Abstract
Context:
IGSF1 deficiency is a recently discovered syndrome consisting of congenital central hypothyroidism (CeH) and macroorchidism. Here, we report on a patient presenting with short stature, who was found to carry a pathogenic mutation in the IGSF1 gene.
Case Description:
A 14-year-old Israeli boy was referred to the Academic Medical Center in Amsterdam, The Netherlands, for follow-up on short stature ascribed to constitutional delay of growth and puberty, and familial hypercholesterolemia. Primary hypothyroidism had previously been excluded by a normal thyroid-stimulating hormone (TSH) concentration. However, in follow-up, plasma free thyroxine (FT4) concentrations were repeatedly low, and the patient was diagnosed with CeH. Because of coexistent relative macroorchidism, IGSF1 gene analysis was performed, revealing a mutation (c.2588C>G; p.Ser863Cys). The mutant IGSF1 protein was retained mainly in the endoplasmic reticulum and reached the plasma membrane with poor efficiency compared with wild-type protein. After starting thyroxine treatment, plasma cholesterol almost normalized.
Conclusions:
This case illustrates the necessity of measuring both FT4 and TSH when hypothyroidism is suspected, or needs to be ruled out. In addition, this case suggests that the presence of childhood hypercholesterolemia may be an indication of undiagnosed hypothyroidism.
Keywords: IGSF1, central hypothyroidism, short stature, hypercholesterolemia
A teenager’s diagnosis of constitutional growth delay and hypercholesterolemia was corrected to central hypothyroidism because of IGSF1 deficiency syndrome. Levothyroxine improved both growth and lipids.
Loss-of-function mutations in the immunoglobulin superfamily, member 1 (IGSF1) gene are a cause of X-linked congenital central hypothyroidism (CeH) and macroorchidism [1]. Here we present a patient who was referred for follow-up on short stature, but who was eventually diagnosed with IGSF1 deficiency syndrome (IDS).
1. The Case
A 14-year and 4-month-old Israeli boy was referred to the Academic Medical Center of the University of Amsterdam for short stature ascribed to constitutional delay of growth and puberty (CDGP), and hypercholesterolemia, previously diagnosed in Israel. He was the second child of healthy parents, and was born full-term after an uneventful pregnancy. His neonatal congenital hypothyroidism screening result was normal. During early childhood, there were no health problems, and growth and development proceeded normally.
At the age of 11 years, parents noticed the boy was shorter than his peers, which was monitored by his general practitioner. The boy was first evaluated for short stature by a pediatric endocrinologist at age 13 years. His height and weight were 142.5 [−1.8 standard deviation (SD); 2.4 SD below midparental height] and 40 kg [body mass index (BMI), 19.8 kg/m2, +0.6 SD], according to World Health Organization reference values [2]. Given the normal physical examination and laboratory results [including thyroid-stimulating hormone (TSH)], this was attributed to CDGP. Because total and low-density lipoprotein (LDL)–cholesterol were increased, and his father was previously diagnosed with hypercholesterolemia, he was diagnosed with (probable) familial hypercholesterolemia, and started on simvastatin. At follow-up, lipids decreased to near-normal levels. At the last visit (age 13 years and 8 months), height was 147 cm (−1.8 SD), testicular volume was 10 mL by Prader orchidometer, but pubic hair was absent. Bone age was 2 to 2.5 years delayed (Greulich and Pyle).
When he was first seen in the Academic Medical Center (age 14 years and 4 months), height and weight were 152.4 cm (−1.7 SD) and 44.9 kg (BMI 19.3 kg/m2, 0.0 SD) and 44.9 kg (BMI, 19.3 kg/m2). Except for mild constipation, the medical history revealed no abnormalities. Simvastatin was discontinued 6 months earlier. Physical examination showed normal body proportions. Tanner stage was G3, P2-3; testicular volume by Prader orchidometer was 20mL. Parental heights were 179.6 cm (father, +0.4 SD) and 168.6 cm (mother, +0.8 SD). Both parents and the older sister reported to have had a normal timing and progression of puberty. The discrepancy between the large testicular volume and just recent virilization prompted laboratory testing. Plasma free thyroxine (FT4) and total thyroxine (T4) concentrations were below the reference interval, combined with a normal TSH concentration (Table 1). Testicular volume by ultrasound (age 15 years and 5 months) was 20 mL (2.6 SD; by Prader orchidometer >30 mL). [3]. Plasma testosterone (2.6 nmol/L) was within the age-specific reference interval [4], but relatively low considering testicular volume. Other hypothalamic-pituitary axes were intact, but prolactin was undetectable. Total cholesterol and LDL-cholesterol were elevated (Table 1).
Table 1.
Progress of Thyroid Hormones and Lipids Before and After Start of Treatment
| Plasma Levels | Before Treatment | 1 mo | 3 mo | 4 mo | 11 mo | 13 mo | 14 mo | 16 mo | 19 mo | 25 mo |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 14 y, 5 mo | 14 y, 6 mo | 14 y, 8 mo | 14 y, 9 mo | 15 y, 3 mo | 15 y, 5 mo | 15 y, 6 mo | 15 y, 8 mo | 15 y, 11 mo | 16 y, 5 mo |
| TSH, mU/L (0.5–5.0) | 2.60 | 0.06 | <0.01 | <0.01 | 0.10 | 0.09 | 0.01 | 0.02 | <0.01 | <0.01 |
| FT4, pmol/L (10–23) | 6.2a | 12.1 | 15.8 | 18.7 | 13.9 | 14.7 | 19.1 | 17.7 | 13.7 | 18.0 |
| T4, nmol/L (70–150) | 50a | 60a | 70 | 75 | 70 | x | 90 | x | x | 90 |
| Total cholesterol, mmol/L (3.3–6.5) | 6.66a | 5.89 | 5.73 | 4.72 | 7.13a | 6.61a | 5.93 | 6.37 | 5.80 | 6.50 |
| HDL, mmol/L (0.78–1.94) | 1.45 | 1.54 | 1.59 | 1.23 | 1.44 | 1.80 | 1.64 | 1.51 | 1.41 | 1.62 |
| LDL, mmol/L (1.76–3.52) | 4.71a | 3.55a | 3.56a | 3.02 | 5.25a | 4.26a | 3.87a | 4.46a | 3.91a | 4.44a |
| Triglyceride, mmol/L (0.5–2.0) | 1.12 | 1.77 | 1.29 | 1.04 | 0.97 | 1.22 | 0.94 | 0.90 | 1.07 | 0.97 |
Columns show the laboratory reports in the months after the initiation of treatment. Reference intervals for thyroid hormones are shown in parentheses (all in-house reference intervals). Age-specific in-house reference intervals for total cholesterol, HDL, LDL, and triglycerides are shown in parentheses.
Abbreviations: HDL, high-density lipoprotein; x, missing data.
Abnormal values.
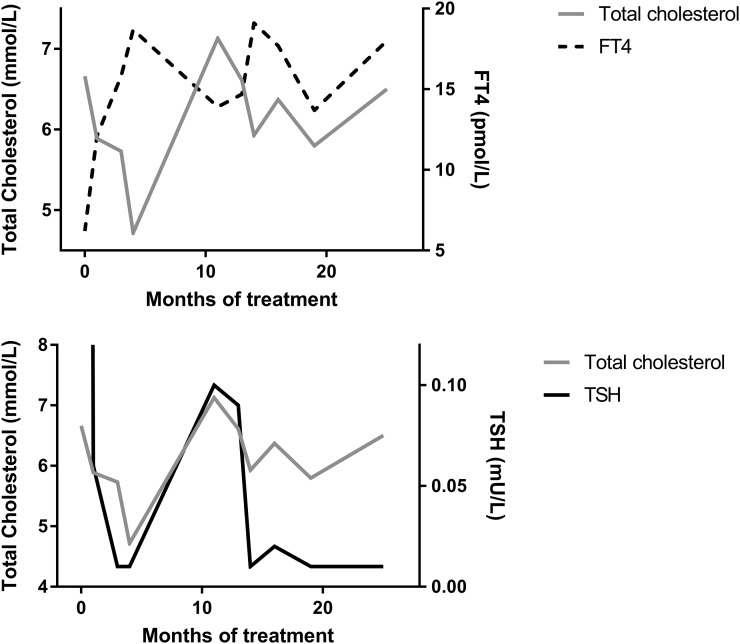
CeH was diagnosed and levothyroxine (LT4) treatment was started. After 1 month, FT4 had increased to just within reference interval, with a concurrent decrease in TSH and total cholesterol. LDL-cholesterol normalized only after FT4 reached the upper half of reference interval. Prolactin levels remained undetectable. After 10 months, there was clear pubertal progression and growth velocity increased from −1.0 to 6.8 SD (Dutch reference values) [5]. Within 2 years, the LT4 dose had to be increased several times, most likely because of pubertal growth. During this time, FT4 had a striking inverse relationship with total cholesterol (Fig. 1). Despite adequate treatment, LDL-cholesterol returned to values above reference interval (Table 1).
Figure 1.
Graphic representation of the course of biochemical findings over time. The inverse relationship between plasma FT4 and total cholesterol concentrations (top panel) and the direct relationship between plasma TSH and total cholesterol concentrations (bottom panel) are shown.
2. Genetic Studies
The combination of CeH, delayed puberty, hypoprolactinemia, and macroorchidism was suggestive of IDS; therefore, IGSF1 Sanger sequencing was performed. A probably deleterious, hemizygous c.2588C>G (p.Ser863Cys; transcription accession number NM_001170961.1) mutation was found. The same (heterozygous) mutation was found in the patient's mother (who had a normal plasma TSH, but low normal FT4 concentration). A similar mutation (p.Ser863Phe) was previously described [1]. Because the patient’s father was being treated for hypercholesterolemia, and considering the patient’s elevated LDL-cholesterol despite optimal FT4 concentrations, genetic testing for familial hypercholesterolemia was performed. Analysis of the LDLR, APOB, and PCSK9 genes yielded no pathogenic mutations [6]. Written informed consent was obtained for this case report.
3. Protein Studies
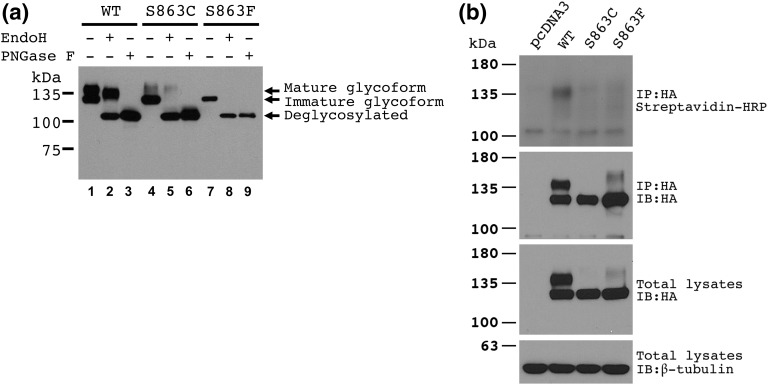
We examined expression and posttranslational regulation of the mutant protein in heterologous HEK293 cells. Cells were transfected with pcDNA3 or vectors expressing the IGSF1 wild type, the current Ser863Cys protein, or the previously described Ser863Phe protein. The protein lysates were extracted and analyzed as reported previously [1]. Compared with the wild-type protein, Ser863Phe migrates primarily as an immature glycoform. Although a similar migratory pattern was seen for Ser863Cys, it additionally exhibited trace amounts of the mature glycoform [Fig. 2(a)]. These glycosylation patterns suggested that Ser863Cys is largely retained in the endoplasmic reticulum, which was confirmed by dramatic reductions in plasma membrane expression relative to wild type, as determined by cell surface biotinylation analysis [Fig. 2(b)] [1].
Figure 2.
Alterations in IGSF1 impair its plasma membrane trafficking. HEK293 cells were transfected with pcDNA3 (empty vector) or with vectors expressing the indicated wild-type (WT) and mutant IGSF1 proteins. (a) Protein lysates were deglycosylated with either PNGase F or EndoH, resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and immunoblotted using an antibody specific to the IGSF1 C-terminal domain (CTD) (RRID AB_2631165). (b) Membrane expression of IGSF1 CTD was analyzed by cell surface biotinylation. HA, hemagglutinin; HRP, horseradish peroxidase; IB, immunoblot; IP, immunoprecipitation.
4. Discussion
Here we report on a teenager initially diagnosed with CDGP and probable familial hypercholesterolemia. After finding a low FT4 in combination with a normal TSH concentration, the diagnosis was corrected to CeH because of IDS. Although CeH and macroorchidism are the main clinical features of IDS, patients may also have prolactin deficiency, delayed puberty combined with normal timing of testicular growth, growth hormone deficiency, and increased BMI [1]. Our patient had CeH, prolactin deficiency, disharmonious pubertal development, and relative macroorchidism, whereas his BMI and insulin-like growth factor 1 concentrations were normal.
CeH is defined as hypothyroidism caused by quantitative or qualitative TSH deficiency [7]. Although congenital CeH is regarded as less severe than congenital primary hypothyroidism, based on initial FT4 values, over half of CeH cases can be classified as moderate to severe [8]. Because of the central nature of CeH, TSH concentrations may be low, normal, or slightly elevated [7]. Although TSH measurement is an excellent diagnostic test for primary hypothyroidism, it alone is not adequate for detecting CeH. Diagnosing CeH therefore depends on concurrent FT4 measurement. Although most countries have neonatal screening programs for congenital hypothyroidism, most are TSH-based and only detect primary hypothyroidism. In contrast, (F)T4-based screening programs detect both primary hypothyroidism and CeH. Consequently, most known IDS index cases were detected through neonatal screening. However, among the 11 original index cases, three teenagers were diagnosed after presenting with short stature [1]. Our patient also presented with short stature, but was diagnosed with CDGP. As the most common cause of delayed puberty, CDGP is associated with slow growth, delayed bone age, and a positive family history. When there are clues for disturbed growth, but not for a specific diagnosis, further investigations are advised [9]. When delayed pubertal growth acceleration is accompanied by large testes, IDS should be suspected. Although hypothyroidism was considered in our patient, it was assumed to be excluded by finding a normal TSH. Unfortunately, this led to a delayed correct diagnosis. Diagnostic testing for hypothyroidism, particularly CeH, must therefore always include measurement of both TSH and FT4.
Previously, the patient was diagnosed with probable familial hypercholesterolemia based on both his and his father’s elevated lipids and was treated with simvastatin. Although this improved his lipid profile, total cholesterol and LDL-cholesterol remained above reference interval. After stopping simvastatin, and subsequently starting LT4, his lipid profile again improved, initially to within reference values. Both primary hypothyroidism and CeH are associated with hypercholesterolemia, and treatment with LT4 can decrease lipid levels [10]. Although not formally included in the syndrome definition, seven out of 69 known male IDS patients were diagnosed with dyslipidemia, two of whom while on LT4 treatment [11]. In this case, lipid levels showed an inverse relationship with FT4 concentrations. However, eventually, LDL-cholesterol did not normalize despite LT4 replacement. Genetic testing for familial hypercholesterolemia yielded no abnormalities. Whether this increased LDL-cholesterol is because of an unknown genetic variant, an uncommon consequence of IDS, or a reflection of hypothalamic deregulation of hepatic lipid metabolism is unclear at present [12].
5. Conclusions
This clinical case demonstrates the importance of determining both FT4 and TSH, especially when CeH is suspected or needs to be ruled out, and illustrates the important role of thyroid hormones in lipid metabolism.
Acknowledgments
We are grateful for the help and advice of Dr. A. Wiegman, Department of Pediatrics, Emma Children’s Hospital, and Dr. J.C. Defesche, Department of Clinical Genetics, Academic Medical Center, Amsterdam, The Netherlands.
Acknowledgments
This work was supported by a grant from the AMC Foundation to C.A.H., and by CIHR operating Grant MOP-133557 and NSERC Discovery Grant 2015-05178 to D.J.B.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CDGP
- constitutional delay of growth and puberty
- CeH
- central hypothyroidism
- FT4
- free thyroxine
- IDS
- IGSF1 deficiency syndrome
- IGSF1
- immunoglobulin superfamily, member 1
- LDL
- low-density lipoprotein
- LT4
- levothyroxine
- T4
- thyroxine
- TSH
- thyroid-stimulating hormone.
References and Notes
- 1.Sun Y, Bak B, Schoenmakers N, van Trotsenburg AS, Oostdijk W, Voshol P, Cambridge E, White JK, le Tissier P, Gharavy SN, Martinez-Barbera JP, Stokvis-Brantsma WH, Vulsma T, Kempers MJ, Persani L, Campi I, Bonomi M, Beck-Peccoz P, Zhu H, Davis TM, Hokken-Koelega AC, Del Blanco DG, Rangasami JJ, Ruivenkamp CA, Laros JF, Kriek M, Kant SG, Bosch CA, Biermasz NR, Appelman-Dijkstra NM, Corssmit EP, Hovens GC, Pereira AM, den Dunnen JT, Wade MG, Breuning MH, Hennekam RC, Chatterjee K, Dattani MT, Wit JM, Bernard DJ. Loss-of-function mutations in IGSF1 cause an X-linked syndrome of central hypothyroidism and testicular enlargement. Nat Genet. 2012;44(12):1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joustra SD, van der Plas EM, Goede J, Oostdijk W, Delemarre-van de Waal HA, Hack WW, van Buuren S, Wit JM. New reference charts for testicular volume in Dutch children and adolescents allow the calculation of standard deviation scores. Acta Paediatr. 2015;104(6):e271–e278. [DOI] [PubMed] [Google Scholar]
- 4.Kushnir MM, Blamires T, Rockwood AL, Roberts WL, Yue B, Erdogan E, Bunker AM, Meikle AW. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56(7):1138–1147. [DOI] [PubMed] [Google Scholar]
- 5.Fredriks AM, van Buuren S, Wit JM, Verloove-Vanhorick SP. Body index measurements in 1996-7 compared with 1980. Arch Dis Child. 2000;82(2):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Graaf A, Avis HJ, Kusters DM, Vissers MN, Hutten BA, Defesche JC, Huijgen R, Fouchier SW, Wijburg FA, Kastelein JJ, Wiegman A. Molecular basis of autosomal dominant hypercholesterolemia: assessment in a large cohort of hypercholesterolemic children. Circulation. 2011;123(11):1167–1173. [DOI] [PubMed] [Google Scholar]
- 7.Persani L. Clinical review: central hypothyroidism: pathogenic, diagnostic, and therapeutic challenges. J Clin Endocrinol Metab. 2012;97(9):3068–3078. [DOI] [PubMed] [Google Scholar]
- 8.Zwaveling-Soonawala N, van Trotsenburg AS, Verkerk PH. The severity of congenital hypothyroidism of central origin should not be underestimated. J Clin Endocrinol Metab. 2015;100(2):E297–E300. [DOI] [PubMed] [Google Scholar]
- 9.Stalman SE, Hellinga I, Wit JM, Hennekam RC, Kamp GA, Plötz FB. Growth failure in adolescents: etiology, the role of pubertal timing and most useful criteria for diagnostic workup. J Pediatr Endocrinol Metab. 2016;29(4):465–473. [DOI] [PubMed] [Google Scholar]
- 10.Ferretti E, Persani L, Jaffrain-Rea ML, Giambona S, Tamburrano G, Beck-Peccoz P. Evaluation of the adequacy of levothyroxine replacement therapy in patients with central hypothyroidism. J Clin Endocrinol Metab. 1999;84(3):924–929. [DOI] [PubMed] [Google Scholar]
- 11.Joustra SD, Heinen CA, Schoenmakers N, Bonomi M, Ballieux BE, Turgeon MO, Bernard DJ, Fliers E, van Trotsenburg AS, Losekoot M, Persani L, Wit JM, Biermasz NR, Pereira AM, Oostdijk W, IGSF1 Clinical Care Group . IGSF1 deficiency: lessons from an extensive case series and recommendations for clinical management. J Clin Endocrinol Metab. 2016;101(4):1627–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruinstroop E, Fliers E, Kalsbeek A. Hypothalamic control of hepatic lipid metabolism via the autonomic nervous system. Best Pract Res Clin Endocrinol Metab. 2014;28(5):673–684. [DOI] [PubMed] [Google Scholar]