Abstract
Context:
Adrenal insufficiency (AI) is an important medical concern for clinicians when normocortisolemia is achieved during treatment of endogenous Cushing syndrome (CS).
Objective:
To examine symptoms of potential AI in a large population of normocortisolemic patients without CS treated with mifepristone, a glucocorticoid receptor antagonist indicated for the treatment of patients with CS.
Methods:
We conducted a pooled safety analysis of five phase 3, placebo-controlled clinical trials of normocortisolemic adults without CS but diagnosed with psychotic depression (n = 1460). Patients were treated with once-daily mifepristone 300 mg (n = 110), 600 mg (n = 471), or 1200 mg (n = 252), or placebo (n = 627) administered for 7 consecutive days. All study investigators were trained and instructed to assess for the development of AI and to report all adverse events (AEs) at each clinic visit. The incidence of (1) AI or similar terminologies and that of (2) ≥3 concurrent symptoms that could be associated with AI was evaluated.
Results:
Mean serum cortisol and adrenocorticotropic hormone levels increased dose dependently with mifepristone treatment. There were no reports of AI and no significant differences between the mifepristone-treated and placebo groups in the incidence of patients having ≥3 AEs that could be associated with AI.
Conclusions:
This large pooled analysis of normocortisolemic patients without CS found no cases of AI and no differences between mifepristone therapy and placebo in the incidence of symptom combinations mimicking AI, even at the highest (1200 mg) dose. These findings further add clinically important insights to the safety and tolerability profile of mifepristone therapy.
Keywords: mifepristone, Cushing syndrome, adrenal insufficiency, adrenal crisis, safety
A pooled analysis of five studies of mifepristone in normocortisolemic patients found no significant symptom combinations mimicking adrenal insufficiency reported vs placebo.
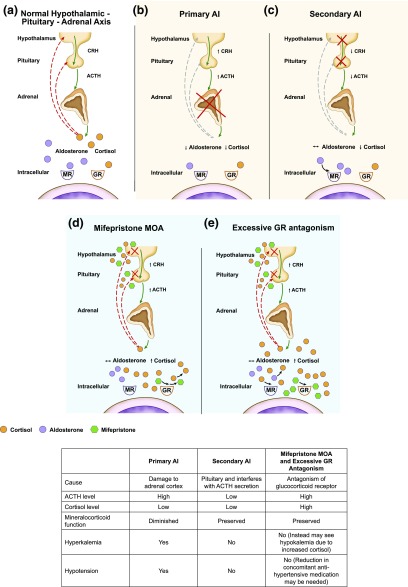
The development of adrenal insufficiency (AI), especially acute adrenal crisis, is a major medical concern following successful surgical treatment of endogenous Cushing syndrome (CS) and the subsequent attainment of normocortisolemia, and, as such, patients typically require glucocorticoid replacement therapy until the hypothalamic-pituitary-adrenal (HPA) axis [Fig. 1(a)] recovers. Effective medical treatment that achieves normocortisolemia in patients with CS can also increase susceptibility to developing AI, as seen with adrenal steroidogenesis inhibitors (e.g., ketoconazole, mitotane, metyrapone) [1–4] and drugs that inhibit adrenocorticotropic hormone (ACTH) secretion (e.g., pasireotide). Assessment of AI during medical treatment of CS is performed mainly on a clinical basis, and, when clinically suspected, may require temporary dose interruption and treatment with glucocorticoid therapy to prevent progression to adrenal crisis [5].
Figure 1.
(a) The HPA axis under normal physiological circumstances regulates the production of cortisol from the adrenals. Stimulation of the hypothalamus and pituitary to release CRH and ACTH leads to increased production of cortisol. Excess cortisol in turn provides inhibitory (negative) feedback at the hypothalamus and pituitary, leading to decreased release of CRH and ACTH and decreased production of cortisol. (b) In primary AI, damage to the adrenal glands leads to decreased production of cortisol and aldosterone; CRH and ACTH are increased due to ongoing production in the hypothalamus and pituitary and lack of negative feedback. (c) In secondary AI, there is damage to either the hypothalamus or pituitary, resulting in decreased release of CRH and/or ACTH, which leads to decreased production of cortisol. Mineralocorticoid (aldosterone) function is preserved. (d) and (e) Mifepristone competes with cortisol to bind to glucocorticoid receptors. By antagonizing these receptors in the hypothalamus and pituitary gland, mifepristone interferes with the feedback mechanism that regulates cortisol production, causing an increase in ACTH, which stimulates the production of more cortisol. Although excess antagonism of GR receptors can mimic features of AI, elevated cortisol levels and mineralocorticoid activation make AI unlikely. CRH, corticotropin-releasing hormone; GR, glucocorticoid receptor; MOA, mechanism of action; MR, mineralocorticoid receptor.
Primary AI occurs when the adrenal cortex is impaired or destroyed, such as by an infection, bleeding, or autoimmune disease (e.g., Addison disease), and is characterized by signs of both glucocorticoid and mineralocorticoid deficiency [6] [Fig. 1(b)]. Acute episodes of primary AI leading to adrenal crisis—a life-threatening medical emergency—are associated with laboratory abnormalities, including hyponatremia, hyperkalemia, and hypoglycemia, and signs and symptoms, including hypotension, severe weakness, anorexia, acute abdominal pain, nausea/vomiting, diarrhea, and confusion [7]. Secondary AI occurs when ACTH secretion (pituitary source) or corticotropin-releasing hormone (hypothalamus source) decreases, causing a reduction in adrenal cortisol production [6, 8] [Fig. 1(c)]. Unlike in primary AI, mineralocorticoid (aldosterone) function is preserved in secondary AI [6]. Therefore, some of the key electrolyte and clinical abnormalities (e.g., hyperkalemia, hypotension, hypovolemia, postural dizziness, salt craving) associated with mineralocorticoid deficiency are less likely to occur. The onset of secondary AI is usually more gradual than primary AI [6, 8]. Successful treatment of CS can also cause glucocorticoid withdrawal syndrome, a constellation of nonspecific symptoms typically characterized by nausea, weight loss, decreased appetite, fatigue, lethargy, and myalgia [5, 9–12].
Mifepristone (Korlym®; Corcept Therapeutics, Menlo Park, CA) is a competitive glucocorticoid receptor antagonist approved by the US Food and Drug Administration for the treatment of hyperglycemia in patients with endogenous CS who have failed surgery or are not candidates for surgery [13]. It binds to the glucocorticoid receptor with >10 times the affinity of endogenous cortisol, while having no affinity for the mineralocorticoid receptor [Fig. 1(d)] [14, 15]. By competitively antagonizing the glucocorticoid receptor at the level of the hypothalamus and pituitary gland, mifepristone disrupts the central negative HPA feedback mechanism, resulting in increased ACTH and, subsequently, cortisol levels. Therefore, efficacy and safety monitoring must be based on clinical assessment, not by measuring serum cortisol levels.
Some of the signs and symptoms of glucocorticoid withdrawal syndrome, which indicate effective treatment of CS in patients receiving mifepristone, may overlap with those of AI. In the phase 3 SEISMIC (Study of the Efficacy and Safety of Mifepristone in the Treatment of Endogenous Cushing Syndrome) trial of mifepristone, two patients were reported as having adrenal insufficiency [16]. In these patients, symptoms of nausea and decreased appetite were observed, whereas blood pressure, glucose, and potassium levels remained normal [16]. The patients’ symptoms were managed with temporary drug discontinuation and/or a short duration of dexamethasone therapy. A post hoc analysis of the most common adverse events (AEs) during SEISMIC noted that several AEs, including nausea, fatigue, and headache, declined over time during treatment, despite increases in mifepristone doses, suggesting that these symptoms were not dose dependent and, instead, were temporary symptoms of glucocorticoid withdrawal, which should be anticipated during the initial mifepristone dose titration phase of therapy [17]. The authors thus suggested that it is more appropriate to characterize the reported AI events as “excessive glucocorticoid receptor antagonism” [Fig. 1(e)] [17]. Further analysis of mifepristone in patients with CS would help support these findings; however, assessment of safety outcomes requires large patient populations, which are difficult to obtain for patients with CS because of the relative rarity of the disease. Other controlled clinical trials of mifepristone, in patients without CS, may offer additional insights into its safety profile.
A number of open-label and double-blind clinical studies have been conducted to assess mifepristone treatment in patients with psychotic depression [18–25]. HPA axis dysregulation, a feature of psychotic depression [26, 27], has been thought to contribute to its psychotic symptoms [25]. Five placebo-controlled, phase 3 studies were conducted to assess 7 days of treatment with the competitive glucocorticoid receptor antagonist, mifepristone, in patients with psychotic depression [18–22]. Although the differences between treatment groups were of variable significance, results of the published studies found that mifepristone therapy was associated with more rapid improvement in psychotic symptoms at day 7 vs placebo, and that this improvement in symptoms was sustained for several weeks posttreatment [18–20]. Therefore, the objective of this analysis was to retrospectively examine the incidence of AI and symptoms mimicking AI in a large pooled analysis of these patients treated with mifepristone therapy at daily doses ranging from 300 mg up to 1200 mg for 7 days.
1. Methods
A. Study Design
Data from five placebo-controlled phase 3 studies evaluating mifepristone therapy in nonacutely stressed patients with psychotic depression were available for pooled analysis. All five studies used similar study designs and patient populations; details have been previously published (NCT00637494, NCT00146523, NCT00130676, NCT00128479) [18–22]. Adults meeting the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, and Text Revision and Structured Clinical Interview for DSM Disorders criteria for psychotic depression were eligible for enrollment in study centers across the United States and Eastern Europe. All patients had blood collections to measure serum cortisol and ACTH levels. None of the patients had clinical or biochemical evidence of CS. The study protocols were approved by an institutional review board at each study center, and all patients provided written informed consent before participation.
Patients were randomized in a 1:1 ratio to receive 7 days of double-blind treatment with mifepristone or placebo. Mifepristone doses varied across studies (300, 600, or 1200 mg per day), and clinical assessments were made at baseline and at days 7, 14, 28, 42, and 56. The investigators of these studies were trained and specifically required to clinically assess signs and symptoms of AI at each study visit and report all AEs, including spontaneous AEs. Blood samples were collected for determination of serum cortisol and ACTH levels at baseline, treatment day 7, and during follow-up as specified according to the study protocols. Additional safety assessments included physical examinations and laboratory assessments.
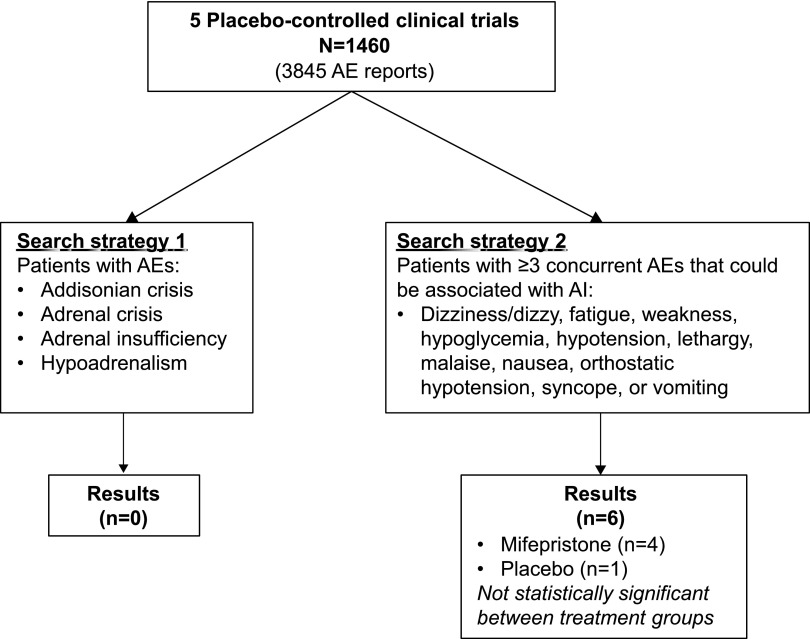
For this pooled analysis, we conducted a systematic search of the studies’ safety databases for the incidence of AI using two different search strategies (Fig. 2). The first strategy searched for patients with AEs using specific AI terminologies (Addisonian crisis, adrenal crisis, adrenal insufficiency, or hypoadrenalism), and the second strategy searched for patients with ≥3 concurrent AEs that could be associated with AI (dizziness/dizzy, fatigue, weakness, hypoglycemia, hypotension, lethargy, malaise, nausea, orthostatic hypotension, syncope, and vomiting).
Figure 2.
Search strategy diagram. Investigators were trained and required to specifically assess for AI at each clinic visit and report all AEs. Search strategies included searching for AI-related terms (search strategy 1) and concurrent AEs associated with AI (search strategy 2).
B. Statistical Analysis
Data from all five phase 3 studies of mifepristone therapy for psychotic depression were available for statistical analyses. Safety database search results are presented by mifepristone dose group and/or all mifepristone dose groups combined (300 mg + 600 mg + 1200 mg) vs placebo. Baseline characteristics, changes in mean ACTH and serum cortisol, and AE frequencies are summarized using descriptive statistics. Differences between all mifepristone doses combined and placebo were analyzed using Fisher’s exact test. P <0.05 defined statistical significance.
2. Results
A. Patient Disposition
The pooled analysis included a total of 1460 patients, 833 who received mifepristone (300 mg, n = 110; 600 mg, n = 471; 1200 mg, n = 252) and 627 who received placebo. At baseline, there were no differences in age, male-to-female ratio, body mass index, or systolic or diastolic blood pressure between the mifepristone-treated and placebo-treated groups (Table 1).
Table 1.
Baseline Characteristics of Study Participants
| Mifepristone (n = 833) | Placebo (n = 627) | |
|---|---|---|
| Age (y) | 44.7 ± 11.6 | 44.7 ± 11.2 |
| Gender (% female) | 57.9 | 60.5 |
| ACTH (pg/mL) | n = 778 | n = 572 |
| 19.6 ± 12.5 | 22.5 ± 15.1 | |
| Serum cortisol (µg/dL) | n = 807 | n = 601 |
| 12.1 ± 5.4 | 12.4 ± 5.4 | |
| BMI (kg/m2) | n = 534 | n = 370 |
| 28.8 ± 5.2 | 28.7 ± 5.8 | |
| Systolic BP (mm Hg) | 122.6 ± 12.9 | 123.4 ± 13.9 |
| Diastolic BP (mm Hg) | 78.2 ± 8.7 | 78.7 ± 9.4 |
| Potassium (mEq/L) | n = 776 | n = 603 |
| 4.2 ± 0.4 | 4.3 ± 0.4 | |
| Sodium (mEq/L) | n = 777 | n = 607 |
| 140.4 ± 2.9 | 140.7 ± 2.8 |
Data are mean ± standard deviation unless stated otherwise. To convert the values for ACTH to picomoles per liter, multiply by 0.22. To convert the values for serum cortisol to nanomoles per liter, multiply by 27.6.
Abbreviations: BMI, body mass index; BP, blood pressure.
B. Laboratory and Physical Assessments
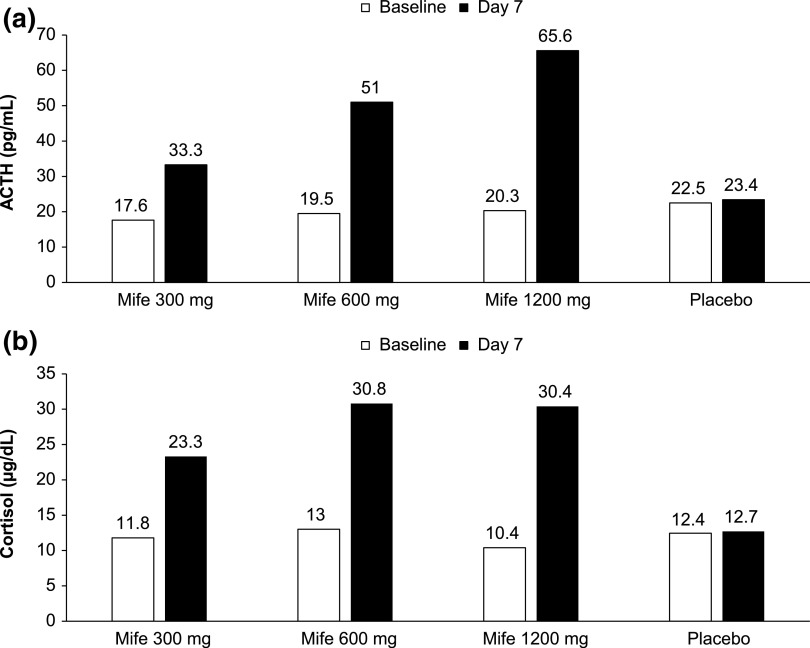
All patients were normocortisolemic at baseline based on serum cortisol assessments (Table 1). After 7 days of mifepristone therapy, mean ACTH and serum cortisol levels increased significantly in a dose-dependent manner (Fig. 3). Mean sodium levels were maintained (140.4 mEq/L) and potassium levels decreased slightly (by <0.2 to 4.04 mEq/L) following 7 days of mifepristone therapy. Sodium and potassium levels remained relatively unchanged in the placebo group. Mean ± standard deviation systolic/diastolic blood pressure remained stable on day 7 for the mifepristone (123.5 ± 12.2/78.2 ± 7.9 mm Hg) and placebo groups (123.3 ± 12.9/78.4 ± 8.6 mm Hg).
Figure 3.
(a) Change in mean ACTH after 7 days; (b) Change in mean serum cortisol after 7 days. To convert the values for ACTH to picomoles per liter, multiply by 0.22. To convert the values for serum cortisol to nanomoles per liter, multiply by 27.6. Mife, mifepristone.
C. Adverse Events
AEs from all five studies (n = 1460) were evaluated by dose group and by all doses combined vs placebo. Treatment-emergent AEs were reported in 556/833 (66.7%) of mifepristone-treated patients and 386/627 (61.6%) of placebo-treated patients (Table 2). The AE profile was comparable between mifepristone and placebo groups, with similar numbers of events noted in the two groups (Table 2).
Table 2.
AEs With Frequency of ≥5% in Mifepristone Studies in Patients With Psychotic Depression (n = 1460)
|
Mifepristone |
Placebo (n = 627) | ||||
|---|---|---|---|---|---|
| Adverse Event (%) | 300 mg (n = 110) | 600 mg (n = 471) | 1200 mg (n = 252) | All Doses (n = 833) | |
| Subjects with ≥1 TEAE | 66.4 | 62.2 | 75.4 | 66.7 | 61.6 |
| Nausea | 18.2 | 14.0 | 17.5 | 15.6 | 11.2 |
| Headache | 18.2 | 9.3 | 23.8 | 14.9 | 11.6 |
| Insomnia | 3.6 | 5.7 | 5.2 | 5.3 | 6.2 |
| Constipation | 2.7 | 4.2 | 9.5 | 5.6 | 5.7 |
| Dizziness | 8.2 | 6.6 | 11.5 | 8.3 | 5.4 |
| Dry mouth | 4.5 | 5.1 | 11.5 | 7.0 | 5.4 |
| Diarrhea | 9.1 | 4.0 | 9.1 | 6.2 | 5.1 |
| Somnolence | 7.3 | 2.8 | 3.2 | 3.5 | 3.8 |
| Anxiety | 5.5 | 3.0 | 5.2 | 4.0 | 3.7 |
| Fatigue | 8.2 | 3.6 | 5.6 | 4.8 | 3.3 |
| Dyspepsia | 1.8 | 2.1 | 9.5 | 4.3 | 3.2 |
| Back pain | 6.4 | 1.7 | 2.8 | 2.6 | 2.7 |
| Vomiting | 4.5 | 3.8 | 7.5 | 5.0 | 2.6 |
| Rash | 0.9 | 1.7 | 5.2 | 2.6 | 1.9 |
Multiple AEs for specific AE preferred terms are counted only once for each patient. AEs are listed by highest percentage order in last column. Preferred term is listed if a frequency of at least 5% in any column is calculated.
Abbreviation: TEAE, treatment-emergent adverse event.
In addition, no events of AI or similar terminologies (search strategy 1) were reported in any mifepristone-treated groups (Fig. 2). Analysis of patients with ≥3 concurrent AEs that potentially could be associated with AI (search strategy 2) revealed no statistically significant differences between the placebo-treated group vs mifepristone, with only a few affected patients in either of these groups (Fig. 2; Table 3).
Table 3.
Patients With ≥3 Concurrent AEs Potentially Associated With Adrenal Insufficiency
| Mifepristone (n = 833) | Placebo (n = 627) | |
|---|---|---|
| 3 Concurrent AEsa | 4 (0.48) | 0 |
| 4 Concurrent AEsa | 0 | 1 (0.16) |
| Totala | 4 (0.48) | 1 (0.16) |
No significant differences (P <0.05) in AEs were noted between mifepristone and placebo groups, as assessed using Fisher’s exact test.
Among the 252 normocortisolemic patients in this analysis who received the highest dose (1200 mg) of mifepristone, there were no patients who reported ≥3 concurrent AEs associated with possible AI, and the frequency of AEs was similar to that in the placebo group.
3. Discussion
This pooled analysis of normocortisolemic patients without CS found no cases of AI or similar terminologies and no differences between escalating mifepristone dose groups and placebo in the incidence of symptom combinations mimicking AI. Mean ACTH and serum cortisol levels increased significantly during treatment in a dose-dependent manner, indicating adequate glucocorticoid receptor binding.
The overall frequency of AEs was high in the placebo group; however, this was to be expected, given the patient population and the protocol, which specified the proactive solicitation of AEs in addition to spontaneously recorded events. Assessments of AEs by solicitation in clinical studies have been associated with higher rates of AEs compared with unstructured (spontaneous) AE assessments [28].
In patients with CS, surgery and effective medical treatment with adrenal steroidogenesis or ACTH synthesis inhibitors may increase the predisposition to AI and/or glucocorticoid withdrawal syndrome. Mifepristone, an effective medical treatment of patients with CS [16], is a competitive glucocorticoid receptor antagonist [14]. Because patients with CS have established physical dependence/tolerance on supraphysiological glucocorticoid levels, receptor antagonism with mifepristone can be expected to produce symptoms of glucocorticoid withdrawal. However, with no affinity for the mineralocorticoid receptor, hypotension and hyperkalemia are unlikely to be observed with mifepristone therapy. In fact, over time, the excess cortisol created by the perturbation of the negative feedback mechanism in the HPA axis may activate mineralocorticoid receptors.
During treatment with mifepristone in patients with CS, it is important to monitor glucose and blood pressure soon after the start of mifepristone treatment because a reduction in concomitant antidiabetic medications (especially insulin) and/or antihypertensive medications may be required to prevent hypoglycemia and/or hypotension as the core etiology of their CS is being treated [17].
The findings from this pooled analysis provide additional insights into the safety/tolerability of mifepristone. Although the generalizability of these results to other patient populations, including patients with CS, is uncertain, the pooled patient population in this analysis was large, increasing the likelihood of discovering rare AEs associated with mifepristone. In addition, the trials included in the pooled analysis were all placebo controlled, another strength of the analysis. In contrast, despite the large number of patients who received the highest dose of mifepristone, the treatment duration of 7 days may not have been long enough for signs and symptoms of AI to develop and could be considered a limitation to the study. Another potential limitation of the study is that the HPA axis of the patients was not formally evaluated using a Cosyntropin or insulin tolerance test because performing these tests would have been challenging in such a large cohort and was not one of the objectives of these studies. Therefore, cortisol collection times differed somewhat among studies, with the majority of samples collected outside of the morning hours (7:00 am to 10:00 am). Despite these limitations, patients in this analysis had mean baseline serum cortisol levels of 12.2 ± 5.4 µg/dL; thus, it is very unlikely that patients with undiagnosed AI or CS were included in the analysis of this study. Furthermore, several publications of prospective, double-blind, randomized, placebo-controlled studies of mifepristone administered in healthy (non-CS subjects) populations at dosages of 600 mg daily for 14 to 28 days also did not note any AI events, which support the findings of the current analysis [29–31].
The lack of AI and the low incidence of symptom combinations mimicking AI with the escalating doses of mifepristone in this study are clinically important, especially as improved glucose tolerance [16] and progressive global clinical improvement [17, 32] have been observed with dose escalations of the drug in patients with CS. Furthermore, a recent follow-up analysis of clinical trial data of patients with CS treated with mifepristone at an average daily dose of 758 ± 290 mg found clinically meaningful weight loss (≥5% of body weight) that was achieved after 24 weeks of therapy, and this weight loss persisted for ~2 additional years with continued mifepristone therapy [33]. More recently, a case report was published that described the use of mifepristone to restore the HPA axis in a woman with prolonged secondary AI following unilateral adrenalectomy [34]. In this case, the patient’s HPA axis failed to recover after 6 years, and she required maintenance glucocorticoids throughout this time. Multiple attempts to decrease the dosage of replacement glucocorticoid failed. The patient was started on a very low dose of mifepristone (150 mg 3 times per week), which was gradually titrated up to 600 mg daily, all while continuing the same dose of glucocorticoid replacement. Recovery of the HPA axis was biochemically observed (increases in ACTH and cortisol), with both hydrocortisone and mifepristone doses gradually tapered and discontinued after 8 months of treatment. Remarkably, the patient did not experience any side effects related to glucocorticoid withdrawal or AI, which the authors proposed may have been related to the measured titration schedule of mifepristone [34]. Together these data are reassuring; however, given the limitations of the current analysis, we caution that clinicians treating patients with CS should still continue to carefully monitor for clinical signs and symptoms of glucocorticoid withdrawal during mifepristone treatment and, when necessary, consider treatment of excessive glucocorticoid receptor antagonism with temporary withdrawal of mifepristone and, if needed, a stress dose with short-term dexamethasone therapy [35].
In summary, this large pooled analysis of controlled phase 3 trials found no cases of AI or similar terminologies and no significant differences compared with placebo in the incidence of symptom combinations mimicking AI during mifepristone treatment in nonacutely stressed normocortisolemic patients with psychotic depression. Together, these data further substantiate the overall safety and tolerability of mifepristone therapy.
Acknowledgments
We thank David Cram, formerly of Corcept Therapeutics, for his contributions to the study concept and outline, and Sarah Mizne, of MedVal Scientific Information Services, for providing professional writing and editorial assistance. This manuscript was prepared according to the International Society for Medical Publication Professionals’ “Good Publication Practice for Communicating Company-Sponsored Medical Research: the GPP3 Guidelines” and the International Committee of Medical Journal Editors’ “Uniform Requirements for Manuscripts Submitted to Biomedical Journals.”
Acknowledgments
This work was supported by Corcept Therapeutics. The authors did not receive any grants in support of writing this manuscript.
Clinical trial registry: ClinicalTrials.gov no. NCT00637494 (registered 11 March 2008), NCT00146523 (registered 2 September 2005), NCT00130676 (registered 12 August 2005), NCT00128479 (registered 8 August 2005).
Disclosure Summary: K.C.J.Y. has served on advisory boards for Pfizer and Corcept Therapeutics. A.M. and D.N. are employees of Corcept Therapeutics.
Footnotes
- ACTH
- adrenocorticotropic hormone
- AE
- adverse event
- AI
- adrenal insufficiency
- CS
- Cushing syndrome
- HPA
- hypothalamic-pituitary-adrenal.
References and Notes
- 1.Moncet D, Morando DJ, Pitoia F, Katz SB, Rossi MA, Bruno OD. Ketoconazole therapy: an efficacious alternative to achieve eucortisolism in patients with Cushing’s syndrome. Medicina (B Aires). 2007;67(1):26–31. [PubMed] [Google Scholar]
- 2.Castinetti F, Guignat L, Giraud P, Muller M, Kamenicky P, Drui D, Caron P, Luca F, Donadille B, Vantyghem MC, Bihan H, Delemer B, Raverot G, Motte E, Philippon M, Morange I, Conte-Devolx B, Quinquis L, Martinie M, Vezzosi D, Le Bras M, Baudry C, Christin-Maitre S, Goichot B, Chanson P, Young J, Chabre O, Tabarin A, Bertherat J, Brue T. Ketoconazole in Cushing’s disease: is it worth a try? J Clin Endocrinol Metab. 2014;99(5):1623–1630. [DOI] [PubMed] [Google Scholar]
- 3.Baudry C, Coste J, Bou Khalil R, Silvera S, Guignat L, Guibourdenche J, Abbas H, Legmann P, Bertagna X, Bertherat J. Efficiency and tolerance of mitotane in Cushing’s disease in 76 patients from a single center. Eur J Endocrinol. 2012;167(4):473–481. [DOI] [PubMed] [Google Scholar]
- 4.Verhelst JA, Trainer PJ, Howlett TA, Perry L, Rees LH, Grossman AB, Wass JA, Besser GM. Short and long-term responses to metyrapone in the medical management of 91 patients with Cushing’s syndrome. Clin Endocrinol (Oxf). 1991;35(2):169–178. [DOI] [PubMed] [Google Scholar]
- 5.Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, Tabarin A; Endocrine Society . Treatment of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing’s syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014;4(2):739–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman AB. Clinical Review#: the diagnosis and management of central hypoadrenalism. J Clin Endocrinol Metab. 2010;95(11):4855–4863. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya A, Kaushal K, Tymms DJ, Davis JR. Steroid withdrawal syndrome after successful treatment of Cushing’s syndrome: a reminder. Eur J Endocrinol. 2005;153(2):207–210. [DOI] [PubMed] [Google Scholar]
- 10.Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocr Rev. 2003;24(4):523–538. [DOI] [PubMed] [Google Scholar]
- 11.Margolin L, Cope DK, Bakst-Sisser R, Greenspan J. The steroid withdrawal syndrome: a review of the implications, etiology, and treatments. J Pain Symptom Manage. 2007;33(2):224–228. [DOI] [PubMed] [Google Scholar]
- 12.Dixon RB, Christy NP. On the various forms of corticosteroid withdrawal syndrome. Am J Med. 1980;68(2):224–230. [DOI] [PubMed] [Google Scholar]
- 13.Korlym® (Mifepristone) [package insert] Menlo Park, CA: Corcept Therapeutics; 2016. Available at http://www.korlym.com/docs/KorlymPrescribingInformation.pdf. Accessed August 26, 2016. [Google Scholar]
- 14.Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129–156. [DOI] [PubMed] [Google Scholar]
- 15.Heikinheimo O, Kontula K, Croxatto H, Spitz I, Luukkainen T, Lähteenmäki P. Plasma concentrations and receptor binding of RU 486 and its metabolites in humans. J Steroid Biochem. 1987;26(2):279–284. [DOI] [PubMed] [Google Scholar]
- 16.Fleseriu M, Biller BM, Findling JW, Molitch ME, Schteingart DE, Gross C; SEISMIC Study Investigators . Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J Clin Endocrinol Metab. 2012;97(6):2039–2049. [DOI] [PubMed] [Google Scholar]
- 17.Yuen KC, Williams G, Kushner H, Nguyen D. Association between mifepristone dose, efficacy, and tolerability in patients with Cushing syndrome. Endocr Pract. 2015;21(10):1087–1092. [DOI] [PubMed] [Google Scholar]
- 18.DeBattista C, Belanoff J, Glass S, Khan A, Horne RL, Blasey C, Carpenter LL, Alva G. Mifepristone versus placebo in the treatment of psychosis in patients with psychotic major depression. Biol Psychiatry. 2006;60(12):1343–1349. [DOI] [PubMed] [Google Scholar]
- 19.Blasey CM, Block TS, Belanoff JK, Roe RL Efficacy and safety of mifepristone for the treatment of psychotic depression. J Clin Psychopharmacol 2011;31(4):436–440. NCT00128479. [DOI] [PubMed] [Google Scholar]
- 20.Blasey CM, DeBattista C, Roe R, Block T, Belanoff JK A multisite trial of mifepristone for the treatment of psychotic depression: a site-by-treatment interaction. Contemp Clin Trials 2009;30(4):284–288. NCT00130676. [DOI] [PubMed] [Google Scholar]
- 21.Clinicaltrials.gov. A study of mifepristone vs. placebo in the treatment of patients with major depression with psychotic features. Available at: https://clinicaltrials.gov/ct2/show/NCT00637494. Accessed 30 August 2016.
- 22.Clinicaltrials.gov. An international study of the safety and tolerability of corlux for psychotic symptoms in psychotic major depression. Available at: https://clinicaltrials.gov/ct2/show/NCT00146523. Accessed 30 August 2016.
- 23.Flores BH, Kenna H, Keller J, Solvason HB, Schatzberg AF. Clinical and biological effects of mifepristone treatment for psychotic depression. Neuropsychopharmacology. 2006;31(3):628–636. [DOI] [PubMed] [Google Scholar]
- 24.Simpson GM, El Sheshai A, Loza N, Kingsbury SJ, Fayek M, Rady A, Fawzy W. An 8-week open-label trial of a 6-day course of mifepristone for the treatment of psychotic depression. J Clin Psychiatry. 2005;66(5):598–602. [DOI] [PubMed] [Google Scholar]
- 25.Belanoff JK, Flores BH, Kalezhan M, Sund B, Schatzberg AF. Rapid reversal of psychotic depression using mifepristone. J Clin Psychopharmacol. 2001;21(5):516–521. [DOI] [PubMed] [Google Scholar]
- 26.Nelson JC, Davis JM. DST studies in psychotic depression: a meta-analysis. Am J Psychiatry. 1997;154(11):1497–1503. [DOI] [PubMed] [Google Scholar]
- 27.Posener JA, DeBattista C, Williams GH, Chmura Kraemer H, Kalehzan BM, Schatzberg AF. 24-Hour monitoring of cortisol and corticotropin secretion in psychotic and nonpsychotic major depression. Arch Gen Psychiatry. 2000;57(8):755–760. [DOI] [PubMed] [Google Scholar]
- 28.Rief W, Nestoriuc Y, von Lilienfeld-Toal A, Dogan I, Schreiber F, Hofmann SG, Barsky AJ, Avorn J. Differences in adverse effect reporting in placebo groups in SSRI and tricyclic antidepressant trials: a systematic review and meta-analysis. Drug Saf. 2009;32(11):1041–1056. [DOI] [PubMed] [Google Scholar]
- 29.Page ST, Krauss RM, Gross C, Ishida B, Heinecke JW, Tang C, Amory JK, Schaefer PM, Cox CJ, Kane J, Purnell JQ, Weinstein RL, Vaisar T. Impact of mifepristone, a glucocorticoid/progesterone antagonist, on HDL cholesterol, HDL particle concentration, and HDL function. J Clin Endocrinol Metab. 2012;97(5):1598–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross C, Blasey CM, Roe RL, Allen K, Block TS, Belanoff JK. Mifepristone treatment of olanzapine-induced weight gain in healthy men. Adv Ther. 2009;26(10):959–969. [DOI] [PubMed] [Google Scholar]
- 31.Gross C, Blasey CM, Roe RL, Belanoff JK. Mifepristone reduces weight gain and improves metabolic abnormalities associated with risperidone treatment in normal men. Obesity (Silver Spring). 2010;18(12):2295–2300. [DOI] [PubMed] [Google Scholar]
- 32.Katznelson L, Loriaux DL, Feldman D, Braunstein GD, Schteingart DE, Gross C. Global clinical response in Cushing’s syndrome patients treated with mifepristone. Clin Endocrinol (Oxf). 2014;80(4):562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fein HG, Vaughan TB III, Kushner H, Cram D, Nguyen D. Sustained weight loss in patients treated with mifepristone for Cushing’s syndrome: a follow-up analysis of the SEISMIC study and long-term extension. BMC Endocr Disord. 2015;15:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohan P. Mifepristone accelerates HPA axis recovery in secondary adrenal insufficiency. Case Rep Endocrinol. 2016;2016:4709597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleseriu M, Molitch ME, Gross C, Schteingart DE, Vaughan TB III, Biller BM. A new therapeutic approach in the medical treatment of Cushing’s syndrome: glucocorticoid receptor blockade with mifepristone. Endocr Pract. 2013;19(2):313–326. [DOI] [PubMed] [Google Scholar]