Abstract
Purpose:
Intramuscular (IM) testosterone is the most common modality for testosterone therapy of both male hypogonadism and female-to-male (FTM) gender transition. However, IM injections can be painful and often are not self-administered by the patient. The objective of this study was to further characterize subcutaneous (SC) administration of testosterone as an effective and safe alternative to IM injections by evaluating the pharmacodynamics of serum total and free testosterone concentrations between weekly testosterone injections.
Methods:
Eleven FTM transgender patients already receiving weekly SC testosterone cypionate with documented therapeutic levels prior to enrollment had free and total serum testosterone levels measured at eight different time points during a 1-week dosing interval.
Results:
Mean levels of total and free testosterone were stable and remained well within the normal range between injections. Overall mean ± standard deviation levels for the seven samples taken between injections were 627 ± 206 ng/dL (range, 205 to 1410) for total testosterone and 146 ± 51 pg/mL (range, 38 to 348) for free testosterone. No adverse effects were encountered.
Conclusions:
The results of this study support use of SC testosterone to achieve therapeutic and stable serum testosterone levels for the purpose of gender transition. It is anticipated that these results can be extended to hypogonadal men. This route may be preferred over IM testosterone because it is relatively painless and easy to self-inject thus allowing for the convenience and economy of patient self-administration.
Keywords: pharmacokinetics, subcutaneous testosterone
Serum testosterone (T) concentrations following weekly SC injections of T cypionate remained stable. These results further support the use of SC T injections as an alternative to IM injections.
For treatment of hypogonadal men and female-to-male (FTM) transgender patients, intramuscular (IM) injection of testosterone esters is standard care and is the most commonly used modality in the United States [1]. While on average serum testosterone levels remain within the target range, if the dosing interval is greater than 1 week, levels of testosterone rise above the normal range within the first 48 hours after the injection and fall below the normal range in the days prior to the next dose [2, 3]. Variations in serum testosterone levels may be associated with concomitant fluctuation in mood, energy level, and sexual function [4, 5]. Furthermore, IM injections are often painful and may require administration by a trained professional, making this modality less than ideal. Another option for IM injections, testosterone undecanoate, although administered infrequently, is painful and is available only through a risk evaluation and mitigation program because of the risk of pulmonary oil microembolism [6, 7]. Alternatives to IM testosterone ester administration each have their own disadvantages. Transdermal testosterone formulations (patches, gels) can also have limitations, such as local reactions, poor adhesion, fear of skin-to-skin transmission, unpleasant odor, lack of insurance coverage or high copays, and limited patient acceptance [8–10]. Subcutaneous (SC) insertion of testosterone pellets is available but has been limited by the need for surgery, the possibility of infection, fibrosis or pellet extrusion, limited data regarding efficacy, inflexibility of dosing and limited acceptance [11, 12].
Recent reports indicate that SC administration of testosterone esters may be an acceptable alternative to IM injections in hypogonadal men and FTM transgender patients [13–16]. Only one pharmacokinetic study of SC testosterone ester injection has been reported [14]. That study used fixed doses from an autoinjector and allowed pretreatment serum concentrations as high as 300 ng/dL in hypogonadal men thus providing a confounding factor of significant endogenous testosterone secretion to data interpretation. To further characterize SC testosterone as a practical and acceptable alternative to IM administration, we evaluated the pharmacokinetics of testosterone following manual SC injections of testosterone cypionate to patients undergoing FTM gender transition. Genetic females undergoing gender transition provide an ideal opportunity to assess pharmacokinetics because their low endogenous secretion of testosterone contributes minimally to serum measurements of testosterone. Providing information regarding the pharmacokinetics of testosterone ester injections will further characterize its efficacy and reliability, as well as provide information regarding appropriate timing of serum sampling to monitor therapy.
1. Materials and Methods
A. Patients
FTM transgender patients between 18 and 50 years old already receiving SC therapy with testosterone cypionate were recruited from the Maine Medical Center outpatient Reproductive Endocrinology and Infertility clinic. Inclusion criteria were as follows: (1) good general health with normal hepatic and renal function, (2) serum total testosterone concentrations <50 ng/dL prior to therapy and no hyperandrogenism, (3) normal thyroid function, (4) normal hematocrit, and (5) no concomitant administration of drugs or supplements known to affect the hypothalamic-pituitary-ovarian axis. All patients had been on a stable dose of testosterone cypionate for >2 months prior to completing study blood draws and had serum free and total testosterone concentrations documented to be within the normal adult male range on that dose. The study was approved by the Maine Medical Center Institutional Review Board, and all subjects provided written informed consent.
B. Protocol
Testosterone cypionate (Hikma Farmaceutica®, Portugal) was self-administered into the abdominal SC tissue by all patients using a 1-mL luer lock syringe with a 25-gauge 5/8” needle. Injection sites were contained within an area 2 inches above or below a horizontal line extending from 2 to 6 inches lateral to the umbilicus. No patients reported any difficulty with the injections. Patients were required to inject doses on Mondays to allow for study draws to be completed during normal laboratory business hours (Monday through Saturday). On the study week, patients presented on Monday prior to self-injecting their weekly dose of testosterone cypionate. Blood was drawn just prior to the injection (day 0), followed by blood draws at 6 hours and 1, 2, 3, 4, 5, and 7 days post injection. The final blood sample (day 7) was drawn just prior to the patient’s next dose of testosterone. Patients were instructed to return each day for blood draws at the same time that they had administered their dose on day 0. They were allowed a 2-hour window on either side of the target draw time to complete the blood draw. SC injections were administered by patients between 0800 hours and 1100 hours using their own syringes, needles, and testosterone vials but were observed by study staff to ensure proper technique. If patients missed a scheduled blood draw, they continued with the remainder of scheduled blood draws for the week, continued their Monday dosing schedule, and made up the missed blood draw on the same day in the following week at the originally scheduled time frame. Self-injection by patients with their own materials allowed for a real-life situation for our study.
Blood was drawn and processed with separation of serum within 1 hour of each blood draw. Serum was divided into two identical aliquots; one to be used for analysis, the second as a back-up sample. These were immediately frozen then stored at −70°C. Samples were shipped in batches on dry ice for measurement of serum total and free testosterone concentrations at Quest Diagnostics (Valencia, CA).
C. Hormone Assays
Serum total testosterone was measured by liquid chromatography/mass spectrometry, and free testosterone was measured using equilibrium dialysis (Quest Diagnostics). Proficiency tests for these assays were concordant with the Centers for Disease Control and Prevention reference method assays. The normal adult male range for total testosterone was 250 to 1100 ng/dL and for free testosterone was 46 to 224 pg/mL. Interassay precision for very low (11.64 ng/dL), low (53.16 ng/dL), medium (229.37 ng/dL), and high (1073.54 ng/dL) total testosterone concentration quality control sera, respectively, expressed as percent coefficient of variation (CV), was as follows: 9.94%, 5.28%, 3.02%, and 3.05%. Interassay precision free testosterone (% buffer/serum ratio of 2.11) was 11.64%. The lower limit of detection for total testosterone was 0.84 ng/dL and for free testosterone was 0.1 pg/mL. Total serum estradiol (E2) was measured by liquid chromatography/mass spectrometry (LabCorp, Calabasas, CA). Interassay precisions for low, medium, and high E2 were 4.4%, 3.5%, and 3.3%. The lower limit of detection for E2 was 1 pg/mL.
D. Clinical Efficacy and Safety
These patients were also included in a larger longer-term evaluation of clinical safety and efficacy of SC testosterone administration [16]. To provide context for this pharmacokinetic study, we included the clinical efficacy and safety data for the patients in this report. Efficacy was assessed by suppression of menses and E2 concentrations, as well as appearance of facial hair and deepening of the voice. Safety was assessed by history and physical examination of injections sites, as well as monitoring chemistry panels and hematocrits. In addition, during this study subjective cyclical changes in symptoms related to the injections were assessed by asking if the patients noted any difference in symptoms the 2 days after an injection compared with the 2 days before an injection. This evaluation included energy levels, mood, and well-being, as well as providing for an open-ended response.
E. Data Analysis
Continuous data were described as mean (standard deviation) or as median (range), as appropriate; categorical data were summarized as frequencies (n, %). Intra- and interindividual variation in hormone measurements was assessed as CV (%). Hormone levels were compared between the two preinjection samples (7 days apart), as well as the first preinjection sample and the 6-hour sample by two-sided paired t test. Changes in serum total and free testosterone levels over time between injections were evaluated by mixed models analysis with maximum likelihood estimation. Time was entered into the model and post hoc analyses comparing serum total testosterone between sequential time points were performed by paired t tests. Bivariate relationships between variables were evaluated by Pearson correlation, and standard multiple regression was used to examine these relationships after taking covariates in account. Significance was accepted at P = 0.05. All analyses were performed using SPSS Statistical Software, version 24 (IBM SPSS, Inc., Armonk, NY).
2. Results
A. Patient Characteristics
Fourteen patients met inclusion criteria and signed consent to participate. One patient decided to discontinue hormone therapy for gender transition prior to initiating study blood draws. One patient moved out of state prior to initiating study blood draws. One patient had a change in work schedule that prohibited participation in the study. Eleven patients ultimately completed study blood draws. All eight blood samples were collected in all 11 patients. Patient characteristics are displayed in Table 1. The individual doses of testosterone varied from 50 to 100 mg per week (median 75 mg).
Table 1.
Demographic and Clinical Characteristics of Study Group
|
Variable |
Mean ± Standard Deviation (Range) or Median (Range) |
|---|---|
| n | 11 |
| Age, y | 30.6 ± 8.5 (21–48) |
| BMI, kg/m2 | 28.5 ± 7.4 (20.3–39.3) |
| Duration of T therapy, wk | 119 ± 126 (8–434) |
| Duration of SC T therapy, wk | 47.6 ± 19.3 (8–72) |
| SC T dose, mg | 75 (50–100) |
| Pretherapy hormone levelsa | |
| Total testosterone | 52.8 ± 26.3 (27–103, n = 8) |
| Free testosterone | 3.7 ± 3.7 (0.6–11.0, n = 6) |
| Prestudy hormone levelsb | |
| Total testosterone, ng/dL | 671 ± 238 (340–1184) |
| Free testosterone, pg/mL | 119 ± 49 (41–199) |
| Estradiol, pg/mL | 39.0 ± 18.4 (14–68) |
Abbreviation: T, testosterone.
Prior to initiation of testosterone therapy (performed at LabCorp).
Most recent serum testosterone measurement while on therapy and prior to the study week (performed at LabCorp).
B. Pharmacokinetics
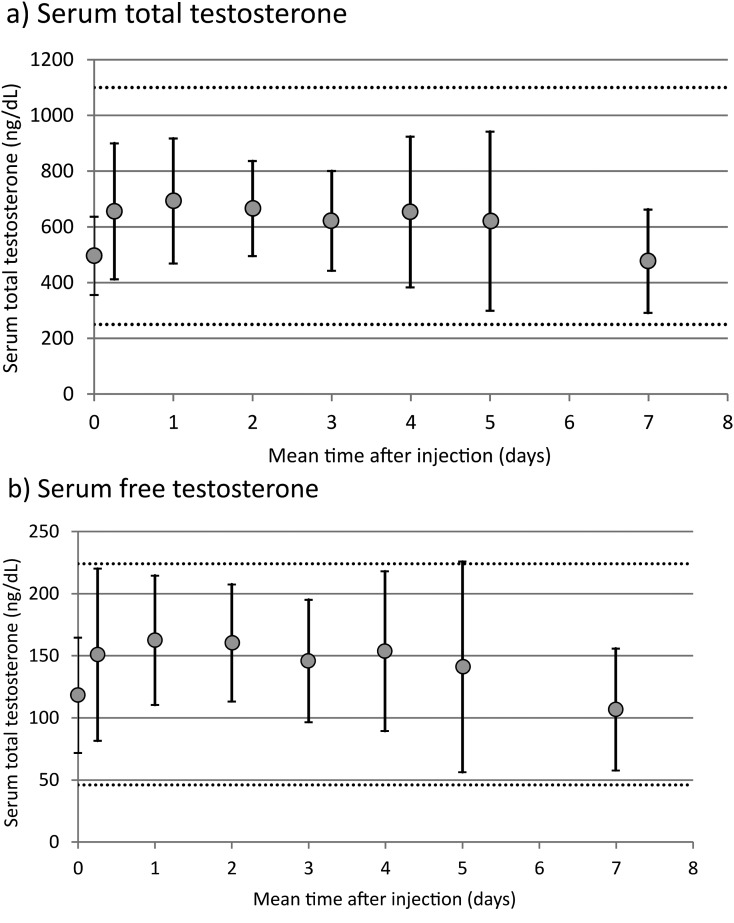
Mean serum concentrations of total testosterone and free testosterone over 1 week are displayed for the 11 patients in Fig. 1(a) and 1(b), respectively. Mean serum total testosterone remained within the normal range throughout the 7-day interval between doses. There was a significant effect of time on serum total testosterone level (ptime = 0.006 by mixed models analysis) and on free testosterone level (ptime = 0.003 by mixed models analysis) during the 7 days between injections.
Figure 1.
Serum total and free testosterone over a 1-week treatment cycle. Serum measurements taken at 0 and 7 days were from samples drawn immediately prior to SC testosterone injection. Dashed lines represent the lower and upper values of normal range for each total free testosterone. Data are shown as mean ± standard deviation.
In post hoc analysis, paired comparisons between time points indicated no significant difference among serum total testosterone levels measured 6 hours to 5 days after injection; there was, however, a significant decrease at 7 days, compared both with the initial postinjection measurement (656 ± 244 ng/dL and 477 ± 185 ng/dL, P = 0.012) and the 5-day measurement (621 ± 321 ng/dL and 477 ± 185 ng/dL, P = 0.023). Compared with baseline preinjection values, serum concentrations increased significantly at 6 hours after injection for both total testosterone (497 ± 140 and 656 ± 244, P = 0.02) and free testosterone 118 ± 46 pg/mL and 151 ± 69 pg/mL, P = 0.003). There was no significant difference in serum levels between the two samples drawn 7 days apart immediately prior to testosterone injections for both total testosterone (497 ± 140 and 477 ± 185 ng/dL, P = 0.58) and free testosterone (118 ± 46 and 107 ± 49 pg/mL, P = 0.25).
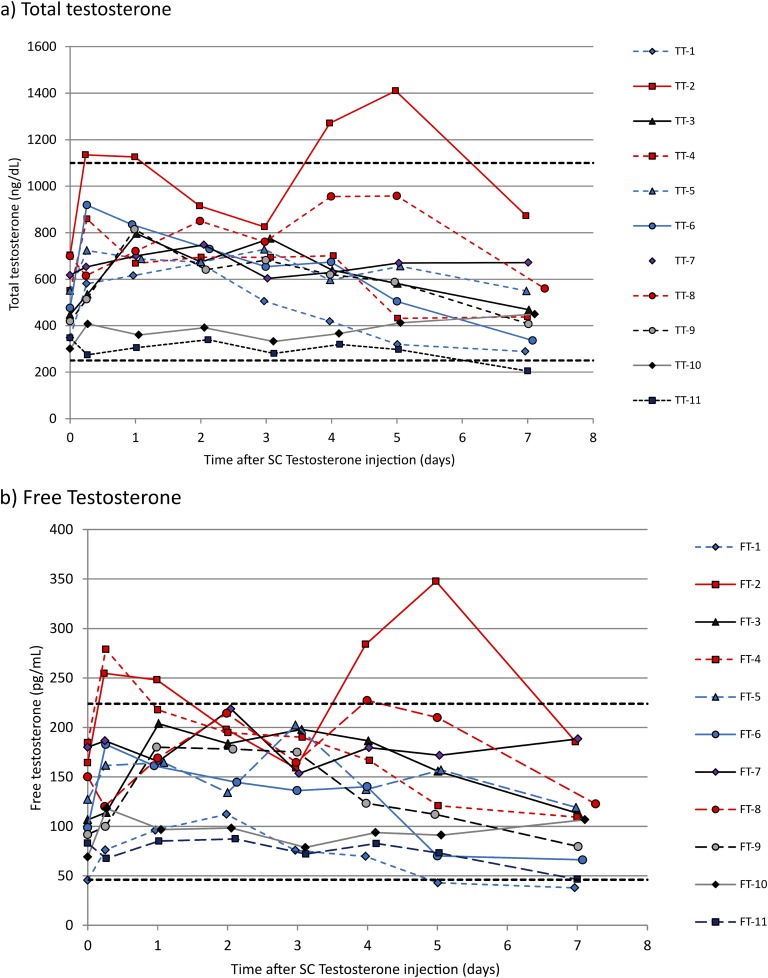
Individual total testosterone and free testosterone data are shown in Fig. 2(a) and 2(b), respectively. One patient (testosterone dose, 50 mg) had one total testosterone measurement below normal range (immediately prior to subsequent dose) and one patient (testosterone dose, 100 mg) had four measurements above normal range [Fig. 2(a)]. The rise in serum testosterone levels above the normal range was confirmed by duplicate serum testosterone measurements using the reserve samples. We confirmed with this patient and all others that they did not take any testosterone (injection or otherwise) in addition to the injections prescribed at 7-day intervals. All patients with serum testosterone levels within the lower half of the normal range were receiving a dose of 50 mg per week. The median (range) interindividual CV for serum total testosterone was 37.2% (25.6% to 51.7%) and the intraindividual CV was 20.2% (7.0% to 30.4%). There was a significant inverse relationship between intraindividual CV and body mass index (BMI; r2 = 0.486, P = 0.017).
Figure 2.
Individual hormone levels over time after SC testosterone injection: 11 female-to-male transgender patients. Individual patients are identified by number in the key. Horizontal dashed lines represent the lower and upper limits of the normal male range for total and free testosterone. The three patients with the lowest serum testosterone concentrations were all receiving 50-mg doses, and the patient with the highest concentration was receiving a 100-mg dose. FT, free testosterone; TT, total testosterone.
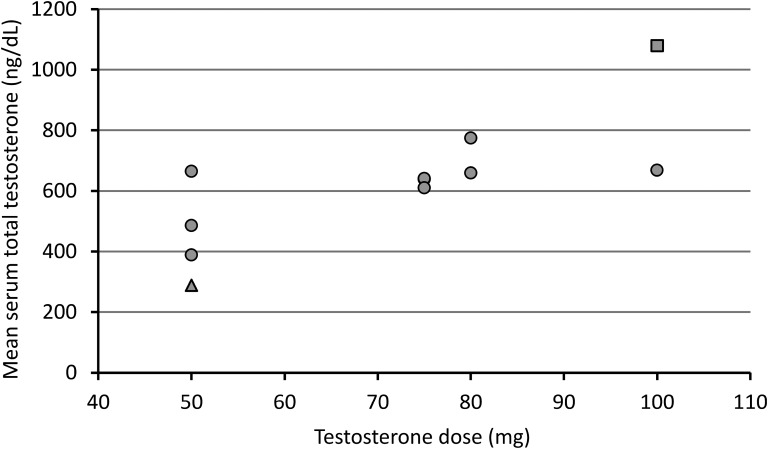
C. Dose Effects
The relationship between mean serum total testosterone and SC testosterone dose is shown in Fig. 3. There was a significant correlation between mean serum total testosterone and SC testosterone dose (r2 = 0.59, P = 0.006). In a regression model that included BMI as a covariate, only total testosterone dose was a significant independent predictor of serum mean total testosterone (standardized β coefficient, 0.77; P = 0.005), explaining 58.9% of the variance in mean serum total testosterone.
Figure 3.
The relationship between testosterone dose and mean serum total testosterone. Data are shown as mean serum total testosterone among measurements taken between testosterone injections. The closed triangle represents a patient with one serum total testosterone measurement below the normal male range during the study; the closed square represents one patient with four serum total testosterone measurements above the normal male range during the study.
D. Clinical Efficacy and Safety
Seven of 11 patients had serum E2 levels <50 pg/mL. Four had E2 levels >50 pg/mL (52.8, 53.0, 57.0, and 68.0 pg/mL). All 11 patients developed amenorrhea and reported deepening of the voice and appearance of facial hair. None of the 11 patients had local reactions to the injections by either history or physical examination. None of the 11 patients reported any subjective decline in symptoms of energy, well-being, or mood in the 2 days prior to the injection. None reported any symptoms of increased aggression, anger, or irritability in general or in the 2 days following an injection. Hematocrit and chemistry panel values remained within the normal range throughout treatment.
3. Discussion
Our data demonstrate that when testosterone cypionate is injected SC at a weekly interval, mean serum levels remain stable and within the normal range. A similar pattern of mean serum testosterone levels has been reported with weekly IM injections of testosterone [2, 3]. A slight decline in serum testosterone concentrations occurred in the 2 days prior to the injection. Near identical serum concentrations of total and free testosterone 7 days apart just prior to injections further supports consistency of serum testosterone levels with SC injections. The patients with testosterone levels within the lower half of the normal range were all receiving relatively small doses of testosterone. Thus, increasing the dose of testosterone would reasonably be anticipated to maintain serum testosterone levels well within the normal range throughout the week between injections. Our data indicate that the dose can be monitored by a single serum total testosterone measurement 1 to 5 days after an injection.
With respect to individual serum testosterone concentrations, overall levels were reasonably stable. Only one patient had a single serum testosterone level slightly below the normal range and because their serum testosterone levels were fairly stable and consistently in the lower range of normal through the week, this could easily be corrected by an increase in the dose. One patient had several serum testosterone levels slightly above the normal range. Whether the variability observed in serum testosterone concentrations in our study is more or less than that with IM testosterone injections is uncertain because pharmacodynamic studies of IM injections reported only mean and not individual data [2, 3]. Additional studies in larger populations of patient receiving either IM or SC testosterone could further define the degree of variability. However, the data in our study indicate that serum levels of testosterone are reasonably sustained between SC injections. Adverse effects are not anticipated in the patient whose levels serum testosterone concentrations rose slightly above the normal range nor in the one patient whose serum testosterone concentrations were fairly steady but fell slightly below the normal range just prior to another dose. As mentioned previously, in this latter patient, a single serum testosterone measurement 1 to 5 days after an injection would lead to a dose increase that would be anticipated to maintain serum testosterone levels within the normal range throughout the dosing interval.
In our patients BMI ranged from 20.3 to 39.3, and increased BMI did not appear to have an adverse effect on the ability to maintain serum testosterone levels within the normal range. We observed an inverse correlation between BMI and intraindividual variability in serum testosterone levels across the week further indicating that an increased BMI does not present a barrier to effective SC administration of testosterone. These findings regarding the lack of an effect of BMI on pharmacokinetics of SC injection of testosterone need to be further evaluated in a larger study.
The slight decline in mean serum testosterone levels just prior to an injection was not accompanied by a decline in subjective symptoms of energy or mood and is thus unlikely to have clinical significance. In the context of longer-term therapy in these patients [16], the SC route of testosterone administration was effective in producing deepening of the voice and appearance of facial hair and produced no adverse effects in these 11 patients, including no local reactions at the injections sites.
These data in combination with previous reports of efficacy and safety of the SC route of testosterone injections in hypogonadal men and FTM transgender patients confirm that the SC route is an acceptable alternative to the IM route [13–16]. Our study, although conducted in a small cohort of patients similar in size to pharmacokinetic studies of IM testosterone [2, 3] indicates that serum testosterone levels remain reasonably steady between SC injections. Larger studies could be undertaken to obtain more detailed information regarding variability of serum concentrations or differences in serum levels following injections at different sites, or possible effects of different BMIs. We have previously reported greater patient acceptability of the SC route compared with the IM route [16]. The SC route appears to be more economical than the IM route. The median dose of SC testosterone in this study (75 mg/week) and in previous studies [13–16] is less than the commonly recommended dose of 100 mg/week for IM dosing [1, 4]. SC injections are easily self-administered. An auto injector as used in a previous pharmacokinetic study of SC injection that may increase the expense of this therapy [14] was not necessary to produce stable levels of serum testosterone in our current study or normal levels of serum testosterone in our previous report [16].
Acknowledgments
We thank Jennie Robichaud, MA, for assistance in collecting data and Angela Cartin for assistance in preparation of the manuscript.
Acknowledgments
This work was supported by a Mentored Research Grant from Maine Medical Center.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CV
- coefficient of variation
- E2
- serum estradiol
- FTM
- female-to-male
- IM
- intramuscular
- SC
- subcutaneous.
References and Notes
- 1.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society . Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–2559. [DOI] [PubMed] [Google Scholar]
- 2.Snyder PJ, Lawrence DA. Treatment of male hypogonadism with testosterone enanthate. J Clin Endocrinol Metab. 1980;51(6):1335–1339. [DOI] [PubMed] [Google Scholar]
- 3.Sokol RZ, Palacios A, Campfield LA, Saul C, Swerdloff RS. Comparison of the kinetics of injectable testosterone in eugonadal and hypogonadal men. Fertil Steril. 1982;37(3):425–430. [DOI] [PubMed] [Google Scholar]
- 4.Gooren LJ, Bunck MC. Androgen replacement therapy: present and future. Drugs. 2004;64(17):1861–1891. [DOI] [PubMed] [Google Scholar]
- 5.Nieschlag E. Testosterone treatment comes of age: new options for hypogonadal men. Clin Endocrinol (Oxf). 2006;65(3):275–281. [DOI] [PubMed] [Google Scholar]
- 6.Middleton T, Turner L, Fennell C, Savkovic S, Jayadev V, Conway AJ, Handelsman DJ. Complications of injectable testosterone undecanoate in routine clinical practice. Eur J Endocrinol. 2015;172(5):511–517. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Harnett M, Dobs AS, Swerdloff RS. Pharmacokinetics and safety of long-acting testosterone undecanoate injections in hypogonadal men: an 84-week phase III clinical trial. J Androl. 2010;31(5):457–465. [DOI] [PubMed] [Google Scholar]
- 8.Abadilla KA, Dobs AS. Topical testosterone supplementation for the treatment of male hypogonadism. Drugs. 2012;72(12):1591–1603. [DOI] [PubMed] [Google Scholar]
- 9.de Ronde W. Hyperandrogenism after transfer of topical testosterone gel: case report and review of published and unpublished studies. Hum Reprod. 2009;24:425–428. [DOI] [PubMed] [Google Scholar]
- 10.Kovac JR, Rajanahally S, Smith RP, Coward RM, Lamb DJ, Lipshultz LI. Patient satisfaction with testosterone replacement therapies: the reasons behind the choices. J Sex Med. 2014;11(2):553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCullough AR, Khera M, Goldstein I, Hellstrom WJ, Morgentaler A, Levine LA. A multi-institutional observational study of testosterone levels after testosterone pellet (Testopel(®)) insertion. J Sex Med. 2012;9(2):594–601. [DOI] [PubMed] [Google Scholar]
- 12.Kelleher S, Howe C, Conway AJ, Handelsman DJ. Testosterone release rate and duration of action of testosterone pellet implants. Clin Endocrinol (Oxf). 2004;60(4):420–428. [DOI] [PubMed] [Google Scholar]
- 13.Al-Futaisi AM, Al-Zakwani IS, Almahrezi AM, Morris D. Subcutaneous administration of testosterone. A pilot study report. Saudi Med J. 2006;27(12):1843–1846. [PubMed] [Google Scholar]
- 14.Kaminetsky J, Jaffe JS, Swerdloff RS. Pharmacokinetic profile of subcutaneous testosterone enanthate delivered via a novel, prefilled single‐use autoinjector: a phase ii study. Sex Med. 2015;3(4):269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson J, Schrager SM, Clark LF, Dunlap SL, Belzer M. Subcutaneous testosterone: an effective delivery mechanism for masculinizing young transgender men. LGBT Health. 2014;1(3):165–167. [DOI] [PubMed] [Google Scholar]
- 16.Spratt DI, Stewart I, Savage C, Craig W, Spack NP, Chandler DW, Spratt LV, Eimicke T, Olshan JS. Subcutaneous injection of testosterone is an effective and preferred alternative to intramuscular injection: demonstration in female-to-male transgender patients [published online ahead of print April 3, 2017]. J Clin Endocrinol Metab. [DOI] [PubMed]