Abstract
Multiple endocrine neoplasia type 1 (MEN1) and von Hippel-Lindau (VHL) are autosomal-dominant diseases caused by germline mutations in tumor-suppressor genes. A patient with a germline MEN1 mutation and a somatic VHL mutation in the tumor has not been reported. Herein, we report on a patient with MEN1 and a metastatic nonfunctioning pancreatic neuroendocrine tumor (PNET) with a somatic VHL mutation. This patient underwent a pancreaticoduodenectomy for a grade 2 PNET obstructing her pancreatic duct. The patient developed liver and regional lymph node metastases as well as growth of a PNET in the remnant pancreas. As part of a clinical trial for mutation-targeted therapy, a biopsy of the metastatic tumor was obtained. The clinical diagnosis, confirmed by OncoVAR-NET and molecular profiling analysis, revealed MEN1 with a germline deletion in exon 2 and a c.402 deletion C, p.Phe134LeufsX51. In addition, a somatic mutation in the VHL gene—a nonsense mutation, c.529A>T, p.Arg177Ter—was identified by hybrid capture sequencing. The mutations were confirmed by Sanger sequencing. Comparative genomic hybridization showed loss of heterozygosity in both the MEN1 and VHL genes. The patient was treated with sunitinib and had a partial response to treatment. This case illustrates not only that a second hit occurs in tumor suppressor genes but that somatic mutations are also possible in additional tumor suppressor genes. This suggests that targeted therapy selection should include analysis of somatic mutations even when the susceptibility gene is known.
Keywords: multiple endocrine neoplasia, sunitinib, type 1, von Hippel Lindau
The case report presents a rare finding in a patient with MEN1-associated metastatic PNET who had a somatic VHL mutation and responded to sunitinib treatment.
The manuscript Somatic VHL mutation in a patient with MEN1-associated metastatic pancreatic neuroendocrine tumor responding to sunitinib treatment: A Case Report presents a rare finding in a patient with metastatic PNET. After operative resection, mutational analysis was conducted using tumor DNA. Loss of heterozygosity was noted in VHL and MEN1.
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal-dominant disease caused by a mutation in the MEN1 tumor-suppressor gene. Somatic mutations in MEN1 also occur in 40% of nonfunctioning pancreatic neuroendocrine tumors (PNETs) and may be associated with disease aggressiveness [1]. The prevalence of MEN1 in postmortem studies was 0.25%. By the fifth decade of life, biochemical manifestations are present in 98% of patients with MEN1, and clinical manifestations are present in 80% [2]. The penetrance of pancreatic tumors is 30% to 70% [2]. Common manifestations seen in MEN1 include primary hyperparathyroidism, pituitary adenomas, and neuroendocrine tumors of the gastro-entero-pancreatic tract [2]. MEN1 is diagnosed on the basis of one of the following three criteria: (1) occurrence of two or more MEN1-associated tumors (pituitary, parathyroid, or gastro-entero-pancreatic tumors), (2) occurrence of one of the MEN1-associated tumors in a first-degree relative of a patient with a clinical diagnosis of one of the MEN1 manifestations, or (3) the presence of a germline MEN1 mutation [2]. The MEN1 gene locus is on chromosome 11q13 and encodes the menin protein [3]. As in other tumor suppressor–inherited cancer syndromes, a multistep model of “two hits” occurs, causing inactivating mutations in MEN1.
von Hippel-Lindau (VHL) disease is an autosomal-dominant disorder caused by a germline mutation in the tumor-suppressor gene VHL. It is located on chromosome 3p25.5 [4, 5]. The incidence of VHL is 1 in 36,000 live births with a penetrance of 95% at 65 years of age [5]. The clinical manifestation of VHL includes retinal hemangioblastomas, central nervous system hemangioblastomas, renal cystic and solid tumors, endolymphatic sac tumors, pheochromocytomas, cystic pancreatic lesions, and PNETs. PNETs are present in 11% to 17% of patients with VHL [5, 6]. Although both MEN1 and VHL may manifest with PNETs, no germline MEN1 mutation with a coexisting somatic VHL mutation has been reported in the literature.
In sporadic PNETs, rates of somatic MEN1 mutations have been reported as 13% to 44%, whereas rates of somatic VHL mutations have been reported as <1% [7, 8]. With advances in next-generation sequencing, it is becoming possible to efficiently analyze driver mutations in cancer for precision medicine with targeted therapy. In patients with low- and intermediate-grade PNETs, there are various treatment options, including somatostatin analogues, sunitinib, everolimus, and peptide receptor radionuclide therapy. Sunitinib is a tyrosine kinase inhibitor that is active against multiple tyrosine kinase receptors, including VEGFR, PDGFR, KIT, RET, and FMS-like tyrosine kinase 3 (FLT3) [9]. Everolimus is a mammalian target of the rapamycin (mTOR) signaling pathway inhibitor and a derivative of rapamycin. It inhibits the PI3K/mTOR-raptor pathway, which regulates tumor cell proliferation and angiogenesis [9]. Both sunitinib and everolimus have shown significantly improved progression-free survival in patients with progressive metastatic neuroendocrine tumors, including PNETs, and both drugs are approved by the US Food and Drug Administration for this indication [10, 11]. However, it is unclear which patients with metastatic PNETs benefit from which agent.
Thus, we are conducting a phase II trial of mutation-targeted therapy with sunitinib or everolimus in patients with advanced low- or intermediate-grade neuroendocrine tumors of the gastrointestinal tract and pancreas with or without cytoreductive surgery (NCT02315625) to test whether the tumor genotype determines response to treatment [9]. As part of our clinical trial, tumor biopsy and mutational analysis by next-generation sequencing is performed. Patients with somatic or germline mutations in MEN1/PDGFR/KIT/FLT3 or wild-type tumor are treated with sunitinib [9]. Patients with somatic or germline mutations in NF1/PTEN/P13K/AKT/mTOR/VHL are treated with everolimus. When there is disease progression, subjects cross over to treatment with the other medication. Treatment is discontinued in the case of disease progression after crossover, unacceptable toxicity, or withdrawal of consent [9]. Herein, we report on a patient who enrolled in this trial with a germline MEN1 mutation and metastatic PNET who was also found to have a somatic VHL mutation that responded to sunitinib therapy.
1. Case Description
A 45-year-old woman with MEN1 presented with abdominal pain, distention, bloating, and diarrhea. Her medical history was notable for primary hyperparathyroidism, which required a 3.5-gland parathyroidectomy, a nonfunctioning pituitary adenoma (for which she had transphenoidal resection because of growth and associated mass effect on the internal carotid artery), and Zollinger-Ellison syndrome, for which she was treated with pantoprazole twice daily. She also had a history of well-differentiated metastatic pulmonary neuroendocrine tumors, for which she had a video-assisted thoracoscopic resection. Her family history was notable for a father, a brother, two paternal uncles, one paternal aunt, a paternal grandmother, and a son all diagnosed with MEN1. In June 2010, she presented with symptoms and biochemical evidence of pancreatitis.
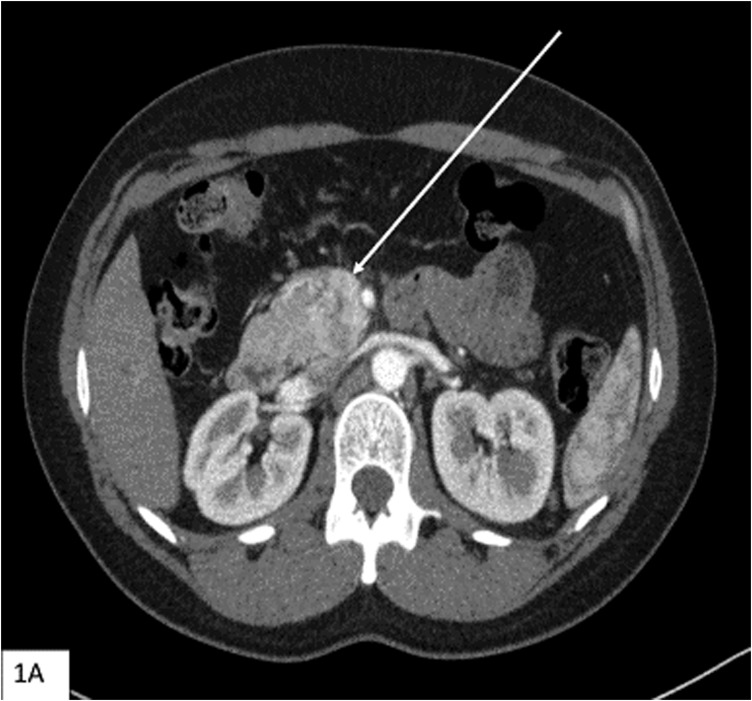
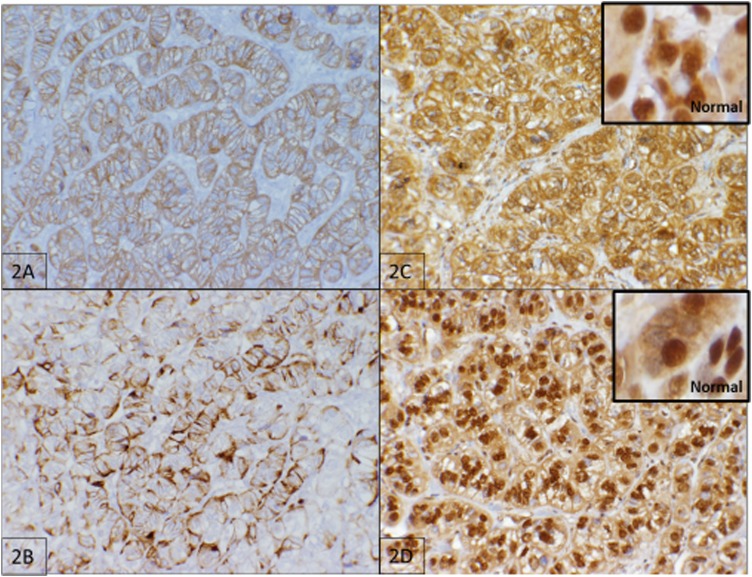
A workup revealed an obstructing complex mass in the head of the pancreas and lesions in the tail of the pancreas and duodenum [Fig. 1(A)]. She underwent a pancreaticoduodenectomy and had an uncomplicated postoperative course. Pathology showed duodenal and retroperitoneal gastrinomas as well as a PNET. Lesions were well differentiated with a WHO grade 1 tumor and a pT2N0MX stage. Immunohistochemical staining of the tumor was positive for synaptophysin and chromogranin [Fig. 2(A) and 2(B)]. Immunohistochemistry was also performed for VHL (ab140989; Abcam, Cambridge, MA) and menin (ab2605; Abcam, Cambridge, MA) [Fig. 2(C) and 2(D)]. Although there was positive staining in the menin protein, menin is a nuclear protein, and localization to the cellular membrane, as shown in Fig. 2(C), is evidence of an abnormal protein [12]. VHL staining appears predominantly cytoplasmic in normal tissue [13]. As shown in Fig. 2(D), the neoplastic cells showed both nuclear and cytoplasmic staining.
Figure 1.
(A) Computed tomography scan (axial section) with contrast showing mass in the head of the pancreas (arrow) that abuts the superior mesenteric vein and the second/third portion of the duodenum.
Figure 2.
Tumor immunohistochemistry. (A) Immunohistochemistry for synaptophysin (×20 magnification, brown color). (B) Immunohistochemistry for chromogranin (×20 magnification, brown color). (C) Immunohistochemistry for menin (×20 magnification, brown color) with an inset of normal pancreatic tissue (×40 magnification, brown color). (D) Immunohistochemistry for VHL (×20 magnification, brown color) with an inset of normal pancreatic tissue (×40 magnification, brown color).
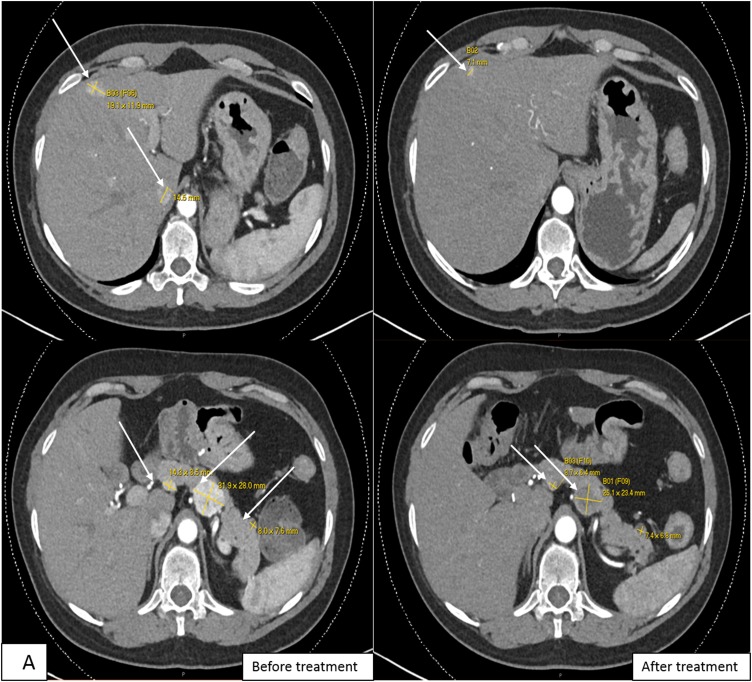
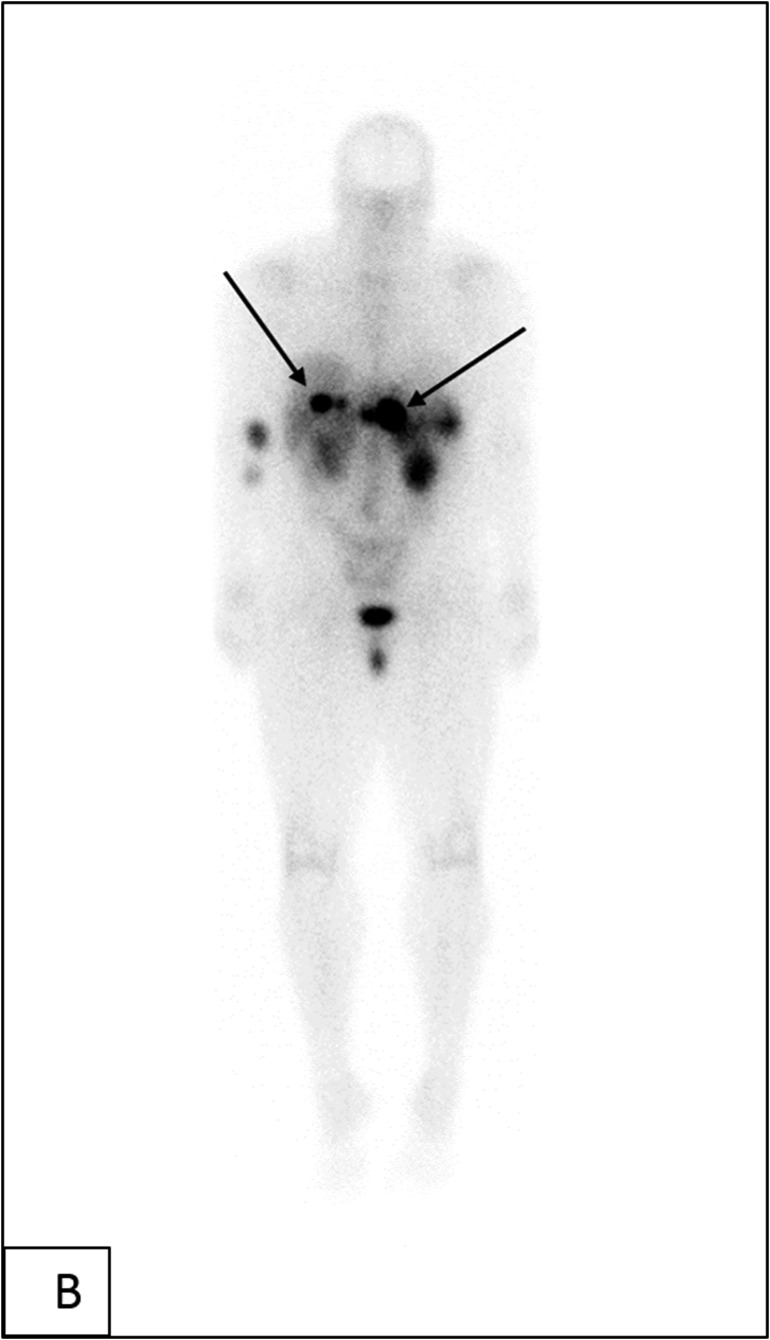
Follow-up imaging in June 2014 showed growth of the tumor in the body and tail of the pancreas, mesenteric lymph node, and three arterial-phase enhancing liver lesions [Fig. 3(A) and 3(B)]. She underwent transarterial chemoembolization procedures in August 2014, October 2014, and January 2015 for the liver lesions without response to treatment. Her pancreatic body tumor grew from 2.6 to 3.2 cm in greatest diameter.
Figure 3.
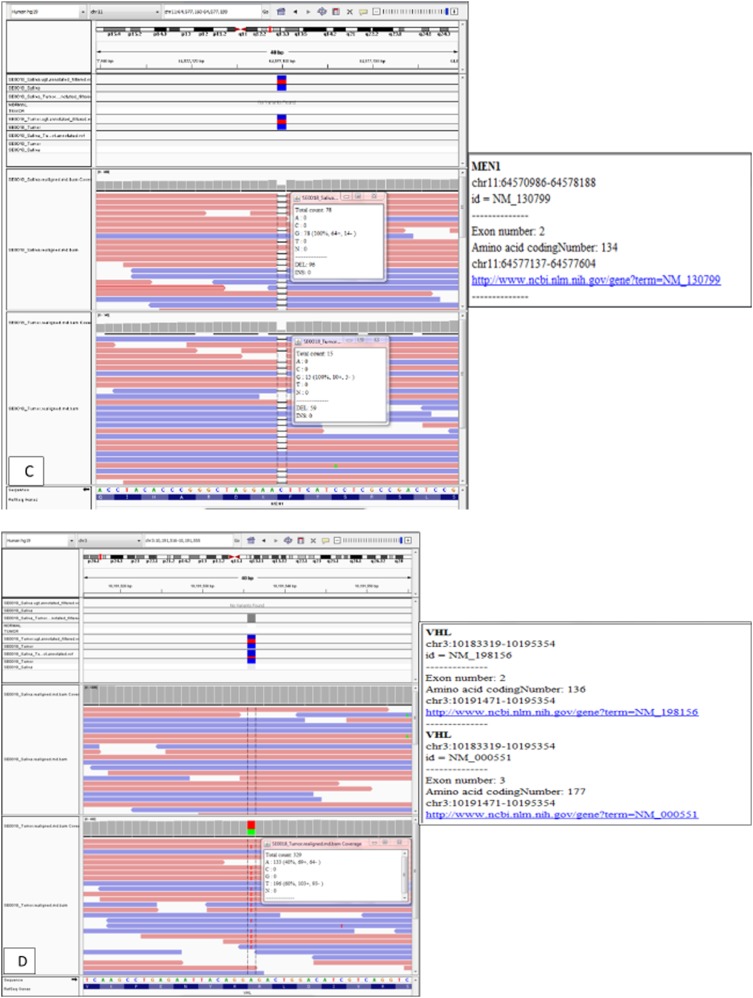
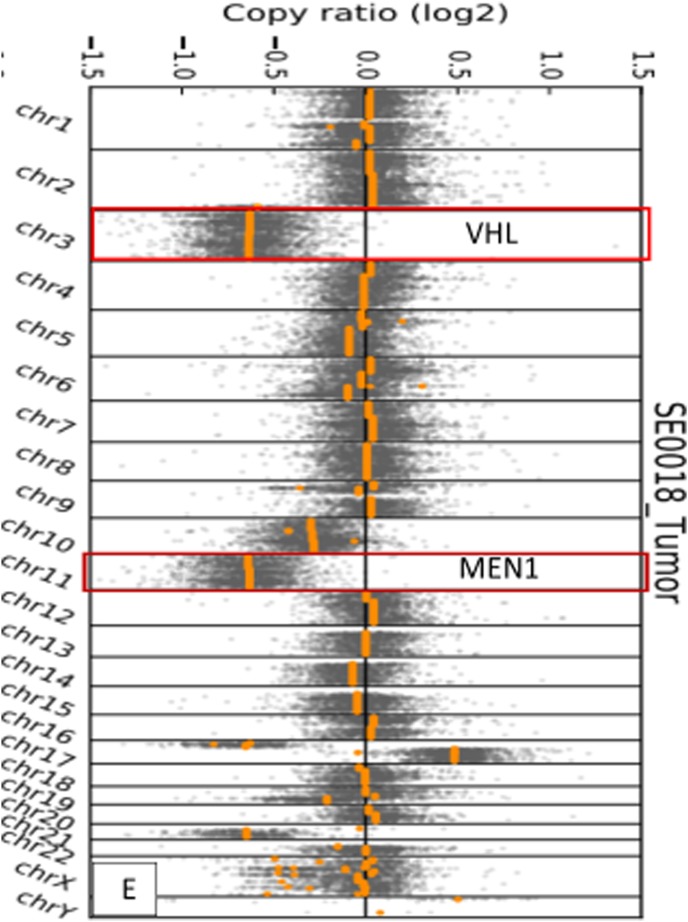
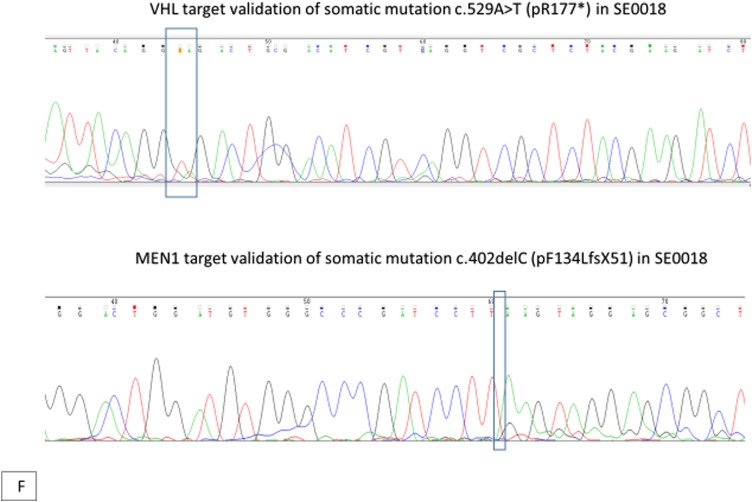
(A) Computed tomography scans (axial section) of the abdomen with contrast showing enhancing mass along the dorsal aspect of the pancreas before and after treatment, decreasing from 3.1 cm at the greatest diameter to 2.5 cm, as well as a decrease in representative hepatic lesions (arrows). (B) Octreotide scan showing liver and peripancreatic uptake consistent with metastatic tumors (arrows). (C) DNA sequencing showing the same MEN1 germline and somatic mutations. (D) VHL somatic nonsense mutation, c. 529A>T. (E) Comparative genomic hybridization array showing LOH in chromosomes 3 and 11. (F) Sanger sequencing confirming the presence of c.402 deletion C, p.Phe134LeufsX51 MEN1 and c.529A>T, p.Arg177Ter VHL mutations.
In July 2016, the patient was enrolled in the phase 2 trial Mutation-Targeted Therapy With Sunitinib or Everolimus in Patients With Advanced Low- or Intermediate-Grade Neuroendocrine Tumors of the Gastrointestinal Tract and Pancreas With or Without Cytoreductive Surgery (NCT02315625). As part of the clinical trial, the patient’s tumor DNA and RNA were sequenced and compared with peripheral blood DNA and RNA, respectively, for 500 known tumor suppressor and oncogenes. The OncoVar-NET assay (an oncogene panel sequencing analysis) was designed for the patient’s GI NET tumor tissue by hybrid capture sequencing to validate somatic mutations identified in the coding regions of the following genes: PTEN, PIK3CA, AKT1, MTOR, VHL, TSC1, TSC2, NF1, TP53, MEN1, FLT3, PDGFRA, ATM, KIT, and ATRX. Peripheral blood served as a germline reference.
The sequencing results confirmed a germline MEN1 mutation (deletion in exon 2, c.402 deletion C, p.Phe134LeufsX51), loss of heterozygosity (LOH) in the tumor tissue, and the same mutation in the other allele [Fig. 3(C) and 3(E)]. A somatic mutation in the VHL gene was identified, specifically a nonsense mutation c.529A>T, p.Arg177Ter [Fig. 3(D) and 3(E)]. No other genetic alterations in genes associated with PNETs, such as PTEN, PIK3CA, AKT1, MTOR, TSC1, TSC2, NF1, TP53, FLT3, PDGFRA, ATM, KIT, and ATRX, were identified. Sanger sequencing confirmed the MEN1 and VHL mutations noted in the original molecular profiling [Fig. 3(F)]. The patient was treated with sunitinib and had response to treatment by November 2016 [Fig. 3(A)]. Of note, between the initiation of therapy and the response to treatment, our patient received no other therapy for her neuroendocrine tumors outside of sunitinib, and she remains on the same medication at the same dose.
2. Discussion
MEN1 and VHL are autosomal-dominant diseases with known susceptibility genes that cause PNETs. In both of these inherited syndromes, a second hit (somatic mutation) occurs in addition to the germline, resulting in multiorgan disease manifestation. Although secondary somatic mutations may occur in inherited tumor-suppressor cancer syndromes, a coexisting germline mutation in the tumor-suppressor gene with a somatic mutation in another tumor-suppressor gene responsible for an inherited syndrome has not been reported. Our patient presented with a known germline mutation in the MEN1 gene and a family history of MEN1. She developed a metastatic PNET. She was enrolled in a clinical trial to assess whether the tumor genotyping was associated with a response to sunitinib and everolimus. Her previous clinical diagnosis of MEN1 was supported by her family history as well as by tumor genotyping using tumor tissue from her previous operative intervention, confirming the presence of a c.402 deletion C, p.Phe134LeufsX51 in MEN1 in exon 2 with LOH in the MEN1 locus in the tumor tissue; thus, she was started on sunitinib therapy. In addition, she had a coexisting somatic nonsense mutation in VHL gene c.529A>T, p.Arg177Ter. A germline MEN1 mutation with a somatic mutation in the tumor suppressor VHL has not been observed and may be a tumor genotype that is associated with aggressive PNETs.
Somatic VHL mutations in PNETs may be associated with metastatic progression [14–17]. In a study by Hessman et al. [18], LOH at 3p was observed in 45% of nonfamilial PNETs and in 36% of MEN1-associated PNETs. They also found that 92% of malignant tumors had allelic loss at 3p and 11q13 [18]. Possible VHL mutations associated with well-differentiated PNETs were found in one of 60 cases, according to a review of LOH studies in PNETs and gastrointestinal neuroendocrine tumors performed by Furlan et al. [17]. Lott et al. [16] found involvement of chromosome 3p in sporadic pancreatic islet cell tumorigenesis, with associated progression to malignancy. Chung et al. [19] demonstrated that both 3p and 11q (associated with VHL and MEN1, respectively) were commonly deleted loci in PNETs. Therefore, the combination of germline MEN1 mutation and somatic VHL mutation may be associated with more aggressive disease. Furthermore, even in patients with a known germline mutation in either VHL or MEN1, tumor genotyping may reveal a somatic mutation that would allow the selection of the most appropriate agent to target the altered pathway(s).
In the case described, sunitinib therapy was selected because the patient had MEN1 and preclinical studies showed response to sunitinib therapy in transgenic mouse models of MEN1-associated PNETs [20]. The presence of a somatic mutation in VHL is associated with activation of the mTOR pathway; thus, everolimus treatment could be effective. However, VHL is associated with major tumor angiogenesis, which may explain why the patient had a response to sunitinib therapy [21, 22].
The case described illustrates that therapy selection based on tumor genotype in practice needs to be tested in clinical trials. Although “precision” treatment is selected according to the driver mutation(s) present and the expected pathway to be activated, response to treatment may be determined by the presence of coexisting mutations. Thus, in the current clinical trial in which the patient is enrolled, subjects who do not respond to one agent on the basis of the tumor genotype or presence of germline mutation are crossed over to treatment with the other agent to determine the important tumor genotype that predicts response to a selected agent.
Acknowledgments
We acknowledge Dr. Sudheer Gara of the Endocrine Oncology Branch at the National Cancer Institute as well as Jennifer Walling, David Petersen, and Marbin Pineda of the Genetics Branch of the Center for Cancer Research at the National Cancer Institute.
Acknowledgments
This research was supported by the intramural research program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- FLT3
- FMS-like tyrosine kinase 3
- LOH
- loss of heterozygosity
- MEN1
- multiple endocrine neoplasia type 1
- mTOR
- mammalian target of the rapamycin
- PNET
- pancreatic neuroendocrine tumor
- VHL
- von Hippel-Lindau.
References and Notes
- 1.Jiao Y, Shi C, Edil BH, de Wilde RF, Klimstra DS, Maitra A, Schulick RD, Tang LH, Wolfgang CL, Choti MA, Velculescu VE, Diaz LA Jr, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331(6021):1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97(9):2990–3011. [DOI] [PubMed] [Google Scholar]
- 3.Alexakis N, Connor S, Ghaneh P, Lombard M, Smart HL, Evans J, Hughes M, Garvey CJ, Vora J, Vinjamuri S, Sutton R, Neoptolemos JP. Hereditary pancreatic endocrine tumours. Pancreatology. 2004;4(5):417–433, discussion 434–435. [DOI] [PubMed] [Google Scholar]
- 4.Bender BU, Gutsche M, Gläsker S, Müller B, Kirste G, Eng C, Neumann HP. Differential genetic alterations in von Hippel-Lindau syndrome-associated and sporadic pheochromocytomas. J Clin Endocrinol Metab. 2000;85(12):4568–4574. [DOI] [PubMed] [Google Scholar]
- 5.Cassol C, Mete O. Endocrine manifestations of von Hippel-Lindau disease. Arch Pathol Lab Med. 2015;139(2):263–268. [DOI] [PubMed] [Google Scholar]
- 6.Keutgen XM, Hammel P, Choyke PL, Libutti SK, Jonasch E, Kebebew E. Evaluation and management of pancreatic lesions in patients with von Hippel-Lindau disease. Nat Rev Clin Oncol. 2016;13(9):537–549. [DOI] [PubMed] [Google Scholar]
- 7.Moore PS, Missiaglia E, Antonello D, Zamò A, Zamboni G, Corleto V, Falconi M, Scarpa A. Role of disease-causing genes in sporadic pancreatic endocrine tumors: MEN1 and VHL. Genes Chromosomes Cancer. 2001;32(2):177–181. [DOI] [PubMed] [Google Scholar]
- 8.Görtz B, Roth J, Krähenmann A, de Krijger RR, Muletta-Feurer S, Rütimann K, Saremaslani P, Speel EJ, Heitz PU, Komminoth P. Mutations and allelic deletions of the MEN1 gene are associated with a subset of sporadic endocrine pancreatic and neuroendocrine tumors and not restricted to foregut neoplasms. Am J Pathol. 1999;154(2):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neychev V, Steinberg SM, Cottle-Delisle C, Merkel R, Nilubol N, Yao J, Meltzer P, Pacak K, Marx S, Kebebew E. Mutation-targeted therapy with sunitinib or everolimus in patients with advanced low-grade or intermediate-grade neuroendocrine tumours of the gastrointestinal tract and pancreas with or without cytoreductive surgery: protocol for a phase II clinical trial. BMJ Open. 2015;5(5):e008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao JC, Lombard-Bohas C, Baudin E, Kvols LK, Rougier P, Ruszniewski P, Hoosen S, St Peter J, Haas T, Lebwohl D, Van Cutsem E, Kulke MH, Hobday TJ, O’Dorisio TM, Shah MH, Cadiot G, Luppi G, Posey JA, Wiedenmann B. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai A, Murakami A, Sano K, Uchino S, Fukushima Y. Unusual clinical and pathological presentation of a neuroendocrine tumor in a patient with multiple endocrine neoplasia type 1. Endocr J. 2009;56(7):887–895. [DOI] [PubMed] [Google Scholar]
- 13.Dannenberg H, De Krijger RR, van der Harst E, Abbou M, IJzendoorn Y, Komminoth P, Dinjens WN. Von Hippel-Lindau gene alterations in sporadic benign and malignant pheochromocytomas. Int J Cancer. 2003;105(2):190–195. [DOI] [PubMed] [Google Scholar]
- 14.Schmitt AM, Schmid S, Rudolph T, Anlauf M, Prinz C, Klöppel G, Moch H, Heitz PU, Komminoth P, Perren A. VHL inactivation is an important pathway for the development of malignant sporadic pancreatic endocrine tumors. Endocr Relat Cancer. 2009;16(4):1219–1227. [DOI] [PubMed] [Google Scholar]
- 15.Barghorn A, Komminoth P, Bachmann D, Rütimann K, Saremaslani P, Muletta-Feurer S, Perren A, Roth J, Heitz PU, Speel EJ. Deletion at 3p25.3-p23 is frequently encountered in endocrine pancreatic tumours and is associated with metastatic progression. J Pathol. 2001;194(4):451–458. [DOI] [PubMed] [Google Scholar]
- 16.Lott ST, Chandler DS, Curley SA, Foster CJ, El-Naggar A, Frazier M, Strong LC, Lovell M, Killary AM. High frequency loss of heterozygosity in von Hippel-Lindau (VHL)-associated and sporadic pancreatic islet cell tumors: evidence for a stepwise mechanism for malignant conversion in VHL tumorigenesis. Cancer Res. 2002;62(7):1952–1955. [PubMed] [Google Scholar]
- 17.Furlan D, Cerutti R, Uccella S, La Rosa S, Rigoli E, Genasetti A, Capella C. Different molecular profiles characterize well-differentiated endocrine tumors and poorly differentiated endocrine carcinomas of the gastroenteropancreatic tract. Clin Cancer Res. 2004;10(3):947–957. [DOI] [PubMed] [Google Scholar]
- 18.Hessman O, Lindberg D, Einarsson A, Lillhager P, Carling T, Grimelius L, Eriksson B, Akerström G, Westin G, Skogseid B. Genetic alterations on 3p, 11q13, and 18q in nonfamilial and MEN 1-associated pancreatic endocrine tumors. Genes Chromosomes Cancer. 1999;26(3):258–264. [PubMed] [Google Scholar]
- 19.Chung DC, Brown SB, Graeme-Cook F, Tillotson LG, Warshaw AL, Jensen RT, Arnold A. Localization of putative tumor suppressor loci by genome-wide allelotyping in human pancreatic endocrine tumors. Cancer Res. 1998;58(16):3706–3711. [PubMed] [Google Scholar]
- 20.Allen E, Wakters IB, Hanahan D. Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition. Clin Cancer Res. 2011;17(16):5299–5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jimenez C, Cabanillas ME, Santarpia L, Jonasch E, Kyle KL, Lano EA, Matin SF, Nunez RF, Perrier ND, Phan A, Rich TA, Shah B, Williams MD, Waguespack SG. Use of the tyrosine kinase inhibitor sunitinib in a patient with von Hippel-Lindau disease: targeting angiogenic factors in pheochromocytoma and other von Hippel-Lindau disease-related tumors. J Clin Endocrinol Metab. 2009;94(2):386–391. [DOI] [PubMed] [Google Scholar]
- 22.Yao JC. Molecular targeted therapy for carcinoid and islet-cell carcinoma. Best Pract Res Clin Endocrinol Metab. 2007;21(1):163–172. [DOI] [PubMed] [Google Scholar]