Abstract
Objective:
Androgen deprivation therapy (ADT) given to men with prostate cancer is associated with metabolically adverse changes in body composition leading to insulin resistance, but the underlying mechanisms are not fully understood. We investigated prospectively whether androgen deprivation or its consequences may be associated with alterations in thyroid function in men.
Design:
We performed a prespecified secondary analysis of a prospective case control study.
Methods:
We prospectively followed men with nonmetastatic prostate cancer newly commencing ADT (n = 34) and age-matched controls (n = 29) for 12 months. We assessed secondary outcomes on thyrotropin (TSH) and thyroid hormones using a linear mixed model to determine mean adjusted differences (MADs) between groups.
Results:
After a 12-month follow-up period, TSH increased in cases compared with control subjects [MAD, 0.69 mIU/L; 95% confidence interval (CI), 0.58–0.82; P < 0.001]. This was accompanied by a rise in FT4 (MAD, 2.2 pmol/L; 95% CI, 1.1–3.2; P < 0.001), reduced FT3-FT4 conversion (MAD, −0.07; 95% CI, −0.10 to −0.4; P < 0.001), and stable FT3. TSH change correlated significantly with changes in weight, body mass index, and fat mass in cases but not with waist circumference, lean mass, visceral fat, insulin resistance, testosterone, sex hormone binding globulin, and estradiol. The rise in TSH after 12 months was strongly associated with changes in leptin.
Conclusions:
A profound rise in TSH in the absence of peripheral hypothyroidism under ADT suggests set point adaptations of the hypothalamic-pituitary-thyroid axis. This appears to be mediated by body composition changes and by the fat-associated hormone leptin rather than by androgen deficiency. Further studies are required to determine the causality and biological implications of these findings.
Keywords: TSH, thyroid hormones, set point, body composition, androgen deprivation, prostate cancer
We found a profound rise in TSH in the absence of hypothyroidism in androgen-deprived men compared with control subjects, suggesting set-point adaptations of the hypothalamic-pituitary-thyroid axis.
Androgen deprivation treatment (ADT) is a mainstay therapy for prostate cancer and improves survival in men with high-risk disease [1]. However, ADT has been associated with metabolically adverse changes in body composition leading to increased insulin resistance [2]. The underlying mechanisms are largely unknown but may include direct androgen receptor-mediated effects on somatic tissues or indirect effects such as reduced motivation to engage in physical activity [2]. Given the good prognosis of men receiving ADT, understanding how ADT affects metabolism is important.
Some of these changes occurring in men on ADT resemble clinical features frequently seen in hypothyroidism. Similar to ADT, hypothyroidism is associated with increases in fat mass, dyslipidemia, and changes in glucose homeostasis [3–5]. Reversed causality has also been suggested, linking obesity to profound changes in thyroid function, particularly a rise in thyrotropin (TSH) [6–8]. Moreover, in association with a decline in circulating concentrations of sex hormone binding globulin (SHBG) and sex hormones, clinical manifestations of hypothyroidism in adult men include gonadal dysfunction and impaired fertility [9, 10].
In view of the importance of thyroid hormones in metabolic regulation and preclinical models reporting a relationship between androgen status and thyroid function [11–13], we took advantage of a case control study to prospectively explore whether the set point of the pituitary-thyroid axis may be altered under ADT, particularly as a consequence of changes in body composition. Patients with nonmetastatic prostate cancer initiating ADT were compared with an age- and body mass index (BMI)-matched prostate cancer control group.
1. Materials and Methods
This study is a prespecified secondary analysis from a prospective 12-month case-control study of 63 age-matched and radiotherapy-matched, ADT-naive men with localized nonmetastatic prostate cancer. All patients were treated as outpatient at a tertiary referral hospital (Austin Health, Melbourne, Australia). The primary outcome measure of the study was biomechanical and video-based functional gait assessment [14]. Additional predefined secondary outcome measures such as body composition, insulin resistance (HOMA2-IR), and quality of life have also been reported [15, 16]. Here, we further evaluated thyroid function in cases and control subjects at initiation and during follow-up assessments after 6 and 12 months. This study was approved by the Human Research Ethics Committee, Austin Health (H2011/04224). All participants provided written informed consent after full explanation of the purpose and nature of the research study protocol.
The trial protocol has been described in detail previously [14]. Briefly, participants were recruited from the hospital outpatient clinics for men with prostate cancer. Inclusion criteria were localized nonmetastatic prostate cancer, no prior ADT, and unrestricted activity with unimpaired Eastern Co-operative Oncology Group performance status of 0. Recruitment was restricted to men newly initiating ADT. Men were excluded if they had any illnesses or other factors predisposing them to androgen deficiency or if they had relevant renal, liver, cardiac, or neuromuscular disease. Case subjects were newly commencing long-term ADT with different brands of gonadotropin-releasing hormone agonists, and control subjects were men with prostate cancer not receiving ADT who were matched for age, cancer diagnosis, and radiotherapy treatment. None of the patients had a history of thyroid disease or was receiving thyroid hormone replacement or antithyroid medication. In eight patients, baseline laboratory tests were delayed by 2 to 8 weeks, but there was no significant association between thyroid parameters, particularly FT4 values and delay. All men received general lifestyle education for prostate cancer, with written advice to exercise regularly and to maintain healthy dietary habits.
A. Body Composition
Dual x-ray absorptiometry was used to measure body composition, including fat mass and lean tissue mass, at 0 and 12 months (Prodigy version 7.51; GE Lunar, Madison, WI). The coefficient of variation was <2% for repeated scans [15]. As a surrogate for total body skeletal muscle mass, appendicular skeletal muscle mass was calculated from the sum of the fat-free and bone mineral content–free mass in both arms and legs [15]. Visceral adipose tissue was quantified using the enCore software (version 16; GE Health Care, Madison, WI) algorithm for dual x-ray absorptiometry, which correlates well with gold-standard magnetic resonance imaging volumetric measurements [17].
B. Laboratory Methods
Morning fasting blood samples were taken for all biochemical tests. Thyroid function tests were performed by a single laboratory. Thyroid hormone measurements were performed by Austin Health Pathology by electrochemiluminescence immunoassay on the Roche Cobas 6000 platform (Roche, North Ryde, NSW, Australia). The third-generation TSH has functional sensitivity (coefficient of variation, 20%) of 0.01 mIU/L and interassay variations of <3% at 0.46 and 32 mIU/L. The standard curve was calibrated with the third International Standard of the World Health Organization for human TSH (IRP 81/565). Coefficients of interassay variation were 2.7% for free thyroxine (FT4) at a concentration of 11 pmol/L and 3.6% for free triiodothyronine (FT3) at 4.4 pmol/L. Laboratory-evaluated reference intervals used for routine diagnostics were 0.4 to 4 mIU/L for TSH, 3.1 to 6.8 pmol/L for FT3, and 10 to 23 pmol/L for FT4.
The calculated FT3/FT4 ratio served as an estimate of conversion efficiency or global deiodinase activity.
For serum total testosterone, an electrochemiluminescence immunoassay (Cobas C8000; Roche Diagnostics, North Ryde, NSW, Australia) was used with a minimum detection limit of 0.4 nmol/L and interassay variation between 5.0% and 6.9%. The same system was used for SHBG and estradiol [14]. Insulin resistance was estimated from fasting plasma glucose and c-peptide using the updated homeostatic model assessment of insulin resistance (HOMA2-IR) [15]. Serum leptin concentrations were measured by a commercial human enzyme-linked immunosorbent assay (Thermo Fisher Scientific, Scoresby, VIC, Australia). Coefficients of interassay variation were 3.9%, 5.6%, and 4.6% at leptin concentrations of 151, 241, and 905 pg/ml, respectively. Samples exceeding the upper limit of the calibration curve were diluted accordingly.
C. Statistical Analysis
Median and interquartile range are reported, unless otherwise stated, because data were mostly non-normally distributed. TSH was naturally logarithmically transformed for calculations. Between-group comparisons of baseline characteristics were made using Wilcoxon rank sum test for continuous variables or χ2 test for frequencies (substituted with Fisher’s exact test in cases of low frequencies). Correlations were based on Kendall’s tau rank correlation. Outcomes were treated as explanatory and not adjusted for multiple testing. To depict the change for each individual, a scatter plot of their baseline measurement vs 12-month follow-up is shown, and the regression line was stratified by group. For the longitudinal analysis, we used a linear mixed model fitted by restricted maximum likelihood [18]. Fixed effects included baseline values of the variable assessed, group (cases and control subjects), visit (categorical time points at 0, 6, and 12 months), and the interaction term of visit × group. The latter term reflects the parameter of interest, which is between-group change during follow-up. As a random effect, we added the repeated measurements by subject. The mean adjusted difference (MAD) between the groups from 0 to 12 months is reported as a quantitative measure of change, surrounded by its profiled 95% confidence interval (CI). P values refer to the overall significance of the change between groups during follow-up. Two-sided P values <0.05 were considered significant. Statistical analyses were performed using the R statistical base version 3.3.1 for Mac with the added packages JGR 1-7.18, Deducer 0.7-9, effects 3.1-2 and lme4 1.1-12 [18–20].
2. Results
Patient baseline characteristics are shown in Table 1. Participants were well matched between groups for age, BMI, testosterone, FT3, TSH, and comorbidities, except for FT4 values, which differed slightly but significantly between cases and control subjects (Table 1). Although testosterone levels were within the reference range in all participants at baseline, they declined after treatment in the ADT group and remained unchanged over 12 months in the control group [0.40 nmol/L (95% CI, 0.30–0.50) vs 14.8 nmol/L (95% CI, 11.2–15.6); MAD, −13.0 nmol/L (95% CI, −15.4 to −10.7)]; P value between groups <0.001). This was accompanied by distinct changes in body composition, metabolic markers, and thyroid hormones (Table 2). BMI and fat mass increased, whereas insulin resistance, lean mass, and appendicular muscle mass decreased. Body weight, waist circumference, visceral fat mass, and HbA1c did not significantly change (Table 2).
Table 1.
Baseline Characteristics of the Study Participants
| Parameter | ADT Group, Median [IQR] (n = 34) | Control Group, Median [IQR] (n = 29) | P Value |
|---|---|---|---|
| Age, y | 67.6 [64.6–72.0] | 70.6 [65.3–72.9] | 0.48 |
| Weight, kg | 83.6 [72.2–96.2] | 83.1 [76.3–91.4] | 0.95 |
| BMI, kg/m2 | 27.8 [25.4–31.5] | 27.2 [26.0–31.8] | 0.75 |
| Waist circumference, cm | 102 [96–111] | 100 [96–108] | 0.38 |
| Leptin, ng/ml | 6.74 [4.62–11.7] | 7.01 [4.62–11.1] | 0.99 |
| TSH, mIU/L | 1.69 [1.17–2.23] | 1.55 [1.42–2.15] | 0.66 |
| FT4, pmol/L | 12.0 [10.8–13.2] | 15.2 [13.4–16.5] | <0.001 |
| FT3, pmol/L | 4.90 [4.70–5.20] | 4.90 [4.50–5.10] | 0.66 |
| Testosterone, nmol/L | 14.1 [10.2–17.6] | 15.0 [11.1–16.9] | 0.91 |
| SHBG, nmol/L | 50.0 [41.0–62.0] | 44.0 [33.0–49.0] | 0.046 |
| Estradiol, pmol/L | 105 [73–143] | 86 [76–104] | 0.07 |
| Medical comorbidities, % | |||
| Ischemic heart disease | 17.6 | 17.2 | 1.00 |
| Diabetes mellitus | 14.7 | 17.2 | 1.00 |
| Liver disease | 0 | 0 | 1.00 |
| Chronic kidney disease | 0 | 0 | 1.00 |
| Hypertension | 58.8 | 58.6 | 1.00 |
Abbreviation: IQR, interquartile range.
Table 2.
Changes in Metabolic Markers and Thyroid Function in Cases vs Controls
| Parameter | ADT Group, Median [IQR] (n = 34) | Controls, Median [IQR] (n = 29) | MAD (95% CI) | P Value |
|---|---|---|---|---|
| Weight, kg | ||||
| 0 mo | 83.6 [72.2–96.2] | 83.1 [76.3–91.4] | ||
| 6 mo | 83.6 [74.9–97.3] | 84.1 [76.5–94.5] | ||
| 12 mo | 85.5 [76.3–98.0] | 84.1 [75.7–94.1] | −1.7 (−3.2 to −0.19) | 0.08 |
| Waist circumference, cm | ||||
| 0 mo | 102 [96–111] | 100 [96–108] | ||
| 12 mo | 108 [100–111] | 102 [96–109] | −1.1 (−3.1 to 1.0) | 0.49 |
| BMI, kg/m2 | ||||
| 0 mo | 27.8 [25.4–31.5] | 27.2 [26.0–31.8] | ||
| 6 mo | 27.8 [26.3–31.7] | 27.3 [25.4–31.2] | ||
| 12 mo | 28.3 [26.6–32.3] | 27.1 [25.4–31.6] | 0.65 (0.14 – 1.15) | 0.03 |
| Fat mass, g | ||||
| 0 mo | 24,318 [19,160–35,118] | 23,857 [20,396–29,710] | ||
| 12 mo | 29,425 [23,946–35,980] | 23,709 [19,077–29,416] | 3421 (2035 – 4807) | <0.001 |
| Lean mass, g | ||||
| 0 mo | 55,029 [50,571–60,589 | 55,302 [51,380–60,516] | ||
| 12 mo | 53,187 [49,423–55,785] | 54,485 [51,551–58,669] | −1453 (−190 to −2716) | 0.03 |
| Appendicular skeletal muscle mass, g | ||||
| 0 mo | 24,700 [22,892–27,848] | 24,923 [23,019–27,339] | ||
| 12 mo | 23,563 [22003–26,087] | 24,372 [21,990–27,090] | −941 (−468 to −1414) | <0.001 |
| Visceral fat, g | ||||
| 0 mo | 2022 [1736–2532] | 1628 [1356–2425] | ||
| 12 mo | 2040 [1697–2456] | 1674 [1289–2184] | 39 (−134 to 213) | 0.66 |
| Leptin, ng/ml | ||||
| 0 mo | 6.74 [4.62–11.7] | 7.01 [4.62–11.1] | ||
| 12 mo | 12.6 [7.86–23.1] | 7.96 [3.93–10.7] | 8.13 (4.43 – 11.8) | <0.001 |
| TSH, mIU/L | ||||
| 0 mo | 1.69 [1.17–2.23] | 1.55 [1.42–2.15] | ||
| 6 mo | 1.81 [1.34–2.22] | 1.61 [1.21–2.07] | ||
| 12 mo | 1.99 [1.30–2.78] | 1.57 [1.14–2.12] | 0.69 (0.58–0.82) | <0.001 |
| FT4, pmol/L | ||||
| 0 mo | 12.0 [10.8–13.2] | 15.2 [13.4–16.5] | ||
| 6 mo | 14.2 [12.4–15.3] | 15.5 [14.1–16.5] | ||
| 12 mo | 15.0 [13.9–16.1] | 15.3 [14.0–16.1] | 2.2 (1.1 – 3.2) | <0.001 |
| FT3, pmol/L | ||||
| 0 mo | 4.90 [4.70–5.20] | 4.90 [4.50–5.10] | ||
| 6 mo | 4.70 [4.23–5.15] | 4.70 [4.50–5.25] | ||
| 12 mo | 4.80 [4.55–5.40] | 4.90 [4.60–5.20] | −0.12 (−0.40 to 0.16) | 0.20 |
| FT3/FT4 ratio | ||||
| 0 mo | 0.42 [0.39–0.46] | 0.32 [0.30–0.37] | ||
| 6 mo | 0.33 [0.31–0.37] | 0.30 [0.29–0.35] | ||
| 12 mo | 0.32 [0.31 – 0.36] | 0.33 [0.29–0.35] | −0.07 (−0.10 to −0.40) | <0.001 |
| Testosterone, nmol/L | ||||
| 0 mo | 14.1 [10.2–17.6] | 15.0 [11.1–16.9] | ||
| 6 mo | 0.40 [0.30–0.57] | 14.3 [9.90–17.2] | ||
| 12 mo | 0.40 [0.30–0.50] | 14.8 [11.2–15.6] | −13.0 (−15.4 to −10.7) | <0.001 |
| Estradiol, pmol/L | ||||
| 0 mo | 105 [73–143] | 86 [76–104] | ||
| 6 mo | 25 [19–38] | 80 [71–95] | ||
| 12 mo | 19 [19–25] | 72 [56–93] | −86.5 (−98.9 to −62.5) | <0.001 |
| SHBG, nmol/L | ||||
| 0 mo | 50 [41–62] | 44 [33–49] | ||
| 6 mo | 46 [35–71] | 40 [35–50] | ||
| 12 mo | 40 [33–64] | 45 [38–50] | 3.9 (0.8–8.6) | 0.05 |
| HOMA2-IR | ||||
| 0 mo | 2.15 [1.65–2.62] | 1.89 [1.40–2.76] | ||
| 6 mo | 2.15 [1.72–2.87] | 1.77 [1.50–2.57] | ||
| 12 mo | 2.59 [1.99–3.19] | 1.71 [1.43–2.21] | 0.59 (0.24–0.94) | 0.02 |
Parameters of body composition have been reported and discussed in detail elsewhere [14–16] but are retabulated here for convenience because they were associated with thyroid function.
Abbreviation: IQR, interquartile range.
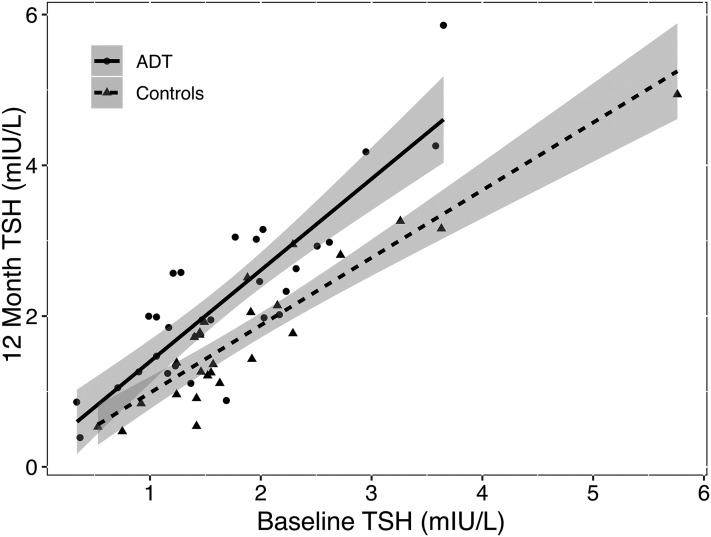
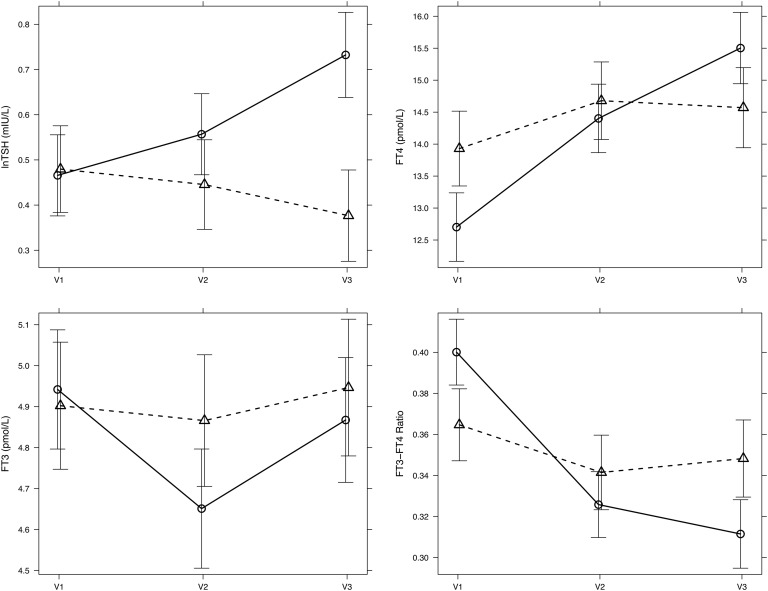
Case subjects showed a broad based and pronounced increase in TSH after 12 months, which was absent in control subjects (Fig. 1). Between the groups, the MAD during follow-up was 0.69 mIU/L TSH (95% CI, 0.58–0.82; P < 0.001) (Table 2; Fig. 2). Whereas FT3 levels remained unchanged, FT4 concentrations rose more strongly in cases, and the FT3/FT4 ratio was reduced after 12 months in cases compared with control subjects (Table 2; Fig. 2).
Figure 1.
Change in TSH in individual patients on ADT vs controls after 12-month follow-up. In the case of no change, all points would be expected to scatter around the diagonal representing the imaginary equality line, and the regression lines fitted to the points of each group would coincide. In contrast, the figure shows a nearly parallel line shift between the ADT and control group. The mean between-group difference was then determined more precisely with a linear mixed model (see Results and Fig. 2).
Figure 2.
Adjusted mean difference in TSH, FT4, FT3, and the FT3/FT4 ratio between cases (solid line) and control subjects (broken line) during 12-month follow-up. Effect plots were derived using a linear mixed model. For details refer to Materials and Methods and Results. Error bars indicate the 95% confidence limit of the mean. V1, baseline visit; V2, 6-month; V3, 12-month follow-up.
TSH changes in cases over 12 months were inversely and strongly correlated with changes in weight and BMI, weakly correlated with changes in fat mass and lean mass (the latter narrowly missing significance) but were not associated with waist circumference, visceral fat, and insulin resistance (Table 3). They were uncorrelated with changes in testosterone, SHBG, or estradiol concentrations (Table 3). We found no associations of the changes in other thyroid hormone parameters with any of these parameters, except for a negative correlation between the changes in SHBG and FT4 (tau, −0.27; P = 0.046) (Table 3).
Table 3.
Associations Between Changes in Thyroid Function, Body Composition, and Sex Steroids in Cases
| Parameter | TSH tau, P Value | FT4 tau, P Value | FT3 tau, P Value | FT3/FT4 Ratio tau, P Value |
|---|---|---|---|---|
| Weight, kg | 0.33, 0.01 | −0.01, 0.91 | 0.05, 0.72 | 0.04, 0.75 |
| BMI, kg/m2 | 0.39, 0.003 | 0.03, 0.82 | 0.0, 0.96 | −0.03, 0.86 |
| Waist circumference, cm | −0.07, 0.59 | −0.09, 0.48 | 0.16, 0.24 | 0.18, 0.17 |
| Fat mass, g | 0.29, 0.04 | −0.05, 0.71 | 0.08, 0.58 | 0.15, 0.32 |
| Lean mass, g | 0.27, 0.06 | −0.17, 0.24 | −0.26, 0.07 | −0.09, 0.53 |
| Visceral fat mass, g | 0.01, 0.90 | 0.03, 0.81 | 0.22, 0.10 | 0.09, 0.51 |
| HOMA-IR | 0.07, 0.57 | −0.06, 0.61 | 0.13, 0.35 | 0.16, 0.24 |
| Testosterone, nmol/L | −0.02, 0.89 | 0.04, 0.75 | 0.02, 0.86 | −0.09, 0.50 |
| SHBG, nmol/L | −0.08, 0.60 | −0.27, 0.046 | −0.16, 0.23 | 0.13, 0.33 |
| Estradiol, pmol/L | −0.02, 0.85 | −0.04, 0.75 | −0.04, 0.76 | −0.04, 0.76 |
Tau refers to Kendall’s tau rank correlation.
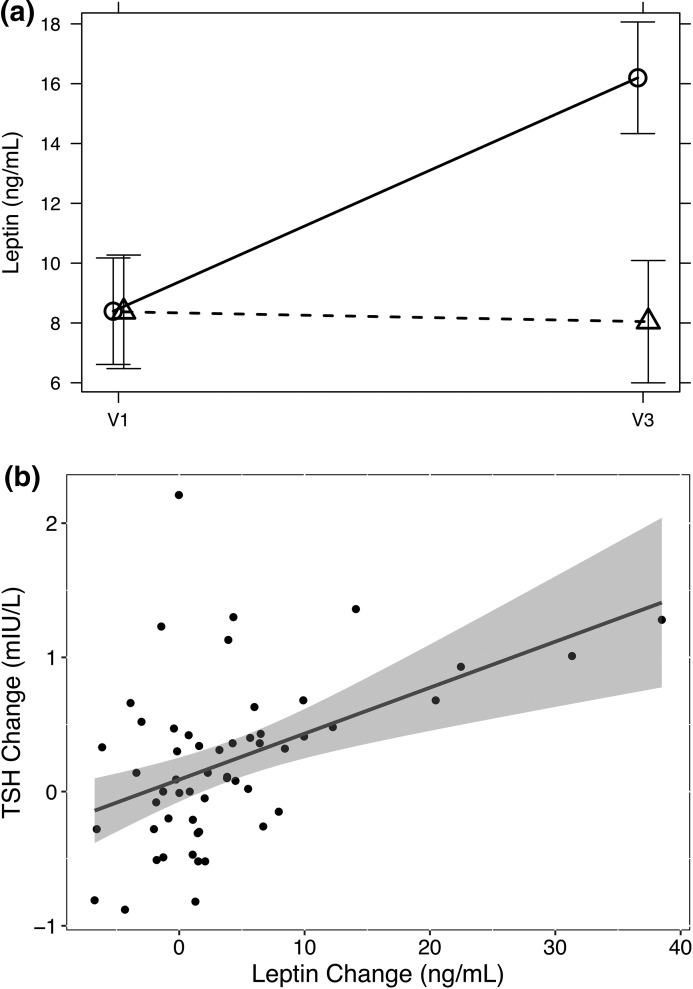
Despite showing similar levels at baseline, concentrations of the fat-associated hormone leptin were significantly increased after 12 months in cases compared with control subjects [Table 2; Fig. 3(a)]. The change observed in TSH after 12 months was significantly positively correlated with the 12-month leptin difference (tau, 0.33; P < 0.001) [Fig. 3(b)].
Figure 3.
Differential changes in leptin in cases and control subjects (a) and their association with TSH increase (b). (a) Adjusted mean difference between cases (solid line) and controls (broken line) derived by a mixed model, similar to Fig. 2. (b) Relationship between the 12-month changes in leptin and TSH. The regression line and its 95% confidence limit are indicative only because significance was based on Kendall’s tau (see Results). V1, baseline visit; V3, 12-month follow-up.
3. Discussion
Because both androgen deficiency and hypothyroidism have been associated with increased fat mass and adverse metabolic sequelae and given possible cross-talk between the gonadal and thyroid axis in men, we used men with prostate cancer initiating ADT as a model to assess the direct and indirect consequences of androgen deprivation on thyroid function in a controlled fashion. In this prespecified secondary analysis of a prospective case control study, we show that, during 12-month follow-up, ADT is associated with a pronounced rise in pituitary TSH, unaccompanied by peripheral hypothyroidism, indicating a set point change in the hypothalamic-pituitary-thyroid axis. These changes in thyroid function during follow-up in androgen-deprived men were uncorrelated to ADT-associated decrements in serum testosterone or estradiol concentrations but strongly related to changes in weight, BMI, fat mass, and leptin levels. Therefore, we interpret our findings to indicate that ADT-associated adverse metabolic outcomes are not due to androgen deprivation–mediated changes in thyroid dysfunction; rather, ADT-associated changes in body composition lead to progressively adaptive changes in the thyroid axis.
To our knowledge, no comparable data from controlled trials on thyroid function and body composition under ADT are available to compare our findings, and few studies have investigated changes in thyroid function during androgen deprivation, mostly in animal models [12, 13, 21–26]. Salminen et al. [23] reported no significant change in TSH under ADT in a prospective study, but potentially confounding changes in body composition were not assessed during follow-up in their study. The cross-sectional study of Morote et al. [24] contradicts these authors, reporting a statistically significant increase in TSH, similar to the present findings. Reports on FT4 during ADT are also discordant, although the reason for the discrepancies is not clear. Hypothalamic-pituitary-thyroid axis dynamics may vary according to baseline health or metabolic and body composition changes. Our cohort consisted of relatively well men with nonmetastatic prostate cancer receiving adjuvant ADT with curative intent. In castrated mice, both basal and TSH-stimulated thyroid hormone synthesis was unaltered [12]. In the rat, orchidectomy had no effect on serum T4 and TSH, but adaptive changes in the activities of deiodinase types 1 and 2 and local T3 production were observed [13]. Although data are limited, there is no evidence suggesting that gonadotropin-releasing hormone agonists may directly interfere with thyroid function testing, for instance by exerting TRH-like cross-reactivity [26].
Strengths of our study are its prospective controlled design and the availability of a multitude of explanatory variables [14–16]. Confidence intervals were narrow despite the relatively small study size. Secondary (although prespecified) outcomes for thyroid hormones are a limitation. A slight, but significant baseline imbalance for FT4 was apparent despite the controlled design, but this was adjusted for when assessing between-group change in a linear mixed model. The adjusted mean difference between the groups was further supported by the majority of individual changes observed in the ADT group but not in the control subjects (Fig. 1). Baseline imbalances between groups as observed for FT4 and SHBG may be anticipated in a nonrandomized design. However, due to strict and inherent indications of ADT, such a study cannot be randomized. Based on anthropometry and body composition measurements by dual-energy x-ray absorptiometry, nutritional status was similar between groups. The prevalence of comorbidities was overall low and similar in both groups among this relatively healthy cohort of men, consistent with their normal testosterone level. Therefore, nonthyroidal illness is unlikely to be a major confounder. The study is controlled, but observational and therefore cannot determine whether the changes in thyroid function are causally related to adverse metabolic changes in these patients.
In long-term survivors of prostate cancer on ADT, various adverse effects have been reported, including sexual complaints, hot flushes, changes in body composition with increased fat and reduced lean mass despite little or no weight gain, musculoskeletal decline leading to increased frailty, unfavorable metabolic consequences for glucose and insulin homeostasis, and increased cardiovascular risks [2, 14–16]. The current study extends those findings to distinct alterations in thyroid function during ADT, which accompany the metabolically adverse changes in body composition.
The present findings shed further light on the complex interaction of thyroid function, weight gain, and obesity, which have been linked in a mutual relationship [27–29]. Hypothyroidism has long been recognized to cause weight gain and metabolically unfavorable consequences that, if not treated adequately, increase mortality. However, distinct changes in thyroid parameters after either weight gain or weight loss suggested that reversed causality may also exist [27–29]. TSH elevation associated with obesity has been termed “hyperthyrotropinemia” because the rise in the pituitary hormone has not typically been accompanied by peripheral hypothyroidism and a decrease in free thyroid hormones [27]. We used ADT as a model to prospectively assess possible differential effects of changes in body composition on the thyroid axis in a controlled design because, unlike other obesity-related models, ADT is weight neutral. It increases fat mass but at the same time reduces lean and muscle mass [2, 14, 15, 30]. Our findings of a pronounced rise in TSH under ADT over control subjects after 12 months, unaccompanied by a shift in FT4 or FT3 toward hypothyroidism, support the notion that ADT-associated changes in fat mass may be directly linked with alterations in thyroid homeostasis. The significant associations with the changes in weight, BMI, and fat mass suggest that TSH may increase as a consequence of the changes in body composition. Hormonal factors such as leptin that are directly released from fat tissue are likely candidates for modulating the pituitary set point of TSH [27, 28]. In the current study, we observed an increase in leptin in the ADT group after 12 months compared with control subjects. This confirms earlier reports by our group and others that leptin levels increase significantly with ADT [30] and conversely are decreased by testosterone treatment [31]. Change in TSH was strongly associated with a change in leptin. These findings are consistent with reports suggesting that leptin can stimulate pituitary TSH secretion directly or indirectly by raising hypothalamic TRH [27]. However, there is also evidence that TSH can stimulate leptin release via a direct action on human adipocytes expressing a functional TSH receptor [32]. Therefore, it is tempting to speculate that this bidirectional link may not only inform the central control system on the fat and energy reserves of the body but also provide adaptive feedforward correction to energy expenditure.
Our findings in the ADT model indicate a crosstalk and close pathophysiological integration between thyroid function, energy metabolism, and body composition [33–36]. This involves both adaptations in the central hypothalamic-pituitary feedback regulation by thyroid hormones and the feedforward control of deiodinase activity by TSH [37]. Negative feedback control by thyroid hormones at the pituitary level is mediated via type 2 deiodinase and intrapituitary T3 concentration, which, in turn, suppresses pituitary TSH secretion [37]. Long-feedback regulation by thyroid hormones on TRH is achieved by the interaction of hypophysiotropic TRH neurons and specialized glial cells termed “tancytes” [34–37]. These cells, unlike other brain cells such as astrocytes, express the enzyme deiodinase type 2, which enables them to convert circulating T4 into T3. Together with their responsiveness to other humoral and neuronal inputs, tancytes provide a regulatory interface for adjusting the set point of the hypothalamic-pituitary-thyroid axis in response to changes in the energetic and metabolic needs of the body [11, 25–27, 33–37]. The sensitivity of deiodinase type 1 and type 2 activities to nutritional factors, fasting, and weight gain facilitates both central and local regulation of T3 conversion and thyroid hormone utilization by various organs [33–42]. Achieving T3 stability appears to be an overarching goal of the central set point adaptation and peripheral deiodinase regulation [43]. FT3 levels and T4 to T3 conversion rate are disproportionally low, although their reduction is less pronounced than in the low-T3 syndrome. Adjustments to the pituitary set point have been recognized as important mechanisms in the syndrome of TACITUS (thyroid allostasis in critical illness, tumors, uremia, and starvation) [37, 44, 45]. According to the present data, allostatic regulation may play an important role in the early response of the system when energetically challenged by minor disturbances, suggesting a gradual transition from homeostatic control to the more dramatic changes in allostatic thyroid regulation and the low-T3 syndrome.
In summary, androgen deprivation in men with nonmetastatic prostate cancer is associated with profound changes in body composition and adverse metabolic effects. The current study extends those findings in a controlled design to the regulation of thyroid function. A rise in TSH in the absence of peripheral hypothyroidism suggests that the set point of the hypothalamic-pituitary-thyroid axis may be reset under ADT, in association with changes in body composition, but not directly due to androgen deficiency. Adipokines produced by fat cells such as leptin may be directly involved in this adaptive response. Despite these moderate alterations, none of the patients developed clinical and/or biochemical thyroid dysfunction during follow-up. When facing energetic and metabolic challenges, central and peripheral adaptations appear to promote T3 stability, which takes priority over set point maintenance. Further studies are required to investigate the causality and biological implications of these findings.
Acknowledgments
This work was supported by National Health and Medical Research Council (NHMRC) of Australia Project Grant 1006407, by a NHMRC Medical and Dental Postgraduate Research Scholarship 1017233 (to A.C.), and by NHMRC Career Development Fellowship 1024139 (to M.G.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADT
- androgen deprivation treatment
- BMI
- body mass index
- CI
- confidence interval
- FT3
- free triiodothyronine
- FT4
- free thyroxine
- MAD
- mean adjusted difference
- SHBG
- sex hormone binding globulin
- TSH
- thyrotropin.
References and Notes
- 1.Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, Gez E, Kil P, Akdas A, Soete G, Kariakine O, van der Steen-Banasik EM, Musat E, Piérart M, Mauer ME, Collette L; EORTC Radiation Oncology Group and Genito-Urinary Tract Cancer Group . Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516–2527. [DOI] [PubMed] [Google Scholar]
- 2.Grossmann M, Zajac JD. Management of side effects of androgen deprivation therapy. Endocrinol Metab Clin North Am. 2011;40(3):655–671, x. [DOI] [PubMed] [Google Scholar]
- 3.Rochon C, Tauveron I, Dejax C, Benoit P, Capitan P, Fabricio A, Berry C, Champredon C, Thieblot P, Grizard J. Response of glucose disposal to hyperinsulinaemia in human hypothyroidism and hyperthyroidism. Clin Sci (Lond). 2003;104(1):7–15. [DOI] [PubMed] [Google Scholar]
- 4.Brenta G. Why can insulin resistance be a natural consequence of thyroid dysfunction? J Thyroid Res. 2011;2011:152850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tagliaferri V, Romualdi D, Guido M, Mancini A, De Cicco S, Di Florio C, Immediata V, Di Segni C, Lanzone A. The link between metabolic features and TSH levels in polycystic ovary syndrome is modulated by the body weight: an euglycaemic-hyperinsulinaemic clamp study. Eur J Endocrinol. 2016;175(5):433–441. [DOI] [PubMed] [Google Scholar]
- 6.Reinehr T, de Sousa G, Andler W. Hyperthyrotropinemia in obese children is reversible after weight loss and is not related to lipids. J Clin Endocrinol Metab. 2006;91(8):3088–3091. [DOI] [PubMed] [Google Scholar]
- 7.de Moura Souza A, Sichieri R. Association between serum TSH concentration within the normal range and adiposity. Eur J Endocrinol. 2011;165(1):11–15. [DOI] [PubMed] [Google Scholar]
- 8.Roelfsema F, Veldhuis JD. Thyrotropin secretion patterns in health and disease. Endocr Rev. 2013;34(5):619–657. [DOI] [PubMed] [Google Scholar]
- 9.Wagner MS, Wajner SM, Maia AL. The role of thyroid hormone in testicular development and function. J Endocrinol. 2008;199(3):351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wajner SM, Wagner MS, Maia AL. Clinical implications of altered thyroid status in male testicular function. Arq Bras Endocrinol Metabol. 2009;53(8):976–982. [DOI] [PubMed] [Google Scholar]
- 11.Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res. 2006;153:209–235. [DOI] [PubMed] [Google Scholar]
- 12.Bagchi N, Shivers B, Brown TR. Effects of castration and sex steroids on the thyroid response to thyrotropin. Endocrinology. 1984;114(5):1652–1656. [DOI] [PubMed] [Google Scholar]
- 13.Sosic-Jurjevic B, Filipovic B, Renko K, Ajdzanovic V, Manojlovic-Stojanoski M, Milosevic V, Köhrle J. Orchidectomy of middle-aged rats decreases liver deiodinase 1 and pituitary deiodinase 2 activity. J Endocrinol. 2012;215(2):247–256. [DOI] [PubMed] [Google Scholar]
- 14.Cheung AS, Gray H, Schache AG, Hoermann R, Lim Joon D, Zajac JD, Pandy MG, Grossmann M. Androgen deprivation causes selective deficits in the biomechanical leg muscle function of men during walking: a prospective case-control study. J Cachexia Sarcopenia Muscle. 2017;8(1):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung AS, Hoermann R, Dupuis P, Joon DL, Zajac JD, Grossmann M. Relationships between insulin resistance and frailty with body composition and testosterone in men undergoing androgen deprivation therapy for prostate cancer. Eur J Endocrinol. 2016;175(3):229–237. [DOI] [PubMed] [Google Scholar]
- 16.Cheung AS, de Rooy C, Hoermann R, Lim Joon D, Zajac JD, Grossmann M. Quality of life decrements in men with prostate cancer undergoing androgen deprivation therapy. Clin Endocrinol (Oxf). 2017;86(3):388–394. [DOI] [PubMed] [Google Scholar]
- 17.Cheung AS, de Rooy C, Hoermann R, Gianatti EJ, Hamilton EJ, Roff G, Zajac JD, Grossmann M. Correlation of visceral adipose tissue measured by Lunar Prodigy dual X-ray absorptiometry with MRI and CT in older men. Int J Obes. 2016;40(8):1325–1328. [DOI] [PubMed] [Google Scholar]
- 18.Bates D, Mächler M, Bolker B, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 19.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Available at: http://www.R-project.org/. Accessed 21 October 2016.
- 20.Fellows I. Deducer: a data analysis GUI for R. J Stat Softw. 2012;49:1–15. [Google Scholar]
- 21.LeRoith D, Liel Y, Sack J, Livshin Y, Laufer N, Schenker J, Spitz IM. The TSH response to TRH is exaggerated in primary testicular failure and normal in the male castrate. Acta Endocrinol (Copenh). 1981;97(1):103–108. [DOI] [PubMed] [Google Scholar]
- 22.Barreca T, Martorana G, Franceschini R, Giberti C, Brancadoro MT, Messina V, Rolandi E. Suppression of testicular androgenesis by D-tryptophan-6-luteinizing hormone-releasing hormone does not affect TSH secretion in male subjects. Horm Res. 1986;23(3):181–184. [DOI] [PubMed] [Google Scholar]
- 23.Salminen E, Koskinen A, Backman H, Nurmi M. Effect of adjuvant androgen deprivation on thyroid function tests in prostate cancer patients. Anticancer Drugs. 2004;15(4):351–356. [DOI] [PubMed] [Google Scholar]
- 24.Morote J, Esquena S, Orsola A, Salvador C, Trilla E, Cecchini L, Raventós CX, Planas J, Catalán R, Reventós J. Effect of androgen deprivation therapy in the thyroid function test of patients with prostate cancer. Anticancer Drugs. 2005;16(8):863–866. [DOI] [PubMed] [Google Scholar]
- 25.Lehrer S, Diamond EJ, Stone NN, Stock RG. Serum thyroid-stimulating hormone is elevated in men with Gleason 8 prostate cancer. BJU Int. 2005;96(3):328–329. [DOI] [PubMed] [Google Scholar]
- 26.Chantilis SJ, Barnett-Hamm C, Byrd WE, Carr BR. The effect of gonadotropin-releasing hormone agonist on thyroid-stimulating hormone and prolactin secretion in adult premenopausal women. Fertil Steril. 1995;64(4):698–702. [DOI] [PubMed] [Google Scholar]
- 27.Reinehr T. Thyroid function in the nutritionally obese child and adolescent. Curr Opin Pediatr. 2011;23(4):415–420. [DOI] [PubMed] [Google Scholar]
- 28.Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biondi B. Thyroid and obesity: an intriguing relationship. J Clin Endocrinol Metab. 2010;95(8):3614–3617. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton EJ, Gianatti E, Strauss BJ, Wentworth J, Lim-Joon D, Bolton D, Zajac JD, Grossmann M. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clin Endocrinol (Oxf). 2011;74(3):377–383. [DOI] [PubMed] [Google Scholar]
- 31.Sih R, Morley JE, Kaiser FE, Perry HM III, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82(6):1661–1667. [DOI] [PubMed] [Google Scholar]
- 32.Menendez C, Baldelli R, Camiña JP, Escudero B, Peino R, Dieguez C, Casanueva FF. TSH stimulates leptin secretion by a direct effect on adipocytes. J Endocrinol. 2003;176(1):7–12. [DOI] [PubMed] [Google Scholar]
- 33.Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18(2):141–144. [DOI] [PubMed] [Google Scholar]
- 34.Herwig A, Ross AW, Nilaweera KN, Morgan PJ, Barrett P. Hypothalamic thyroid hormone in energy balance regulation. Obes Facts. 2008;1(2):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014;35(2):159–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joseph-Bravo P. Hypophysiotropic thyrotropin-releasing hormone neurons as transducers of energy homeostasis. Endocrinology. 2004;145(11):4813–4815. [DOI] [PubMed] [Google Scholar]
- 37.Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Homeostatic control of the thyroid–pituitary axis: perspectives for diagnosis and treatment. Front Endocrinol (Lausanne). 2015;6:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danforth E Jr, Horton ES, O’Connell M, Sims EA, Burger AG, Ingbar SH, Braverman L, Vagenakis AG. Dietary-induced alterations in thyroid hormone metabolism during overnutrition. J Clin Invest. 1979;64(5):1336–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr. 2000;71(6):1421–1432. [DOI] [PubMed] [Google Scholar]
- 40.Tagliaferri M, Berselli ME, Calò G, Minocci A, Savia G, Petroni ML, Viberti GC, Liuzzi A. Subclinical hypothyroidism in obese patients: relation to resting energy expenditure, serum leptin, body composition, and lipid profile. Obes Res. 2001;9(3):196–201. [DOI] [PubMed] [Google Scholar]
- 41.Knudsen N, Laurberg P, Rasmussen LB, Bülow I, Perrild H, Ovesen L, Jørgensen T. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90(7):4019–4024. [DOI] [PubMed] [Google Scholar]
- 42.Müller MJ, Enderle J, Bosy-Westphal A. Changes in energy expenditure with weight gain and weight loss in humans. Curr Obes Rep. 2016;5(4):413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoermann R, Midgley JE, Larisch R, Dietrich JW. Relational stability in the expression of normality, variation and control of thyroid function. Front Endocrinol (Lausanne). 2016;7:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mebis L, Debaveye Y, Ellger B, Derde S, Ververs EJ, Langouche L, Darras VM, Fliers E, Visser TJ, Van den Berghe G. Changes in the central component of the hypothalamus-pituitary-thyroid axis in a rabbit model of prolonged critical illness. Crit Care. 2009;13(5):R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maia AL, Wajner SM. New insights toward the acute non-thyroidal illness syndrome. Front Endocrinol. 2012;3(8):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]