Abstract
Background:
Soy protein with isoflavones appears to have an adverse effect on thyroid function, but it is not known whether it is the protein or isoflavone component that is deleterious. The effect of isoflavone-free soy on thyroid function was determined in patients with subclinical hypothyroidism, with a secondary aim of assessing its effect on cardiovascular risk indices.
Methods:
This was a randomized, double-blind, crossover study involving 80 patients with subclinical (compensated) hypothyroidism. Patients were randomly assigned to either isolated soy (isoflavone-free) protein (SP) or casein protein (CP) supplementation for 8 weeks, washed out for 8 weeks, and then crossed over for a further 8-week period.
Results:
Thyroid function was unaffected by either a SP or CP. There were significant decreases in fasting glucose (4.7 ± 0.6 vs 5.5 ± 1.4, P < 0.01), insulin resistance (3.3 ± 3.0 vs 3.8 ± 3.4, P = 0.05), total cholesterol (4.4 ± 0.9 vs 5.3 ± 1.2, P < 0.01), triglycerides (0.9 ± 0.5 vs 1.7 ± 0.9, P < 0.1), and highly sensitive C-reactive protein (hsCRP; 0.8 ± 0.7 vs 2.6 ± 2.8, P < 0.01) in the SP group compared with the CP group. Blood pressure, low-density lipoprotein, and high-density lipoprotein remained unchanged in both groups.
Conclusion:
SP alone had no effect on thyroid function in patients with subclinical hypothyroidism and resulted in a significant reduction in fasting glucose, insulin resistance, total cholesterol, triglycerides, and hsCRP compared with CP.
Keywords: soy protein, isoflavone, subclinical hypothyroidism, cardiovascular risk
Patients treated with isolated soy (isoflavone-free) or casein protein in a crossover study resulting in a reduction in fasting glucose, insulin resistance, total cholesterol, triglycerides, and hsCRP.
Soy foods have become popular in Western countries through the suggestion that they have health benefits, including protection against coronary heart disease [1–3], breast and prostate cancer [4–6], osteoporosis [7], and alleviation of hot flushes [8]. This has led to the development of isoflavone supplements and the fortification of foods with soy constituents [9, 10]. There are concerns that soy may adversely affect thyroid function in susceptible individuals [11, 12], and in vitro studies have demonstrated that isoflavones inhibit thyroid peroxidase, an enzyme involved in the synthesis of triiodothyronine (T3) and thyroxine (T4) [13, 14].
The two main components of soy thought to be responsible for most of the proposed health benefits of soy are soy protein and the soy isoflavones, of which genistein is more biologically potent that daidzein and glycitein. It is unclear which component of soy (the protein, the isoflavones, or an interaction between the two) may have an effect on thyroid function [12–15]. It has not previously been possible to determine the relative effective contribution of each component, as there are no studies either in vitro or in vivo with an isoflavone-free soy preparation.
Studies have shown that soy protein with isoflavones has an adverse effect on thyroid function [16, 17], and another study in subclinical hypothyroidism was associated with a threefold increased risk of developing overt hypothyroidism [15]. These studies have also highlighted the positive effect of soy on cardiovascular risk, reducing insulin resistance, highly sensitive C-reactive protein (hsCRP), and blood pressure. However, it is not known whether the effects observed were due to isoflavones alone or due to the isoflavones in combination with the soy protein, although a study performed with isoflavones alone showed no beneficial effects, whereas the same isoflavone preparation with soy protein was effective in improving glycemic control and reducing cardiovascular risk parameters [18], suggesting that soy protein alone may have inherent activity. Therefore, this double-blind, crossover trial was undertaken to determine the effect of soy protein alone on thyroid parameters in patients with compromised thyroid function (subclinical hypothyroidism), with a secondary outcome to evaluate cardiovascular risk markers, by comparing 30 g isolated soy (isoflavone-free) protein (SP) with 30 g casein protein (CP).
1. Subjects and Methods
Eighty patients, 27 males and 53 female subjects with subclinical hypothyroidism (age range, 20 to 81 years) [thyrotropin (TSH) value between 5 and 15 mU/L; normal range 0.5 to 4.7 mU/L, with a normal free T4 (fT4)], were recruited by identification from the routine biochemical testing performed at Hull Royal Infirmary, United Kingdom, over a 12-month period. The screening thyroid function tests were done 4 to 8 weeks after the initial biochemical testing (Table 1). Exclusion criteria included patients taking thyroxine or drugs that may interfere with thyroid function, antihypertensive agents, insulin-sensitizing agents, lipid-lowering medications, as well as antibiotic use within the last 6 months. Women who were contemplating pregnancy were also excluded. Subjects were instructed, at randomization and then at each subsequent visit, to maintain their level of physical activity throughout the study and avoid food products containing soy, alcohol, vitamin or mineral supplementation, and over-the-counter medications. Ethics approval was obtained from Hull and East Yorkshire Ethics Committee.
Table 1.
Thyroid Function Tests at the Start and 2 Months After Supplementation
| Soy Protein (SP) | Casein Protein (CP) | Postactive–Postplacebo | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 8 Weeks | P Value | Baseline | 8 Weeks | P Value | P Value | |
| fT3 | 4.44 ± 0.51 | 4.37 ± 0.46 | 0.32 | 4.40 ± 0.58 | 4.43 ± 0.42 | 0.70 | 0.88 |
| fT4 | 12.60 ± 1.26 | 11.06 ± 1.63 | 0.08 | 12.11 ± 1.40 | 11.93 ± 1.77 | 0.48 | 0.32 |
| TSH | 5.70 ± 1.01 | 5.86 ± 2.08 | 0.56 | 5.93 ± 2.16 | 5.71 ± 2.16 | 0.12 | 0.54 |
fT3, pmol/L; fT4, pmol/L; TSH, mU/L (mean ± standard deviation).
Abbreviation: fT3, free triiodothyronine.
Forty patients were initiated after randomization with a cereal bar containing 30 g isolated SP and 40 patients with a cereal bar containing 30 g CP per day for 8 weeks (first phase). After an 8-week washout period, the participants received the alternative supplementation for 8 weeks (second phase). An eight-week time period was chosen for each arm as this was the minimum time of exposure that might have been expected to affect thyroid function, given that the half-life of thyroxine is ~7 days. Compliance was calculated by counting the returned bars.
Soy was supplied by Solbar Industries (Ashdod, Israel). Isoflavones were removed by serial alcohol washing (Dishman, Ahmedabad, India) to a level ˂300 parts per billion (assayed by FERA, Sand Hutton, United Kingdom). Casein bars were prepared from a single batch, and each bar contained the same amount of protein. Soy and casein bars were prepared by Halo Foods (Swindon, United Kingdom), and the study product was then randomized by Essential Nutrition (Brough, United Kingdom) using a computer-generated block randomization list.
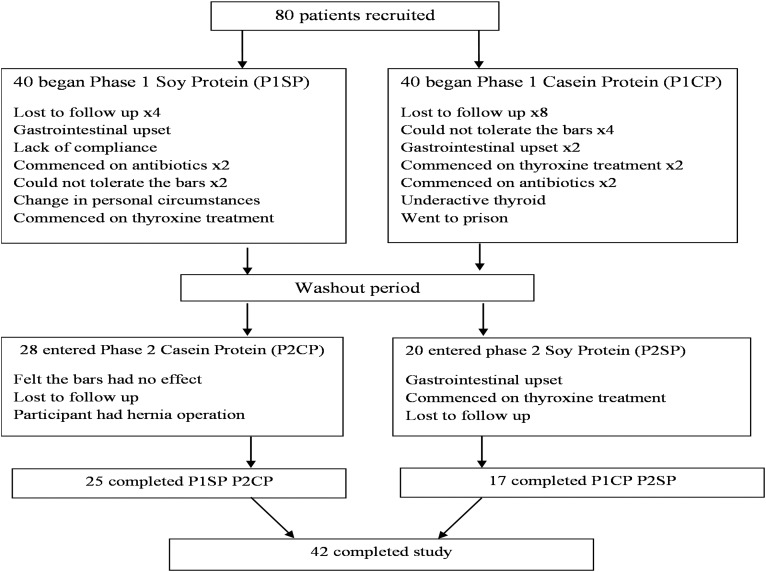
The primary outcome of the study was a change in serum-free thyroxine, whereas secondary outcome measures were blood pressure, homeostasis model assessment of insulin resistance (HOMA-IR), lipids, and hsCRP. Overt hypothyroidism was defined as a combination of TSH >4.7 mU/L and fT4 <9 pmol/L. A flow chart of patients through the study is given in Fig. 1.
Figure 1.
Flow chart describing the progress of patients through the trial.
A. Study Measurements
The weight and blood pressure of participants were measured, and blood samples were collected at the beginning and end of each phase, following an overnight fast. Blood pressure was measured after the patients had been seated quietly for at least 5 minutes with the right arm supported at heart level. Blood pressure measurements were performed using an automated device (NPB-3900; Nellcor Puritan Bennett, Pleasanton, CA) during each study visit. Two readings were obtained at the beginning of each visit at least 1 minute apart, and the mean value was taken. Fasting venous blood samples were collected and separated by centrifugation at 2000g for 15 minutes at 4°C, and the aliquots were stored at −80°C within 1 hour of collection. Plasma glucose was measured using a Synchron LX20 analyzer (Beckman-Coulter, High Wycombe, United Kingdom), and serum insulin was assayed using a competitive chemiluminescent immunoassay performed using the DPC Immulite 2000 analyzer (Euro/DPC, Llanberis, United Kingdom). The coefficient of variation of this method was 8%, calculated using duplicate study samples. The analytical sensitivity was 2 µU/mL. Insulin resistance was calculated using HOMA-IR (insulin × glucose)/22.5) [19]. Total cholesterol, triglycerides, and high-density lipoprotein (HDL) cholesterol levels were measured enzymatically using a Synchron LX20 analyzer (Beckman-Coulter). Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation. All thyroid assays were performed on an Abbott Architect i4000 immunoassay analyzer (Abbott Diagnostics Division, Maidenhead, United Kingdom). The reference range for TSH was 0.5 to 4.7 mU/L; fT4, 9 to 24 pmol/L; and free T3, 2.5 to 5.3 pmol/L. Urinary iodine measurements in 24-hour urine collections were undertaken by inductively coupled plasma–mass spectrometry to monitor for iodine repletion, and plasma phytoestrogen measurement was undertaken by liquid chromatography–tandem mass spectrometry (HFL Sport Science Laboratory, Cambridge, United Kingdom).
B. Statistical Analysis
For a significant reduction in fT4, a sample size of 40 patients in a crossover design was calculated giving 80% power to detect a mean decrease of 0.4 pmol/L fT4, with a two-sided α error of 0.05 [18]. Given the high dropout rate with this type of study, it was elected to recruit 80 patients and accept a dropout rate of 50%. Mean percentage changes obtained at the end of supplementation with 30 g SP were compared with the results at the end of placebo CP using the paired Student t test for biochemical data and Wilcoxon’s signed–rank test for clinical observations. Baseline parameters were not compared statistically because this was a randomized controlled study [20, 21].
Wilcoxon’s signed–rank test was applied to biochemical data that violated the assumptions of normality when tested using the Kolmogorov–Smirnov test. The period and the carryover effect that may have occurred from the crossover design were also tested using the appropriate Student t test. Intention to treat analysis was done. Statistical correction for multiple testing since was not done because it can severely reduce power to detect important effects due to the fact that the general null hypothesis (that all the null hypotheses are true) is not of interest and there is a high probability of type II errors [22].
Statistical analysis was performed using SPSS for windows version 22.0. An arbitrary level of 5% statistical significance (two-tailed) was assumed. The data are reported as mean ± standard deviation.
2. Results
Mean age of patients was 55.1 ± 14.2 years. The mean body mass index (BMI) of patients was 28.8 ± 5.9 kg/m2; mean systolic blood pressure was 134.2 ± 22.2 mm Hg; and mean diastolic blood pressure was 81.5 ± 14.2 mm Hg. Thyroid peroxidase antibodies were positive (>75 U/mL) in 27 (33.7%) patients. Compliance was 98% in both groups. Of the 27 male and 53 female subjects with subclinical hypothyroidism that started the study, 16 males and 26 females completed.
The mean TSH, free T3, and fT4 levels remained unchanged before and after supplementation with either soy protein or CP. No patient developed overt hypothyroidism during the study.
Weight and BMI were unchanged after either SP or CP supplementation. There were no differences in mean systolic or diastolic blood pressures between the groups. There was a significant decrease in mean glucose (P < 0.01) that reflected in a decrease in HOMA-IR (P = 0.05) in patients who received SP supplementation compared with CP (Table 2).
Table 2.
Subjects’ Characteristics and Effects on Cardiovascular Risk at the Start of the Trial and 2 Months After Supplementation
| Soy Protein (SP) | Casein Protein (CP) | Postactive–Postplacebo | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 8 Weeks | P Value | Baseline | 8 Weeks | P Value | P Value | |
| Weight | 84.0 ± 20.4 | 84.4 ± 20.4 | 0.09 | 84.4 ± 20.3 | 84.8 ± 20.5 | 0.1 | 0.99 |
| BMI | 29.0 ± 5.6 | 29.1 ± 5.7 | 0.07 | 29.3 ± 5.6 | 29.4 ± 6.1 | 0.07 | 0.91 |
| SBP | 131.4 ± 18.2 | 130.3 ± 17.9 | 0.5 | 133.5 ± 23.4 | 129.9 ± 17.8 | 0.1 | 0.40 |
| DBP | 81.3 ± 13.9 | 78.6 ± 13.9 | 0.2 | 82.1 ± 13.1 | 77.4 ± 11.6 | 0.01 | 0.47 |
| Glucose | 6.2 ± 0.9 | 4.7 ± 0.6 | <0.01 | 5.4 ± 1.3 | 5.5 ± 1.4 | 0.07 | <0.01 |
| Insulin | 12.4 ± 9.9 | 14.9 ± 13.6 | 0.03 | 13.4 ± 10.3 | 14.3 ± 10.5 | 0.3 | 0.23 |
| HOMA-IR | 3.6 ± 3.0 | 3.3 ± 3.0 | 0.2 | 3.5 ± 3.4 | 3.8 ± 3.4 | 0.2 | 0.05 |
| TC | 5.3 ± 1.2 | 4.4 ± 0.9 | <0.01 | 5.3 ± 1.1 | 5.4 ± 1.2 | 0.2 | <0.01 |
| LDL | 3.3 ± 1.1 | 3.2 ± 1.1 | 0.4 | 3.2 ± 1.2 | 3.3 ± 1.0 | 0.5 | 0.26 |
| HDL | 1.4 ± 0.4 | 1.5 ± 1.6 | 0.5 | 1.3 ± 0.4 | 1.3 ± 0.4 | 0.7 | 0.54 |
| TG | 2.6 ± 0.9 | 0.9 ± 0.5 | <0.01 | 1.6 ± 0.8 | 1.7 ± 0.9 | 0.3 | <0.01 |
| hsCRP | 3.4 ± 2.6 | 0.8 ± 0.7 | <0.01 | 2.4 ± 2.5 | 2.6 ± 2.8 | 0.5 | <0.01 |
BMI, kg/m2; SBP, mm Hg; DBP, mm Hg; glucose, plasma glucose, mmol/L; insulin, µIU/mL; TC, mmol/L; TG, mmol/L; LDL, mmol/L; HDL, mmol/L; hsCRP, mg/L. To convert values for glucose to milligrams per decilitre, divide by 0.056. To convert values for insulin to picomoles per liter, multiply by 6. To convert values for cholesterol to milligrams per decilitre, divide by 0.0259. To convert values for triglycerides to milligrams per decilitre, divide by 0.0113.
Abbreviations: DBP, diastolic blood pressure, SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides.
There was a decrease in the total cholesterol and triglycerides (P < 0.01) in patients who received SP supplementation compared with CP between groups, but HDL cholesterol levels did not differ (Table 2). There was a reduction in LDL of ~3% with soy protein and an increase in LDL of ~3% with CP; this was not found to be statistically significant (Table 2).
There was a significant reduction in mean hsCRP (P < 0.01) in patients who received SP that was not seen with CP (Table 2).
The calculated 10-year cardiovascular risk using the Framingham equation was 13.3% for both SP and CP that fell to 11.2% following SP, a significant 2.1% absolute and a 10% relative fall (P < 0.05) between groups.
3. Discussion
Studies on soy with isoflavones have shown a significant effect on both T4 and TSH levels [16, 17], and these data using an isolated soy protein, isoflavone-free preparation confirm that SP is not responsible for those changes that are therefore likely to be due to the intrinsic isoflavone component of soy. CP has been shown previously to have no effect on thyroid function in postmenopausal women [23].
A significant improvement in plasma glucose and a fall in HOMA after SP were observed, in accordance with a meta-analysis of randomized trials [24], and more recent soy isoflavone studies [16, 17] showed a significant decrease in plasma glucose that may in part be due to the SP alone, as shown in this work.
Insulin resistance (HOMA-IR) significantly improved in patients supplemented with SP compared with CP, which was in accordance with studies that have shown isoflavones in soy protein improve insulin resistance [18, 25]; however, this effect appears to be lost when isoflavones are given alone (without soy protein) [26–28].
There was a significant improvement in total cholesterol and triglyceride values after soy protein supplementation, although LDL and HDL were not altered. Some previous studies have suggested that the lipid-lowering effects are mainly due to the isoflavone content, but this study showed that soy protein alone most likely contributes to the lipid-lowering effects reported. However, the observed effects on lipid lowering have not been consistent; some studies have shown an improvement with the soy preparation [29–32], whereas others have shown no benefit on total cholesterol, LDL cholesterol, and triglycerides [33–35]; likewise, isoflavones alone have shown no lipid benefit [36, 37]. These differences reported by others may be due in part to the differing preparations used.
There was no improvement seen in BMI and weight, but the marker of inflammation, hsCRP, was reduced significantly after soy protein supplementation. In accordance with the lipid data, previous studies have shown an improvement in hsCRP in some [38, 39] but not all studies [40, 41] that most likely reflects the differing soy preparations and study conditions used. Our study suggests that isolated soy protein has anti-inflammatory effects.
Although there was no difference in the protein composition between soy with and without isoflavones following serial alcohol washing and digestion of protein that should have not differed following ingestion, nonetheless the serial alcohol washing could have altered the tertiary structure of the protein and removed other components besides isoflavones.
The study limitations include the increased dropout seen that was mitigated by appropriate powering of the study and its crossover design. The number of participants who completed the study was similar to other studies [15, 42]. Secondly, the ratio of male and female participants who completed the study did not alter, thus alleviating the effect of different response of males and females to soy isoflavones reported previously [43]. Although there was no difference in the protein composition between soy with and without isoflavones following serial alcohol washing, and digestion of protein should have not differed following ingestion, nonetheless the serial alcohol washing could have altered the tertiary structure of the protein and removed other components besides isoflavones.
In conclusion, isolated SP had no effect on thyroid function in patients with subclinical hypothyroidism and resulted in a significant reduction in fasting glucose, insulin resistance, total cholesterol, triglycerides, and hsCRP compared with CP.
Acknowledgments
Views expressed are those of the authors, not those of the Food Standards Agency, United Kingdom.
Acknowledgments
The phytoestrogen standards were produced as part of a Food Standards Agency (Contract T05001) and were donated for use in this project by Dr. Nigel P. Botting (Department of Chemistry, University of St. Andrews, St. Andrews, United Kingdom). This work was supported by Food Standards Agency United Kingdom Contract T05029.
ISRCTN registry: ISRCTN.com no. ISRCTN55827330 (registered 5 September 2006).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CP
- casein protein
- fT4
- free T4
- HDL
- high-density lipoprotein
- HOMA-IR
- homeostasis model assessment of insulin resistance
- hsCRP
- highly sensitive C-reactive protein
- LDL
- low-density lipoprotein
- SP
- soy (isoflavone-free) protein
- T3
- triiodothyronine
- T4
- thyroxine
- TSH
- thyrotropin.
References and Notes
- 1.Weggemans RM, Trautwein EA. Relation between soy-associated isoflavones and LDL and HDL cholesterol concentrations in humans: a meta-analysis. Eur J Clin Nutr. 2003;57(8):940–946. [DOI] [PubMed] [Google Scholar]
- 2.Nestel P. Isoflavones: their effects on cardiovascular risk and functions. Curr Opin Lipidol. 2003;14(1):3–8. [DOI] [PubMed] [Google Scholar]
- 3.Tsugane S, Sawada N. The JPHC study: design and some findings on the typical Japanese diet. Jpn J Clin Oncol. 2014;44(9):777–782. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto S, Sobue T, Kobayashi M, Sasaki S, Tsugane S; Japan Public Health Center-Based Prospective Study on Cancer Cardiovascular Diseases Group . Soy, isoflavones, and breast cancer risk in Japan. J Natl Cancer Inst. 2003;95(12):906–913. [DOI] [PubMed] [Google Scholar]
- 5.Messina MJ. Emerging evidence on the role of soy in reducing prostate cancer risk. Nutr Rev. 2003;61(4):117–131. [DOI] [PubMed] [Google Scholar]
- 6.Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132(3):552S–558S. [DOI] [PubMed] [Google Scholar]
- 7.Messina M, Ho S, Alekel DL. Skeletal benefits of soy isoflavones: a review of the clinical trial and epidemiologic data. Curr Opin Clin Nutr Metab Care. 2004;7(6):649–658. [DOI] [PubMed] [Google Scholar]
- 8.Messina M, Hughes C. Efficacy of soyfoods and soybean isoflavone supplements for alleviating menopausal symptoms is positively related to initial hot flush frequency. J Med Food. 2003;6(1):1–11. [DOI] [PubMed] [Google Scholar]
- 9.Nurmi T, Mazur W, Heinonen S, Kokkonen J, Adlercreutz H. Isoflavone content of the soy based supplements. J Pharm Biomed Anal. 2002;28(1):1–11. [DOI] [PubMed] [Google Scholar]
- 10.Setchell KD. Soy isoflavones–benefits and risks from nature's selective estrogen receptor modulators (SERMs). J Am Coll Nutr. 2001;20(5 Suppl):354S–362S; discussion 381S–383S. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick M. Soy formulas and the effects of isoflavones on the thyroid. N Z Med J. 2000;113(1103):24–26. [PubMed] [Google Scholar]
- 12.Doerge DR, Sheehan DM. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect. 2002;110(Suppl 3):349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Divi RL, Chang HC, Doerge DR. Anti-thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochem Pharmacol. 1997;54(10):1087–1096. [DOI] [PubMed] [Google Scholar]
- 14.Divi RL, Doerge DR. Inhibition of thyroid peroxidase by dietary flavonoids. Chem Res Toxicol. 1996;9(1):16–23. [DOI] [PubMed] [Google Scholar]
- 15.Sathyapalan T, Manuchehri AM, Thatcher NJ, Rigby AS, Chapman T, Kilpatrick ES, Atkin SL. The effect of soy phytoestrogen supplementation on thyroid status and cardiovascular risk markers in patients with subclinical hypothyroidism: a randomized, double-blind, crossover study. J Clin Endocrinol Metab. 2011;96(5):1442–1449. [DOI] [PubMed] [Google Scholar]
- 16.Sathyapalan T, Rigby AS, Bhasin S, Thatcher NJ, Kilpatrick ES, Atkin SL. Effect of soy in men with type 2 diabetes mellitus and subclinical hypogonadism: a randomized controlled study. J Clin Endocrinol Metab. 2017;102(2):425–433. [DOI] [PubMed] [Google Scholar]
- 17.Sathyapalan T, Aye M, Rigby AS, Fraser WD, Thatcher NJ, Kilpatrick ES, Atkin SL. Soy reduces bone turnover markers in women during early menopause: a randomized controlled trial. J Bone Miner Res. 2017;32(1):157–164. [DOI] [PubMed] [Google Scholar]
- 18.Jayagopal V, Albertazzi P, Kilpatrick ES, Howarth EM, Jennings PE, Hepburn DA, Atkin SL. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25(10):1709–1714. [DOI] [PubMed] [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 20.Senn S. Testing for baseline balance in clinical trials. Stat Med. 1994;13(17):1715–1726. [DOI] [PubMed] [Google Scholar]
- 21.de Boer MR, Waterlander WE, Kuijper LD, Steenhuis IH, Twisk JW. Testing for baseline differences in randomized controlled trials: an unhealthy research behavior that is hard to eradicate. Int J Behav Nutr Phys Act. 2015;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Persky VW, Turyk ME, Wang L, Freels S, Chatterton R Jr, Barnes S, Erdman J Jr, Sepkovic DW, Bradlow HL, Potter S. Effect of soy protein on endogenous hormones in postmenopausal women. Am J Clin Nutr. 2002;75(1):145–153. [DOI] [PubMed] [Google Scholar]
- 24.Zhang YB, Chen WH, Guo JJ, Fu ZH, Yi C, Zhang M, Na XL. Soy isoflavone supplementation could reduce body weight and improve glucose metabolism in non-Asian postmenopausal women: a meta-analysis. Nutrition. 2013;29(1):8–14. [DOI] [PubMed] [Google Scholar]
- 25.Goodman-Gruen D, Kritz-Silverstein D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J Nutr. 2001;131(4):1202–1206. [DOI] [PubMed] [Google Scholar]
- 26.Hall WL, Vafeiadou K, Hallund J, Bugel S, Reimann M, Koebnick C, Zunft HJ, Ferrari M, Branca F, Dadd T, Talbot D, Powell J, Minihane AM, Cassidy A, Nilsson M, Dahlman-Wright K, Gustafsson JA, Williams CM. Soy-isoflavone-enriched foods and markers of lipid and glucose metabolism in postmenopausal women: interactions with genotype and equal production. Am J Clin Nutr. 2006;83(3):592–600. [DOI] [PubMed] [Google Scholar]
- 27.Charles C, Yuskavage J, Carlson O, John M, Tagalicud AS, Maggio M, Muller DC, Egan J, Basaria S. Effects of high-dose isoflavones on metabolic and inflammatory markers in healthy postmenopausal women. Menopause. 2009;16(2):395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricci E, Cipriani S, Chiaffarino F, Malvezzi M, Parazzini F. Effects of soy isoflavones and genistein on glucose metabolism in perimenopausal and postmenopausal non-Asian women: a meta-analysis of randomized controlled trials. Menopause. 2010;17(5):1080–1086. [DOI] [PubMed] [Google Scholar]
- 29.Crouse Jr III, Morgan T, Terry JG, Ellis J, Vitolins M, Burke GL. A randomized trial comparing the effect of casein with that of soy protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch Intern Med. 1999;159(17):2070–2076. [DOI] [PubMed] [Google Scholar]
- 30.Wangen KE, Duncan AM, Xu X, Kurzer MS. Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am J Clin Nutr. 2001;73(2):225–231. [DOI] [PubMed] [Google Scholar]
- 31.Teede HJ, Dalais FS, Kotsopoulos D, Liang YL, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001;86(7):3053–3060. [DOI] [PubMed] [Google Scholar]
- 32.Merz-Demlow BE, Duncan AM, Wangen KE, Xu X, Carr TP, Phipps WR, Kurzer MS. Soy isoflavones improve plasma lipids in normocholesterolemic, premenopausal women. Am J Clin Nutr. 2000;71(6):1462–1469. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Jones PJ, Ausman LM, Lichtenstein AH. Soy protein reduces triglyceride levels and triglyceride fatty acid fractional synthesis rate in hypercholesterolemic subjects. Atherosclerosis. 2004;173(2):269–275. [DOI] [PubMed] [Google Scholar]
- 34.Meinertz H, Nilausen K, Hilden J. Alcohol-extracted, but not intact, dietary soy protein lowers lipoprotein(a) markedly. Arterioscler Thromb Vasc Biol. 2002;22(2):312–316. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins DJ, Kendall CW, Jackson CJ, Connelly PW, Parker T, Faulkner D, Vidgen E, Cunnane SC, Leiter LA, Josse RG. Effects of high- and low-isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am J Clin Nutr. 2002;76(2):365–372. [DOI] [PubMed] [Google Scholar]
- 36.Nestel PJ, Pomeroy S, Kay S, Komesaroff P, Behrsing J, Cameron JD, West L. Isoflavones from red clover improve systemic arterial compliance but not plasma lipids in menopausal women. J Clin Endocrinol Metab. 1999;84(3):895–898. [DOI] [PubMed] [Google Scholar]
- 37.Dewell A, Hollenbeck CB, Bruce B. The effects of soy-derived phytoestrogens on serum lipids and lipoproteins in moderately hypercholesterolemic postmenopausal women. J Clin Endocrinol Metab. 2002;87(1):118–121. [DOI] [PubMed] [Google Scholar]
- 38.Azadbakht L, Atabak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: a longitudinal randomized clinical trial. Diabetes Care. 2008;31(4):648–654. [DOI] [PubMed] [Google Scholar]
- 39.Clerici C, Setchell KD, Battezzati PM, Pirro M, Giuliano V, Asciutti S, Castellani D, Nardi E, Sabatino G, Orlandi S, Baldoni M, Morelli O, Mannarino E, Morelli A. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. J Nutr. 2007;137(10):2270–2278. [DOI] [PubMed] [Google Scholar]
- 40.Mangano KM, Hutchins-Wiese HL, Kenny AM, Walsh SJ, Abourizk RH, Bruno RS, Lipcius R, Fall P, Kleppinger A, Kenyon-Pesce L, Prestwood KM, Kerstetter JE. Soy proteins and isoflavones reduce interleukin-6 but not serum lipids in older women: a randomized controlled trial. Nutr Res. 2013;33(12):1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikander E, Metsä-Heikkilä M, Tiitinen A, Ylikorkala O. Evidence of a lack of effect of a phytoestrogen regimen on the levels of C-reactive protein, E-selectin, and nitrate in postmenopausal women. J Clin Endocrinol Metab. 2003;88(11):5180–5185. [DOI] [PubMed] [Google Scholar]
- 42.D'Anna R, Santamaria A, Cannata ML, Interdonato ML, Giorgianni GM, Granese R, Corrado F, Bitto A. Effects of a new flavonoid and Myo-inositol supplement on some biomarkers of cardiovascular risk in postmenopausal women: a randomized trial. Int J Endocrinol. 2014;2014:653561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Sun LL, He LP, Ling WH, Liu ZM, Chen YM. Soy food consumption, cardiometabolic alterations and carotid intima-media thickness in Chinese adults. Nutr Metab Cardiovasc Dis. 2014;24(10):1097–1104. [DOI] [PubMed] [Google Scholar]