Abstract
Context:
Accurate measurements of circulating hormones is essential for the practice of endocrinology. Immunometric assays employing the streptavidin-biotin system are widely used to measure hormones. However, these assays are susceptible to interference in patients taking biotin supplementations. This interference could mimic a coherent hormone profile, leading to misdiagnosis and unnecessary treatment.
Case description:
The patient, a 32-year-old man with X-linked adrenomyeloneuropathy recently diagnosed with Graves disease, was referred to our department to evaluate his response to antithyroid drugs. His thyroid function tests were still consistent with hyperthyroidism while he had been receiving carbimazole 40 mg/d for 6 weeks. We found no signs of thyrotoxicosis on physical examination despite the “frank and severe” biochemical hyperthyroidism. Noticing that all the patient’s assays had been done at the same laboratory, we suspected assay interference. We therefore repeated the thyroid function tests at our hospital laboratory, which uses a different assay platform. Surprisingly, all the results were normal, confirming assay interference. The patient was taking an investigational “vitamin” therapy, which turned out to be biotin, prescribed at a dose of 100 mg tid as part of a trial of high-dose biotin in X-linked adrenomyeloneuropathy.
Conclusions:
This case should encourage physicians to ask their patients about possible biotin intake, especially when laboratory results are not compatible with clinical findings. If biotin interference is suspected, we propose either using a different assay not based on the streptavidin-biotin system or repeating the analyses after stopping biotin supplementation for one week.
Keywords: biotin, hormone assay, interference, Graves disease, hyperthyroidism
Interference of biotin treatment with immunoassays using the biotin-streptavidin system may mimic Graves disease, as shown in a patient treated with high doses of biotin for a neurologic disorder.
Hormone measurement is the cornerstone of diagnostic workup and patient follow-up in endocrinology. Great progress has been made in the past years to allow rapid and inexpensive evaluation of blood samples without compromising the quality and precision of hormone measurements. However, modern techniques have their own caveats, as illustrated by this case report.
1. Case Report
A 32-year-old man with X-linked adrenomyeloneuropathy and chronic depression treated with hydrocortisone 10 mg bid, fampiridine 10 mg bid, paroxetine 20 mg once a day, and three capsules per day of an “investigational drug” consulted his general practitioner because of gradual-onset erectile dysfunction. The general practitioner ordered laboratory tests, which revealed hyperthyroidism with suppressed serum thyrotropin (TSH) (0.012 mIU/L) controlled at 0.017 (normal range, 0.27 to 4.2), elevated serum free thyroxine (fT4), and elevated free triiodothyronine at respectively 79.2 pmol/L (normal range, 12 to 2.9) and 11.5 pmol/L (normal range, 3.1 to 6.8). The general practitioner referred the patient to an endocrinologist, who found no signs of thyrotoxicosis on physical examination. Thyroid function tests repeated in the same laboratory confirmed the markedly elevated free triiodothyronine and fT4 and suppressed serum TSH levels. Positivity for anti-TSH receptor antibodies (>40 IU/L; normal, <1.75 IU/L) (Table 1) established the diagnosis of Graves disease, and the patient was prescribed an antithyroid drug (carbimazole 40 mg/d).
Table 1.
Serial Thyroid Function Tests Performed on the Patient, First on the Roche Cobas e170 Platform Using the Biotin-Streptavidin System and Then on Other Platforms (Siemens ADVIA Centaur XP for Thyroid Hormones and BRAHMS for Anti-TSH Receptor Antibodies)
| Characteristic |
Roche Cobas e170 |
Siemens ADVIA Centaur XP and BRAHMS (TRAK) |
|||
|---|---|---|---|---|---|
| March 31 | May 30 | June 8 | July 12 | July 13 | |
| TSH, mIU/L | 0.012 | 0.017 (N: 0.27-4.2) | 0.052 | 1.83 (N: 0.3-4.5) | |
| fT4, pmol/L | 79.2 (N: 12-21.9) | >100 | 51.6 | 14.4 (N: 10-22.5) | |
| Free triiodothyronine, pmol/L | 11.5 (N: 3.1-6.8) | 17.7 | 9.7 | 5.6 (N: 3.1-6.5) | |
| Antithyroglobulin Ab, IU/mL | 159 (N <115) | <9 (N <60) | |||
| Anti-TSH receptor Ab, IU/L | >40 (N <1.75) | <0.3 (N <1.6) | |||
| Antithyroid drugs | — | — | Carbimazole 40 mg/d | Carbimazole 40 mg/d | Carbimazole 40 mg/d |
Abbreviation: N, normal range.
Follow-up thyroid function tests performed five weeks after carbimazole initiation showed a slight improvement (Table 1). Because his endocrinologist was on holiday, the patient was referred to our department to evaluate his treatment response. We were surprised by the lack of clinical signs of thyrotoxicosis despite the “frank and severe” biochemical hyperthyroidism. Noticing that all the patient’s assays had been done at the same laboratory, which used the Roche Cobas e170 immunoassay platform, we suspected assay interference. We therefore repeated the thyroid function tests at our hospital laboratory, which uses a different assay platform. Surprisingly, all the results were normal: in particular, he tested negative for antithyroglobulin and antithyroid peroxidase antibodies, as well as for anti-TSH receptor antibodies (Table 1).
When we questioned the patient about his medications, he drew our attention to an investigational “vitamin” therapy he was taking. We contacted the clinical research center and discovered that the investigational drug was in fact biotin, prescribed at a dose of 100 mg tid as part of a trial of high-dose biotin in X-linked adrenomyeloneuropathy (EudraCT Number: 2014-000698-38). This confirmed our suspicion that biotin had interfered in the hormone assays. Indeed, the first laboratory had used the streptavidin-biotin system, whereas our laboratory used a different platform. Carbimazole was thus stopped immediately.
2. Discussion
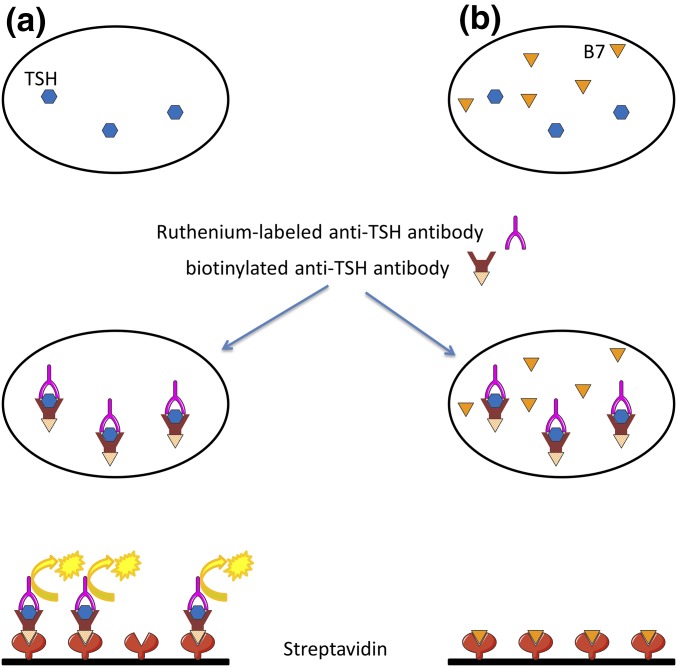
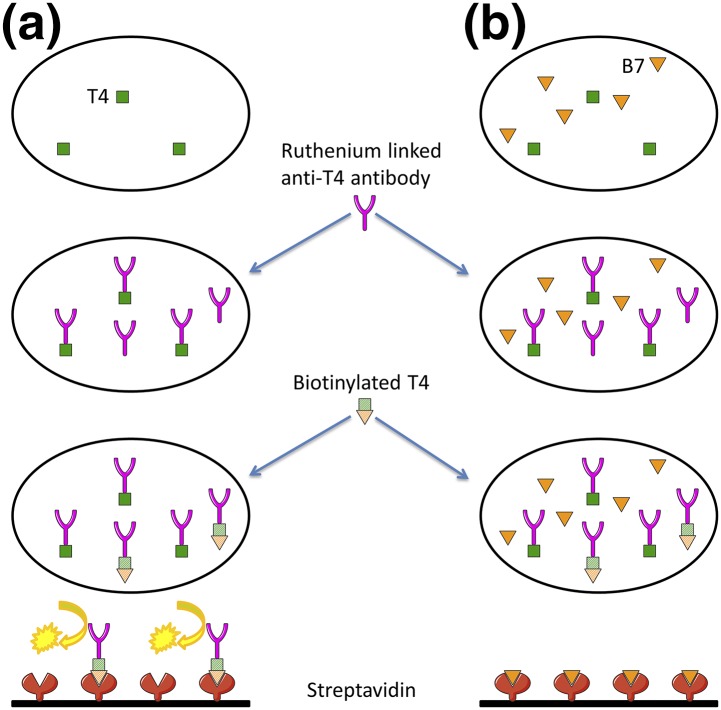
Biotin, also known as vitamin H or B7, is present in the normal diet and is an important cofactor in numerous enzymatic reactions. Its high affinity for streptavidin makes the streptavidin-biotin system very interesting for use in immunoassays. High biotin concentrations can interfere with such assays, leading to incorrect levels of the target ligand. Indeed, when the assay uses a sandwich method (TSH), the serum sample is incubated with a biotinylated monoclonal anti-TSH antibody and a ruthenium-labeled monoclonal anti-TSH antibody. The addition of streptavidin-coated magnetic microparticles causes the resulting immune complexes to bind to the solid phase. Application of a voltage generates chemiluminescence, in direct proportion to the TSH level [Fig. 1(a)]. A high biotin concentration in the sample reduces immune complex binding to the solid phase, yielding a falsely low TSH level [Fig. 1(b)]. When a competitive method is used (fT4), the serum sample is incubated with a ruthenium-linked antithyroxine antibody. Biotinylated thyroxine is then added and binds unoccupied sites on the ruthenium-linked antithyroxine antibody. The immune complexes bind to the streptavidin-coated magnetic microparticles, generating chemiluminescence, which is inversely proportional to the fT4 level [Fig. 2(a)]. A high biotin concentration in the sample saturates streptavidin binding sites, resulting in a falsely high fT4 level [Fig. 2(b)] [1].
Figure 1.
Mechanisms of biotin interference in the TSH assay. (a) The serum sample is incubated with a biotinylated monoclonal anti-TSH antibody and a ruthenium-labeled monoclonal anti-TSH antibody. The addition of streptavidin-coated magnetic microparticles causes the resulting immune complexes to bind to the solid phase. Application of a voltage generates chemiluminescence, in direct proportion to the TSH level. (b) A high biotin concentration in the sample saturates streptavidin binding sites, yielding a falsely low TSH level. B7, biotin.
Figure 2.
Mechanisms of biotin interference in competitive thyroxine (T4) assay. (a) The serum sample is incubated with a ruthenium-linked anti-T4 antibody. Biotinylated T4 is then added and binds unoccupied sites on the ruthenium-linked anti-T4 antibody. The immune complexes bind to the streptavidin-coated magnetic microparticles, generating chemiluminescence (inversely proportional to the fT4 level). (b) In the case of biotin excess, the high biotin concentration in the sample saturates streptavidin binding sites, resulting in a falsely high fT4 level. B7, biotin.
Biotin is commonly used to treat diffuse alopecia (15 mg/d, corresponding to 150 to 500 times the recommended daily intake) and as a nonspecific treatment of various skin and hair disorders. Recently, a pilot study suggested that high biotin doses (100 to 300 mg) might have an impact on disability and progression in multiple sclerosis [2].
Biotin interference with thyroid function immunoassays has already been reported (Table 2). This interference also affects assays of other hormones (parathyroid hormone, estradiol, testosterone, dehydroepiandrosterone sulfate, etc.), as well as some common analytes (β human chorionic gonadotropin, ferritin, troponins, tumor markers, etc.) [3]. Importantly, the minimal dose required for interference to occur, the duration of interference, and the magnitude of error are not known and might be analyte specific. Wijeratne et al. [4] studied the time-response curve after ingestion of 30 mg biotin and found that fT4 levels peaked (sevenfold) around two hours after biotin ingestion and remained elevated for 24 hours. Recently, Elston et al. [5] reported evidence of interference in thyroid function tests 16 hours after the last dose of biotin. Usually, serum TSH and fT4 levels return to normal 24 to 48 hours after biotin discontinuation, but anti-TSH receptor antibodies can take up to seven days to normalize [6].
Table 2.
Summary of Reported Cases of Biotin Interference in Thyroid Function Tests
| Reference | Age | Daily Biotin Dose | Thyroid Function Tests on Biotin |
Assay Method(s) | Consequences | Thyroid Function Tests After Discontinuing Biotina
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSH, mIU/L | fT4, pmol/L | fT3, pmol/L | Anti-TSH Receptor Ab, IU/L | TSH, mIU/L | fT4, pmol/L | fT3, pmol/L | Anti-TSH Receptor Ab, IU/L | |||||
| Elston et al. (5) | 3 d | 10 mg | 38.4 | 77 | ND | ND | Boehringer Mannheim ES700 | Delay in treating hypothyroidism | 140, >209 | 16.3, 11 | ND | ND |
| Elston et al. (5) | 3 y | 40 mg | 0.62 | 15.5 | 4.5 | ND | Roche Cobas e601 | None | 3.96 | 75.9 | 14 | ND |
| Wijeratne et al. (4) | 1 wk | 30 mg | 3.75 | >77.7 | 24.9 | ND | Beckman DxI | None | N | N | N | ND |
| Barbesino (1) | 55 y | 300 mg | 0.02 | >100.4 | ND | 36 | Elecsys, Roche | 123I thyroid scan | 0.78 | 18 | ND | <1.75 |
| Elston et al. (5) | 63 y | 300 mg | 0.02 | >100, 69 | 11.6 | >40 | Roche & Beckman | None | 1.93, 1.9 | 14, 17 | 4.4 | 2.3 |
| Kummer et al. (6) | 9 y | 10 mg/kg | 0.05 | 80.3 | ND | 38.6 | NR | None | 1.8 | 20.3 | ND | <0.3 |
| Kummer et al. (6) | 2 y | 14 mg/kg | 0.02 | >100 | ND | >40 | NR | Antithyroid drugs | 3.75 | 21.9 | ND | ND |
| Kummer et al. (6) | 2 y | 15 mg/kg | 0.04 | >100 | ND | >40 | NR | Antithyroid drugs | 6.07 | 14.9 | ND | 0.7 |
| Kummer et al. (6) | 5 mo | 2 mg/kg | 0.02 | >100 | ND | >40 | NR | None | 2.2 | 14.5 | ND | 1 |
| Kummer et al. (6) | 1 mo | 7 mg/kg | 0.08 | >100 | ND | >40 | NR | None | 8.12 | 23.7 | ND | 0.4 |
| Kummer et al. (6) | 1 mo | 8 mg/kg | 0.03 | >100 | ND | >40 | NR | Antithyroid drugs | 2.87 | 24.6 | ND | <0.3 |
| Trambas et al. (3) | NR | 300 mg | 0.02 | >100 | 17.3 | ND | NR | NR | 1.3 | 11.3 | 4.5 | ND |
| Simó-Guerrero et al. (8) | 38 y | 300 mg | 0.07 | 50.1 | ND | ND | Roche, Modular E170 | None | 2.34 | 13.3 | ND | ND |
| Bülow Pedersen et al. (9) | 4 d | 5 mg | 0.1 | ND | ND | ND | NR | None | 4.3 | ND | ND | Negative |
| Minkovsky et al. (10) | 74 y | 300 mg | 0.02 | >100.4 | ND | ND | Roche | 123I thyroid scan | 4.54 | 19.3, 21.9 | ND | ND |
Abbreviations: fT3, free triiodothyronine; ND, not done; NR, not reported.
Results of thyroid functions tests after using a different assay are in bold.
In practice, although packet inserts for laboratory kits that use the streptavidin-biotin system contain a warning on biotin interference, not all clinicians are aware of this pitfall. The case reported here should encourage physicians to ask their patients about possible biotin intake, especially when laboratory results are not compatible with clinical findings. If biotin interference is suspected, then biotin supplementation should be stopped for two to three days before repeating the assays. Alternatively, such bizarre results should be controlled with a different assay not based on the streptavidin-biotin system or by using a simple procedure designed to suppress biotin interference by means of streptavidin-coated microparticules, which we recently proposed [7].
Acknowledgments
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- fT4
- free thyroxine
- TSH
- thyrotropin.
References and Notes
- 1.Barbesino G. Misdiagnosis of Graves’ disease with apparent severe hyperthyroidism in a patient taking biotin megadoses. Thyroid. 2016;26(6):860–863. [DOI] [PubMed] [Google Scholar]
- 2.Sedel F, Papeix C, Bellanger A, Touitou V, Lebrun-Frenay C, Galanaud D, Gout O, Lyon-Caen O, Tourbah A. High doses of biotin in chronic progressive multiple sclerosis: a pilot study. Mult Scler Relat Disord. 2015;4(2):159–169. [DOI] [PubMed] [Google Scholar]
- 3.Trambas CM, Sikaris KA, Lu ZX. More on biotin treatment mimicking Graves’ disease. N Engl J Med. 2016;375(17):1698. [DOI] [PubMed] [Google Scholar]
- 4.Wijeratne NG, Doery JC, Lu ZX. Positive and negative interference in immunoassays following biotin ingestion: a pharmacokinetic study. Pathology. 2012;44(7):674–675. [DOI] [PubMed] [Google Scholar]
- 5.Elston MS, Sehgal S, Du Toit S, Yarndley T, Conaglen JV. Factitious Graves’ disease due to biotin immunoassay interference: a case and review of the literature. J Clin Endocrinol Metab. 2016;101(9):3251–3255. [DOI] [PubMed] [Google Scholar]
- 6.Kummer S, Hermsen D, Distelmaier F. Biotin treatment mimicking Graves’ disease. N Engl J Med. 2016;375(7):704–706. [DOI] [PubMed] [Google Scholar]
- 7.Piketty ML, Prié D, Sedel F, Bernard D, Hercend C, Chanson P, Souberbielle J-C. High-dose biotin therapy leading to false biochemical endocrine profiles: validation of a simple method to overcome biotin interference [published online ahead of print February 21, 2017]. Clin Chem Lab Med. [DOI] [PubMed] [Google Scholar]
- 8.Simó-Guerrero O, Giménez-Pérez G, Recasens-Gracia A, Villà-Blasco C, Castells-Fusté I. False overt hyperthyroidism by interference in immunoassay [in Spanish]. Endocrinol Nutr. 2016;63(8):431–432. [DOI] [PubMed] [Google Scholar]
- 9.Bülow Pedersen I, Laurberg P. Biochemical hyperthyroidism in a newborn baby caused by assay interaction from biotin intake. Eur Thyroid J. 2016;5(3):212–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minkovsky A, Lee MN, Dowlatshahi M, Angell TE, Mahrokhian LS, Petrides AK, Melanson SE, Marqusee E, Woodmansee WW. High-dose biotin treatment for secondary progressive multiple sclerosis may interfere with thyroid assays. AACE Clin Case Rep. 2016;2(4):e370–e373. [DOI] [PMC free article] [PubMed] [Google Scholar]