Abstract
Several studies suggest that neutralizing acid load in the diet with alkali had favorable effects on intermediate markers of musculoskeletal health. We examined whether alkali supplementation with potassium bicarbonate [(KHCO3); 81 mmol/d; n = 12] vs placebo (n = 12) for 84 days altered serum microRNAs, potential biomarkers associated with innumerable biological processes including bone and muscle metabolism. Serum microRNAs, urinary net acid excretion (UNAE), urinary N-telopeptide (UNTX), urinary calcium (UCa), urinary nitrogen (UN), glomerular filtration rate, serum procollagen type 1 amino-terminal propeptide (P1NP), serum insulin-like growth factor-1 (IGF-1), and its serum binding protein IGFBP3 were measured at baseline and day 84. Baseline characteristics and measurements were similar in the two treatment groups. Eighty-four–day changes in UNAE differed by group (KHCO3, −47 ± 9 mmol; placebo, −5 ± 5 mmol; P < 0.01). KHCO3 significantly reduced UNTX, UCa, and serum P1NP but did not affect UN, serum IGF-1, or IGFBP3 levels compared with placebo over 84 days. Fold change in serum circulating microRNA (c-miR)-133b differed significantly by group (KHCO3, 2.26 ± 0.85; placebo, −1.23 ± 0.69; P < 0.01); there was a similar trend in c-miR-21-5p. Fold changes in c-miR-133b and c-miR-21-5p were inversely associated with changes in UNAE and UNTX; fold change in c-miR-21-5p was inversely associated with change in UCa, with a similar trend with c-miR-133b. In summary, reducing renal acid load with KHCO3 was associated with increased expressions of c-miR-133b and c-miR-21-5p. Furthermore, increases in c-miRNA-133b and c-miR-21-5p were inversely associated with bone resorption markers UNTX and UCa consistent with potential beneficial effects on bone in older adults. However, the broader significance of c-miRNAs as musculoskeletal biomarkers is still under investigation, and larger studies are needed to verify these preliminary results.
Keywords: miR-21-5p, miR-133b, alkali supplement, net acid excretion, bone
Circulating miRNA-133b and miRNA-21-5p increased after 84 days of alkali supplementation and were inversely associated with changes in net acid excretion, N-telopeptide, and calcium excretion.
Acid-producing diets in combination with age-related declines in renal function induce a mild but progressive metabolic acidosis that is thought to contribute to the development of osteoporosis and sarcopenia in older adults. Compromised bone and muscle mass can result in frailty, limited mobility, and loss of independence [1, 2]. A number of intervention studies have shown that supplementation with alkaline salts, potassium bicarbonate (KHCO3) [3, 4], or citrate [5–7] in an amount that neutralizes the acid load of the diet significantly reduced bone resorption biomarkers (N- and C-telopeptides) [3–7], with less consistent effects on bone formation biomarkers, such as osteocalcin, bone-specific alkaline phosphatase, and procollagen type 1 amino-terminal propeptide (P1NP) [4, 5]. Alkaline supplements also had favorable effects on calcium balance [7]. In studies examining skeletal muscle markers, alkaline supplementation has reduced urinary nitrogen (UN) excretion [8–10] and increased serum insulin-like growth factor-1 (IGF-1) concentrations [9]. However, benefit on biomarkers of muscle mass has not been consistent across studies [4].
Small noncoding RNA, known as microRNA (miRNA), has recently demonstrated important functional roles in regulating key processes determining bone and skeletal muscle mass [11–14]. miRNAs are synthesized in the nucleus of cells and then are exported to the cytoplasm where they target specific genes for repression of degradation or translation [15–19]. miRNAs can function within the cytoplasm of their cell of origin, or they can be taken up by membrane-derived vesicles and exported into the circulation to be transferred to recipient cells in the same or other tissue [20, 21]. Being membrane-bound, circulating miRNAs (c-miRNAs) were shown to be stable and reproducible analytes [22, 23], with alterations in c-miRNA profiles reflective of the underlying physiological state of donor tissue, thus offering a unique perspective as noninvasive biomarkers [24]. Multiple recent cross-sectional investigations have found that dysregulation in the expression of c-miRNAs correlates with disease states such as osteopenia [25–28] and sarcopenia [29–31]. However, longitudinal data are lacking on changes in c-miRNA expression profiles in response to interventions aimed at improving bone and skeletal muscle health.
The objective of this pilot study was to examine whether a subset of participants consuming an alkaline salt supplement, oral KHCO3, or matched placebo for 84 days had altered c-miRNA expression profiles: miRNAs 1-3p, 21-5p, 122, 125, 133a-3p, 133b, 206, 422, and 486. The miRNAs assessed in this investigation were chosen because they target molecular pathways involved in the regulation of bone and muscle mass and have changed in the circulation under physiological conditions that impact bone and skeletal muscle (Table 1). Furthermore, this investigation sought to determine the association between changes in the expression of these musculoskeletal-associated c-miRNAs and changes in established indices of bone [urinary N-telopeptide (UNTX); P1NP; urinary calcium (UCa)] and muscle metabolism [UN, serum IGF-1, and its binding protein (IGFBP3)] by treatment group.
Table 1.
microRNA Function
| microRNA | Function | |
|---|---|---|
| miR-21-5p | Generation of osteoclast | Bone |
| miR-122 | BMPR1A, bone remodeling | Bone |
| miR-125 | Generation of osteoblast | Bone |
| miR-422 | Biomarker for BMD level | Bone |
| miR-133a-3p | Generation of muscle fibers, muscle hypertrophy, and generation of osteoblasts | Bone; skeletal muscle |
| miR-133b | Generation of muscle fibers, muscle hypertrophy, and generation of osteoblasts | Bone; skeletal muscle |
| miR-1-3p | Generation of muscle fibers and muscle hypertrophy | Skeletal muscle |
| miR-206 | Generation of muscle fibers and muscle hypertrophy | Skeletal muscle |
| miR-486 | Muscle hypertrophy and atrophy | Skeletal muscle |
Abbreviations: BMD, bone mineral density; BMPR1A, bone morphogenetic protein receptor type 1A; miR, microRNA.
1. Materials and Methods
A. Participants and Study Design
This was an 84-day randomized, double-blind, placebo-controlled trial to determine the optimal dose of KHCO3 (1.0 vs 1.5 mmol/kg/d) vs placebo to maximally suppress bone resorption as measured by UNTX in healthy ambulatory older men and women free of chronic disease. This duration of supplementation was previously reported as sufficient to induce changes in bone and muscle health [10]. The eligibility criteria, design, and methods of the main trial have been described in detail elsewhere [4]. Briefly, inclusion criteria were age ≥60 years and an estimated glomerular filtration rate of at least 50 mL⋅min–1⋅1.73 m2. Participants were encouraged to maintain stable diets and physical activity during the trial. This protocol was approved by the Tufts Medical Center-Tufts University Institutional Review Board and written informed consent was obtained from each subject. All subject visits took place at the US Department of Agriculture (USDA) Human Nutrition Research Center on Aging at Tufts University. The study is registered at Clinicaltrials.gov (http://clinicaltrials.gov/show/NCT1475214).
The current observational study was conducted using serum samples from 24 participants randomized to two of the three arms: placebo (n = 12) and KHCO3 1 mmol/kg/d (n = 12), with average KHCO3 of 81 mmol/d. A priori criteria for selection were based on our main study findings [4] and included the following: (1) baseline urinary net acid excretion (UNAE) of 5 mmol or greater as an indicator of higher endogenous renal net acid status at baseline and (2) equal numbers of men and women in the two groups.
B. Supplements
The KHCO3 capsules contained 13.5 mmol of KHCO3 each, and the matching placebos contained microcrystalline cellulose. In the main trial, KHCO3 (1 mmol/kg/d) or matching placebo was administered as two capsules after each meal three times daily. KHCO3 and placebo capsules were purchased from Life Enhancement Products, Inc. (Petaluma, CA), with independent analysis (Covance, Princeton, NJ) confirming that the treatment capsule contained 13.5 mmol of KHCO3. To reduce side effects (i.e., to reduce risk of gastrointestinal intolerance) of KHCO3, capsule intake was gradually increased daily at the initiation of the study until the full assigned dose was achieved. Serum potassium safety checks were conducted from fasting blood draws on study days 10, 13, 16, 19, 22, and 50. A calcium and vitamin D supplement was supplied to all participants. Adherence was measured by pill counts and UNAE. Adherence with the study capsules averaged 92.2% in the placebo group and 91.3% in the KHCO3 group. Further details are described elsewhere [4].
C. Anthropometrics and Body Composition
Baseline height was measured using a wall-mounted stadiometer, and baseline weight was measured using a calibrated digital scale. Baseline dual-energy x-ray absorptiometry (Hologic, Bedford, MA) was used to assess body composition (fat mass and fat-free mass).
D. Biochemical Measurements
Blood was drawn between 7:00 and 9:30 am after the subjects had fasted for 12 hours. All samples were batched for analyses. Serum P1NP was measured by competitive radioimmunoassay with Uni P1NP RIA kits from Orion Diagnostica (Espoo, Finland), with intra-assay and interassay coefficient of variances (CVs) of 5.0% and 8.1%, respectively. Serum IGF-1 and IGFBP3 levels were measured by chemiluminescent immunoradiometric assay on an automated immunoassay system (IMMULITE 1000; Diagnostic Product Corp., Los Angeles, CA), with CVs of 3% to 9%. Urinary creatinine was measured on an automated clinical chemistry analyzer (Olympus AU400; Olympus America Inc., Melville, NY), with CVs of 3% to 6%. UCa was measured by direct-current plasma emission spectroscopy (Beckman SpectraSpan VI Direct Current Plasma Emission Spectrophotometer; Beckman Instruments, Fullerton, CA) with a CV of 3% to 5%. UNTX was measured by enzyme-linked immunosorbent assay (Wampole, Princeton, NJ), with a CV of 5.6% to 7.7%. UN was measured with a model FP-2000 nitrogen/protein determinator (LECO, St. Joseph, MI), with intra-assay and interassay CVs of 6.5% and 8.6%, respectively [10]. Urinary pH was determined with the Accumet Excel pH meter (Fisher Scientific, Pittsburgh, PA). UNAE in urine (UNAE = titratable acid + NH4+ − HCO3–) was measured in our laboratory by a modification of the Jørgensen titration method [32] as described by Chan [33], with a precision of 10.1% [3].
E. Circulating microRNA Extraction and Expression
From serum, RNA was extracted using an miRVanaTM PARISTM RNA Purification Kit (AM1556; Applied Biosystems, Foster City, CA). From extracted RNA, miRNAs of interest (Table 1) were analyzed using TaqMan® MicroRNA Assays (4427975; Applied Biosystems) following previously described multiplex reverse transcriptase (RT) and preamplification protocol. Briefly, miRNAs were reverse-transcribed using the TaqMan® microRNA RT kit (4366596; Applied Biosystems) with the miRNA-specific stem-loop RT primers pooled in 1× Tris-EDTA buffer for a final dilution of 0.05× for each miRNA RT primer. The RT primer pool (6 μL) was then added to the RT reaction mix (0.3 μL 100 mM deoxynucleoside triphosphate, 3 μL enzyme, 1.5 μL 10× RT buffer, 0.19 μL ribonuclease inhibitor) and 4 μL of serum RNA. A preamplification step was performed to increase the complementary DNA template using a primer pool of 20× Taqman® Small RNA Assay for the miRNA of interest at 0.05× concentration in 1× Tris-EDTA buffer. Preamplification reaction mix was constituted of 3.75 μL primer pool, 2.5 μL complementary DNA, 12.5 μL Taqman® Universal PCR Master Mix (2×), no uracil-N-glycosylase (#4440040; Applied Biosystems), and 6.25 μL nuclease-free H2O. Reverse transcription and preamplification were conducted following the manufacturer’s instructions in a T100TM Thermal Cycler (Bio-Rad, Hercules, CA). After preamplification, quantitative reverse transcription polymerase chain reaction amplifications were conducted following the manufacturer’s instructions using the CFX96 TouchTM Real-Time PCR Detection System (Bio-Rad, Hercules, CA). All miRNAs were normalized to U6 small nuclear RNA. Fold changes were calculated from baseline values using the ∆∆ cycle threshold method.
F. Statistical Analysis
Comparisons between baseline descriptive characteristics were conducted using the independent Student t test. Data were examined to determine if sex impacted outcome variables. There was no effect of sex on variables at the conclusion of the 84-day trial. Mixed-model repeated measures analysis of variance was performed to assess the effects of time (baseline vs day 84), treatment [KHCO3 vs placebo], and time-by-treatment interactions for blood and analytes and c-miRNA expression. On the basis of the Akaike information criterion, unstructured covariance was determined as the appropriate model for analysis. For post hoc pairwise comparisons, Bonferroni correction was performed. Fold change data for c-miRNA were log transformed (log2) for statistical analysis. Multidimensional scaling of significant or near significant c-miRNA (miR-133b and miR-21-5p) was preformed using MetaboAnalyst 3.0 (http://www.metaboanalyst.ca). Spearman’s ρ rank correlation coefficient was used to determine associations between c-miRNA expression and ∆ values of blood and urine analytes. The α level for significance was set at P ≤ 0.05. Data were analyzed using IBM SPSS Statistics for Windows Version 22.0 (IBM Corp., Armonk, NY).
2. Results
A. Study Sample and Intervention
Baseline characteristics did not differ between treatment groups (Table 2). As expected, by the end of the study (day 84), UNAE was significantly reduced with KHCO3 (−47 ± 9 mmol) compared with placebo (−5 ± 5 mmol; P < 0.01; time-by-treatment effect), demonstrating the effect of our intervention.
Table 2.
Baseline Participant Characteristics
| Placebo (n = 12) | KHCO3 (n = 12) | P Valuea | |
|---|---|---|---|
| Age, y | 67 ± 2 | 67 ± 1 | 0.87 |
| Female, % | 50 | 50 | 1.0 |
| Height, cm | 168.7 ± 2.8 | 170.9 ± 4.9 | 0.59 |
| Weight, kg | 69.3 ± 3.1 | 75.8 ± 4.9 | 0.28 |
| Body mass index, kg/m2 | 24.3 ± 0.7 | 25.6 ± 1.1 | 0.33 |
| Fat mass, kg | 19.1 ± 2.1 | 21.9 ± 2.4 | 0.39 |
| Fat-free mass, kg | 48.8 ± 3.1 | 50.2 ± 4.1 | 0.79 |
| GFR, mL/min/1.73 m2 | 73.1 ± 2.8 | 71.0 ± 2.2 | 0.57 |
Values are presented as mean ± standard error of the mean.
Abbreviation: GFR, glomerular filtration rate.
P values were calculated using the Student t test.
B. Traditional Biochemical Parameters
As seen in the larger cohort in the main study [4], KHCO3 altered biomarkers of bone health (Table 3). During the 84-day intervention, KHCO3 supplementation significantly reduced UNTX compared with placebo (KHCO3 = −158 ± 31 nmol; placebo = −47 ± 28 nmol; time-by-treatment interaction P < 0.05) and UCa (KHCO3 = −23 ± 19 mmol; placebo = 38 ± 18 mmol; time-by-treatment interaction P < 0.05). Although there was a time effect for serum P1NP (P < 0.05), there was a trend only for a KHCO3 effect in this small sample (P = 0.10). In addition, similar to our larger cohort in the main study, [4] there were no statistically significant differences in UN (KHCO3 = −7.2 ± 11.2 mg⋅kg−1; placebo = 7.3 ± 17.1 mg⋅kg−1; time-by-treatment interaction P = 0.48) and serum IGF-1 (KHCO3 = −8.4 ± 15.8 mmol⋅L−1; placebo = −9.3 ± 8.3 mmol⋅L−1; time-by-treatment interaction P = 0.33) or IGFBP3 concentration (KHCO3 = 0.0 ± 0.1 mg⋅L−1; placebo = 0.3 ± 0.1 mg⋅L−1; time-by-treatment interaction P = 0.09) in response to KHCO3 compared with placebo in this subset.
Table 3.
Baseline and Day 84 Serum and Urine Biochemical Measurements by Treatment Group
| Time | Placebo | KHCO3 |
P Value |
|||
|---|---|---|---|---|---|---|
| Time | Treatment | T × T | ||||
| Serum | ||||||
| P1NP (nmol⋅L–1) | Baseline | 52.0 ± 5.8 | 45.3 ± 4.3 | |||
| Day 84 | 50.4 ± 4.5 | 37.1 ± 2.3 | 0.03 | 0.10 | 0.12 | |
| IGF-1(mmol⋅L–1) | Baseline | 163.6 ± 25.4 | 150.0 ± 12.9 | |||
| Day 84 | 155.2 ± 17.7 | 159.3 ± 11.1 | 0.96 | 0.84 | 0.33 | |
| IGFBP3 (mg⋅L–1) | Baseline | 5.1 ± 0.4 | 4.9 ± 0.2 | |||
| Day 84 | 5.1 ± 0.4 | 5.2 ± 0.2 | 0.14 | 0.96 | 0.09 | |
| Urine | ||||||
| UNAE (mmol⋅d–1) | Baseline | 38.4 ± 5.1 | 44.7 ± 8.9 | |||
| Day 84 | 33.8 ± 6.5 | −2.0 ± 1.0 | 0.05 | <0.01 | <0.01 | |
| Urinary pH | Baseline | 5.8 ± 0.1 | 5.9 ± 0.1 | |||
| Day 84 | 5.7 ± 0.1 | 7.0 ± 0.1 | <0.01 | <0.01 | <0.01 | |
| UNTX (nmol⋅d–1) | Baseline | 383.3 ± 33.6 | 352.9 ± 47.9 | |||
| Day 84 | 335.9 ± 47.6 | 194.6 ± 28.0 | 0.12 | <0.01 | 0.02 | |
| UCa (mmol⋅d–1) | Baseline | 126.4 ± 17.4 | 117.4 ± 23.9 | |||
| Day 84 | 163.9 ± 25.0 | 94.7 ± 16.3 | 0.16 | 0.58 | 0.03 | |
| UN (mg⋅kg–1⋅d–1) | Baseline | 218.4 ± 16.0 | 225.7 ± 15.8 | |||
| Day 84 | 198.7 ± 19.6 | 191.5 ± 16.3 | 0.28 | 0.23 | 0.48 |
Values are presented as mean ± standard error of the mean. P Values were calculated using mixed-model repeated measures analysis of variance with Bonferroni adjustment for pairwise comparisons.
Abbreviation: T × T, time-by-treatment interaction.
C. Changes in c-miRNAs
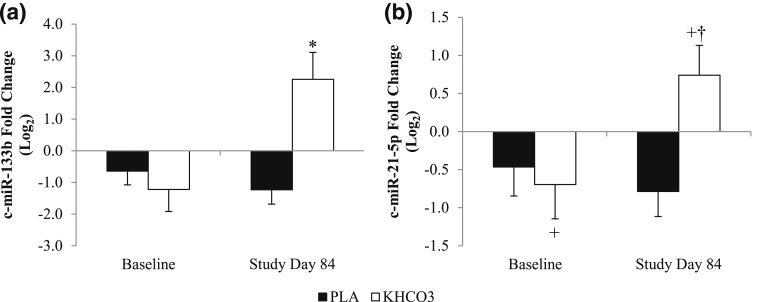
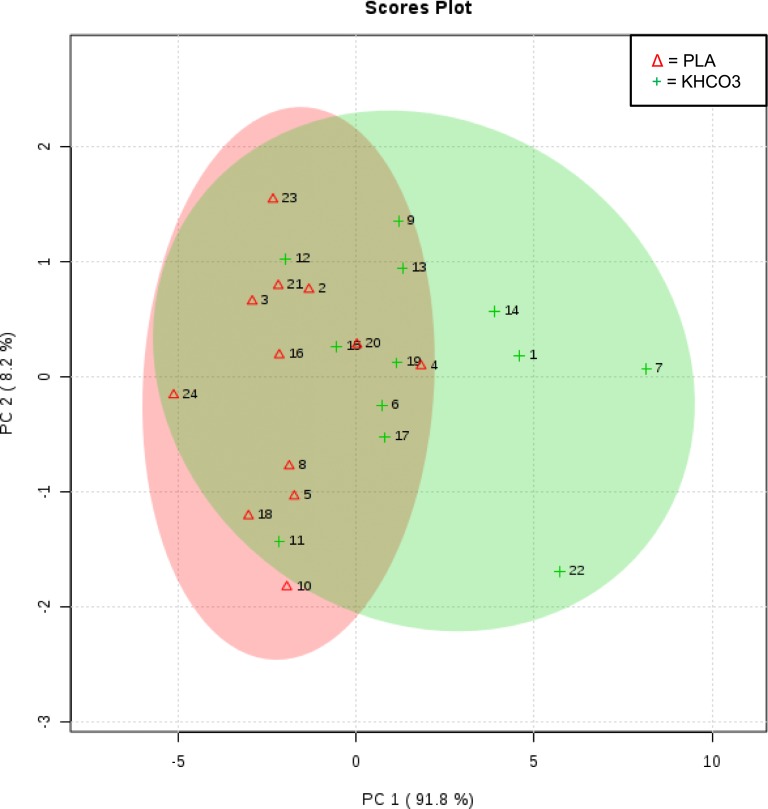
Alterations in the nine c-miRNAs over the 84-day intervention by group are listed in Table 4. Expression of c-miR-133b was significantly upregulated at day 84 in the KHCO3 group vs the placebo group (time-by-treatment interaction P = 0.02) [Fig. 1(a)] with a similar trend noted in expression of c-miR-21-5p (time-by-treatment interaction P = 0.065) [Fig. 1(b)]. Multidimensional scaling of participants by expression of c-miR-133b and c-miR-21-5p on study day 84 showed that those receiving placebo generally clustered together, with negative principal component 1 values (Fig. 2). Participants receiving KHCO3 supplementation had more spread, with partial overlap with participants in the placebo group. This result suggests interindividual variance in the response to KHCO3 supplementation compared with placebo in this pilot study with a limited sample size.
Table 4.
Baseline and Day 84 Circulating microRNA Expression by Treatment Group
| Time | Placebo | KHCO3 |
P Value |
|||
|---|---|---|---|---|---|---|
| Time | Treatment | T × T | ||||
| miR-21-5p | Baseline | −0.46 ± 0.38 | −0.78 ± 0.34 | |||
| Day 84 | −0.70 ± 0.45 | 0.74 ± 0.39 | 0.23 | 0.054 | 0.065 | |
| miR-122 | Baseline | −0.88 ± 0.61 | −0.06 ± 0.62 | |||
| Day 84 | −0.87 ± 0.54 | −0.53 ± 0.46 | 0.44 | 0.43 | 0.75 | |
| miR-125 | Baseline | −0.49 ± 0.39 | −0.39 ± 0.42 | |||
| Day 84 | −0.67 ± 0.50 | 0.03 ± 0.56 | 0.49 | 0.74 | 0.61 | |
| miR-422 | Baseline | −0.63 ± 0.44 | −1.52 ± 0.85 | |||
| Day 84 | −0.91 ± 0.45 | −1.33 ± 0.58 | 0.37 | 0.92 | 0.74 | |
| miR-133a-3p | Baseline | −0.94 ± 0.52 | −0.46 ± 0.35 | |||
| Day 84 | −0.58 ± 0.43 | 0.27 ± 0.44 | 0.14 | 0.23 | 0.67 | |
| miR-133b | Baseline | −0.65 ± 0.43 | −1.23 ± 0.45 | |||
| Day 84 | −1.23 ± 0.69 | 2.26 ± 0.85 | 0.09 | <0.01 | 0.02 | |
| miR-1-3p | Baseline | −1.00 ± 0.66 | −0.91 ± 0.67 | |||
| Day 84 | −1.03 ± 0.68 | 0.08 ± 1.07 | 0.47 | 0.23 | 0.55 | |
| miR-206 | Baseline | −0.71 ± 0.53 | −0.28 ± 0.74 | |||
| Day 84 | −0.57 ± 0.51 | −1.00 ± 0.65 | 0.95 | 0.62 | 0.64 | |
| miR-486 | Baseline | −0.90 ± 0.51 | −1.24 ± 0.34 | |||
| Day 84 | −1.11 ± 0.49 | −0.08 ± 0.55 | 0.56 | 0.19 | 0.25 |
Values are presented as mean ± standard error of the mean (log2). P Values were calculated using mixed-model repeated measures analysis of variance with Bonferroni adjustment for pairwise comparisons. Bold numbers highlight statistical differences.
Abbreviation: T × T, time-by-treatment interaction.
Figure 1.
(a) The 84-day change in expression of c-miR-133b by group. (b) The 84-day change in expression of c-miR-21-5p by group. Values are presented as mean ± standard error of the mean. *Time-by-treatment interaction; P < 0.05. +Trend toward main effect of treatment; P = 0.054. †Trend toward time-by-treatment interaction; P = 0.065. PLA, placebo.
Figure 2.
Multidimensional scaling of participants by expression of c-miR-133b and c-miR-21-5p on study day 84. PC, principal component; PLA, placebo.
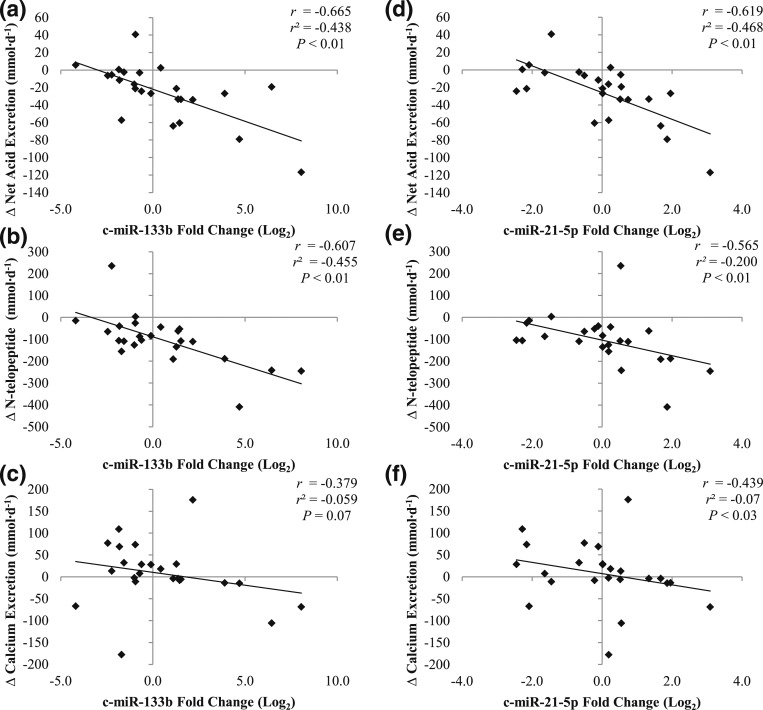
There was a statistically significant inverse association between 84-day change in c-miR-133b expression and changes in UNAE (r = −0.665; P < 0.01) [Fig. 3(a)] and UNTX (r = −0.607; P < 0.01) [Fig. 3(b)] and a trend in UCa (r = −0.379; P = 0.07) [Fig. 3(c)]. Similarly, 84-day change in expression of c-miR-21-5p was inversely associated with 84-day changes in UNAE (r = −0.619; P < 0.01) [Fig. 3(d)], UNTX (r = −0.565; P < 0.01) [Fig. 3(e)], and UCa (r = −0.439; P = 0.03) [Fig. 3(f)]. Lastly, 84-day changes in c-miR-133b and c-miR-21-5p were also inversely correlated with changes in serum P1NP levels, but the associations were not statistically significant (c-miR-133b, r = −0.271; P = 0.20; c-miR-21-5p, r = −0.283; P = 0.18).
Figure 3.
Association between 84-day changes in (a–c) c-miR-133b or (d–f) c-miR-21-5p and 84-day changes in UNAE, UNTX, or UCa.
3. Discussion
Our study demonstrated that after 84 days of KHCO3 supplementation, there was a significant increase in the expression of c-miR-133b compared with placebo in our sample of older men and women with baseline positive renal acid load. Furthermore, upregulation of c-miR-133b expression was associated with reductions in renal acid load and traditional markers of bone resorption. There was a trend toward an increase in c-miR-21-5p in response to KHCO3 supplementation, and as with c-miR-133b, upregulation of c-miR-21-5p expression was significantly associated with lower renal acid load and bone resorption markers including UCa.
A. c-miR-133b
miR-133b is a unique miRNA, being a critical regulator in both bone [34] and skeletal muscle [35]. Through the regulation of bone morphogenetic protein 2, miR-133b has been reported to influence osteogenesis [34]. When miR-133b is overexpressed in mesenchymal cells in bone, it can directly inhibit runt-related transcription factor 2, a transcription factor critical to bone formation, by promotion of osteoblast differentiation [34]. Therefore, an increase in miR-133b expression within bone tissue suggests a reduction in osteoblastic activity. The significance of an increase in levels of c-miR-133b is less clear. We found a strong inverse association between c-miR-133b and UNTX over an 84-day period. There was also a trend for an inverse association between c-miR-133b and serum P1NP. These findings suggest that a rise in c-miR-133b may be indicative of lower bone remodeling in the older adult population, which in turn could be construed as bone sparing. Notably, a cross-sectional case-control study reported that c-miR-133b expression was lower in postmenopausal women who suffered an osteoporotic fracture at the femoral neck than in healthy age-matched controls [36], suggesting that higher c-miR-133b is a marker of reduced bone fragility. To our knowledge, however, there are no published longitudinal controlled data on changes in c-miR-133b from human intervention studies to improve bone health to confirm these findings.
Increased expression of miR-133b in C2C12 myoblasts promotes skeletal muscle mass via myogenesis and hypertrophy by repressing expression of serum response factor and the IGF-1 receptor [35, 37]. Nevertheless, in our small sample of older adults, 84-day change in c-miR-133b expression was not associated with changes in current biomarkers of muscle anabolism and catabolism (UN, IGF-1, or IGFBP3). Notably, compared with current biomarkers of bone turnover, these muscle biomarkers can be highly variable in individuals on self-selected diets. Therefore, it would be useful to reexamine these associations in larger samples.
B. c-miR-21-5p
In bone-marrow macrophages, upregulation of miR-21-5p has enhanced the generation of osteoclasts [38, 39] by way of diminishing total protein expression of programmed cell death 4, a known inhibitor of osteoclastogenesis [40]. Although its upregulation in bone macrophages promoted osteoclastogenesis and bone resorption, the significance of higher circulating levels of this protein has not been studied in randomized controlled intervention studies.
In our study following 84 days of KHCO3 supplementation, we found that higher expressions of c-miR-21-5p were associated with lower bone resorption as measured by UNTX and UCa, suggesting bone sparing in this older population. Similar to our c-miR-133b results, a trend for an inverse association between change in c-miR-21-5p and change in serum P1NP values was observed. In support of our data, a recent cross-sectional study found that postmenopausal women diagnosed with osteoporosis by dual-energy x-ray absorptiometry had lower expression of c-miR-21-5p than healthy controls [25], suggesting that higher levels of c-miR-21-5p favor higher bone mass. However, two other investigations reported that miR-21-5p expression was elevated in the circulation in individuals with osteoporotic fractures compared with expression in individuals with osteoarthritis [26] or nonosteoporotic fractures [27]. In the latter study, however, a limitation was lack of documentation of the time frame of postfracture serum collection across study subjects. Without standardization of collection time postfracture, there may be wide variations in c-miRNA levels similar to what is seen in traditional circulating markers of bone resorption such as UNTX [41]. Thus, discordant results on the direction of c-miR-21-5p expression from previous investigations may be due to study design, population, and methodological differences.
C. Strengths and Limitations
This pilot study had important strengths, mainly a parallel-arm, blinded, placebo-controlled design that examined changes in c-miRNAs involved in bone and muscle homeostasis. Most of the published literature evaluating these miRNAs in the circulation as potential biomarkers was cross-sectional in design. A limitation of our study was a small sample size, which may have prevented us from detecting any additional differences in c-miRNAs between groups. However, this study generated findings that can be pursued in a larger cohort in the future. Another limitation is that we measured total c-miRNA levels rather than those within organ-specific microvesicles. The latter, newer methodology will be an important next step in identifying, for example, whether alterations in c-miR-133b are specific to the effect of KHCO3 on bone, or muscle, or both.
4. Conclusion
This pilot investigation provides preliminary evidence that reductions in bone resorption following 84 days of KHCO3 supplementation were reflected by changes in c-miR-133b and c-miR-21-5p expression. Although previous studies reported that bone and muscle disease were cross-sectionally correlated with c-miRNA expression profiles, the current study reported longitudinal changes in c-miRNA expression with alkali supplementation, altering the endogenous acid load in older adults. Further work in a larger sample is needed to confirm these findings and to clarify with newer techniques whether our two main findings are related to bone, or muscle, or both. In addition, it is important to look at a broader miRNA profile to understand the biological pathways involved.
Acknowledgments
This material is based on work supported by the USDA, under agreement No. 58-1950-0-014. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the USDA. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
Acknowledgments
This study was funded by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 1RO1AR060261, T32 NIDDK Training Grant 5T32DK062032-23 (to L.M.M.), and NIA K01 Award KAG047247A-A1 (to D.A.R.).
Acknowledgments
Author contributions: Study design, conduct, data collection: B.D.-H., R.A.F., and L.C. Data analysis and interpretation: L.M.M., B.D.-H., D.A.R., Y.E., R.A.F., and L.C. Revising manuscript content: L.M.M., B.D.-H., D.A.R., Y.E., R.A.F., and L.C. Approving final manuscript: L.M.M., B.D.-H., D.A.R., Y.E., R.A.F., and L.C.
Clinical trial registry: ClinicalTrials.gov.no. NCT1475214 (registered November 2011).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- c-miRNA
- circulating microRNA
- CV
- coefficient of variance
- IGF-1
- insulin-like growth factor-1
- IGFBP3
- insulin-like growth factor-1 binding protein
- KHCO3
- potassium bicarbonate
- miRNA
- microRNA
- P1NP
- procollagen type 1 amino-terminal propeptide
- RT
- reverse transcriptase
- UCa
- calcium
- UN
- urinary nitrogen
- UNAE
- urinary net acid excretion
- UNTX
- urinary N-telopeptide
- USDA
- US Department of Agriculture.
References and Notes
- 1.Frisoli A Jr, Chaves PH, Ingham SJ, Fried LP. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: results from the Women’s Health and Aging Study (WHAS) II. Bone. 2011;48(4):952–957. [DOI] [PubMed] [Google Scholar]
- 2.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson-Hughes B, Harris SS, Palermo NJ, Castaneda-Sceppa C, Rasmussen HM, Dallal GE. Treatment with potassium bicarbonate lowers calcium excretion and bone resorption in older men and women. J Clin Endocrinol Metab. 2009;94(1):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawson-Hughes B, Harris SS, Palermo NJ, Gilhooly CH, Shea MK, Fielding RA, Ceglia L. Potassium bicarbonate supplementation lowers bone turnover and calcium excretion in older men and women: a randomized dose-finding trial. J Bone Miner Res. 2015;30(11):2103–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jehle S, Zanetti A, Muser J, Hulter HN, Krapf R. Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J Am Soc Nephrol. 2006;17(11):3213–3222. [DOI] [PubMed] [Google Scholar]
- 6.Jehle S, Hulter HN, Krapf R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2013;98(1):207–217. [DOI] [PubMed] [Google Scholar]
- 7.Moseley KF, Weaver CM, Appel L, Sebastian A, Sellmeyer DE. Potassium citrate supplementation results in sustained improvement in calcium balance in older men and women. J Bone Miner Res. 2013;28(3):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frassetto L, Morris RC Jr, Sebastian A. Potassium bicarbonate reduces urinary nitrogen excretion in postmenopausal women. J Clin Endocrinol Metab. 1997;82(1):254–259. [DOI] [PubMed] [Google Scholar]
- 9.Ceglia L, Harris SS, Abrams SA, Rasmussen HM, Dallal GE, Dawson-Hughes B. Potassium bicarbonate attenuates the urinary nitrogen excretion that accompanies an increase in dietary protein and may promote calcium absorption. J Clin Endocrinol Metab. 2009;94(2):645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dawson-Hughes B, Castaneda-Sceppa C, Harris SS, Palermo NJ, Cloutier G, Ceglia L, Dallal GE. Impact of supplementation with bicarbonate on lower-extremity muscle performance in older men and women. Osteoporos Int. 2010;21(7):1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JF, Tao Y, Li J, Deng Z, Yan Z, Xiao X, Wang DZ. microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol. 2010;190(5):867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lian JB, Stein GS, van Wijnen AJ, Stein JL, Hassan MQ, Gaur T, Zhang Y. MicroRNA control of bone formation and homeostasis. Nat Rev Endocrinol. 2012;8(4):212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy JJ, Esser KA. MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J Appl Physiol (1985). 2007;102(1):306–313 [DOI] [PubMed] [Google Scholar]
- 14.Rivas DA, Lessard SJ, Rice NP, Lustgarten MS, So K, Goodyear LJ, Parnell LD, Fielding RA. Diminished skeletal muscle microRNA expression with aging is associated with attenuated muscle plasticity and inhibition of IGF-1 signaling. FASEB J. 2014;28(9):4133–4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409(6818):363–366. [DOI] [PubMed] [Google Scholar]
- 16.Meister G. Argonaute proteins: functional insights and emerging roles. Nat Rev Genet. 2013;14(7):447–459. [DOI] [PubMed] [Google Scholar]
- 17.Schraivogel D, Meister G. Import routes and nuclear functions of Argonaute and other small RNA-silencing proteins. Trends Biochem Sci. 2014;39(9):420–431. [DOI] [PubMed] [Google Scholar]
- 18.Kong YW, Cannell IG, de Moor CH, Hill K, Garside PG, Hamilton TL, Meijer HA, Dobbyn HC, Stoneley M, Spriggs KA, Willis AE, Bushell M. The mechanism of micro-RNA-mediated translation repression is determined by the promoter of the target gene. Proc Natl Acad Sci USA. 2008;105(26):8866–8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillai RS, Artus CG, Filipowicz W. Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA. 2004;10(10):1518–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aoi W, Sakuma K. Does regulation of skeletal muscle function involve circulating microRNAs? Front Physiol. 2014;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. [DOI] [PubMed] [Google Scholar]
- 22.Dhahbi JM. Circulating small noncoding RNAs as biomarkers of aging. Ageing Res Rev. 2014;17:86–98. [DOI] [PubMed] [Google Scholar]
- 23.Margolis LM, Lessard SJ, Ezzyat Y, Fielding RA, Rivas DA. Circulating microRNA are predictive of aging and acute adaptive response to resistance exercise in men [published online ahead of print December 7, 2016]. Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/glw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alevizos I, Illei GG. MicroRNAs as biomarkers in rheumatic diseases. Nat Rev Rheumatol. 2010;6(7):391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Wang Z, Fu Q, Zhang J. Plasma miRNA levels correlate with sensitivity to bone mineral density in postmenopausal osteoporosis patients. Biomarkers. 2014;19(7):553–556. [DOI] [PubMed] [Google Scholar]
- 26.Panach L, Mifsut D, Tarín JJ, Cano A, García-Pérez MA. Serum circulating micrornas as biomarkers of osteoporotic fracture. Calcif Tissue Int. 2015;97(5):495–505. [DOI] [PubMed] [Google Scholar]
- 27.Seeliger C, Karpinski K, Haug AT, Vester H, Schmitt A, Bauer JS, van Griensven M. Five freely circulating miRNAs and bone tissue miRNAs are associated with osteoporotic fractures. J Bone Miner Res. 2014;29(8):1718–1728. [DOI] [PubMed] [Google Scholar]
- 28.Hackl M, Heilmeier U, Weilner S, Grillari J. Circulating microRNAs as novel biomarkers for bone diseases - Complex signatures for multifactorial diseases? Mol Cell Endocrinol. 2016;432:83–95. [DOI] [PubMed] [Google Scholar]
- 29.Donaldson A, Natanek SA, Lewis A, Man WD, Hopkinson NS, Polkey MI, Kemp PR. Increased skeletal muscle-specific microRNA in the blood of patients with COPD. Thorax. 2013;68(12):1140–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karolina DS, Armugam A, Tavintharan S, Wong MT, Lim SC, Sum CF, Jeyaseelan K. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus [published correction appears in PLoS One. 2011;6(9)]. PLoS One. 2011;6(8):e22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyachi M, Tsuchiya K, Yoshida H, Yagyu S, Kikuchi K, Misawa A, Iehara T, Hosoi H. Circulating muscle-specific microRNA, miR-206, as a potential diagnostic marker for rhabdomyosarcoma. Biochem Biophys Res Commun. 2010;400(1):89–93. [DOI] [PubMed] [Google Scholar]
- 32.Jorgensen K. Titrimetric determination of the net excretion of acid/base in urine. Scand J Clin Lab Invest. 1957;9(3):287–291. [DOI] [PubMed] [Google Scholar]
- 33.Chan JC. The rapid determination of urinary titratable acid and ammonium and evaluation of freezing as a method of preservation. Clin Biochem. 1972;5(1-4):94–98. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci USA. 2008;105(37):13906–13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38(2):228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weilner S, Skalicky S, Salzer B, Keider V, Wagner M, Hildner F, Gabriel C, Dovjak P, Pietschmann P, Grillari-Voglauer R, Grillari J, Hackl M. Differentially circulating miRNAs after recent osteoporotic fractures can influence osteogenic differentiation. Bone. 2015;79:43–51. [DOI] [PubMed] [Google Scholar]
- 37.Huang MB, Xu H, Xie SJ, Zhou H, Qu LH. Insulin-like growth factor-1 receptor is regulated by microRNA-133 during skeletal myogenesis. PLoS One. 2011;6(12):e29173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Wijnen AJ, van de Peppel J, van Leeuwen JP, Lian JB, Stein GS, Westendorf JJ, Oursler MJ, Im HJ, Taipaleenmäki H, Hesse E, Riester S, Kakar S. MicroRNA functions in osteogenesis and dysfunctions in osteoporosis. Curr Osteoporos Rep. 2013;11(2):72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujita S, Ito T, Mizutani T, Minoguchi S, Yamamichi N, Sakurai K, Iba H. miR-21 gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J Mol Biol. 2008;378(3):492–504. [DOI] [PubMed] [Google Scholar]
- 40.Sugatani T, Vacher J, Hruska KA. A microRNA expression signature of osteoclastogenesis. Blood. 2011;117(13):3648–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivaska KK, Gerdhem P, Akesson K, Garnero P, Obrant KJ. Effect of fracture on bone turnover markers: a longitudinal study comparing marker levels before and after injury in 113 elderly women. J Bone Miner Res. 2007;22(8):1155–1164. [DOI] [PubMed] [Google Scholar]