Abstract
Context:
Peripubertal hyperandrogenemia—a precursor to polycystic ovary syndrome—is prominent in girls with obesity.
Objective:
We examined sources of overnight testosterone (T) and progesterone (P4) and potential sources of obesity-associated hyperandrogenemia during puberty.
Design:
Cross-sectional.
Setting:
Research unit.
Participants/Interventions:
Fifty girls ages 7 to 18 years—both normal weight (NW) and overweight (OW)—underwent dexamethasone (DEX) suppression (1 mg orally; 10 pm) and cosyntropin stimulation testing (0.25 mg intravenously; 8 am the following day), with blood sampled before and 30 and 60 minutes after cosyntropin. Thirty-nine subjects receiving DEX had frequent blood sampling overnight (every 10 minutes from 10 pm to 7 am) and were compared with 70 historical controls who did not receive DEX.
Outcomes:
Random coefficient regression modeling assessed changes in hormone concentrations after DEX and cosyntropin.
Results:
NW early pubertal controls exhibited early morning increases in free T and P4 levels; NW and OW late pubertal controls exhibited early morning increases in P4. Such changes were not observed in subjects receiving DEX. Post-DEX morning free T levels were fourfold higher in OW vs NW late pubertal girls. Postcosyntropin changes in free T and androstenedione were both 2.3-fold higher in OW vs NW late pubertal girls.
Conclusions:
These data suggest that (1) overnight increases in free T and P4 concentrations in NW early pubertal girls and P4 concentrations in late pubertal girls are of adrenal origin and (2) OW late pubertal girls demonstrate elevated nonadrenal free T levels after DEX and exaggerated adrenal androgen (free T and androstenedione) responses to cosyntropin.
Keywords: androgen; hyperandrogenemia; adolescent, puberty; obesity
Overweight late pubertal girls demonstrated higher free testosterone (T) after dexamethasone suppression and greater free T and androstenedione responses to cosyntropin stimulation.
Adolescent hyperandrogenemia (HA) can be a forerunner to polycystic ovary syndrome (PCOS), the leading cause of female infertility and a contributor to metabolic disarray [1–5]. Childhood obesity also increases PCOS risk and is highly prevalent: approximately 20% of peripubertal US girls have a body mass index-for-age percentile (BMI%) ≥95 [6, 7]. Our data suggest that about 65% of obese girls have HA, even during pre/early puberty [8]; however, the sources of androgen excess in such girls remain unclear. Although the ovary is the primary source of HA in PCOS adolescents and women, some demonstrate adrenal HA as well [9–11]. In addition, the developmental timing of ovarian vs adrenal androgen overproduction in peripubertal girls with HA is uncertain. These represent important gaps in knowledge because PCOS prevention strategies may be most effective during puberty.
Normal daily patterns of sex steroid concentrations observed in early pubertal girls might provide clues to the origins of obesity-associated HA. In normal pre/early pubertal girls, testosterone (T) and progesterone (P4) concentrations increase from late evening to early morning—similar to adrenocorticotropic hormone (ACTH) and cortisol—consistent with an ACTH-dependent adrenal source [12, 13]. Estradiol (E2) levels are low before thelarche, suggesting limited ovarian activity before puberty; however, it remains possible that ovarian androgens may contribute to pre- and early pubertal HA. For example, nonobese girls with premature adrenarche have increased ovarian androgens at thelarche [14]. Regardless, the sources of androgen excess in unselected girls with obesity remain unclear.
We used adrenal suppression and stimulation protocols in normal weight (NW) and overweight (OW) peripubertal girls to examine sources of overnight T and P4 concentrations and to clarify how excess weight influences both adrenal and nonadrenal androgens across puberty. We hypothesized that the adrenal gland is the major source of overnight rises in T and P4 concentrations and a contributor to adiposity-related HA throughout puberty.
1. Subjects and Methods
The University of Virginia (UVA) and University of California San Diego institutional review boards approved all study procedures.
A. Subjects
Healthy and overweight volunteers were recruited for adolescent hormone studies using local advertisements and from clinics at UVA and the University of California San Diego. Exclusion criteria included syndromic obesity, BMI% <5, diabetes, inadequately treated thyroid disorders, central precocious puberty, adrenal disorders (except premature adrenarche), morning follicular phase 17-hydroxyprogesterone (17-OH Prog) >200 ng/dL, hyperprolactinemia, and total T >150 ng/dL [15]. Subjects were not excluded on the basis of clinical androgen excess or biochemical HA. No subject had taken medications affecting the reproductive system, adrenal gland, or glucose metabolism for at least 90 days before the study.
Fifty girls (Table 1) ages 7 to 18 years of age were categorized by pubertal status [pre/early pubertal (Tanner 1 to 2 by breast palpation; “early”) vs mid/late pubertal (Tanner 3 to 5; “late”)] and weight status [NW (BMI% 5 to 84) vs OW (BMI% ≥85)]. We performed adrenal suppression and stimulation protocols in 10 early pubertal girls [4 NW (BMI% 10 to 67), 6 OW (BMI% 98 to 99.9)] and 40 late pubertal girls [10 NW (BMI% 14 to 80), 30 OW (BMI% 88 to 99.9)].
Table 1.
Subject Characteristics
|
Early Pubertal |
Late Pubertal |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
NW |
OW |
NW |
OW |
||||||
| Current Subjects | Historical Controls | Current Subjects | Historical Controls | Current Subjects | Historical Controls | Current Subjects | Historical Controls | ||
| n | 4 | 8 | 6 | 5 | 10 | 32 | 30 | 25 | |
| Anthropometrics/Metabolism | Tanner stage | 1.5 ± 0.3 | 1.4 ± 0.2 | 1.9 ± 0.2 | 1.7 ± 0.2 | 4.2 ± 0.4 | 4.2 ± 0.2 | 4.7 ± 0.1 | 4.8 ± 0.1 |
| Age (y) | 9.8 ± 1.0 | 9.9 ± 0.8 | 9.4 ± 0.7 | 9.3 ± 0.4 | 13.9 ± 0.9 | 14.1 ± 0.4 | 14.9 ± 0.4 | 15.4 ± 0.3 | |
| Bone age (y) | 9.0 ± 1.0 | 9.6 ± 1.1 | 10.2 ± 0.6 | 10.6 ± 0.5 | 14.3 ± 0.8 | 13.8 ± 0.7 | 16.0 ± 0.3 | 16.6 ± 0.5 | |
| BA:CA | 0.93 ± 0.10 | 0.94 ± 0.05 | 1.09 ± 0.06 | 1.13 ± 0.07 | 1.04 ± 0.03 | 1.06 ± 0.03 | 1.10 ± 0.2 | 1.09 ± 0.3 | |
| BMI Z-score | −0.43 ± 0.30 | −0.52 ± 0.25 | 2.25 ± 0.09 | 2.24 ± 0.19 | 0.19 ± 0.17 | 0.15 ± 0.15 | 2.00 ± 0.08 | 1.92 ± 0.09 | |
| BMI % | 38.0 ± 11.8 | 33.2 ± 8.0 | 98.8 ± 0.3 | 97.9 ± 1.1 | 57.8 ± 5.9 | 56.0 ± 5.1 | 96.6 ± 0.7 | 95.7 ± 0.7 | |
| Insulin (mIU/mL) | 3.7 ± 1.0 | 7.6 ± 1.9 | 10.2 ± 2.1 | 20.2 ± 5.4 | 7.0* ±1.8 | 15.8 ± 2.3 | 21.9 ± 3.5 | 26.3 ± 2.4 | |
| Puberty | LH (mIU/mL) | 0.3 ± 0.2 | 1.0 ± 0.5 | 0.2 ± 0.1 | 0.7 ± 0.4 | 3.8 ± 1.1 | 6.5 ± 0.7 | 7.1 ± 2.0 | 5.7 ± 0.7 |
| FSH (mIU/mL) | 2.7 ± 0.6 | 2.0 ± 0.4 | 2.2 ± 0.6 | 1.3 ± 0.5 | 4.7 ± 0.5 | 4.9 ± 0.4 | 4.8 ± 0.6 | 4.1 ± 0.2 | |
| E2 (pg/mL) | 17.3 ± 5.2 | 17.8 ± 4.0 | 27.5 ± 5.4 | 27.5 ± 2.9 | 45.3 ± 10.6 | 56.1 ± 5.9 | 69.0 ± 13.4 | 61.8 ± 5.6 | |
| P4 (ng/mL) | 0.1 ± 0.0 | 0.4 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.0 | 2.2 ± 0.9 | 0.6 ± 0.1 | |
| IGF-1 (ng/mL) | 267.2 ± 42.4 | 282.3 ± 38.6 | 163.3 ± 23.3 | 194.8 ± 25.8 | 446.1 ± 38.1 | 412.5 ± 19.5 | 442.2a ± 31.8 | 292.3 ± 25.0 | |
| Androgens | Free T (pmol/L) | 3.8 ± 0.8 | 4.1 ± 1.3 | 6.1 ± 0.8 | 4.5 ± 1.0 | 9.8 ± 2.2 | 15.4 ± 1.9 | 36.7 ± 5.3 | 35.2 ± 6.9 |
| Total T (ng/dL) | 10.2 ± 0.2 | 8.7 ± 2.4 | 9.2 ± 0.8 | 6.3 ± 1.3 | 23.6 ± 7.1 | 26.4 ± 2.9 | 41.6 ± 5.7 | 47.0 ± 4.8 | |
| SHBG (nmol/L) | 83.0 ± 22.8 | 59.5 ± 6.6 | 31.1 ± 4.0 | 29.9 ± 6.0 | 53.2 ± 6.0 | 41.5 ± 4.3 | 21.1 ± 2.3 | 22.0 ± 2.7 | |
| 17-OH Prog (ng/dL) | 79.0 ± 2.1 | NA | 92.7 ± 5.1 | NA | 116.4 ± 13.1 | NA | 138.4 ± 11.9 | NA | |
| DHEA-S (µg/dL) | 49.5 ± 10.7 | 33.6 ± 8.8 | 53.1 ± 7.4 | 50.4 ± 11.9 | 111.8 ± 14.5 | 116.7 ± 15.3 | 156.8 ± 21.7 | 152.0 ± 15.2 | |
Clinical, anthropometric, and screening laboratory characteristics of subjects receiving DEX and cosyntropin compared with historical controls not receiving DEX. Screening laboratories were not collected based on menstrual cycle timing; some levels of LH, FSH, E2, P4, free/total T, and 17-OH Prog may be affected. Values reported represent mean ± standard error of the mean.
Abbreviations: BA:CA, bone age to chronological age ratio; DHEA-S, DHEA-sulfate; NA, not available.
P < 0.01 for subjects vs controls within each weight and pubertal stage group.
Overnight frequent blood sampling (described in the following section) was performed (at the discretion of individual subjects) in 39 girls (4 NW, 6 OW early pubertal; 10 NW, 19 OW late pubertal). Overnight data from these subjects were compared with 70 historical controls who underwent identical sampling but had not received dexamethasone (DEX; 8 NW, 5 OW early pubertal; 32 NW, 25 OW late pubertal; Table 1) [12]. Ten OW late pubertal controls did not have luteinizing hormone (LH) available for comparison.
B. Study Procedures
Informed consent and assent were obtained from custodial parents and subjects, respectively. Screening medical history, physical examination, and fasting morning blood samples were performed as previously described [12]. A subset of subjects/controls had bone age x-rays evaluated by a single pediatric endocrinologist (C.M.B.S.) using the Greulich-Pyle method [16].
Postmenarchal girls underwent formal study during the mid-follicular phase (cycle days 7 to 10); oligomenorrheic girls were studied ≥60 days after last menstrual period; and premenarcheal girls were studied as convenient. In all subjects, serum P4 ≤2 ng/mL excluded study during the luteal phase.
All subjects received DEX (1 mg orally) at 10 pm the night before adrenal stimulation. Cosyntropin (synthetic ACTH, 0.25 mg intravenously) was administered at 8 am the following morning. Blood was drawn via indwelling intravenous line for cortisol, T, P4, androstenedione (A4), 17-OH Prog, 17-hydroxypregnenolone, dehydroepiandrosterone (DHEA), and E2 levels immediately before (baseline) and 30 and 60 minutes after cosyntropin.
For 39 subjects, frequent blood sampling was performed the night before cosyntropin administration. Blood was obtained via indwelling intravenous line every 10 minutes from 7 pm through 7 am for LH, T, and P4 levels (steroid hormones analyzed in 2-hour pools). Formal “lights out” (11 pm through 7 am) facilitated sleep. Seventy historical controls underwent similar overnight frequent blood sampling but did not receive DEX.
C. Hormone Measurements
The UVA Center for Research in Reproduction Ligand Assay and Analysis Core Laboratory analyzed each subject’s samples in duplicate in the same assay using serum for each hormone. Samples with measured values below an assay’s functional sensitivity [i.e., the lowest concentration with accuracy to a known standard within 20% and intra-assay coefficient of variation (CV) <20%] were assigned a value slightly below the assay’s sensitivity. LH, follicle-stimulating hormone (FSH), and insulin-like growth factor 1 (IGF-1) were measured by chemiluminescence (Immulite 2000, Siemens, Los Angeles, CA; sensitivity, 0.1 IU/L for LH/FSH; 25 ng/mL for IGF-1; intra-assay CVs, 3.2% to 3.5%; interassay, CVs 4.9% to 6.6%). DHEA was measured by enzyme-linked immunosorbent assay (ALPCO, Salem, NH; sensitivity, 0.4 ng/mL; intra-assay, CV 5.7%; interassay CV, 8.0%). Total T, E2, P4, A4, and 17-OH Prog were measured by radioimmunoassay (Siemens; sensitivities, 5 ng/dL, 10 pg/mL, 0.1 ng/mL, 0.1 ng/mL, and 0.1 ng/mL; intra-assay CVs, 4.3% to 6.1%; interassay CVs, 7.1% to 8.1%). The UVA Ligand Laboratory T assay demonstrates good correlation with commercial liquid chromatography tandem mass spectrometry [17]. We adjusted measured P4 concentrations for minor assay cross-reaction with cortisol (0.03%) [18]. Sex hormone–binding globulin (SHBG), DHEA-sulfate, cortisol, and insulin were measured by chemiluminescence (Immulite 2000; sensitivities 0.2 nmol/L, 7 µg/dL, 1 µg/dL, and 2.6 µIU/mL; intra-assay CVs, 2.5% to 4.8%; interassay CVs, 5.2% to 7.9%). Free T was calculated from total T and SHBG levels, assuming an albumin concentration of 4.3 g/dL [19].
D. Statistical Methods
D-1. LH characteristics and screening data
LH pulses were identified using the computer algorithm Cluster 7 [20]. Mean LH concentration and pulse frequency (interpulse interval) were analyzed in 4-hour time blocks: 7:00 pm to 10:50 pm (represents time awake); 11:00 pm to 2:50 am and 3:00 am to 6:50 am (represents time asleep, time block values averaged for analysis) [12, 21]. LH secretory characteristics and screening data for each pubertal-weight group (see the following section) were compared between subjects receiving DEX and historical controls using two-tailed Student t tests with unequal variance.
D-2. DEX suppression
Overnight changes in free T and P4 levels were analyzed via random coefficient piecewise regression using loge picomole/L and nanogram/mL scales, respectively, to satisfy homoscedastic variance and normality assumptions. Predictor variables were DEX exposure, pubertal status, weight status, and sampling time; the latter was assigned as the midpoint of each 2-hour pool (e.g., 1:55 am for the 1:00 am to 2:50 am pool). To account for an expected morning increase in endogenous ACTH, a change point was incorporated into the model at 1:55 am; this allowed us to compare evening (7:55 pm to 1:55 am) vs early morning (1:55 am to 5:55 am) changes in free T and P4 levels. The random coefficient piecewise regression model was decomposed into eight submodels (early pubertal NW ± DEX, early pubertal OW ± DEX, late pubertal NW ± DEX, and late pubertal OW ± DEX). For each model, the regression intercept coefficient represents the estimated mean hormone concentration at sampling start (i.e., 7:55 pm [before DEX administration]) and the regression slope coefficients represents the average linear rate of change in hormone levels during evening hours (7:55 pm to 1:55 am, with DEX given at 10:00 pm) and early morning hours (1:55 am to 6:55 am). For each study group (i.e., early pubertal NW, early pubertal OW, late pubertal NW, late pubertal OW), we compared regression coefficients (i.e., intercepts, evening slopes, and early morning slopes) between girls who did and did not receive DEX using Student t tests. We tested for nonzero linear slopes and differential linear trends related to DEX exposure, puberty status, and weight status. A Bonferroni-corrected two-sided P < 0.05 criterion was used to reject the null hypothesis.
D-3. Cosyntropin stimulation
Hormone levels were analyzed via random coefficient regression, using pubertal status, weight status, sampling time (0, 30, and 60 minutes), and the square of the sampling time (0, 900, and 3600 minutes2) as predictor variables, assessing for linear and quadratic trends in hormone concentration response to cosyntropin. Type III F tests were used to assess the influences of pubertal and weight statuses on hormone concentration vs time relationships. To control type 1 error, global F tests were performed to assess the null hypothesis that all random coefficient regression model parameters (i.e., intercept, linear slope, and quadratic coefficient) were the same irrespective of pubertal or weight status. If the global test yielded a P value ≤0.1, then we compared parameters between groups (NW early pubertal, OW early pubertal, NW late pubertal, and OW late pubertal) using Student t tests with a two-sided P < 0.05 criterion.
2. Results
A. Screening Characteristics
When considered according to pubertal and weight groupings, current subjects and historical controls were generally well-matched for biochemical and anthropometric data (Table 1). Of the controls, 2 NW and 5 OW late pubertal girls had hirsutism alone; 2 NW and 4 OW late pubertal girls had oligomenorrhea (defined as <9 menses/y if ≥2 years postmenarche) alone; and 1 NW and 13 OW late pubertal girls had both. Of subjects, seven OW late pubertal girls had hirsutism alone; five OW late pubertal girls had oligomenorrhea alone; and one NW and five OW late pubertal girls had both. Of all participants (current subjects and historical controls), one OW early pubertal girl (current subject) had adrenarche at age 7.
B. Overnight LH Concentrations and Pulse Frequency
Within each pubertal-weight grouping, mean LH concentrations and interpulse intervals were similar regardless of DEX exposure, with one exception: NW early pubertal girls receiving DEX had longer interpulse intervals while asleep compared with controls (344 ± 31 minutes [mean ± standard error of the mean] vs 199 ± 27 minutes, P = 0.009).
C. DEX Suppression
In subjects, median morning cortisol concentration after DEX [average of 5:55 am pool (if available) and 7:00 am samples] was <1 µg/dL (interquartile range, <1 to 1), confirming adequacy of ACTH suppression.
C-1. Free T
Evening baseline free T concentrations (i.e., intercept values; ~7:55 pm) were higher in each late pubertal group compared with corresponding early pubertal groups, both in the presence and absence of DEX treatment (P < 0.001 for all comparisons) (Fig. 1; Supplemental Table 1 (14.9KB, docx) ). Similarly, evening baseline free T values were higher in each OW group compared with corresponding NW groups, regardless of DEX treatment (P ≤ 0.001 for all comparisons).
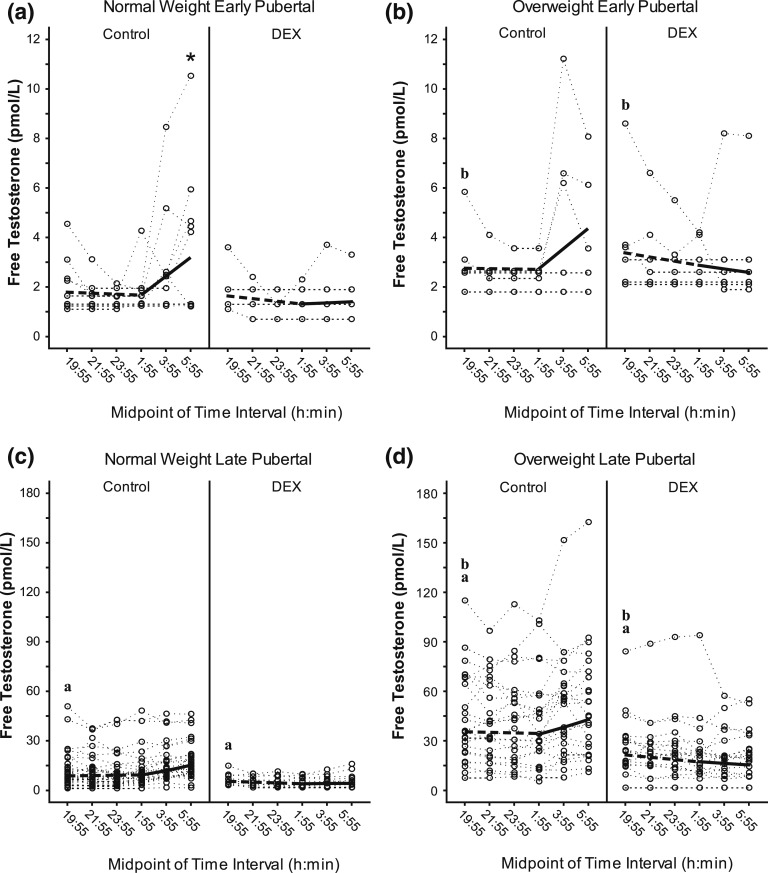
Figure 1.
Free T during DEX suppression test. The figure shows overnight free T (pmol/L) in early pubertal—(a) normal weight, (b) overweight—and late pubertal—(c) normal weight, (d) overweight—girls who did and did not receive DEX (i.e., DEX and control, respectively). Dotted lines represent individual subject responses. Dashed (first 6 hours) and solid lines (last 4 hours) identify predicted free T. Different y-axis scales are used for early vs late pubertal subjects. Darker dotted lines represent overlap of individual responses. The letter “a” in the graphs designates a significant difference in intercept between late vs early puberty among girls with the same weight and DEX status (P < 0.001); “b” designates a significant difference in intercept between OW vs NW among girls with the same pubertal and DEX status (P ≤ 0.001). *Both (a) an early morning slope significantly different from zero (P = 0.014) and (b) a significant difference between evening vs morning slope (P < 0.001).
In NW early pubertal controls, free T levels rose in the early morning (1:55 to 5:55 am; P = 0.014), and the early morning slope was greater than evening slope (7:55 pm to 1:55 am; P < 0.001) [Fig. 1(a)]. However, NW early pubertal subjects receiving DEX demonstrated no early morning increase in free T concentrations, and evening and early morning slopes were not different. Although other subgroups exhibited similar patterns [Fig. 1(b–d)], neither evening nor early morning free T slopes significantly differed according to DEX, pubertal, or weight status.
Together, these data suggest that (1) evening free T values increase across pubertal development and with excess weight and (2) free T levels increase during early morning hours in NW early pubertal girls, but DEX administration prevents this increase. A potential caveat is that apparently flat slopes for some girls may partly reflect total testosterone values near functional assay sensitivity.
C-2. Progesterone
Evening baseline (intercept) P4 values were higher in late vs early pubertal NW girls not receiving DEX and in late vs early pubertal OW girls regardless of DEX exposure (P < 0.001 for each) (Fig. 2; Supplemental Table 1 (14.9KB, docx) ). In NW early pubertal controls (i.e., not receiving DEX), early morning slope trended to be positive (unadjusted P = 0.037; Bonferroni-corrected P = 0.111) and was greater than the evening slope (Bonferroni-corrected P = 0.034). P4 concentrations rose in early morning for both late pubertal control groups (NW girls, P = 0.005; OW girls, P = 0.002), with slopes greater than evening (P < 0.001 for both). However, corresponding subjects receiving DEX demonstrated no early morning P4 level changes.
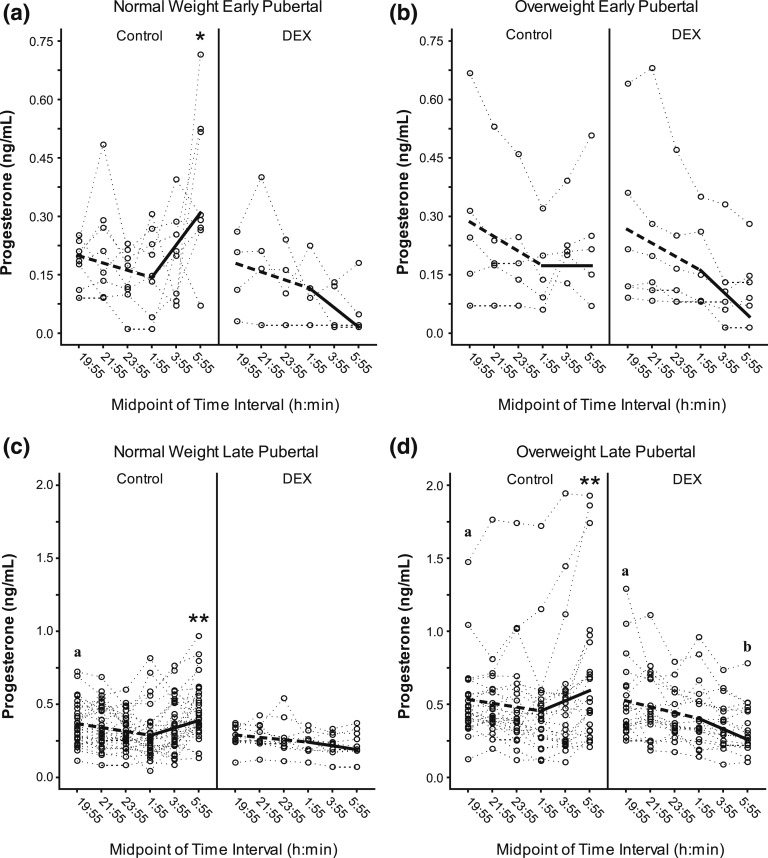
Figure 2.
Progesterone during DEX suppression test. The figure shows overnight P4 (in nanograms per milliliter) in early pubertal—(a) normal weight, (b) overweight—and late pubertal—(c) normal weight, (d) overweight—girls who did and did not receive dexamethasone (i.e., DEX and control, respectively). Dotted lines represent individual subject responses. Dashed (first 6 hours) and solid lines (last 4 hours) identify predicted P4. Different y-axis scales are used for early vs late pubertal subjects. Darker dotted lines represent overlap of individual responses. The letter “a” in the graphs designates a significant difference in intercept between late vs early puberty among girls with the same weight and DEX statuses (P < 0.001). Asterisks designate significant differences in evening vs morning slopes (*P < 0.05, **P < 0.001). The letter “b” in the graphs designates a significant difference between early morning slope in DEX vs no DEX among girls with the same pubertal and weight status (P < 0.05).
Among OW late pubertal girls, DEX administration was associated with significantly lower early morning P4 slope (P = 0.010) [Fig. 2(d)]. Although other subgroups exhibited similar patterns [Fig. 2(a–c)], DEX-related differences were not statistically demonstrable. There were no demonstrable weight- or puberty-related differences in slopes.
Overall, these data suggest that evening P4 values increase across pubertal development in most girls and DEX prevents early morning increases in P4 concentrations, particularly in OW late pubertal girls.
D. Cosyntropin Stimulation
Cortisol concentrations appropriately increased after cosyntropin in every group (P < 0.001), with no differences among groups.
D-1. Free T
Intercept values were significantly greater than zero in late pubertal girls only, implying a nonadrenal (ovarian) contribution to DHEA levels in late puberty only (Fig. 3, Supplemental Table 2 (15.5KB, docx) ). Mean DEX-suppressed free T levels (i.e., regression intercept)—ostensibly from a nonadrenal source (e.g., ovary)—were significantly greater than zero only in OW late pubertal girls. DEX-suppressed free T levels were 3.7-fold higher in late vs early pubertal NW girls; this was not statistically significant, possibly reflecting small numbers of early pubertal NW girls (n = 4). OW late pubertal girls had 8.3-fold higher DEX-suppressed free T levels compared with OW early pubertal girls (P = 0.002). These findings imply increased nonadrenal (ovarian) free T values across puberty, at least in OW girls. Although mean DEX-suppressed free T levels were 84% higher in OW vs NW early pubertal girls, this was not statistically significant. DEX-suppressed free T level was fourfold higher in OW vs NW late pubertal girls (P = 0.001), suggesting that overweight status is associated with increased nonadrenal (ovarian) androgens in late pubertal girls.
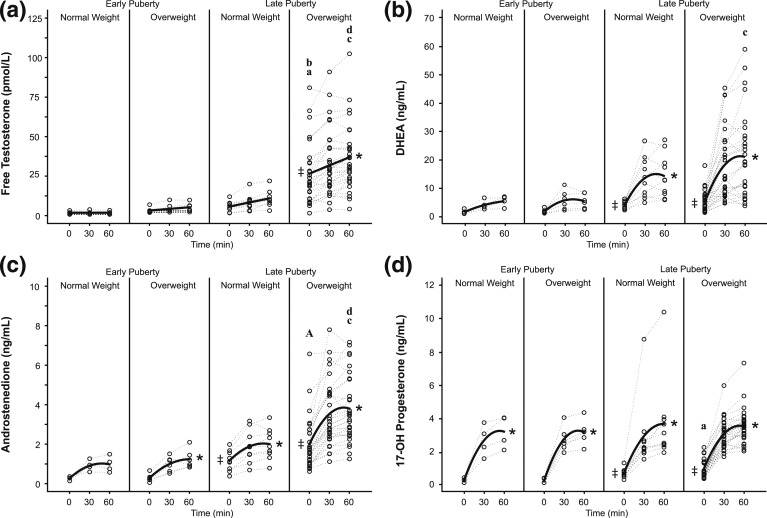
Figure 3.
Cosyntropin stimulation test. Regression analyses of adrenal hormones—(a) free testosterone, (b) dehydroepiandrosterone, (c) androstenedione, (d) 17-OH progesterone—are shown. Dotted and solid lines identify observed individual and predicted hormone levels, respectively. ‡Statistically positive mean DEX-suppressed baseline values [i.e., 95% confidence interval (CI) of regression intercept does not include zero]. Asterisks designate statistically significant slopes [i.e., 95% CI of response rate (per hour) does not include zero]. In the graphs, the letter “a” designates a significant difference in intercept between late vs early puberty among girls with the same weight status (aP < 0.05, AP < 0.001); “b” designates a significant difference in intercept between OW vs NW among girls with the same pubertal status (P ≤ 0.001); “c” designates a significant difference in slope between late vs early puberty among girls with the same weight status (P < 0.05); and “d” designates a significant difference in slope between OW vs NW among girls with the same pubertal status (P < 0.05).
Changes in free T levels after cosyntropin (i.e., regression slopes)—representing an adrenal source—were not different from zero in either early pubertal group. Changes in free T levels were not different between NW and OW early pubertal girls or between early and late pubertal NW girls. After cosyntropin, free T levels trended toward an increase in NW late pubertal girls (P = 0.053), whereas free T levels markedly increased in OW late pubertal girls (P < 0.001), with a 2.3-fold greater slope in the OW vs NW late pubertal groups (P = 0.033). The increase in free T level after cosyntropin was 5.2-fold greater in late vs early pubertal OW girls (P = 0.013). These data suggest that OW late pubertal girls exhibit elevated adrenal free T levels in response to ACTH.
Total T concentration results are reported in the Supplemental Methods and Materials (19.6KB, docx) .
D-2. Progesterone
DEX-suppressed (intercept) P4 values, representing nonadrenal sources, were greater than zero in late pubertal OW girls only (Supplemental Table 2 (15.5KB, docx) ). Although DEX-suppressed P4 concentrations appeared to be fourfold higher in late vs early pubertal OW girls, this difference was not statistically significant (P = 0.052). P4 values increased after cosyntropin in every group (P < 0.001), suggesting an adrenal contribution to P4 levels. Slopes did not differ among groups, except for an 18% decrease in late vs early OW girls (P = 0.013).
D-3. DHEA
DEX-suppressed (intercept) DHEA concentrations did not differ by weight status. Intercept values were significantly greater than zero in late pubertal girls only, implying a nonadrenal (ovarian) contribution to DHEA levels in late puberty only (Fig. 3; Supplemental Table 2 (15.5KB, docx) ). DEX-suppressed DHEA trended toward 3.2-fold higher levels in late vs early pubertal OW girls (P = 0.052). DHEA levels increased after cosyntropin in both late pubertal groups (P < 0.001 for both), with a threefold higher slope in late vs early pubertal OW girls (P = 0.013), but no significant differences according to weight status.
D-4. A4
DEX-suppressed (intercept) A4 values did not differ by weight status. DEX-suppressed A4 intercept values were greater than zero in late pubertal girls only and were 6.9-fold higher in late vs early pubertal OW girls (P = 0.001), suggesting increased nonadrenal (ovarian) contribution to A4 concentrations in late puberty (Fig. 3, Supplemental Table 2 (15.5KB, docx) ). A4 levels significantly increased after cosyntropin in all groups except for early pubertal NW girls (early OW, P = 0.050; late NW, P = 0.012; late OW, P < 0.001), with 2.3-fold higher response rates in OW vs NW late pubertal girls (P = 0.005) and in late vs early pubertal OW girls (P = 0.020). Overall, these data suggest that adrenal A4 levels in response to ACTH are elevated in OW late pubertal girls.
D-5. 17-OH Prog
DEX-suppressed (intercept) 17-OH Prog values were greater than zero in late pubertal girls only, with 6.3-fold higher levels in late vs early pubertal OW girls (P = 0.003), suggesting increased ovarian levels in late puberty (Fig. 3, Supplemental Table 2 (15.5KB, docx) ). 17-OH Prog levels increased after cosyntropin in all groups (P ≤ 0.001), but slopes did not differ according to either pubertal or weight status.
D-6. Additional analyses
Baseline (DEX-suppressed) and cosyntropin-stimulated 17OH Preg and E2 concentrations are described in Supplemental Materials (19.6KB, docx) . Also, we performed a secondary analysis of cosyntropin-stimulated free T, A4, and 17-OH Prog levels after excluding data from one NW late pubertal girl who had an elevated 17-OH Prog response (peak 10.42 ng/mL) despite a normal screening level (1.23 ng/mL) and no clinical signs of HA (Supplemental Table 3 (13.6KB, docx) ). Briefly, this secondary analysis suggested that OW late pubertal girls had 70% higher DEX-suppressed A4 levels (P = 0.052), a 2.0-fold higher free T slope (P = 0.066) after cosyntropin, a 2.1-fold higher A4 slope (P = 0.011), and a 35% higher 17-OH Prog slope (P = 0.033) compared with NW late pubertal girls.
3. Discussion
The sources of androgen overproduction—a risk factor for PCOS—across pubertal maturation remain unclear [1, 2]. The purpose of our study was twofold. First, we aimed to test whether the adrenal gland is a major contributor to the diurnal patterns of serum T and P4 concentrations during puberty in girls, using DEX to suppress overnight ACTH and comparing resulting T and P4 levels to girls who had not received DEX. Second, we aimed to assess how excess weight influences adrenal androgens in peri-pubertal girls by examining sex steroid concentration responses to DEX suppression and cosyntropin stimulation. Although we did not directly assess ovarian sex steroids, we inferred that DEX-suppressed sex steroid concentrations primarily reflected a nonadrenal source.
In the overnight studies, DEX abolished the previously observed early morning increases of free T and P4 levels in early pubertal girls and in late pubertal girls, respectively, implying an adrenal (ACTH-dependent) source [12, 13]. T and P4 are important regulators of gonadotropin-releasing hormone (GnRH) pulsatility with elevated T concentrations impairing P4 inhibition of GnRH pulses and P4 exhibiting day-night differences in inhibitory effects during puberty [21, 22]. Thus, we hypothesize that diurnal changes in concentrations of P4 and T may contribute to diurnal changes of pulsatile GnRH release during puberty.
DEX suppression and cosyntropin stimulation testing suggested that the adrenal capacity to secrete androgens (free T, A4, DHEA) increases across puberty, especially in OW girls, with higher free T and A4 concentration responses after cosyntropin observed related to obesity. Differences in 17-OH Prog levels were not demonstrable between NW and OW peripubertal girls. Our results suggest that A4 and free T concentration responses after cosyntropin may be more sensitive indicators of adrenal HA than 17-OH Prog levels in OW girls.
Although ovarian function was not studied directly, our pre-DEX evening and post-DEX morning data suggest a nonadrenal (e.g., ovarian) origin for (1) increasing levels of serum free T and P4 across puberty in most girls and (2) increasing levels of A4, 17-OH Prog, and DHEA across puberty in OW girls. Fourfold higher free T levels after DEX in OW vs NW older girls suggest nonadrenal (ovarian) sources of obesity-associated HA. More direct examination of ovarian androgens in OW girls is in progress.
Because SHBG levels were lower in OW girls, higher DEX-suppressed free T levels or greater free T concentration responses to cosyntropin do not prove that T production is necessarily increased in OW girls. With lower SHBG, free T levels or responses to cosyntropin can be slightly higher even with slightly reduced production levels. Regardless, serum free T levels represent the most physiologically relevant fraction of circulating T. Accordingly, our results suggest that excess weight during late puberty is associated with increased bioactive T exposure from both adrenal and ovarian sources.
We note several limitations in our current study. We did not directly assess adrenal and ovarian sex steroid production via venous catheterization. We used changes in serum hormone levels after cosyntropin stimulation as a measure of adrenal androgen capacity. Similarly, we used serum hormone levels after DEX suppression as a measure of nonadrenal (non-ACTH–dependent) hormones, and we inferred that such sex steroid concentrations primarily reflected an ovarian source. Overall, our data suggested that DEX did not influence LH secretion. More direct assessments of adrenal and ovarian hormone production are difficult and/or not ethically feasible in adolescent girls.
Additionally, we had relatively small sample sizes for healthy girls, which prompted our use of historical controls. Despite lack of randomization, characteristics of current subjects (who received DEX) and historical controls (who did not receive DEX) were similar in most respects. Compared with corresponding historical controls, late pubertal NW subjects had lower fasting insulin levels and late pubertal OW subjects had higher IGF-1 levels. [Note that we used a more rigorous P value criterion for significance (P < 0.01) given the high number of screening characteristics compared.] The potential physiological relevance of such apparent differences is unclear. Similarly, although there was an apparent difference in sleep-associated LH interpulse intervals between NW early pubertal girls according to DEX status, we doubt that this is specifically related to DEX administration because it was observed in one group only.
Recruitment was not based on clinical androgen excess or biochemical HA, but we recognize that girls concerned about androgen excess may have preferentially volunteered. However, analyses after exclusion of girls with clinical androgen excess (data not shown) were not substantially different from the primary analyses, with one exception: in this secondary analysis, late pubertal OW controls no longer had an early morning rise in P4. Importantly, in an investigation of the origins of obesity-related HA during puberty, we argue that it would be inappropriate to systematically exclude girls with clinical signs of androgen excess. Nonetheless, given the potential for recruitment bias, these data cannot be used to estimate the prevalence of HA in NW and/or OW peri-pubertal girls.
Our study explores the influence of increased weight on the relative adrenal and ovarian sources of androgens across puberty in younger girls. Our findings suggest that both adrenal and nonadrenal (e.g., ovarian) androgens increase across puberty, especially in those with excess weight. Exaggerated androgen production related to peripubertal obesity may negatively influence normal hypothalamic-pituitary-ovarian system maturation during this critical developmental window [3, 23].
Most previous studies evaluated PCOS women, who primarily exhibit ovarian HA, although some also have adrenal HA [9, 10]. Although exaggerated serum 17-OH Prog responses to ACTH and GnRH agonist (GnRHa) are commonly used markers for adrenal and ovarian HA, respectively, some studies suggest that 17-OH Prog may not be the best marker for adrenal HA [9]. In 60 PCOS women, increased cosyntropin-stimulated DHEA levels defined adrenal HA, whereas increased 17-OH Prog levels after GnRHa or elevated T levels after short-term DEX suppression defined ovarian HA [24]. In these women, 5% had adrenal HA, 63% had ovarian HA, 23% had both ovarian and adrenal HA, and 8% had neither (adipose source proposed). In 42 adolescent women with clinical HA (mean age, 18 years), 14% had abnormal 17-OH Prog concentration responses to cosyntropin, 58% to GnRHa, and 12% to both [11]. Among OW girls in our study, 13% had adrenal HA (increased postcosyntropin DHEA levels), 30% had ovarian HA (elevated DEX-suppressed free T levels), and 17% had both. Thus, the predominance of ovarian dysfunction in OW girls appears similar to that in PCOS women and adolescents. Other studies in adults found increased A4 levels in response to cosyntropin [9, 25–27]. If we define adrenal HA as increased A4 concentrations after cosyntropin, then the prevalence of adrenal HA in our OW girls would increase to 53%. In 15 obese, glucose-intolerant PCOS adolescents (mean age, 14 years), elevated cosyntropin-related A4 concentrations were ameliorated by insulin sensitization with metformin (−32%) and rosiglitazone (−26%) [28, 29]. These match our findings of increased adrenal A4 concentration responses in adolescent obesity and link adrenal androgen hyperresponsiveness to insulin resistance. Yet, some studies found that A4 represented adrenal HA in nonobese subjects only, whereas others suggested A4 and T were mostly ovarian (elevated basal levels but normal cosyntropin responses) [9, 30–32]. Although our late pubertal OW girls demonstrated exaggerated cosyntropin-stimulated free T level responses, the increases were small relative to DEX-suppressed (likely ovarian) levels.
Formal assessment of androgen sources in unselected early to mid-pubertal girls, particularly with obesity, has not been previously performed. Nonobese Spanish girls with premature adrenarche—another putative risk factor for PCOS [33]—exhibited adrenal HA using cosyntropin (mean age, 6.7 years) and ovarian HA using GnRHa (mean age, 9.8 years) during early puberty [14, 34]. Because early adrenal HA is linked to ovarian HA and PCOS, adrenal androgens may accelerate the development of ovarian HA in mid to late puberty, possibly by prematurely increasing daytime LH pulse secretion. Low-dose glucocorticoids have been used with lifestyle modification and metformin in adult PCOS to ameliorate adrenal HA without worsening metabolic parameters [35, 36]. Our data suggest that adrenal-origin HA begins by mid to late puberty in overweight girls, providing an opportunity for interventions to prevent subsequent GnRH pulse abnormalities leading to PCOS.
4. Conclusion
In summary, our data suggest that overnight increases in free T levels in NW early pubertal girls and P4 levels in late pubertal girls are likely of adrenal origin, and that OW late pubertal girls demonstrate elevated serum androgen levels from both adrenal sources (i.e., increased serum A4 and free T responses to cosyntropin) and nonadrenal sources (i.e., increased free T levels after DEX). These data add to what is known about the developmental timing and sources of excess androgen in OW peripubertal girls. Current therapy targets postmenarchal girls with established PCOS, but we suggest that preventive strategies against early HA may help prevent abnormal LH regulation and subsequent PCOS. Future studies in younger girls are required to explore the plausibility and efficacy of such strategies.
Acknowledgments
We gratefully acknowledge Lauren Lockhart, Michelle Abshire, Anne Gabel, Katherine Ehrlich, and Deborah Sanderson, for research coordination; the nurses and staff of the University of Virginia Clinical Research Unit for protocol implementation; and Daniel Haisenleder and the Center for Research in Reproduction Ligand Core Laboratory for assay performance.
Acknowledgments
This research was supported by the Eunice Kennedy Shriver National Institutes of Health (NIH)/National Institute of Child Health and Human Development Grants K23 HD070854 (to C.M.B.S.) and T32 HD07383 (to K.D.H.); the National Centers for Translational Research in Reproduction and Infertility Grants P50 HD28934 (to C.M.B.S., C.R.M., J.C.M.) and P50 HD12303 (to R.F.S., H.L.C.-A., R.J.C.); NIH Women’s Reproductive Health Research program Grants K12 HD001259 (to H.L.C.-A.); and the University of Virginia Children’s Hospital Grant-in-Aid (to C.M.B.S.).
Author contributions: C.M.B.S., K.D.H., R.F.S., H.L.C.-A., R.J.C., .C.RM., and J.C.M. participated in design of the study, recruitment of subjects, performance of experiments, and analysis of data. J.T.P. designed, performed, and created the figures for the statistical analyses. C.M.B.S., C.R.M., and J.C.M. wrote the manuscript.
Clinical trial registry: ClinicalTrials.gov no. NCT01421797 (registered 19 August 2011).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- A4
- androstenedione
- ACTH
- adrenocorticotropic hormone
- BMI%
- body mass index-for-age percentile
- CV
- coefficient of variation
- DEX
- dexamethasone
- DHEA
- dehydroepiandrosterone
- E2
- estradiol
- FSH
- follicle-stimulating hormone
- GnRH
- gonadotropin-releasing hormone
- GnRHa
- gonadotropin-releasing hormone agonist
- HA
- hyperandrogenemia
- IGF-1
- insulin-like growth factor 1
- LH
- luteinizing hormone
- NW
- normal weight
- 17-OH Prog
- 17-hydroxyprogesterone
- OW
- overweight
- P4
- progesterone
- PCOS
- polycystic ovary syndrome
- SHBG
- sex hormone–binding globulin
- T
- testosterone.
References and Notes
- 1.Apter D, Vihko R. Endocrine determinants of fertility: serum androgen concentrations during follow-up of adolescents into the third decade of life. J Clin Endocrinol Metab. 1990;71(4):970–974. [DOI] [PubMed] [Google Scholar]
- 2.Venturoli S, Porcu E, Fabbri R, Magrini O, Gammi L, Paradisi R, Flamigni C. Longitudinal evaluation of the different gonadotropin pulsatile patterns in anovulatory cycles of young girls. J Clin Endocrinol Metab. 1992;74(4):836–841. [DOI] [PubMed] [Google Scholar]
- 3.Apter D, Bützow T, Laughlin GA, Yen SS. Accelerated 24-hour luteinizing hormone pulsatile activity in adolescent girls with ovarian hyperandrogenism: relevance to the developmental phase of polycystic ovarian syndrome. J Clin Endocrinol Metab. 1994;79(1):119–125. [DOI] [PubMed] [Google Scholar]
- 4.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF; Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society . The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–488. [DOI] [PubMed] [Google Scholar]
- 5.Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90(8):4650–4658. [DOI] [PubMed] [Google Scholar]
- 6.Laitinen J, Taponen S, Martikainen H, Pouta A, Millwood I, Hartikainen AL, Ruokonen A, Sovio U, McCarthy MI, Franks S, Järvelin MR. Body size from birth to adulthood as a predictor of self-reported polycystic ovary syndrome symptoms. Int J Obes Relat Metab Disord. 2003;27(6):710–715. [DOI] [PubMed] [Google Scholar]
- 7.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudsen KL, Blank SK, Burt Solorzano C, Patrie JT, Chang RJ, Caprio S, Marshall JC, McCartney CR. Hyperandrogenemia in obese peripubertal girls: correlates and potential etiological determinants. Obesity (Silver Spring). 2010;18(11):2118–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasquali R, Patton L, Pocognoli P, Cognigni GE, Gambineri A. 17-hydroxyprogesterone responses to gonadotropin-releasing hormone disclose distinct phenotypes of functional ovarian hyperandrogenism and polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(11):4208–4217. [DOI] [PubMed] [Google Scholar]
- 10.Ehrmann DA, Rosenfield RL, Barnes RB, Brigell DF, Sheikh Z. Detection of functional ovarian hyperandrogenism in women with androgen excess. N Engl J Med. 1992;327(3):157–162. [DOI] [PubMed] [Google Scholar]
- 11.Ibáñez L, Potau N, Zampolli M, Prat N, Gussinyé M, Saenger P, Vicens-Calvet E, Carrascosa A. Source localization of androgen excess in adolescent girls. J Clin Endocrinol Metab. 1994;79(6):1778–1784. [DOI] [PubMed] [Google Scholar]
- 12.McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94(1):56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitamura R, Yano K, Suzuki N, Ito Y, Makita Y, Okuno A. Diurnal rhythms of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol secretion before the onset of female puberty in short children. J Clin Endocrinol Metab. 2000;85(3):1074–1080. [DOI] [PubMed] [Google Scholar]
- 14.Ibáñez L, Potau N, Zampolli M, Street ME, Carrascosa A. Girls diagnosed with premature pubarche show an exaggerated ovarian androgen synthesis from the early stages of puberty: evidence from gonadotropin-releasing hormone agonist testing. Fertil Steril. 1997;67(5):849–855. [DOI] [PubMed] [Google Scholar]
- 15.Armengaud JB, Charkaluk ML, Trivin C, Tardy V, Bréart G, Brauner R, Chalumeau M. Precocious pubarche: distinguishing late-onset congenital adrenal hyperplasia from premature adrenarche. J Clin Endocrinol Metab. 2009;94(8):2835–2840. [DOI] [PubMed] [Google Scholar]
- 16.Acheson RM, Fowler G, Fry EI, Janes M, Koski K, Urbano P, Werfftenboschjj VA. Studies in the reliability of assessing skeletal maturity from x-rays. I. Greulich-Pyle atlas. Hum Biol. 1963;35:317–349. [PubMed] [Google Scholar]
- 17.Legro RS, Schlaff WD, Diamond MP, Coutifaris C, Casson PR, Brzyski RG, Christman GM, Trussell JC, Krawetz SA, Snyder PJ, Ohl D, Carson SA, Steinkampf MP, Carr BR, McGovern PG, Cataldo NA, Gosman GG, Nestler JE, Myers ER, Santoro N, Eisenberg E, Zhang M, Zhang H; Reproductive Medicine Network . Total testosterone assays in women with polycystic ovary syndrome: precision and correlation with hirsutism. J Clin Endocrinol Metab. 2010;95(12):5305–5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, Yoo R, Chang RJ, Foster CM, Caprio S, Marshall JC. Obesity and sex steroid changes across puberty: evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. J Clin Endocrinol Metab. 2007;92(2):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666–3672. [DOI] [PubMed] [Google Scholar]
- 20.McCartney CR, Gingrich MB, Hu Y, Evans WS, Marshall JC. Hypothalamic regulation of cyclic ovulation: evidence that the increase in gonadotropin-releasing hormone pulse frequency during the follicular phase reflects the gradual loss of the restraining effects of progesterone. J Clin Endocrinol Metab. 2002;87(5):2194–2200. [DOI] [PubMed] [Google Scholar]
- 21.Collins JS, Marshall JC, McCartney CR. Differential sleep-wake sensitivity of gonadotropin-releasing hormone secretion to progesterone inhibition in early pubertal girls. Neuroendocrinology. 2012;96(3):222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85(11):4047–4052. [DOI] [PubMed] [Google Scholar]
- 23.McGee WK, Bishop CV, Bahar A, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum Reprod. 2012;27(2):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenfield RL, Mortensen M, Wroblewski K, Littlejohn E, Ehrmann DA. Determination of the source of androgen excess in functionally atypical polycystic ovary syndrome by a short dexamethasone androgen-suppression test and a low-dose ACTH test. Hum Reprod. 2011;26(11):3138–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moran C, Reyna R, Boots LS, Azziz R. Adrenocortical hyperresponsiveness to corticotropin in polycystic ovary syndrome patients with adrenal androgen excess. Fertil Steril. 2004;81(1):126–131. [DOI] [PubMed] [Google Scholar]
- 26.Colak R, Keleştimur F, Unlühizarci K, Bayram F, Sahin Y, Tutuş A. A comparison between the effects of low dose (1 microg) and standard dose (250 microg) ACTH stimulation tests on adrenal P450c17alpha enzyme activity in women with polycystic ovary syndrome. Eur J Endocrinol. 2002;147(4):473–477. [DOI] [PubMed] [Google Scholar]
- 27.Cinar N, Cetinozman F, Aksoy DY, Elcin G, Yildiz BO. Comparison of adrenocortical steroidogenesis in women with post-adolescent severe acne and polycystic ovary syndrome. J Eur Acad Dermatol Venereol. 2015;29(5):875–880. [DOI] [PubMed] [Google Scholar]
- 28.Arslanian SA, Lewy V, Danadian K, Saad R. Metformin therapy in obese adolescents with polycystic ovary syndrome and impaired glucose tolerance: amelioration of exaggerated adrenal response to adrenocorticotropin with reduction of insulinemia/insulin resistance. J Clin Endocrinol Metab. 2002;87(4):1555–1559. [DOI] [PubMed] [Google Scholar]
- 29.Tfayli H, Ulnach JW, Lee S, Sutton-Tyrrell K, Arslanian S. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96(5):1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran C, Arriaga M, Arechavaleta-Velasco F, Moran S. Adrenal androgen excess and body mass index in polycystic ovary syndrome [published online ahead of print January 7, 2017]. J Clin Endocrinol Metab. [DOI] [PubMed] [Google Scholar]
- 31.White D, Leigh A, Wilson C, Donaldson A, Franks S. Gonadotrophin and gonadal steroid response to a single dose of a long-acting agonist of gonadotrophin-releasing hormone in ovulatory and anovulatory women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 1995;42(5):475–481. [DOI] [PubMed] [Google Scholar]
- 32.Farah-Eways L, Reyna R, Knochenhauer ES, Bartolucci AA, Azziz R. Glucose action and adrenocortical biosynthesis in women with polycystic ovary syndrome. Fertil Steril. 2004;81(1):120–125. [DOI] [PubMed] [Google Scholar]
- 33.Ibáñez L, de Zegher F, Potau N. Anovulation after precocious pubarche: early markers and time course in adolescence. J Clin Endocrinol Metab. 1999;84(8):2691–2695. [DOI] [PubMed] [Google Scholar]
- 34.Ibáñez L, Potau N, Marcos MV, De Zegher F. Adrenal hyperandrogenism in adolescent girls with a history of low birthweight and precocious pubarche. Clin Endocrinol (Oxf). 2000;53(4):523–527. [DOI] [PubMed] [Google Scholar]
- 35.Jones GE, Howard JE, Langford H. The use of cortisone in follicular phase disturbances. Fertil Steril. 1953;4(1):49–62. [DOI] [PubMed] [Google Scholar]
- 36.Vanky E, Salvesen KA, Carlsen SM. Six-month treatment with low-dose dexamethasone further reduces androgen levels in PCOS women treated with diet and lifestyle advice, and metformin. Hum Reprod. 2004;19(3):529–533. [DOI] [PubMed] [Google Scholar]