Abstract
Laboratory interference is a drawback in hormonal testing, and clinicians should have a high index of suspicion when faced with biochemical results discordant with the patient's clinical manifestations. A 62-year-old postmenopausal woman initially consulted her primary care physician for mood lability; laboratory workup showed markedly elevated levels of total serum estradiol, progesterone, testosterone, and cortisol as measured by immunoassay. Further investigation demonstrated no evidence of estrogen effect on uterus, no adrenal or adnexal mass, and no evidence of Cushing syndrome. Conventional techniques to unmask laboratory interference, such as dilution, antigen precipitation, and using a different immunoassay did not unveil a potential laboratory interference. The patient had no apparent risk factor for analytic interference, such as absent rheumatoid factor and heterophilic antibodies, but had only mild monoclonal IgG hypergammaglobulinemia. In this case, mass spectrometry unmasked the false elevation in steroid hormones. Interference of gammaglobulins or antibodies with the labeling and separation process of the assay could be the culprits. In conclusion, we report a unique case of multiple steroid hormones elevations due to laboratory interference unmasked by mass spectrometry.
Keywords: steroid measurement, estradiol, testosterone, mass spectrometry, laboratory techniques
Multiple steroid hormones may be affected by aberrant biochemical results measured by immunoassay. Hypergammaglobulinemia may be implicated, and mass spectrometry can uncover the error.
Laboratory evaluation is essential for the accurate assessment and care of patients in endocrinology. When laboratory results contradict the clinical picture, one must suspect analytic interference, a challenging problem faced by clinicians. Pre- or postanalytical errors, as well as direct analytical errors caused by endogenous or exogenous substances, can affect the measurable analyte in the sample, leading to erroneous levels of hormones measured by immunoassay. Each type of assay is susceptible to different interferences, and clinicians need to have a high index of suspicion to uncover them.
1. Case
We report the case of a 62-year-old woman, referred to our endocrinology division for assessment of marked elevation of steroid hormones, including estradiol, progesterone, testosterone, and cortisol. Her primary care physician ordered testing in the context of mood lability and anxiety. The patient’s medical history includes well-controlled type 2 diabetes and hypertension. She does not have liver or autoimmune disease; has been postmenopausal since age 50 years; and did not take any form of hormonal replacement therapy, hormonal substance, or dietary supplement. Menarche occurred at age 12 years, and she had one pregnancy in her early 30s. She was nearly asymptomatic apart from a recent mood change and did not present any vaginal bleeding or symptoms of hypercortisolism. Her physical examination was unremarkable except for moderate generalized obesity; she had no palmar erythema, no clinical hyperandrogenism, and no signs of Cushing syndrome.
Repeated samples at our center confirmed multiple abnormalities (Table 1): serum estradiol, 3073 pmol/L (normal range, 18 to 201 pmol/L); serum progesterone, 30 nmol/L (normal range, 0 to 5 nmol/L); and bioavailable testosterone, 1.75 nmol/L (normal range, 0 to 0.43 nmol/L). She had a low normal sex hormone–binding globulin [(SHBG); 34 nmol/L; normal range, 20 to 130 nmol/L]. Her laboratory results also showed mildly elevated luteinizing hormone (LH) and follicle-stimulating hormone (FSH) (LH, 16 and FSH, 27 U/L, respectively; postmenopausal ranges are as follows: LH, 8 to 59 U/L, and FSH, 67 to 135 U/L) and dehydroepiandrosterone-S levels slightly above normal (10.3 µmol/L; normal range, 0.26 to 6.68 µmol/L). Her serum cortisol was 1250 nmol/L at 10 am and 1433 nmol/L at 3 pm. Results on a 1-mg dexamethasone suppression test were also abnormal (247 nmol/L; normal range < 50 nmol/L), but her late-night salivary cortisol, 24-hour urinary cortisol, and adrenocorticotropic-releasing hormone levels were normal at multiple occasions, which ruled out Cushing syndrome. Abdominal computed tomography, magnetic resonance imaging, and pelvic/transvaginal ultrasonography showed no evidence of adnexal or adrenal mass, and the endometrium was thin. CA-125, α-fetoprotein, and 17-OH-progesterone were normal, and inhibin and anti-Müllerian hormone were appropriately low for her postmenopausal status. Immunoassays used for steroids were electro-chemiluminescence immunoassays done by using Roche Cobas e602 (Roche Diagnostic Canada, Laval, Quebec, Canada).
Table 1.
Summary of the Laboratory Investigations in Current Case
| Hormonal Testing | Matrix | Result | Age-Adjusted Reference Range | Method |
|---|---|---|---|---|
| Estradiol, pmol/L | Serum | 3073 | 18–201 | Immunoassay, Roche Cobas 602 |
| Progesterone, nmol/L | Serum | 30 /L | 0–5 | Immunoassay, Roche Cobas 602 |
| Total testosterone, nmol/L | Serum | 4.1 | 0.1–1.4 | Immunoassay, Roche Cobas 602 |
| Bioavailable testosterone, nmol/L | Serum | 1.75 | 0–0.43 | Calculated using SHBG, total testosterone, and albumin; done with Roche Cobas 602 and 702 |
| Cortisol, nmol/L | Serum | 1250 at 10 am | 100–450 | Immunoassay, Roche Cobas 602 |
| 1433 at 3 pm | 50–300 | |||
| DHEA-S, µmol/L | Serum | 10.3 | 0.26–6.68 | Immunoassay, Roche Cobas 602 |
| 17-OH-progesterone, nmol/L | Serum | 2.5 | 0.6–5.2 | Immunoassay, Roche Cobas 602 |
| SHBG, nmol/L | Serum | 34 | 20–130 | Immunoassay, Roche Cobas 602 |
| LH, U/L | Serum | 16 | 8–59 | Immunoassay, Roche Cobas 602 |
| FSH, U/L | Serum | 27 | 67–135 | Immunoassay, Roche Cobas 602 |
| AMH, ng/mL | Serum | <0.16 | <0.16 | ELISA (Beckman Coulter) done with GEMINI |
| Inhibin B | Serum | <10 | <10 | ELISA (Beckman Coulter) done with Tecan Sunrise plate reader |
| IgG, g/L | Serum | 17.7 | 7–16 | Roche Cobas 602 |
| Clone IgG κ, g/L | Serum | 9.9 | None | Sebia Capillarys2 (electrophoresis and clone quantification) plus Sebia Hydrasys for immunofixation |
| Free estradiol, pg/mL | Serum | <0.5 | <0.5 | LC/MS-MS, by Arup Laboratories |
| Total testosterone, nmol/L | Serum | <0.2 | 0.3–2.1 | LC/MS-MS, at Centre Hospitalier Universitaire de Sherbrooke |
| Free testosterone, pmol/L | Serum | <4 | 0–25 | LC/MS-MS, at Centre Hospitalier Universitaire de Sherbrooke |
Abbreviations: AMH, anti-Müllerian hormone; DHEA-S, dehydroepiandrosterone-S; ELISA, enzyme-linked immunosorbent assay; LC/MS-MS, liquid chromatography–tandem mass spectometry.
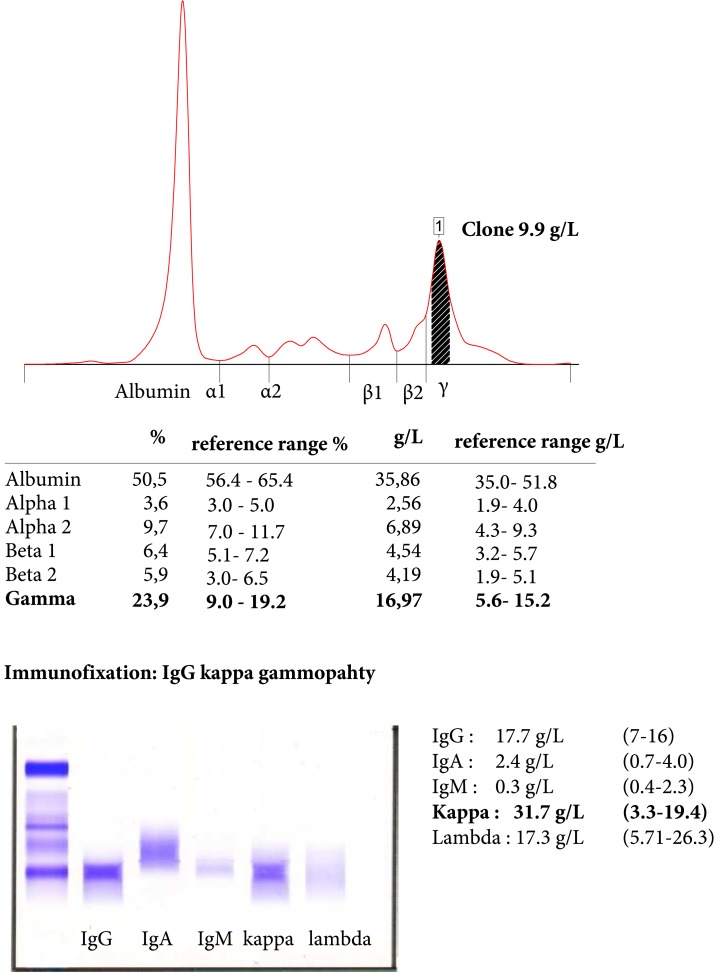
Because of the discrepancy among the biochemical results and the clinical picture, laboratory interference was investigated by using dilution, a different immunoassay, and pretreating the sample with a commercial blocking agent. All rendered similar results and levels showed even higher values after conversion of the 1:10 dilution test. Heterophilic antibody levels and rheumatoid factor were absent, but IgG κ gammopathy was identified, with a clone concentration of 10 g/L (Fig. 1).
Figure 1.
Capillary electrophoresis of serum proteins and immunofixation.
To pursue further investigation, blood samples were analyzed at a referral center to measure testosterone by liquid chromatography–tandem mass spectrometry; no testosterone was detected. Free estradiol was also analyzed by using this method at another specialized laboratory, and result was also nil.
Meanwhile, the patient underwent a total hysterectomy and bilateral ovariectomy for fibromyomas. No pathologic abnormality was described in the ovarian tissue.
2. Discussion
This case highlights the complex difficulties of determining laboratory interference in immunoassays. The prevalence of the problem varies and depends on the assay, ranging from 0.05% to 6% [1].
Clinicians must be aware that such interference is a shortcoming of many hormonal testing and must have a high index of suspicion when the clinical picture is inconsistent with biochemical results. Analytic interference can result in erroneous values that can lead to costly investigations, misdiagnosis, and unnecessary treatments.
The most frequent interference is described with dosage of thyroid hormones and TSH [2], but it has also been reported with other hormones, such as gonadotrophin, prolactin, and, rarely, estrogen [3]. It has never been reported with multiple steroid hormones.
The mechanisms underlying analytic interference in hormonal measurement are multiple and not fully understood. The critical step is the preanalytic phase, where most erroneous results arise [4]. In this case, samples were repeated under close supervision by the biochemist to assure adequate sampling, handling, and processing of the tubes.
Two phenomena can alter the measurable total concentration in a sample: hormone-binding proteins, which could not account for the high result in this case because SHBG was normal; and autoantibodies, which can result in unreliable levels of hormones (e.g., antithyroglobulin or antiprolactin antibodies resulting in a polymeric form of hormone). Autoanalyte antibodies have been described with testosterone [5], but none has been described with other steroid hormones. Cross-reactivity with an endogenous molecule of similar structure is less a concern in recent years because of improvement in antibodies specificity. In our case, even if molecular similarities exist among cortisol, estradiol, progesterone, and testosterone, cross-reactivity is improbable because the assay for each steroid hormone is very specific. Although the lower than expected gonadotrophin levels could raise the possibility of an unmeasured ovarian metabolite, this cannot explain the multiple abnormalities found in this case.
Alteration of antibody binding in the immunoassay can occur with heterophilic antibodies, rheumatoid factor, and human or animal antibodies. In our case, those antibody levels were absent, should have been neutralized with the blocking agent, and should not persist with use of a different type of immunoassay. High-dose hook effect can occur when a very high analyte saturates antibody interaction with antigen and can lead to falsely low results. In this case, we did dilute the sample to try to unmask a nonlinear curve, but results were even higher after dilution. Interestingly, other proteins, such as lysozyme and paraproteins, can also affect antibody binding. IgG κ paraprotein has been reported as giving a falsely elevated d-dimer level by immunoassay [6] and also to block binding of TSH and assay antibody, leading to falsely low results [7]. Furthermore, only one case reported false hyperestrogenism in a young woman [3], who also exhibited monoclonal gammopathy as in our case. This elevated immunoglobulin may be a marker, if not the source, of an interfering antibody responsible for the multiple analytic interferences. More specifically, we assume that because Roche electro-chemiluminescence immunoassays use ruthenium labeling and separation is done by streptavidin-coated microparticles, antibodies to either of those agents could explain the interference [8, 9]. High-dose biotin supplements have been shown to interfere with streptavidin conjugates [10], but our patient did not use biotin at the time of testing.
In the present case, mass spectrometry could unmask the interference. This method is currently available only in selected laboratories, is costlier, and takes longer to process; thus, it cannot be widely recommended. Nonimmunometric methods and measuring direct analytes are helpful in challenging cases but must be used judiciously.
3. Conclusion
This patient had false elevation of multiple steroid hormones due to analytic interference. Analytic errors may be involved in a patient without risk factors other than hypergammaglobulinemia, which may be a potential marker of immune interference. This case demonstrates the complexity of the problem because conventional methods were unable to unmask the problem and mass spectrometry could confirm the error.
Acknowledgments
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- FSH
- follicle-stimulating hormone
- LH
- luteinizing hormone
- SHBG
- sex hormone–binding globulin.
References and Notes
- 1.Sturgeon CM, Viljoen A. Analytical error and interference in immunoassay: minimizing risk. Ann Clin Biochem. 2011;48(Pt 5):418–432. [DOI] [PubMed] [Google Scholar]
- 2.Zaninotto M, Tognon C, Venturini R, Betterle C, Plebani M. Interference in thyroid hormones with Roche immunoassays: an unfinished story. Clin Chem Lab Med. 2014;52(12):e269–e270. [DOI] [PubMed] [Google Scholar]
- 3.Kairemo KJA, Kahn JA, Taipale PJ. Monoclonal gammopathy may disturb oestradiol measurement in the treatment and monitoring of in-vitro fertilization: case report. Hum Reprod. 1999;14(11):2724–2726. [DOI] [PubMed] [Google Scholar]
- 4.Raff H, Sluss PM. Pre-analytical issues for testosterone and estradiol assays. Steroids. 2008;73(13):1297–1304. [DOI] [PubMed] [Google Scholar]
- 5.Kuwahara A, Kamada M, Irahara M, Naka O, Yamashita T, Aono T. Autoantibody against testosterone in a woman with hypergonadotropic hypogonadism. J Clin Endocrinol Metab. 1998;83(1):14–16. [DOI] [PubMed] [Google Scholar]
- 6.Mugler K, Lefkowitz JB. False-positive D-dimer result in a patient with Castleman disease. Arch Pathol Lab Med. 2004;128(3):328–331. [DOI] [PubMed] [Google Scholar]
- 7.Luzzi VI, Scott MG, Gronowski AM. Negative thyrotropin assay interference associated with an IgGkappa paraprotein. Clin Chem. 2003;49(4):709–710. [DOI] [PubMed] [Google Scholar]
- 8.Gessl A, Blueml S, Bieglmayer C, Marculescu R. Anti-ruthenium antibodies mimic macro-TSH in electrochemiluminescent immunoassay. Clin Chem Lab Med. 2014;52(11):1589–1594. [DOI] [PubMed] [Google Scholar]
- 9.Rulander NJ, Cardamone D, Senior M, Snyder PJ, Master SR. Interference from anti-streptavidin antibody. Arch Pathol Lab Med. 2013;137(8):1141–1146. [DOI] [PubMed] [Google Scholar]
- 10.Samarasinghe S, Meah F, Singh V, Basit A, Emanuele N, Emanuele MA, Mazhari A, Holmes EW. Biotin interference with routine clinical immunoassays: understand the causes and mitigate the risks [published online ahead of print May 23, 2017]. Endocr Pract. doi: 10.4158/EP171761.RA. [DOI] [PubMed]