Abstract
Context:
Cabergoline (CAB) is very effective in the treatment of macroprolactinomas, but there are few data on the CAB dose necessary to achieve and maintain normal prolactin (PRL) levels.
Design and Patients:
We retrospectively studied 260 patients. CAB was introduced at a mean dose of 0.83 ± 0.52 mg/wk. When the PRL level had normalized, the patient's physician chose to either maintain the CAB dose (fixed-dose group) or to taper it (de-escalation group) until the minimal effective dose required to maintain a normal PRL level was established.
Results:
PRL normalized in 157 patients (60.8%) during CAB treatment. CAB de-escalation was attempted in 84 (53.5%) of these 157 patients and was successful in 77 (91.7%) cases. The mean CAB dose was reduced from 1.52 ± 1.17 mg/wk to 0.56 ± 0.44 mg/wk at the last visit (P < 1 × 10−4). De-escalation was also possible in some “CAB-resistant” patients, namely those requiring ≥2 mg/wk to normalize PRL. CAB de-escalation had no negative long-term effect on tumor size. At the last visit, maximal diameter was 8.8 ± 8.8 mm in the de-escalation group and 13.4 ± 8.5 mm in the fixed-dose group (P < 0.01).
Conclusion:
In patients with macroprolactinomas, the CAB dosage required to maintain a normal PRL level long term is lower than the initial dosage necessary to normalize the PRL level. After PRL normalization, CAB tapering was almost always successful, even when very high initial doses were necessary. CAB tapering does not undermine tumor control and may attenuate the potential adverse effects of CAB, which appear to be dependent on the cumulative dose.
Keywords: hypothalamus/pituitary, hyperprolactinemia, pituitary disease, prolactinoma
Even if high doses of cabergoline were necessary to achieve normal PRL levels in patients with macroprolactinomas, it was possible to taper cabergoline to very low doses in 90% of cases.
Patients with macroprolactinomas require treatment for two main reasons: to reverse the deleterious consequences of hyperprolactinemia [1] and to reduce tumor mass effects [2]. Cabergoline (CAB) is currently the dopamine agonist (DA) of choice in this setting [3, 4], having been found to be more effective than other DAs [5]. In a compilation of data from 14 prospective studies of CAB in patients with hyperprolactinemic disorders, the hormonal response rate was 73% to 96%, and tumor size was reduced in 50% to 100% of patients [5]. CAB is also better tolerated, with less nausea and dizziness [6], and treatment adherence is improved by the once-per-week dosing schedule. In general, prolactin (PRL) levels are normalized by a low weekly CAB dose of 0.5 to 1 mg [5]. According to Endocrine Society guidelines, once the PRL level has normalized and tumor volume has decreased, DA therapy should be continued for a minimum of two years [4]. Between 24% and 75% of patients maintain a normal PRL level after DA withdrawal. Two meta-analyses suggest that remission persists after CAB withdrawal in about one-third of patients, on average [7, 8].
Cases of cardiac valve disorders in patients with Parkinson disease treated with high-dose CAB [9, 10] have raised concerns about lengthy CAB exposure in patients with prolactinomas, particularly at high doses. Although clinically serious valve disease has seldom or never been observed in patients with prolactinoma receiving DA therapy [11], the risk may not be nonexistent, particularly when the prolactinoma is DA resistant.
When the PRL level has normalized and the prolactinoma has shrunken, sometimes during receipt of high-dose CAB (>2 mg/wk), the same CAB dose may be maintained for a few years before attempting treatment withdrawal, or it may be gradually tapered to the minimum required to maintain both a normal PRL level and tumor volume control. The tapering strategy is mentioned in some guidelines [4] but, to our knowledge, has never been evaluated in a large series of patients, particularly those with macroprolactinomas. Given that both approaches have been used for many years in our center, and because there are few data on the CAB dosage necessary to achieve and maintain a normal PRL level, we retrospectively studied a large series of CAB-treated patients with macroprolactinomas.
1. Patients and Methods
A. Clinical and Biochemical Data
We included all patients with macroprolactinomas (maximal diameter ≥10 mm) managed in our department (Service d’Endocrinologie et des Maladies de la Reproduction, Hospital Bicêtre, France) between 1980 and 2014. We excluded patients with mixed adenomas (PRL + growth hormone or PRL + adrenocorticotropic hormone) and patients with pituitary carcinoma. Our criteria for considering that a macroadenoma was a prolactinoma were the PRL levels: when they were >200 ng/mL and were associated with a large tumor, the diagnosis was considered obvious; when the macroadenoma was associated with PRL levels <200 ng/mL, the diagnosis of macroprolactinoma was considered if the tumor volume was moderate and/or the tumor was cystic. In these cases, the diagnosis of macroprolactinoma was confirmed either by PRL immunostaining, if the patient underwent surgery, or if a substantial tumoral shrinkage was achieved after a short course (3 months) of dopamine agonists.
The following data were retrieved from the patients' records: clinical, biological, and imaging characteristics at diagnosis, the different drugs prescribed, the initial dose of CAB, the CAB dose necessary to normalize the PRL level, and the CAB dose necessary to maintain a normal PRL level.
Some physicians in our center choose to maintain the initially effective CAB dose, whereas others prefer a de-escalation strategy: Once PRL has normalized, they reduce the CAB dose in a stepwise manner and continue to do so as long as the PRL level remains normal, in decrements of 0.5 mg/wk (every 6 months in general); when the dose level of 0.5 mg/wk is reached, they increase the interval between two CAB intakes from once every two weeks to once every 4, 8, 12 weeks, etc., until they find the minimal dose required to maintain a normal PRL level. Each de-escalation step is considered successful if the PRL remains normal 6 months after a dose decrement and/or an increase in the dosing interval. Macroadenoma maximal diameter is checked by magnetic resonance imaging (MRI) every year during the tapering strategy.
Resistance to CAB is defined as a failure to reduce the serum PRL level below the upper limit of normal, together with persistent hyperprolactinemia and/or pituitary tumor mass despite a weekly CAB dose of at least 2.0 mg [12, 13].
PRL was measured with commercial immunoassays. Since 1997, PRL measurements have been performed on a Kryptor analyzer (Brahms Instruments, Hennigsdorf, Germany) with an assay based on time-resolved amplified cryptate emission technology. Briefly, it is a fluoroimmunoassay in homogeneous phase. The detection limit is 0.24 ng/mL (5 mIU/L) (1 ng = 21 mIU, 84/500 preparation based on the Third International Standard for Prolactin of the World Health Organization), and intra- and interassay are 3.5% and 3.7%, respectively, for a concentration of 5.7 ng/mL (120 mIU/L) and 0.6% and 2.1%, respectively, for a concentration of 54.7 ng/mL (1150 mIU/L). Before 1997, PRL measurement was performed with an enzyme-linked immunosorbent assay. The upper limit of normal of the two assays was similar.
The maximal diameter of the adenoma was measured in most cases by MRI, and by computed tomography in some cases before 1990 or when MRI was contraindicated.
Written consent was not necessary for chart-review studies in France before 2004. After 2004, all patients invited to participate in clinical research were asked to provide written informed consent.
B. Genetic Analysis
Information on genetic or familial cases was systematically sought from the patients’ files (MEN1, Carney complex, families with pituitary adenomas, or McCune-Albright syndrome). The entire AIP coding region (exons 1 to 6) and intron-exon junctions were amplified and sequenced in more than 60% of the patients, as reported elsewhere [14]. Younger patients were also screened for large deletions or duplication of the AIP and MEN1 genes by multiplex ligation-dependent probe amplification [14–16]. All of the patients concerned provided written informed consent for genetic analysis.
C. Statistical Analysis
Categorical data are reported as numbers and percentages, and continuous variables as mean ± standard deviation, range, and median. Continuous variables were compared with Student's t test and categorical data with the χ2 test. StatView software (Cary, NC) was used for statistical analyses. Differences were considered statistically significant at P < 0.05.
2. Results
A. Baseline Characteristics
We studied 260 patients, including 125 women (Table 1). Men were older than women (P < 1 × 10−4), their PRL levels were higher, and their maximal tumor diameters were larger (both P < 1 × 10−4). One young male patient with a macroprolactinoma revealed by pituitary apoplexy had a low postoperative PRL level (6 ng/mL) and did not require DA treatment. The lactotroph nature of the macroadenoma was ascertained by immunohistochemistry showing positivity for prolactin. PRL levels in this patient before surgery (performed as emergency surgery) were not available. One female patient had a moderately increased PRL level (25 ng/mL) due to a cystic macroprolactinoma revealed by infertility that decreased in size with DA treatment. Visual impairment and cavernous sinus invasion were more prevalent in men than in women (both P < 1 × 10−4), as was panhypopituitarism (P < 0.01).
Table 1.
Characteristics of Patients With Macroprolactinoma at Diagnosis
| Characteristics | All (n = 260) | Women (n = 125) | Men (n = 135) | Pa |
|---|---|---|---|---|
| Age at diagnosis, y | 36.2 ± 16.2 | 30.6 ± 14.9 | 41.5 ± 15.7 | <1.10−4 |
| 32.7 (10.6–83.1) | 26.8 (10.6–83.1) | 40.5 (13.7–79.0) | ||
| PRL level at diagnosis, ng/mL | 2099 ± 4434 | 934 ± 2305 | 3100 ± 5472 | <1.10−4 |
| 680 (6b–38,000) | 359 (25c–20,000) | 1119 (6b–38,000) | ||
| Maximal tumor diameter at diagnosis, mm | 23.8 ± 14.1 | 18.8 ± 13.2 | 28.0 ± 13.3 | <1.10−4 |
| 20.0 (10.0–110.0) | 15.0 (10.0–110.0) | 24.5 (10.0–67.0) | ||
| Impaired vision | 68 (26.2) | 17 (13.6) | 51 (37.8) | <1.10−4 |
| Cavernous sinus invasion | 99 (38.1) | 32 (25.6) | 67 (49.6) | <1.10−4 |
| Pituitary deficiencies | ||||
| Limited to gonadotropic deficiency | 72/120 (60.0) | 34/46 (73.9) | 38/74 (51.4) | 0.01 |
| Growth hormone deficiencyd | 27/120 (22.5) | 10/46 (21.7) | 17/74 (23.0) | 0.88 |
| Central hypothyroidisme | 23/212 (10.8) | 7/96 (7.3) | 16/116 (13.8) | 0.13 |
| Central hypocortisolisme | 16/212 (7.5) | 6/96 (6.3) | 10/116 (8.6) | 0.52 |
| Panhypopituitarisma | 13/212 (6.1) | 1/96 (1.0) | 12/116 (10.3) | 0.005 |
| AIP mutation | 21/157 (13.4) | 7/66 (10.6) | 14/91 (15.4) | 0.39 |
| MEN1 mutation | 6 (2.3) | 1 (0.8) | 5 (3.7) | 0.24 |
Values are given as mean ± standard deviation, median, and range, or as numbers and percentages.
Statistical significance of comparison between men and women. Statistically significant differences are indicated in bold.
Low PRL level at presentation as a result of apoplexy of a macroprolactinoma proven on immunocytochemistry.
Mildly increased PRL level associated with a cystic macroprolactinoma (see text for details).
Data available in 120 patients.
Data available in 212 patients.
B. Clinical Circumstances of Macroprolactinoma Diagnosis
In women, the presenting signs and symptoms were related to hyperprolactinemia (amenorrhea or oligomenorrhea, usually with galactorrhea). Amenorrhea and galactorrhea were both present in 33 women (26.4%). A mass effect was present at diagnosis in a minority of female patients. In men, most clinical manifestations were due to a mass effect, although various other signs and symptoms (sexual dysfunction, incidental findings) also led to the diagnosis (Supplemental Table 1 (85.2KB, docx) ).
C. Response to CAB
CAB was prescribed to 225 patients, at an average starting dose of 0.83 ± 0.52 mg/wk (median, 0.5 mg/wk), and the dose was gradually increased. The PRL level normalized in 157 cases (60.8%). The time interval between starting DA and the achievement of normal PRL was 22.15 ± 32.51 months (median, 9.96 months). CAB was the only treatment in 116 patients, whereas the other patients had previously received bromocriptine and/or quinagolide. Among these 116 patients, PRL levels normalized in 83 cases (71.6%). In the remaining 33 patients, the most common causes of failure were CAB resistance (n = 11; 16.9%), cerebrospinal fluid leakage as a result of marked tumor shrinkage in patients who also had erosion of the skull base (n = 8; 12.3%), nonadherence to CAB therapy (n = 6; 9.2%), and CAB intolerance (n = 5; 7.7%). However, even if normal PRL levels were not achieved, CAB was considered effective in 17 of these 33 patients, either because menstrual cycles resumed or natural conception occurred (n = 7), or because the PRL level decreased markedly (>95%), and tumor shrinkage was considered very satisfactory (n = 10).
D. Patients Resistant to Standard CAB Doses (≤2 mg/wk)
Forty-four patients (19.6%) received CAB doses >2 mg/wk. The average interval between each dose increment was 7.6 ± 10.0 months (0.96 to 59.0 months) with a median of 5 months. In 22 (50%) of these 44 patients, CAB resistance was only partial, given that dosages >2 mg/wk eventually normalized the PRL. In the 22 remaining patients, 21 had partial response to CAB (even if PRL did not normalize), and only one patient did not respond at all.
Patients with partial or total CAB resistance are compared with CAB-sensitive patients in Table 2. Compared with patients who were sensitive to CAB, resistant patients had higher PRL levels at diagnosis, a larger maximum tumor diameter at diagnosis, and more frequent pituitary deficiencies. Their initial CAB dose was higher, and the time required for CAB to normalize the serum PRL was longer. In addition, resistant patients were slightly more likely to have received quinagolide or undergone surgery. Finally, the duration of CAB therapy was longer in resistant patients, but this may have been related to longer follow-up.
Table 2.
Comparison Between CAB-Sensitive and CAB-Resistant Patients
| Characteristic | Sensitive (n = 135) | Resistant (n = 44) | Pa |
|---|---|---|---|
| Sex ratio, F/M | 62/73 | 21/23 | 0.49 |
| Age at diagnosis, y | 38.2 ± 16.6 | 29.7 ± 13.6 | 0.13 |
| 34.5 (10.6–79.0) | 26.5 (13.3–68.6) | ||
| PRL level at diagnosis, ng/mL | 1292 ± 2182 | 3174 ± 4972 | <1.10−2 |
| 534 (25–16,000) | 1100 (130–22,700) | ||
| Maximal tumor diameter at diagnosis, mm | 22.0 ± 10.3 | 30.7 ± 21.0 | 0.02 |
| 20.2 (10.0–55.0) | 23.0 (10.0–110.0) | ||
| Impaired vision | 31 (23.0) | 12 (27.3) | 0.42 |
| Invasion in cavernous sinus | 46 (34.1) | 21 (47.7) | 0.44 |
| Gonadotropic deficiency only | 43 (31.9) | 12 (27.3) | <1.10−2 |
| AIP mutation | 14 (10.4) | 6 (13.6) | 0.92 |
| MEN1 mutation | 2 (1.5) | 3 (6.8) | 0.24 |
| Initial dose of CAB, mg/wk | 0.73 ± 0.30 | 1.36 ± 0.86 | <1.10−4 |
| 0.50 (0.13–2.00) | 1.00 (0.50–3.50) | ||
| CAB dose at time of PRL normalization, mg/wk | 0.97 ± 0.50 | 3.81 ± 1.28 | <1.10−4 |
| 1.00 (0.25–3.00) | 3.50 (2.50–8.00) | ||
| Duration of CAB treatment, y | 5.8 ± 4.6 | 8.9 ± 4.7 | <1.10−4 |
| 5.1 (0.1–25.4) | 9.0 (1.0–18.6) | ||
| Duration of follow-up, y | 9.6 ± 8.3 | 13.2 ± 8.3 | <1.10−2 |
| 6.3 (0.0–37.3) | 11.9 (1.2–35.0) | ||
| Time between CAB initiation and PRL normalization, y | 1.5 ± 2.3 | 5.5 ± 4.1 | <1.10−4 |
| 0.6 (0.0–14.3) | 4.3 (0.7–12.4) | ||
| Bromocriptine treatment | 41 (30.4) | 18 (40.9) | 0.20 |
| Quinagolide treatment | 22 (16.3) | 16 (36.4) | <1.10−2 |
| Surgery | 16 (11.9) | 15 (34.1) | <1.10−2 |
| Radiotherapy | 3 (2.2) | 6 (13.6) | 0.37 |
Values are given as mean ± standard deviation, median, and range, or as numbers and percentages.
Statistical significance of comparison between males and females. Statistically significant differences are indicated in bold.
E. CAB Tapering
E-1. In patients sensitive to CAB
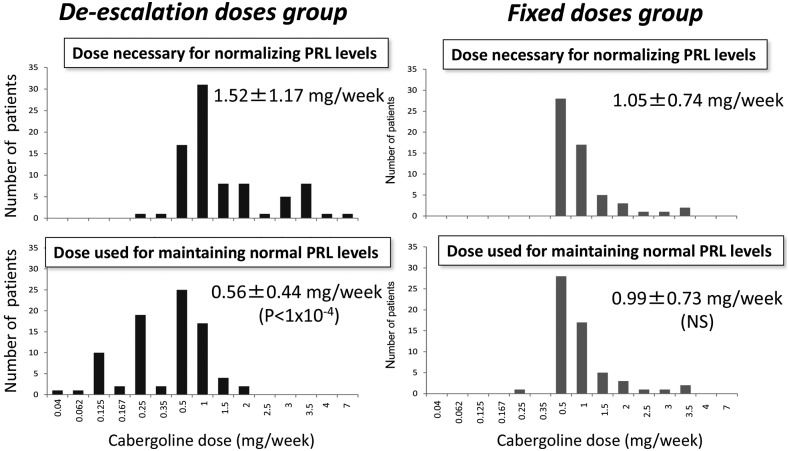
Once PRL levels had normalized (n = 157), at a mean CAB dose of 1.30 ± 1.01 mg/wk (median 1.00 mg/wk) and after a median of 9.9 months of treatment (22.1 ± 32.5 months), the CAB dose was maintained at the same level in 73 patients (fixed-dose group; 46.5%) and was tapered in 84 patients (de-escalation group; 53.5%), as decided by the patients' physicians. CAB de-escalation was successful in 77 of these 84 cases (91.7%); normal PRL levels were maintained at lower doses than those initially necessary. CAB de-escalation was unsuccessful in the remaining seven patients, in whom the PRL reincreased immediately, necessitating a return to the dose necessary to normalize PRL initially. In the 84 patients in the de-escalation group, the weekly CAB dose was reduced from 1.52 ± 1.17 mg at PRL normalization to 0.56 ± 0.44 mg at the last visit (P < 1 × 10−4). Thirty-four patients were tapered to 0.25 mg once per week. Of these 34 patients, 14 were further tapered to lower doses, by progressively increasing the interval between the doses. As a first step, doses were tapered to 0.5 mg every 21 days in two patients, to 0.5 mg every month in 10 patients, and to 0.25 mg every 14 days in two remaining patients. Then, a new trial of interval increase was attempted and was successful in three patients, leading to the administration of 0.5 mg of CAB every 45 days (n = 1) or 0.5 mg every 2 months (n = 2). Finally, the dose was tapered to 0.5 mg every 3 months in one of these three patients. In the 73 patients in the fixed-dose group, the CAB dose was 1.05 ± 0.74 mg/wk at PRL normalization and 0.99 ± 0.73 mg/wk at the last visit (no significant difference). Figure 1 shows the distribution of patients according to the doses of CAB that were necessary to normalize PRL and those required to maintain normal PRL levels in each group.
Figure 1.
Distribution of the patients in the de-escalation group (left panels, dark gray) and the fixed-dose group (right panels, light gray) according to the dose of CAB necessary to normalize the PRL level (upper panels) and the dose of CAB used to maintain a normal PRL level (lower panels).
E-2. In patients with partial CAB resistance
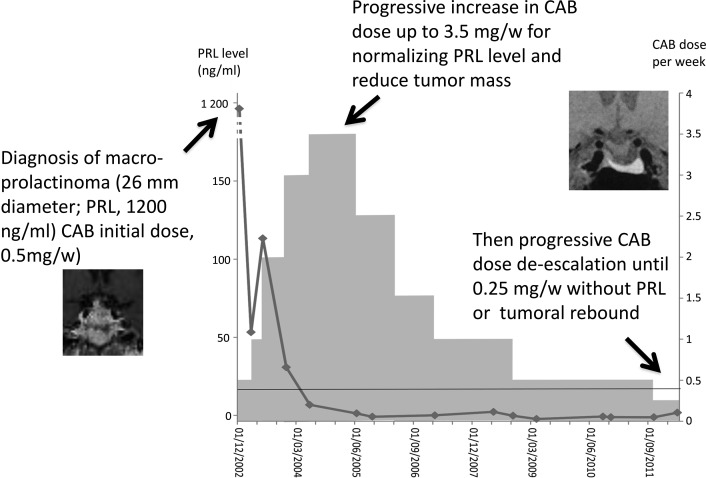
In the subgroup of 22 patients with partial CAB resistance (those who required >2 mg/wk to normalize PRL), CAB de-escalation was attempted in 17 cases (77.3%) and was successful in every case. The CAB dose was tapered from a mean of 3.57 ± 1.02 mg/wk at PRL normalization to 0.93 ± 0.58 mg/wk at the last visit (P < 1 × 10−4). Figure 2 shows the example of a patient requiring 3.5 mg/wk of CAB for PRL normalization and in whom de-escalation to 0.25 mg/wk was possible without a PRL reincrease or tumor regrowth.
Figure 2.
Example of a patient with a macroprolactinoma and initial PRL level of 1200 ng/mL who required 3.5 mg/wk of CAB to normalize the PRL level and in whom de-escalation to 0.25 mg/wk was possible without reincrease in PRL levels or tumor regrowth.
E-3. Effects of CAB tapering on tumor size
CAB de-escalation had no long-term negative consequences for tumor size. Indeed, at last visit, the largest diameter, which was similar in the two groups before CAB initiation, was smaller in the de-escalation group than in the fixed-dose group (8.8 ± 8.8 vs 13.4 ± 8.5 mm; P < 0.01).
E-4. Comparison between the fixed-dose and de-escalation groups
We found no prescription bias (Supplemental Table 2 (95KB, docx) ). Indeed, there was no significant difference between the fixed-dose and de-escalation groups in terms of the sex ratio, the PRL level at diagnosis, maximal tumor diameter at diagnosis, impaired vision or gonadotropic deficiency, the time between CAB initiation and PRL normalization, or the PRL level at PRL normalization. The patients in the de-escalation group were younger, had less frequent cavernous sinus invasion, and had slightly smaller tumors at PRL normalization. They also received higher initial CAB doses, higher CAB doses at PRL normalization, longer CAB therapy, and longer follow-up.
F. CAB Discontinuation
CAB discontinuation was attempted in 35 of the 157 patients whose PRL normalized during receipt of CAB. During a mean follow-up of 30.1 ± 30.6 months [median, 21.5 (range, 0 to 109 months)], hyperprolactinemia did not recur in 21 patients (60%), whereas CAB resumption was required in the other 14 cases (40%) because of recurrent hyperprolactinemia. CAB discontinuation was attempted in 10 patients of the fixed-dose group and was successful in six patients.
Combining these figures with those for the 15 patients in whom CAB tapering was associated with a reincrease in the PRL level and necessitated a return to the dose initially required, the failure rate of CAB discontinuation (or reduction) was 36.8% of patients, in whom either discontinuation or reduction of the dose was associated with recurrence of hyperprolactinemia. Among the 22 patients with partial CAB resistance (those who required >2 mg/wk for PRL normalization), CAB could be discontinued without hyperprolactinemia recurrence in two (9.1%) cases.
3. Discussion
In this retrospective study of a large cohort of patients with macroprolactinoma, we found that even when high CAB doses were necessary to normalize the PRL level, it was almost always possible to taper the dose and/or to increase the dosing interval while maintaining a normal PRL level. This tapering strategy has two potential benefits: it reduces the possible risks associated with long-term CAB exposure, and it identifies patients in whom hyperprolactinemia is likely to recur if CAB is discontinued. Importantly, CAB de-escalation does not result in renewed tumor growth.
The characteristics of our patients at diagnosis are similar to those of previous study populations [17–20]. The only noteworthy difference is that women were overrepresented in our cohort (48%), whereas macroprolactinoma mostly occurs in men [21–23]. The presenting symptoms of macroprolactinoma were also similar to those reported in other series, and were mainly related to hormone hypersecretion in women and to tumor mass effects in men [17–20].
A minority of our patients required CAB doses >2 mg/wk to normalize their PRL levels, a dose level that is widely considered to define CAB resistance [12, 13]. In keeping with previous reports, these patients were younger and had higher PRL levels and larger tumors at diagnosis than CAB-sensitive patients [13, 24]. Half of our “resistant” patients finally achieved normal PRL levels, which were accompanied by a reduction in tumor maximal diameter, with CAB doses up to 8 mg/wk, reached in increases once every 6 months of 0.5 mg/wk. Such “partial resistance” has already been described [13, 24–29], but the relevant publications do not mention whether these high doses were maintained in the long term.
Interestingly, CAB could be tapered in three-fourths of our patients with partial resistance, sometimes to very low doses similar to those required by sensitive patients. Thus, even if high initial CAB doses are required to achieve a normal PRL level, CAB may then be tapered or, in some cases, discontinued. This shows that initial partial CAB resistance does not rule out subsequent CAB tapering once the PRL level has normalized.
Current guidelines on CAB treatment of macroprolactinomas recommend that “with careful clinical and biochemical follow-up, therapy may be tapered and perhaps discontinued in patients who have been treated with dopamine agonists for at least two years, who no longer have elevated serum prolactin, and who have no visible tumor remnant on MRI” [4]. A low effective initial dose of CAB seems to be a good predictor of successful subsequent CAB withdrawal [7, 30, 31]. In our experience, when a very low maintenance dose and/or a long dosing interval has been achieved, complete CAB withdrawal can be attempted. However, lengthy follow-up is necessary, because CAB has a very long half-life [32].
Cases of cardiac valve disease have been described in CAB-treated patients with Parkinson disease [9, 10]. Likewise, rare subclinical valve lesions have been described in some series in patients receiving chronic CAB therapy for prolactinomas [11]. Our strategy, that of using the lowest effective dose required to maintain a normal PRL level and small tumor volume, should minimize this risk.
Such a tapering strategy has previously been evaluated in patients with microprolactinomas [33]. After PRL normalization, different maintenance doses (1 mg, 0.5 mg, or 0.25 mg of CAB weekly) had a similar impact on PRL levels; if hyperprolactinemia recurred after CAB discontinuation, the PRL reincreased, regardless of the previous maintenance dose [33].
This CAB tapering strategy can be proposed to all patients whose PRL levels normalize. Indeed, we found no differences between patients in whom tapering was successful and those in whom it was unsuccessful, or between those in whom dose de-escalation was attempted and those who were maintained on high CAB doses.
Although physicians are more reluctant to reduce the CAB dose in patients with macroprolactinomas than in patients with microprolactinomas, our results show that the de-escalation strategy is acceptable with respect to the risk of tumor regrowth. The success rate of CAB withdrawal in our patients (60%) is high relative to previous reports (between 8% and 47%) [8], probably because we only attempted CAB discontinuation when tapering was not associated with a reincrease in PRL.
The main limitation of our study is its retrospective design: although there were no major differences between patients in whom CAB was and was not tapered, a prescription bias cannot be ruled out.
In conclusion, in patients with macroprolactinomas, the CAB dose necessary to maintain a normal PRL level in the long term is lower than the initial dose necessary for PRL normalization. CAB tapering after PRL normalization is almost always successful in patients with macroprolactinomas, including some patients who require very high initial doses. This CAB de-escalation is not deleterious in terms of tumor size and may help to avoid some of the drug's potential cumulative, dose-dependent adverse effects. Finally, successful tapering helps to define the patients in whom CAB may be safely withdrawn without recurrence of hyperprolactinemia.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CAB
- cabergoline
- DA
- dopamine agonist
- MRI
- magnetic resonance imaging
- PRL
- prolactin
References and Notes
- 1.Bernard V, Young J, Chanson P, Binart N. New insights in prolactin: pathological implications. Nat Rev Endocrinol. 2015;11(5):265–275. [DOI] [PubMed] [Google Scholar]
- 2.Chanson P, Maiter D. Prolactinoma. In: Melmed S, ed. The Pituitary. 4th ed. San Diego, CA: Elsevier; 2017:467–514. [Google Scholar]
- 3.Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, Brue T, Cappabianca P, Colao A, Fahlbusch R, Fideleff H, Hadani M, Kelly P, Kleinberg D, Laws E, Marek J, Scanlon M, Sobrinho LG, Wass JA, Giustina A. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006;65(2):265–273. [DOI] [PubMed] [Google Scholar]
- 4.Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA; Endocrine Society . Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273–288. [DOI] [PubMed] [Google Scholar]
- 5.Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev. 2006;27(5):485–534. [DOI] [PubMed] [Google Scholar]
- 6.Webster J. A comparative review of the tolerability profiles of dopamine agonists in the treatment of hyperprolactinaemia and inhibition of lactation. Drug Saf. 1996;14(4):228–238. [DOI] [PubMed] [Google Scholar]
- 7.Dekkers OM, Lagro J, Burman P, Jørgensen JO, Romijn JA, Pereira AM. Recurrence of hyperprolactinemia after withdrawal of dopamine agonists: systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95(1):43–51. [DOI] [PubMed] [Google Scholar]
- 8.Hu J, Zheng X, Zhang W, Yang H. Current drug withdrawal strategy in prolactinoma patients treated with cabergoline: a systematic review and meta-analysis. Pituitary. 2015;18(5):745–751. [DOI] [PubMed] [Google Scholar]
- 9.Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med. 2007;356(1):29–38. [DOI] [PubMed] [Google Scholar]
- 10.Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med. 2007;356(1):39–46. [DOI] [PubMed] [Google Scholar]
- 11.Caputo C, Prior D, Inder WJ. The need for annual echocardiography to detect cabergoline-associated valvulopathy in patients with prolactinoma: a systematic review and additional clinical data. Lancet Diabetes Endocrinol. 2015;3(11):906–913. [DOI] [PubMed] [Google Scholar]
- 12.Molitch ME. Pharmacologic resistance in prolactinoma patients. Pituitary. 2005;8(1):43–52. [DOI] [PubMed] [Google Scholar]
- 13.Vroonen L, Jaffrain-Rea ML, Petrossians P, Tamagno G, Chanson P, Vilar L, Borson-Chazot F, Naves LA, Brue T, Gatta B, Delemer B, Ciccarelli E, Beck-Peccoz P, Caron P, Daly AF, Beckers A. Prolactinomas resistant to standard doses of cabergoline: a multicenter study of 92 patients. Eur J Endocrinol. 2012;167(5):651–662. [DOI] [PubMed] [Google Scholar]
- 14.Cazabat L, Bouligand J, Salenave S, Bernier M, Gaillard S, Parker F, Young J, Guiochon-Mantel A, Chanson P. Germline AIP mutations in apparently sporadic pituitary adenomas: prevalence in a prospective single-center cohort of 443 patients. J Clin Endocrinol Metab. 2012;97(4):E663–E670. [DOI] [PubMed] [Google Scholar]
- 15.Salenave S, Ancelle D, Bahougne T, Raverot G, Kamenický P, Bouligand J, Guiochon-Mantel A, Linglart A, Souchon PF, Nicolino M, Young J, Borson-Chazot F, Delemer B, Chanson P. Macroprolactinomas in children and adolescents: factors associated with the response to treatment in 77 patients. J Clin Endocrinol Metab. 2015;100(3):1177–1186. [DOI] [PubMed] [Google Scholar]
- 16.Thevenon J, Bourredjem A, Faivre L, Cardot-Bauters C, Calender A, Murat A, Giraud S, Niccoli P, Odou MF, Borson-Chazot F, Barlier A, Lombard-Bohas C, Clauser E, Tabarin A, Parfait B, Chabre O, Castermans E, Beckers A, Ruszniewski P, Le Bras M, Delemer B, Bouchard P, Guilhem I, Rohmer V, Goichot B, Caron P, Baudin E, Chanson P, Groussin L, Du Boullay H, Weryha G, Lecomte P, Penfornis A, Bihan H, Archambeaud F, Kerlan V, Duron F, Kuhn JM, Vergès B, Rodier M, Renard M, Sadoul JL, Binquet C, Goudet P. Higher risk of death among MEN1 patients with mutations in the JunD interacting domain: a Groupe d’etude des Tumeurs Endocrines (GTE) cohort study. Hum Mol Genet. 2013;22(10):1940–1948. [DOI] [PubMed] [Google Scholar]
- 17.Touraine P, Plu-Bureau G, Beji C, Mauvais-Jarvis P, Kuttenn F. Long-term follow-up of 246 hyperprolactinemic patients. Acta Obstet Gynecol Scand. 2001;80(2):162–168. [DOI] [PubMed] [Google Scholar]
- 18.Berinder K, Stackenäs I, Akre O, Hirschberg AL, Hulting AL. Hyperprolactinaemia in 271 women: up to three decades of clinical follow-up. Clin Endocrinol (Oxf). 2005;63(4):450–455. [DOI] [PubMed] [Google Scholar]
- 19.Di Somma C, Colao A, Di Sarno A, Klain M, Landi ML, Facciolli G, Pivonello R, Panza N, Salvatore M, Lombardi G. Bone marker and bone density responses to dopamine agonist therapy in hyperprolactinemic males. J Clin Endocrinol Metab. 1998;83(3):807–813. [DOI] [PubMed] [Google Scholar]
- 20.Noel GL, Suh HK, Frantz AG. Prolactin release during nursing and breast stimulation in postpartum and nonpostpartum subjects. J Clin Endocrinol Metab. 1974;38(3):413–423. [DOI] [PubMed] [Google Scholar]
- 21.Nishioka H, Haraoka J, Akada K Growth potential of prolactinomas in men: is it really different from women? Surg Neurol 2003;59:386–390; discussion 390-381. [DOI] [PubMed] [Google Scholar]
- 22.Ramot Y, Rapoport MJ, Hagag P, Wysenbeek AJ. A study of the clinical differences between women and men with hyperprolactinemia. Gynecol Endocrinol. 1996;10(6):397–400. [DOI] [PubMed] [Google Scholar]
- 23.Delgrange E, Trouillas J, Maiter D, Donckier J, Tourniaire J. Sex-related difference in the growth of prolactinomas: a clinical and proliferation marker study. J Clin Endocrinol Metab. 1997;82(7):2102–2107. [DOI] [PubMed] [Google Scholar]
- 24.Delgrange E, Daems T, Verhelst J, Abs R, Maiter D. Characterization of resistance to the prolactin-lowering effects of cabergoline in macroprolactinomas: a study in 122 patients. Eur J Endocrinol. 2009;160(5):747–752. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee A, Wynne K, Tan T, Hatfield EC, Martin NM, Williamson C, Meeran K. High dose cabergoline therapy for a resistant macroprolactinoma during pregnancy. Clin Endocrinol (Oxf). 2009;70(5):812–813. [DOI] [PubMed] [Google Scholar]
- 26.Ono M, Miki N, Kawamata T, Makino R, Amano K, Seki T, Kubo O, Hori T, Takano K. Prospective study of high-dose cabergoline treatment of prolactinomas in 150 patients. J Clin Endocrinol Metab. 2008;93(12):4721–4727. [DOI] [PubMed] [Google Scholar]
- 27.Di Sarno A, Landi ML, Cappabianca P, Di Salle F, Rossi FW, Pivonello R, Di Somma C, Faggiano A, Lombardi G, Colao A. Resistance to cabergoline as compared with bromocriptine in hyperprolactinemia: prevalence, clinical definition, and therapeutic strategy. J Clin Endocrinol Metab. 2001;86(11):5256–5261. [DOI] [PubMed] [Google Scholar]
- 28.Pascal-Vigneron V, Weryha G, Bosc M, Leclere J. Hyperprolactinemic amenorrhea:treatment with cabergoline versus bromocriptine. Results of a national multicenter randomized double-blind study [in French]. Presse Med. 1995;24(16):753–757. [PubMed] [Google Scholar]
- 29.Webster J, Piscitelli G, Polli A, Ferrari CI, Ismail I, Scanlon MF. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. Cabergoline Comparative Study Group. N Engl J Med. 1994;331(14):904–909. [DOI] [PubMed] [Google Scholar]
- 30.Colao A, Di Sarno A, Cappabianca P, Di Somma C, Pivonello R, Lombardi G. Withdrawal of long-term cabergoline therapy for tumoral and nontumoral hyperprolactinemia. N Engl J Med. 2003;349(21):2023–2033. [DOI] [PubMed] [Google Scholar]
- 31.Kharlip J, Salvatori R, Yenokyan G, Wand GS. Recurrence of hyperprolactinemia after withdrawal of long-term cabergoline therapy. J Clin Endocrinol Metab. 2009;94(7):2428–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreotti AC, Pianezzola E, Persiani S, Pacciarini MA, Strolin Benedetti M, Pontiroli AE. Pharmacokinetics, pharmacodynamics, and tolerability of cabergoline, a prolactin-lowering drug, after administration of increasing oral doses (0.5, 1.0, and 1.5 milligrams) in healthy male volunteers. J Clin Endocrinol Metab. 1995;80(3):841–845. [DOI] [PubMed] [Google Scholar]
- 33.Buyukbayrak EE, Karageyim Karsidag AY, Kars B, Balcik O, Pirimoglu M, Unal O, Turan C. Effectiveness of short-term maintenance treatment with cabergoline in microadenoma-related and idiopathic hyperprolactinemia. Arch Gynecol Obstet. 2010;282(5):561–566. [DOI] [PubMed] [Google Scholar]