [26]. In humans, NTX and other opioid antagonists have been shown to stimulate adrenocorticotropic hormone and cortisol release, but this has only been studied acutely after a single dose [34]. We now report that this increase persisted at 7 days and was accompanied by a significant increase in CSF cortisol levels. The increases in plasma and CSF cortisol levels were similar when lean and OW/OB subjects were compared. This longer-term increase in plasma cortisol levels and the increase in CSF cortisol levels may impact energy balance and could be another factor that mitigates the effects of POMC stimulation by NTX on food intake. Of note, there was a significant positive correlation between the changes in plasma AgRP levels with changes in both plasma cortisol and CSF cortisol levels after NTX treatment. This positive correlation is consistent with prior animal studies showing that corticosterone stimulates AgRP gene expression in the rodent hypothalamus [35]. We have also observed that plasma AgRP levels correlate with hypothalamic AgRP gene expression in rats (unpublished data) and increase in humans with elevated cortisol levels and Cushing disease [36]. Although AgRP levels were measured in both CSF and plasma, only changes in plasma AgRP levels correlated with changes in cortisol. The explanation for this is unclear but may relate to anatomical differences in AgRP fiber tracks that gain access to CSF and blood [37, 38]. Emerging evidence suggests there are distinct populations of AgRP neurons within the arcuate and one of these populations is outside the blood-brain barrier [39]. It is possible that the population that resides outside the blood-brain barrier is the source of AgRP in blood.
There were no changes in plasma and CSF leptin levels in the study groups as a whole, but both plasma and CSF leptin levels decreased significantly in the lean subjects. The percent changes in leptin levels were significantly different when the lean and obese groups were compared. The decreased leptin levels in the lean group may maintain energy balance in the setting of POMC stimulation. Food intake was not documented in this study, but subjects were instructed to maintain their current level of food intake and exercise. In addition, no significant differences on VAS assessing hunger and satiety between NTX and placebo treatments were noted. No correlations between VAS ratings and neuropeptide levels were noted.
In summary, opioid antagonism with NTX stimulates POMC peptide release into CSF in humans, consistent with the effects on brain POMC peptide release in rodents. No effect on POMC prohormone levels was seen after 1 week. The increase in the CSF β-EP-to-POMC ratio is consistent with selective release of the processed peptide or an effect on POMC processing. Furthermore, a potential stimulatory effect of NTX on AgRP levels and the persistent increase in both plasma and CSF cortisol levels may mitigate the stimulatory effects on POMC peptide release with respect to decreasing food intake. It remains to be determined if biomarkers in CSF and plasma could be used to predict responses to obesity pharmacotherapy targeting the melanocortin system.
Acknowledgments
We thank the study subjects for their participation and the staff of the Irving Institute for Clinical and Translational Research.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant RO1-DK093920 (S.L.W.); National Center for Advancing Translational Sciences NIH Grants UL1 TR000040 and T32DK065522 (R.J.G.); the Russell Berrie Foundation Naomi Berrie Fellowship (D.A.) and the Atkins Foundation (S.L.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AgRP
- agouti-related protein
- α-MSH
- α-melanocyte-stimulating hormone
- β-EP
- β-endorphin
- CSF
- cerebrospinal fluid
- ELISA
- enzyme-linked immunosorbent assay
- HPA
- hypothalamic-pituitary-adrenal
- LP
- lumbar puncture
- MC4-R
- melanocortin receptor
- MOR
- µ-opioid receptor
- NTX
- naltrexone
- POMC
- proopiomelanocortin
- OW/OB
- overweight or obese
- RIA
- radioimmunoassay
- RRID
- Research Resource Identifier
- SEM
- standard error of the mean
- sOB-R
- soluble leptin receptor
- VAS
- visual analog scale.
References and Notes
- 1.Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev. 2002;26:713–728. [DOI] [PubMed] [Google Scholar]
- 2.Bodnar RJ. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25:697–725. [DOI] [PubMed] [Google Scholar]
- 3.Bodnar RJ. Endogenous opioids and feeding behavior: a decade of further progress (2004-2014). A Festschrift to Dr. Abba Kastin. Peptides. 2015;72:20–33. [DOI] [PubMed] [Google Scholar]
- 4.Dutia R, Meece K, Dighe S, Kim AJ, Wardlaw SL. beta-Endorphin antagonizes the effects of alpha-MSH on food intake and body weight. Endocrinology. 2012;153:4246–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grossman HC, Hadjimarkou MM, Silva RM, Giraudo SQ, Bodnar RJ. Interrelationships between mu opioid and melanocortin receptors in mediating food intake in rats. Brain Res. 2003;991:240–244. [DOI] [PubMed] [Google Scholar]
- 6.Bouret S, Prevot V, Croix D, Jegou S, Vaudry H, Stefano GB, Beauvillain JC, Mitchell V. Mu-opioid receptor mRNA expression in proopiomelanocortin neurons of the rat arcuate nucleus. Brain Res Mol Brain Res. 1999;70:155–158. [DOI] [PubMed] [Google Scholar]
- 7.Barnes MJ, Argyropoulos G, Bray GA. Preference for a high fat diet, but not hyperphagia following activation of mu opioid receptors is blocked in AgRP knockout mice. Brain Res. 2010;1317:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee M, Wardlaw SL. The central melanocortin system and the regulation of energy balance. Front Biosci. 2007;12:3994–4010. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Elmquist JK, Fukuda M. Central nervous control of energy and glucose balance: focus on the central melanocortin system. Ann N Y Acad Sci. 2011;1243:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mercer AJ, Hentges ST, Meshul CK, Low MJ. Unraveling the central proopiomelanocortin neural circuits. Front Neurosci. 2013;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farooqi IS, O’Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab. 2008;4:569–577. [DOI] [PubMed] [Google Scholar]
- 12.Wardlaw SL. Hypothalamic proopiomelanocortin processing and the regulation of energy balance. Eur J Pharmacol. 2011;660:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. [DOI] [PubMed] [Google Scholar]
- 14.Kelly MJ, Loose MD, Ronnekleiv OK. Opioids hyperpolarize beta-endorphin neurons via mu-receptor activation of a potassium conductance. Neuroendocrinology. 1990;52:268–275. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaffe SB, Sobieszczyk S, Wardlaw SL. Effect of opioid antagonism on beta-endorphin processing and proopiomelanocortin-peptide release in the hypothalamus. Brain Res. 1994;648:24–31. [DOI] [PubMed] [Google Scholar]
- 17.Markowitz CE, Berkowitz KM, Jaffe SB, Wardlaw SL. Effect of opioid receptor antagonism on proopiomelanocortin peptide levels and gene expression in the hypothalamus. Mol Cell Neurosci. 1992;3:184–190. [DOI] [PubMed] [Google Scholar]
- 18.Panigrahi SK, Meece K, Wardlaw. SL. Effects of naltrexone on energy balance and hypothalamic melanocortin peptides in response to a high fat diet. 99th Annual Meeting of the Endocrine Society; April 1–4, 2017; Orlando, FL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiegel TA, Stunkard AJ, Shrager EE, O’Brien CP, Morrison MF, Stellar E. Effect of naltrexone on food intake, hunger, and satiety in obese men. Physiol Behav. 1987;40:135–141. [DOI] [PubMed] [Google Scholar]
- 20.Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, Cowley MA. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab. 2009;94:4898–4906. [DOI] [PubMed] [Google Scholar]
- 21.Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K, Gadde KM, Gupta AK, O’Neil P, Schumacher D, Smith D, Dunayevich E, Tollefson GD, Weber E, Cowley MA. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring). 2009;17:30–39. [DOI] [PubMed] [Google Scholar]
- 22.Page-Wilson G, Meece K, White A, Rosenbaum M, Leibel RL, Smiley R, Wardlaw SL. Proopiomelanocortin, agouti-related protein, and leptin in human cerebrospinal fluid: correlations with body weight and adiposity. Am J Physiol Endocrinol Metab. 2015;309:E458–E465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pritchard LE, Oliver RL, McLoughlin JD, Birtles S, Lawrence CB, Turnbull AV, White A. Proopiomelanocortin-derived peptides in rat cerebrospinal fluid and hypothalamic extracts: evidence that secretion is regulated with respect to energy balance. Endocrinology. 2003;144:760–766. [DOI] [PubMed] [Google Scholar]
- 24.Page-Wilson G, Nguyen KT, Atalayer D, Meece K, Bainbridge HA, Korner J, Gordon RJ, Panigrahi SK, White A, Smiley R, Wardlaw SL. Evaluation of CSF and plasma biomarkers of brain melanocortin activity in response to caloric restriction in humans. Am J Physiol Endocrinol Metab. 2017;312:E19–E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plotsky PM. Opioid inhibition of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation of rats. Regul Pept. 1986;16:235–242. [DOI] [PubMed] [Google Scholar]
- 26.Geer EB, Landman RE, Wardlaw SL, Conwell IM, Freda PU. Stimulation of the hypothalamic-pituitary-adrenal axis with the opioid antagonist nalmefene. Pituitary. 2005;8:115–122. [DOI] [PubMed] [Google Scholar]
- 27.Page-Wilson G, Wardlaw SL, Nguyen KT, Smiley RM. Evaluation of pain and stress in healthy volunteers undergoing research lumbar punctures. Neurology. 2016;87:438–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stovold R, Meredith SL, Bryant JL, Babur M, Williams KJ, Dean EJ, Dive C, Blackhall FH, White A. Neuroendocrine and epithelial phenotypes in small-cell lung cancer: implications for metastasis and survival in patients. Br J Cancer. 2013;108:1704–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlachter LB, Wardlaw SL, Tindall GT, Frantz AG. Persistence of beta-endorphin in human cerebrospinal fluid after hypophysectomy. J Clin Endocrinol. 1983;57:221–224. [DOI] [PubMed] [Google Scholar]
- 30.Xiao E, Kim AJ, Dutia R, Conwell I, Ferin M, Wardlaw SL. Effects of estradiol on cerebrospinal fluid levels of agouti-related protein in ovariectomized rhesus monkeys. Endocrinology. 2010;151:1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burghardt PR, Rothberg AE, Dykhuis KE, Burant CF, Zubieta JK. Endogenous opioid mechanisms are implicated in obesity and weight loss in humans. J Clin Endocrinol Metab. 2015;100:3193–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, Gao XB, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S. Prolylcarboxypeptidase regulates food intake by inactivating alpha-MSH in rodents. J Clin Invest. 2009;119:2291–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Creemers JW, Pritchard LE, Gyte A, Le Rouzic P, Meulemans S, Wardlaw SL, Zhu X, Steiner DF, Davies N, Armstrong D, Lawrence CB, Luckman SM, Schmitz CA, Davies RA, Brennand JC, White A. Agouti-related protein is posttranslationally cleaved by proprotein convertase 1 to generate agouti-related protein (AGRP)83-132: interaction between AGRP83-132 and melanocortin receptors cannot be influenced by syndecan-3. Endocrinology. 2006;147:1621–1631. [DOI] [PubMed] [Google Scholar]
- 34.Lovallo WR, King AC, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Naltrexone effects on cortisol secretion in women and men in relation to a family history of alcoholism: studies from the Oklahoma Family Health Patterns Project. Psychoneuroendocrinology. 2012;37:1922–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savontaus E, Conwell IM, Wardlaw SL. Effects of adrenalectomy on AGRP, POMC, NPY and CART gene expression in the basal hypothalamus of fed and fasted rats. Brain Res. 2002;958:130–138. [DOI] [PubMed] [Google Scholar]
- 36.Page-Wilson G, Freda PU, Jacobs TP, Khandji AG, Bruce JN, Foo ST, Meece K, White A, Wardlaw SL. Clinical utility of plasma POMC and AgRP measurements in the differential diagnosis of ACTH-dependent Cushing’s syndrome. J Clin Endocrinol Metab. 2014;99:E1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. Anatomy of an endogenous antagonist: relationship between agouti- related protein and proopiomelanocortin in brain. J Neurosci. 1999;19:RC26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haskell-Luevano C, Chen P, Li C, Chang K, Smith MS, Cameron JL, Cone RD. Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology. 1999;140:1408–1415. [DOI] [PubMed] [Google Scholar]
- 39.Olofsson LE, Unger EK, Cheung CC, Xu AW. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proc Natl Acad Sci USA. 2013;110:E697–E706. [DOI] [PMC free article] [PubMed] [Google Scholar]