Abstract
The ventromedial hypothalamic nucleus (VMH) regulates glucose production in the liver as well as glucose uptake and utilization in peripheral tissues, including skeletal muscle and brown adipose tissue, via efferent sympathetic innervation and neuroendocrine mechanisms. The action of leptin on VMH neurons also increases glucose uptake in specific peripheral tissues through the sympathetic nervous system, with improved insulin sensitivity. On the other hand, subsets of VMH neurons, such as those that express steroidogenic factor 1 (SF1), sense changes in the ambient glucose concentration and are characterized as glucose-excited (GE) and glucose-inhibited (GI) neurons whose action potential frequency increases and decreases, respectively, as glucose levels rise. However, how these glucose-sensing (GE and GI) neurons in the VMH contribute to systemic glucoregulation remains poorly understood. In this review, we provide historical background and discuss recent advances related to glucoregulation by VMH neurons. In particular, the article describes the role of GE neurons in the control of peripheral glucose utilization and insulin sensitivity, which depend on mitochondrial uncoupling protein 2 of the neurons, as well as that of GI neurons in the control of hepatic glucose production through hypoglycemia-induced counterregulatory mechanisms.
Keywords: ventromedial hypothalamic nucleus, glucose-sensing neuron, leptin-responsive neuron, sympathetic nervous system, counterregulatory response, insulin sensitivity
Our article describes glucoregulation by VMH neurons, in particular the role of GE neurons in the control of peripheral glucose utilization and that of GI neurons in hepatic glucose production.
In 1854, Claude Bernard observed that pricking the floor of the fourth ventricle of the brain in dogs induced transient diabetes, which constituted the first demonstration that the brain is able to influence blood sugar level [1]. Almost 150 years later, it was clear that the brain—in particular, the hypothalamus—plays an important role in the control of glucose metabolism. The ventromedial hypothalamic nucleus (VMH) is now known as one of the key areas of the brain with regard to regulation of glucose metabolism in peripheral tissues such as the liver and skeletal muscle. It was first shown in 1966 that electrical stimulation of the VMH in rabbits induces a rapid increase in blood glucose level accompanied by a marked decrease in glycogen content of the liver as a result of increased glycogenolysis [2]. Sympathetic innervation of the liver and gluconeogenic hormones such as glucagon was found to contribute to the induction of hyperglycemia by VMH stimulation [3–5]. More recently, activation of the transient receptor potential vanilloid 1 (TRPV1) ion channel by electromagnetic manipulation in glucokinase-expressing neurons of the VMH was shown to elicit a hyperglycemic response [6]. Another recent study showed that optogenetic stimulation via channelrhodopsin 2 of VMH neurons that express the transcription factor steroidogenic factor 1 (SF1; also known as adrenal 4-binding protein) induced hyperglycemia and enhanced the counterregulatory response to glucopenia [7]. Furthermore, genetic disruption of glutamate release from SF1 neurons in the VMH of mice attenuated recovery from insulin-induced hypoglycemia [8]. These observations suggest that the VMH controls the counterregulatory response to hypoglycemia.
In contrast to the elicitation of hyperglycemic response, electrical stimulation of the VMH has also been shown to increase glucose utilization in peripheral tissues, including skeletal muscle, heart, and brown adipose tissue (BAT) [9, 10]. In addition, peripheral injection or injection into the VMH of the adipose tissue−derived hormone leptin was found to stimulate glucose utilization in these tissues without affecting the plasma insulin level [11–13]. Moreover, under the hyperinsulinemic-euglycemic condition, hypothalamic injection of leptin augmented both insulin-induced stimulation of glucose uptake in the peripheral tissues and insulin-induced suppression of hepatic glucose production [14]. Together, these findings suggest that VMH neurons have distinct effects on glucose metabolism: These neurons increase hepatic glucose production and control the counterregulatory response to hypoglycemia to maintain the blood glucose level while working to increase glucose utilization in the peripheral tissues as well as augment insulin-induced suppression of hepatic glucose production.
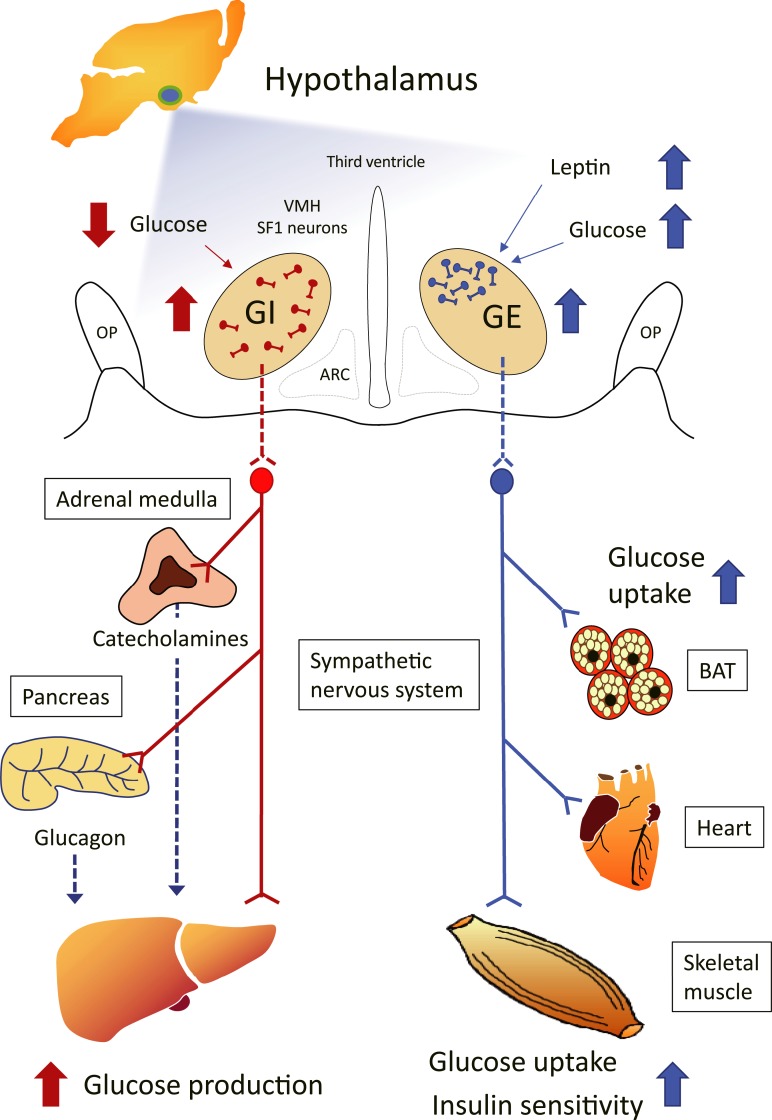
The VMH contains a heterogeneous population of neurons. It is therefore likely that the distinct effects of VMH stimulation on glucose metabolism are mediated by specific types of VMH neurons. A subset of VMH neurons has been shown to sense changes in the concentration of ambient glucose and to include both glucose-excited (GE) and glucose-inhibited (GI) neurons, whose action potential frequency increases or decreases, respectively, as the glucose level rises [15, 16]. In this review, we provide some historical background and describe recent advances regarding the role of VMH neurons in the control of glucose metabolism. In particular, we address the possibility that GE neurons in the VMH control peripheral glucose utilization and insulin sensitivity, whereas GI neurons regulate hepatic glucose production and the hypoglycemia-induced counterregulatory response (Fig. 1).
Figure 1.
Model showing the roles of GE and GI neurons of the VMH in systemic glucoregulation and the possible relation of these neurons to SF1 and leptin-responsive neurons. VMH neurons include glucose-sensing cells, referred to as GE and GI neurons. Subsets of VMH neurons also express SF1 and leptin receptors, with some of these neurons overlapping with GE and GI neurons. Although leptin-activated and leptin-inhibited neurons are distributed among both glucose-sensing and glucose-nonresponsive neurons in the VMH, the number of leptin-activated neurons is nearly twice that of leptin-inhibited neurons among GE neurons. Leptin-activated SF1 neurons are predominantly located within the dorsomedial subdivision of the VMH. The similarity between the effects of GE neuronal activation and those of leptin on glucose metabolism suggests that a subset of VMH neurons that have properties of both GE and leptin-activated neurons mediates the leptin-induced enhancement of glucose uptake and insulin sensitivity in certain peripheral tissues. An increase in blood glucose level triggers the activation of GE neurons in the VMH, which in turn results in activation of the sympathetic nervous system and consequent increases in insulin sensitivity and glucose uptake in BAT, the heart, and skeletal muscle, but not in white adipose tissue. A decrease in blood glucose level elicits the activation of GI neurons in the VMH, which results in activation of the sympathetic nerves innervating the liver, adrenal medulla (stimulating the release of catecholamines), and pancreas (stimulating the release of glucagon) and a consequent increase in hepatic glucose production. ARC, arcuate nucleus of the hypothalamus; OP, optic tract.
1. The VMH and Hepatic Glucose Output
Although electrical stimulation of the VMH in rabbits was shown to induce hyperglycemia accompanied by a reduction in liver glycogen content owing to increased glycogenolysis, stimulation of the lateral hypothalamic nucleus (LH) resulted in a decrease in blood glucose level without a substantial effect on hepatic glycogen [2]. Glycogen metabolism in the liver was shown to be under the control of direct sympathetic innervation, with electrical stimulation of the peripheral end of the splanchnic nerve resulting in rapid and marked increases in the activities of key glycogenolytic enzymes, glycogen phosphorylase and glucose-6-phosphatase, and a concomitant decrease in glycogen content in the liver [3, 4]. Furthermore, given that these effects were still apparent after removal of the adrenal glands and pancreas, it was concluded that they were directly dependent on hepatic sympathetic innervation. By contrast, electrical stimulation of the vagal nerve was shown to enhance hepatic glycogen synthesis [17, 18].
Hepatic gluconeogenesis is also an important contributor to hyperglycemia and maintenance of blood glucose level. VMH stimulation in rats promoted gluconeogenesis in the liver by increasing the activity of phosphoenolpyruvate carboxykinase, a key gluconeogenic enzyme, and by suppressing the activity of pyruvate kinase, a key glycolytic enzyme, whereas LH stimulation suppressed gluconeogenesis [19]. These observations are consistent with more recent findings that the hypothalamic action of insulin suppressed hepatic glucose production and the expression of key gluconeogenic genes through activation of hypothalamic adenosine triphosphate (ATP)-sensitive K+ (KATP) channels and of the efferent vagal nerve innervating the liver [20].
A dual mechanism of hypothalamic control of glucose output from the liver has thus been proposed. One mechanism is direct sympathetic innervation via the presumed VMH-splanchnic nerve pathway that directly controls the glycogenolytic enzymes in the liver. The other mechanism is the neuroendocrine pathway via intervention of pancreatic glucagon or insulin (the hypothalamus-pancreatic axis) and of the adrenomedullary catecholamines epinephrine and norepinephrine (the hypothalamus-adrenal axis), which regulate both glycogenolytic and gluconeogenic enzymes in the liver [5, 21]. Recent anatomic studies have provided support for this dual mechanism by revealing that VMH neurons project caudally to autonomic centers in the brainstem [22, 23]. Notably, studies using transgenic mice combined with viral vectors to trace efferent projections revealed that SF1-positive neurons of the VMH project efferent fibers caudally through the periaqueductal gray to the rostral ventrolateral medulla, nucleus solitary tract, and retrotrapezoid nucleus of the lower brainstem, indicating that the VMH modulates sympathetic nerve activity via synaptic contacts with these regions [22].
Optogenetic suppression of SF1 neurons in the VMH was recently shown to block recovery from insulin-induced hypoglycemia; conversely, activation of these neurons induced diabeteslike hyperglycemia [7]. Optogenetic stimulation of VMH neurons that express the leptin receptor did not induce hyperglycemia, suggesting that VMH neurons are functionally heterogeneous with regard to the control of glucose metabolism. This same study revealed a novel pathway by which SF1 neurons of the VMH stimulate hepatic glucose production: The hyperglycemic response was found to be reproduced by activation of SF1 neurons that project to the anterior bed nucleus of the stria terminalis (aBNST) but not by activation of those that project to other brain areas, including the paraventricular hypothalamic nucleus, central nucleus of the amygdala, or periaqueductal gray [7]. How aBNST neurons stimulate hepatic glucose production and the counterregulatory response remains unknown. Furthermore, this study showed that neurons in the lateral parabrachial nucleus (LPBN), which is implicated in the response to hypoglycemia, form synaptic connections with the specific subset of glucoregulatory SF1 neurons of the VMH that project to the aBNST [7]. It was previously shown that cholecystokinin (CCK) is released from LPBN neurons in response to hypoglycemia and induces a counterregulatory response via activation of VMH neurons [24]. Together, these observations suggest the operation of an ascending LPBN (CCK-expressing neurons) → VMH (SF1-expressing but leptin receptor–negative neurons) → aBNST neuronal circuit in the regulation of glucose metabolism. As discussed later, GI neurons in the VMH regulate hepatic glucose production and the counterregulatory response to hypoglycemia. VMH neurons thus sense changes in blood glucose level directly via an intrinsic glucose-sensing mechanism and indirectly through the ascending neuronal circuit, including the LPBN.
2. The VMH and Leptin-Induced Glucose Utilization in Peripheral Tissues
Glucose uptake and glucose utilization in peripheral tissues are key components of systemic glucose homeostasis. In addition to inducing hyperglycemia, electrical or chemical (glutamate) stimulation of the VMH in rats was found to increase glucose uptake (measured with 2-deoxy-d-[3H]glucose) in the interscapular BAT, heart, and skeletal muscles (soleus, gastrocnemius, quadriceps, and extensor digitorum longus) but not in white adipose tissue or other tissues and organs [9, 10]. Stimulation of the LH had no substantial effect on glucose uptake in any of the tissues examined. The increase in glucose utilization by BAT in response to VMH stimulation was associated with thermogenesis induced by activation of sympathetic nerves innervating the tissue [25]. Glucose uptake in skeletal muscle was increased by VMH stimulation in both anesthetized rats and animals treated with a muscle relaxant, suggesting that muscle contraction was unlikely to serve as a primary regulator of increased glucose uptake in the tissue in response to VMH stimulation [26].
The increased rate of glucose uptake in peripheral tissues induced by VMH stimulation was suppressed by local sympathetic denervation or chemical sympathectomy [9, 26]. The increased glucose uptake was also shown to be dependent on norepinephrine released by sympathetic nerves [10, 27]. On the basis of findings that the VMH–sympathetic nervous system axis enhances not only glucose output from the liver but also its utilization in specific tissues, it was hypothesized that this hypothalamic control of glucose metabolism might constitute a type of feed-forward regulatory system [28].
The role of the VMH in regulation of glucose utilization was further supported by studies examining the effects of leptin on VMH neurons. Leptin is an adipocyte hormone that functions as an afferent signal to the central nervous system (hypothalamus) in a negative feedback loop that regulates adipose tissue mass by affecting food intake and certain peripheral metabolic processes, including glucose utilization [11–13, 29]. Leptin receptors are expressed in several hypothalamic nuclei, including the VMH [29]. In the VMH of adult mice, expression of SF1 defines a specific subset of VMH neurons [30, 31], and the selective loss of leptin receptors in SF1-expressing cells causes increased adiposity and impaired glucose metabolism [32, 33], suggesting that leptin receptors expressed in SF1 neurons are essential for the regulatory action of leptin in energy metabolism.
Recent electrophysiological studies with hypothalamic slice preparations have revealed that leptin either depolarized or hyperpolarized distinct subsets of SF1 neurons in the VMH (neurons activated, inhibited, or unaffected by leptin constituted 17.5%, 17.5%, and 65.0% of SF1-positive cells in the VMH, respectively), whereas insulin hyperpolarized a different subpopulation of SF1 neurons in the VMH (neurons activated, inhibited, or unaffected by insulin constituted 0%, 23.4%, and 76.6% of SF1-positive cells in the VMH, respectively) [34]. Furthermore, most leptin-activated SF1 neurons were located within the dorsomedial subdivision of the VMH, whereas leptin-inhibited SF1 neurons were scattered throughout the VMH [34]. These results suggest that leptin-responsive SF1 neurons are heterogeneous in their location and acute response to leptin. The acute effects of leptin and insulin on neuronal activities were dependent on phosphatidylinositol-3-kinase activity, as their effects were completely abolished by pretreatment of phosphatidylinositol-3-kinase inhibitors or the deletion of both 110-kD catalytic subunits (p110α and p110β) of the enzyme. The leptin-induced depolarization was solely dependent upon the p110β catalytic subunit. On the other hand, the leptin- and insulin-induced hyperpolarization was dependent on the presence of either the p110α or the p110β catalytic subunit [34].
Intracerebroventricular infusion or microinjection of leptin into the VMH in rodents has now been shown to increase glucose uptake in the interscapular BAT, heart, and skeletal muscle but not in white adipose tissue, without significant change in the plasma glucose or insulin level [11, 13]. These effects are thus essentially similar to those of electrical or chemical stimulation of the VMH, although the effect of leptin on glucose uptake in red (slow-twitch oxidative) muscle was more prominent than that in white (fast-twitch glycolytic) muscle [11, 13]. Leptin-stimulated glucose uptake was likewise abolished by sympathetic denervation [11, 12]. In addition, although leptin injection into the VMH increased glucose uptake in skeletal muscle, BAT, and the heart, its injection into the arcuate hypothalamic nucleus increased glucose uptake in BAT alone and its injection into the dorsomedial or paraventricular hypothalamic nucleus had no effect [35].
Hypothalamic leptin and peripheral insulin had a synergistic effect on tissue glucose uptake, suggesting that the leptin-induced activation of the VMH–sympathetic nervous system axis and insulin cooperate in the regulation of glucose uptake by certain peripheral tissues [12]. Consistent with these findings, orexin-induced activation of VMH neurons also increased glucose uptake in skeletal muscle via the action of sympathetic nerves and β2-adrenergic receptors [36]: The effect of orexin on muscle glucose uptake was abolished in skeletal muscle lacking β1-, β2-, and β3-adrenergic receptors (β-less mice) and was rescued by forced expression of β2-adrenergic receptors in the muscle. Microinjection of leptin into the VMH was also shown to enhance insulin sensitivity in the whole body and in red-type skeletal muscle in a manner dependent on the activation of melanocortin receptors and via an intracellular signaling pathway mediated by extracellular signal-regulated kinase in the VMH [14, 35].
3. Glucose-Sensing Neurons in the VMH
The existence of glucose-sensing neurons in the hypothalamus was suggested ~50 years ago by the studies of Oomura et al. [37, 38]. These researchers measured reciprocal changes in spontaneous unit discharges in the VMH and LH (regions classically named the satiety and feeding centers, respectively) in response to intravenous glucose or insulin injection in cats: Glucose increased neuronal activity in the VMH and reduced it in the LH. Single-unit activity of these hypothalamic neurons was also found to be directly regulated by glucose in vitro in rats. The VMH neurons that increased their activity with increasing glucose concentration were termed “glucose responsive,” whereas the LH neurons that decreased their activity with increasing glucose concentration were designated “glucose sensitive.”
Subsequent studies (for reviews, see [15, 16]) have revealed that VMH neurons respond to physiological changes in the extracellular glucose concentration; a subset of VMH neurons increases its electrical activity and the other decreases its activity as the glucose level increases, whereas the converse occurs as the glucose level decreases. These subtypes of glucose-sensing neurons are now more commonly referred to as GE and GI neurons, respectively. Approximately half the glucose-sensing neurons in the VMH are also leptin responsive [39]. Although leptin-activated and leptin-inhibited neurons are distributed among both types of glucose-sensing neurons and glucose-nonresponsive neurons in the VMH, the number of leptin-activated neurons among GE neurons is nearly twice that of leptin-inhibited neurons [39]. GE and GI neurons each constitute ~10% of all SF1 neurons in the VMH [40].
GE neurons increase their electrical activity as glucose levels rise, with glucokinase and KATP channels serving as effectors of glucose-induced signaling in most of these cells [15, 16]. GI neurons also express glucokinase [15, 16], and they are active in the presence of low glucose concentrations as a result of the activation of AMP-activated protein kinase (AMPK), which serves as an intracellular “fuel gauge,” and the AMPK-induced closure of the cystic fibrosis transmembrane conductance regulator Cl– channel [41]. Furthermore, activation of soluble guanylyl cyclase by the gaseous messenger nitric oxide and the consequent production of guanosine 3′,5′-monophosphate contribute to regulation of the AMPK-mediated phosphorylation of the cystic fibrosis transmembrane conductance regulator and activation of GI neurons in the VMH [41, 42]. These observations, using cultured VMH neurons, suggest that AMPK plays an important role in the glucose-sensing mechanism of GI neurons.
4. GI Neurons of the VMH and Systemic Glucoregulation
Since the discovery of glucose-sensing neurons about half a century ago, the role of these neurons in the counterregulatory response to acute hypoglycemia that restores euglycemia has been intensely studied. The counterregulatory response is vital to ameliorate the consequences of hypoglycemia, which often develops incidentally during the course of diabetes and its treatment. Hypoglycemia is thought to be detected and the response integrated predominantly in glucose-sensing neurons within the brain. Focal lesioning of the VMH was shown to abolish the counterregulatory response to systemic hypoglycemia [43], implicating the VMH as the key region in the brain responsible for elicitation of counterregulatory mechanisms through direct activation of sympathetic nerve tone and stimulation of the release of counterregulatory hormones such as glucagon and catecholamines. Moreover, application of a hypoglycemic insulin clamp (to induce systemic hypoglycemia) together with bilateral VMH microdialysis in rats revealed that the counterregulatory hormone response was markedly inhibited by selective prevention of hypoglycemia in the VMH by local perfusion with medium containing d-glucose [44]. In contrast, local perfusion of the VMH with an isotonic solution lacking glucose or with nonmetabolizable l-glucose did not impair counterregulatory hormone release in response to acute systemic hypoglycemia [44]. These results suggest that glucose-sensing neurons in the VMH play a central role in elicitation of the counterregulatory hormone response to hypoglycemia.
The possible role of AMPK in the mechanism by which specialized glucose-sensing neurons within the VMH detect a falling blood glucose level was investigated in rats by chemical activation of the kinase with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) in vivo [45]. Activation of AMPK within the VMH by bilateral microinjection of AICAR resulted in marked reduction in the amount of exogenous glucose required to maintain the plasma glucose level during imposition of the hyperinsulinemic-hypoglycemic clamp. This effect was almost completely accounted for by a pronounced increase in endogenous glucose production (Ra) compared with that obtained in control animals microinjected with saline. The rate of peripheral glucose utilization (Rd) during insulin-induced hypoglycemia did not differ significantly between AICAR- and saline-injected rats. The increase in Ra was most likely due to enhanced hepatic glucose production, resulting from direct sympathetic nerve stimulation and from the counterregulatory hormone response, which is amplified by activation of AMPK in the VMH in animals with recurrent hypoglycemia [45, 46].
AICAR may affect other signaling molecules in addition to AMPK. However, another study indicated that selective downregulation of AMPK in the VMH by bilateral injection of an adeno-associated viral vector encoding a short hairpin RNA specific for AMPK messenger RNA resulted in suppression of the glucagon and epinephrine secretory responses to acute hypoglycemia and significant attenuation of endogenous glucose production [47]. Hence, these results suggest that AMPK in the VMH plays a key role in the detection of acute hypoglycemia and in initiation of the glucose counterregulatory response. Moreover, given that activation of AMPK with AICAR or its inhibition by AMPK inhibitor compound C altered the activity of GI neurons but not that of GE neurons in an ex vivo cell culture preparation derived from the basomedial hypothalamus [48], it seems most likely that GI neurons in the VMH are responsible for detection of falling glucose levels and that the consequent increase in the activity of these neurons triggers the counterregulatory responses (Fig. 1). There is also supporting evidence that CCK-positive neurons of the LPBN are a population of GI neurons and that these neurons project to SF1-expressing neurons of the VMH and form a neurocircuit necessary for counterregulatory responses to hypoglycemia [24].
In a recent study, Stanley et al. [6] exploited an elegant system for noninvasive transient activation or inhibition of the activity of specific neurons in the central nervous system of mice in vivo. They were thus able to achieve neuronal activation remotely with the use of radio waves or magnetic fields to induce opening of the cation channel TRPV1, which is fused to ferritin and tagged with green fluorescent protein and is expressed in a Cre recombinase–dependent manner. Targeted neuronal inhibition with the same stimuli was achieved by mutation of the TRPV1 pore to render it permeable to Cl–. Expression of these constructs was targeted to glucose-sensing neurons in the VMH by placing the Cre coding sequence under the control of the promoter for the glucokinase gene. The acute activation of glucose-sensing neurons in the VMH increased plasma glucose and glucagon concentrations and lowered the circulating insulin level, whereas their inhibition reduced blood glucose and raised insulin levels.
Although these manipulations specifically targeted glucokinase-positive neurons in the VMH, activation and inhibition of this neuronal subpopulation mimicked and blocked the responses to hypoglycemia, respectively. As in the optogenetic stimulation of SF1 neurons of the VMH [7], it thus seems likely that the TRPV1 constructs exert their effects on GI neurons of the VMH. However, given that glucokinase-expressing neurons as well as SF1 neurons include both GE and GI neurons, activation of these glucokinase and SF1 neurons of the VMH may possibly enhance glucose uptake in peripheral tissues, in addition to increasing the blood glucose level. Consistent with this possibility, electrical stimulation of the VMH promotes glucose uptake in certain peripheral tissues, including skeletal muscle, as well as increases the blood glucose level [2, 9]. In addition, the aforementioned studies on electromagnetic stimulation [6] and optogenic stimulation [7] of the VMH neurons did not examine the possible role of the autonomic nerves in producing changes in plasma glucose. Nevertheless, the autonomic nervous system may also contribute directly to the response, as discussed in the preceding section. Altogether, the aforementioned results suggest that GI neurons in the VMH are necessary for the normal regulatory response to hypoglycemia and for maintenance of blood glucose levels during fasting.
5. Role of GE Neurons of the VMH in Glucoregulation
With the exception of a presumed function in the control of food intake, little was known about the physiological role of GE neurons until recently. Recent studies by Toda et al. [40] demonstrated that systemic glucose administration in mice induced mitochondrial fission, which resulted in an increase in mitochondrial number and a reduction in mitochondrial size, and reduced the level of reactive oxygen species in VMH neurons in a manner dependent on uncoupling protein 2 (UCP2). Uncoupling proteins are located in the inner membrane of mitochondria and are important for the bioenergetics of diverse tissues. UCP2 is highly expressed in the hypothalamus, including the VMH. This study also revealed that UCP2 in VMH neurons determines the number of GE neurons in this hypothalamic region and regulates systemic glucose utilization [40]. In brief, whole-body UCP2 knockout (Ucp2KO) mice manifested reduced glucose tolerance compared with control mice, and selective restoration of UCP2 expression in SF1 neurons of the VMH (Ucp2KOKISf1 mice) restored glucose tolerance to a level similar to that of control animals without a change in circulating insulin levels. Application of a hyperinsulinemic-euglycemic clamp revealed that Ucp2KO mice required a significantly lower glucose infusion rate to maintain euglycemia than did control and Ucp2KOKISf1 mice. Whole-body glucose utilization (rate of glucose disappearance during the clamp period) was also significantly lower in Ucp2KO mice than in control and Ucp2KOKISf1 mice, indicating that UCP2 in VMH neurons is required for insulin sensitivity in peripheral tissues. Consistent with this, glucose uptake in skeletal muscle and BAT was significantly lower in Ucp2KO mice than in control mice in vivo, whereas restoration of UCP2 expression in SF1 neurons normalized the levels of glucose uptake. In addition, selective overexpression of UCP2 in SF1 neurons (Ucp2KISf1 mice) increased glucose tolerance and insulin sensitivity, with these effects being mediated by enhanced mitochondrial fission and increased neuronal activity in the VMH. The application of designer receptors exclusively activated by designer drugs technology also revealed that selective and reversible inactivation of SF1 neurons in both Ucp2KISf1 and control mice resulted in impaired glucose metabolism in the periphery in response to a glucose load.
To examine whether UCP2 influences either GE or GI subpopulations of VMH neurons, Toda et al. [40] quantified GE and GI neurons among SF1 neurons in control and Ucp2KISf1 mice. The number of GE neurons was significantly greater (approximately twice) in Ucp2KISf1 mice than in control animals. On the other hand, no difference in the number of GI neurons was apparent between the transgenic and control animals. Collectively, these observations suggest that regulation of systemic glucose utilization by UCP2 in the VMH is mediated by GE neurons. This study also showed that the glucose-induced increase in firing rate of GE neurons in the VMH was significantly reduced by application of diazoxide, a KATP channel opener, in both control and Ucp2KISf1 mice.
Glucose-induced activation of GE neurons in the VMH thus seems to be dependent on UCP2 and mitochondrial fission in these cells and is necessary for the proper control of peripheral glucose metabolism as a result of improved insulin sensitivity. The dorsomedial subdivision of the VMH contains a greater number of GE neurons than does the ventrolateral subdivision of the VMH [40]. These findings are quite similar to those obtained with leptin-induced activation of VMH neurons and suggest that a subset of VMH neurons that are characteristic of GE neurons controls the enhancement of glucose uptake and insulin sensitivity in specific peripheral tissues. Moreover, as already discussed, given that the sympathetic nervous system is implicated in the enhancement of glucose uptake in skeletal muscle, BAT, and the heart in response to VMH stimulation, UCP2-dependent activation of GE neurons in the VMH most likely influences insulin sensitivity in these peripheral tissues via the autonomic nervous system (Fig. 1). Indeed, muscle glucose uptake induced by injection of orexin into the VMH requires β2-adrenergic receptors in both myocytes and nonmyocyte cells, such as those of blood vessels in skeletal muscle, suggesting that dilation of vessels in response to β2-adrenergic receptor stimulation may promote the delivery of insulin to myocytes [36].
Increased expression of UCP2 inhibits glucose-induced insulin secretion in pancreatic β-cells, whereas genetic ablation of UCP2 enhances insulin secretion in pancreatic β-cells of obese diabetic animals [49]. Superoxide-mediated activation of UCP2 was also shown to induce pancreatic β-cell dysfunction [50]. UCP2 may thus have distinct roles in pancreatic β-cells and in GE neurons of the VMH. Further investigation is necessary to clarify the detailed molecular mechanisms by which UCP2 regulates the response to glucose by GE neurons in the VMH.
6. Conclusion
The hypothalamus, in particular the VMH, plays a critical role in the homeostatic control of blood glucose levels by balancing glucose production in the liver and glucose utilization in peripheral tissues, such as skeletal muscle, BAT, and the heart. In addition to leptin action on VMH neurons, the glucose-sensing neurons of the VMH have been shown to contribute to maintenance of this glucose homeostasis. GE neurons in the VMH appear to be responsible for control of peripheral glucose utilization in response to acute hyperglycemia, with UCP2 and mitochondrial fission in GE neurons mediating this control. On the other hand, GI neurons in the VMH control hepatic glucose production through mechanisms associated with the hypoglycemia-induced counterregulatory response. Dysregulation of these physiological functions of GE and GI neurons in the VMH may contribute to the etiology of type 2 diabetes and related diseases, and characterization of such dysregulation may provide a basis for the development of new treatments for these pathogenic conditions.
Acknowledgments
Acknowledgments
Research in Y.M.’s laboratory is supported by a Grant-in-Aid for Scientific Research (B) (24390058) and a Grant-in-Aid for Exploratory Research from the Japan Society for the Promotion of Science; a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Core Research for Evolutional Science and Technology (CREST) Program of the Japan Science and Technology Agency; and the Japan Agency for Medical Research and Development.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aBNST
- anterior bed nucleus of the stria terminalis
- AICAR
- 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside
- AMPK
- AMP-activated protein kinase
- ATP
- adenosine triphosphate
- BAT
- brown adipose tissue
- CCK
- cholecystokinin
- GE
- glucose-excited
- GI
- glucose-inhibited
- KATP
- ATP-sensitive K+ channel
- LH
- lateral hypothalamic nucleus
- LPBN
- lateral parabrachial nucleus
- SF1
- steroidogenic factor 1
- TRPV1
- transient receptor potential vanilloid 1
- UCP2
- uncoupling protein 2
- Ucp2KISf1
- selective overexpression of UCP2 in SF1 neurons
- Ucp2KO
- UCP2 knockout
- Ucp2KOKISf1
- selective restoration of UCP2 expression in SF1 neurons of the VMH
- VMH
- ventromedial hypothalamic nucleus.
References and Notes
- 1.Bernard C. Lecons de Physiologie Experimentale Appliqués â Làmedecine. Paris, France: Baillere et Fils; 1854. [Google Scholar]
- 2.Shimazu T, Fukuda A, Ban T. Reciprocal influences of the ventromedial and lateral hypothalamic nuclei on blood glucose level and liver glycogen content. Nature. 1966;210(5041):1178–1179. [DOI] [PubMed] [Google Scholar]
- 3.Shimazu T, Fukuda A. Increased activities of glycogenolytic enzymes in liver after splanchnic-nerve stimulation. Science. 1965;150(3703):1607–1608. [DOI] [PubMed] [Google Scholar]
- 4.Shimazu T, Amakawa A. Regulation of glycogen metabolism in liver by the autonomic nervous system. II. Neural control of glycogenolytic enzymes. Biochim Biophys Acta. 1968;165(3):335–348. [DOI] [PubMed] [Google Scholar]
- 5.Shimazu T. Reciprocal innervation of the liver: its significance in metabolic control. Adv Metab Disord. 1983;10:355–384. [DOI] [PubMed] [Google Scholar]
- 6.Stanley SA, Kelly L, Latcha KN, Schmidt SF, Yu X, Nectow AR, Sauer J, Dyke JP, Dordick JS, Friedman JM. Bidirectional electromagnetic control of the hypothalamus regulates feeding and metabolism. Nature. 2016;531(7596):647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meek TH, Nelson JT, Matsen ME, Dorfman MD, Guyenet SJ, Damian V, Allison MB, Scarlett JM, Nguyen HT, Thaler JP, Olson DP, Myers MG Jr, Schwartz MW, Morton GJ. Functional identification of a neurocircuit regulating blood glucose. Proc Natl Acad Sci USA. 2016;113(14):E2073–E2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5(5):383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sudo M, Minokoshi Y, Shimazu T. Ventromedial hypothalamic stimulation enhances peripheral glucose uptake in anesthetized rats. Am J Physiol. 1991;261(3 Pt 1):E298–E303. [DOI] [PubMed] [Google Scholar]
- 10.Shimazu T, Sudo M, Minokoshi Y, Takahashi A. Role of the hypothalamus in insulin-independent glucose uptake in peripheral tissues. Brain Res Bull. 1991;27(3-4):501–504. [DOI] [PubMed] [Google Scholar]
- 11.Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48(2):287–291. [DOI] [PubMed] [Google Scholar]
- 12.Haque MS, Minokoshi Y, Hamai M, Iwai M, Horiuchi M, Shimazu T. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes. 1999;48(9):1706–1712. [DOI] [PubMed] [Google Scholar]
- 13.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389(6649):374–377. [DOI] [PubMed] [Google Scholar]
- 14.Toda C, Shiuchi T, Kageyama H, Okamoto S, Coutinho EA, Sato T, Okamatsu-Ogura Y, Yokota S, Takagi K, Tang L, Saito K, Shioda S, Minokoshi Y. Extracellular signal-regulated kinase in the ventromedial hypothalamus mediates leptin-induced glucose uptake in red-type skeletal muscle. Diabetes. 2013;62(7):2295–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes. 2004;53(10):2521–2528. [DOI] [PubMed] [Google Scholar]
- 16.Routh VH, Hao L, Santiago AM, Sheng Z, Zhou C. Hypothalamic glucose sensing: making ends meet. Front Syst Neurosci. 2014;8:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimazu T. Glycogen synthetase activity in liver: regulation by the autonomic nerves. Science. 1967;156(3779):1256–1257. [DOI] [PubMed] [Google Scholar]
- 18.Shimazu T, Fujimoto T. Regulation of glycogen metabolism in liver by the autonomic nervous system. IV. Neural control of glycogen biosynthesis. Biochim Biophys Acta. 1971;252(1):18–27. [DOI] [PubMed] [Google Scholar]
- 19.Shimazu T, Ogasawara S. Effects of hypothalamic stimulation on gluconeogenesis and glycolysis in rat liver. Am J Physiol. 1975;228(6):1787–1793. [DOI] [PubMed] [Google Scholar]
- 20.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434(7036):1026–1031. [DOI] [PubMed] [Google Scholar]
- 21.Shimazu T. Neuronal regulation of hepatic glucose metabolism in mammals. Diabetes Metab Rev. 1987;3(1):185–206. [DOI] [PubMed] [Google Scholar]
- 22.Lindberg D, Chen P, Li C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. J Comp Neurol. 2013;521(14):3167–3190. [DOI] [PubMed] [Google Scholar]
- 23.Uyama N, Geerts A, Reynaert H. Neural connections between the hypothalamus and the liver. Anat Rec A Discov Mol Cell Evol Biol. 2004;280A(1):808–820. [DOI] [PubMed] [Google Scholar]
- 24.Garfield AS, Shah BP, Madara JC, Burke LK, Patterson CM, Flak J, Neve RL, Evans ML, Lowell BB, Myers MG Jr, Heisler LK. A parabrachial-hypothalamic cholecystokinin neurocircuit controls counterregulatory responses to hypoglycemia. Cell Metab. 2014;20(6):1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minokoshi Y, Saito M, Shimazu T. Sympathetic activation of lipid synthesis in brown adipose tissue in the rat. J Physiol. 1988;398:361–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minokoshi Y, Okano Y, Shimazu T. Regulatory mechanism of the ventromedial hypothalamus in enhancing glucose uptake in skeletal muscles. Brain Res. 1994;649(1-2):343–347. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu Y, Satoh S, Yano H, Minokoshi Y, Cushman SW, Shimazu T. Effects of noradrenaline on the cell-surface glucose transporters in cultured brown adipocytes: novel mechanism for selective activation of GLUT1 glucose transporters. Biochem J. 1998;330(1):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimazu T. Hypothalamic control of liver, muscle and adipose tissue metabolism. In: Häussinger D, Jungermann K, eds. Liver and Nervous System. Falk Symposium Vol. 103. Lancaster, UK: Kluwer Academic Publishers; 1998:118–133. [Google Scholar]
- 29.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. [DOI] [PubMed] [Google Scholar]
- 30.Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143(2):607–614. [DOI] [PubMed] [Google Scholar]
- 31.Shima Y, Zubair M, Ishihara S, Shinohara Y, Oka S, Kimura S, Okamoto S, Minokoshi Y, Suita S, Morohashi K. Ventromedial hypothalamic nucleus-specific enhancer of Ad4BP/SF-1 gene. Mol Endocrinol. 2005;19(11):2812–2823. [DOI] [PubMed] [Google Scholar]
- 32.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191–203. [DOI] [PubMed] [Google Scholar]
- 33.Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology. 2008;149(5):2138–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sohn JW, Oh Y, Kim KW, Lee S, Williams KW, Elmquist JK. Leptin and insulin engage specific PI3K subunits in hypothalamic SF1 neurons. Mol Metab. 2016;5(8):669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toda C, Shiuchi T, Lee S, Yamato-Esaki M, Fujino Y, Suzuki A, Okamoto S, Minokoshi Y. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes. 2009;58(12):2757–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiuchi T, Haque MS, Okamoto S, Inoue T, Kageyama H, Lee S, Toda C, Suzuki A, Bachman ES, Kim YB, Sakurai T, Yanagisawa M, Shioda S, Imoto K, Minokoshi Y. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009;10(6):466–480. [DOI] [PubMed] [Google Scholar]
- 37.Oomura Y, Kimura K, Ooyama H, Maeno T, Iki M, Kuniyoshi M. Reciprocal activities of the ventromedial and lateral hypothalamic areas of cats. Science. 1964;143(3605):484–485. [DOI] [PubMed] [Google Scholar]
- 38.Oomura Y, Ono T, Ooyama H, Wayner MJ. Glucose and osmosensitive neurones of the rat hypothalamus. Nature. 1969;222(5190):282–284. [DOI] [PubMed] [Google Scholar]
- 39.Irani BG, Le Foll C, Dunn-Meynell A, Levin BE. Effects of leptin on rat ventromedial hypothalamic neurons. Endocrinology. 2008;149(10):5146–5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toda C, Kim JD, Impellizzeri D, Cuzzocrea S, Liu Z-W, Diano S. UCP2 regulates mitochondrial fission and ventromedial nucleus control of glucose responsiveness. Cell. 2016;164(5):872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy BA, Fakira KA, Song Z, Beuve A, Routh VH. AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. Am J Physiol Cell Physiol. 2009;297(3):C750–C758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canabal DD, Song Z, Potian JG, Beuve A, McArdle JJ, Routh VH. Glucose, insulin, and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2007;292(4):R1418–R1428. [DOI] [PubMed] [Google Scholar]
- 43.Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI. Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycemia. J Clin Invest. 1994;93(4):1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest. 1997;99(2):361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCrimmon RJ, Fan X, Ding Y, Zhu W, Jacob RJ, Sherwin RS. Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus. Diabetes. 2004;53(8):1953–1958. [DOI] [PubMed] [Google Scholar]
- 46.McCrimmon RJ, Fan X, Cheng H, McNay E, Chan O, Shaw M, Ding Y, Zhu W, Sherwin RS. Activation of AMP-activated protein kinase within the ventromedial hypothalamus amplifies counterregulatory hormone responses in rats with defective counterregulation. Diabetes. 2006;55(6):1755–1760. [DOI] [PubMed] [Google Scholar]
- 47.McCrimmon RJ, Shaw M, Fan X, Cheng H, Ding Y, Vella MC, Zhou L, McNay EC, Sherwin RS. Key role for AMP-activated protein kinase in the ventromedial hypothalamus in regulating counterregulatory hormone responses to acute hypoglycemia. Diabetes. 2008;57(2):444–450. [DOI] [PubMed] [Google Scholar]
- 48.Mountjoy PD, Bailey SJ, Rutter GA. Inhibition by glucose or leptin of hypothalamic neurons expressing neuropeptide Y requires changes in AMP-activated protein kinase activity. Diabetologia. 2007;50(1):168–177. [DOI] [PubMed] [Google Scholar]
- 49.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell. 2001;105(6):745–755. [DOI] [PubMed] [Google Scholar]
- 50.Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest. 2003;112(12):1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]