Abstract
Diabetes is a common and important complication of cystic fibrosis, an autosomal recessive genetic disease due to mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Cystic fibrosis–related diabetes (CFRD) is associated with profound detrimental effects on the disease course and mortality and is expected to increase in prevalence as the survival of patients with cystic fibrosis continues to improve. Despite progress in the functional characterization of CFTR molecular defects, the mechanistic basis of CFRD is not well understood, in part because of the relative inaccessibility of the pancreatic tissue and the limited availability of representative animal models. This review presents a concise overview of the current understanding of CFRD pathogenesis and provides a cutting-edge update on novel findings from human and animal studies. Potential contributions from paracrine mechanisms and β-cell compensatory mechanisms are highlighted, as well as functional β-cell and α-cell defects, incretin defects, exocrine pancreatic insufficiency, and loss of islet cell mass. State-of-the-art and emerging treatment options are explored, including advances in insulin administration, CFTR modulators, cell replacement, gene replacement, and gene editing therapies.
Keywords: beta cell, CFRD, CFTR, cystic fibrosis, diabetes, pancreatic insufficiency
Cystic fibrosis–related diabetes is unique and increasing in prevalence. In this review, the latest insights into its mechanism from new animal models and human studies are discussed.
Cystic fibrosis is an autosomal recessive disorder caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. It is the most common life-limiting genetic condition in people of Caucasian ancestry and affects about one in 3000 newborns in Europe, North America, and Australia [1]. The incidence is lower in other parts of the world, such as in Africa (Cape Town, South Africa, one in 12,000) and Asia (Japan, one in 350,000). More than 2000 variants of the CFTR gene have been identified to date, and close to 300 are known cystic fibrosis–causing variants [2]. On the basis of functional consequence, the mutations are broadly grouped into six classes (Table 1) [3, 4]. The CFTR protein acts as an anion (primarily Cl−) channel that controls ion movement across the cell membrane and is regulated by cyclic adenosine monophosphate-dependent phosphorylation. In pancreatic ductal cells, the Cl− secretion via CFTR is functionally coupled to a Cl−-bicarbonate exchanger, producing net bicarbonate secretion, and CFTR itself may also secrete bicarbonate [5, 6]. Defective CFTR function reduces the volume of pancreatic secretions, predisposing to plugging of small ducts, and increases acidity, promoting premature activation of digestive enzymes [7]. In the digestive, respiratory, and reproductive systems, dysfunction of the CFTR protein leads to inspissated secretions and obstruction of epithelium-lined ducts, eventually resulting in inflammation and tissue damage [8]. Pulmonary infections, sinus disease, exocrine and endocrine pancreatic insufficiency, hepatobiliary disease, and male infertility are commonly observed in individuals with cystic fibrosis, with respiratory failure being the primary cause of death. The median predicted survival of patients with cystic fibrosis has improved dramatically because of advances in therapeutics and nutrition, and currently stands at around 40 years [9]. If the mortality continues to decline at the current rate, the median life span of children born and diagnosed in 2010 is projected to reach >50 years. Studies of specific CFTR mutants have proved instrumental in linking the underlying molecular defects to the disease phenotypes and have aided recent developments in targeted small-molecule therapy [10].
Table 1.
Six Classes of CFTR Gene Mutations Grouped According to Their Functional Consequence
| Mutation Class | Molecular Consequence | Representative Genotype | Correct Protein Targeting | Channel Activity | Disease Severity |
|---|---|---|---|---|---|
| I | Premature termination due to nonsense or frameshift mutation | G542X | No | No | Severe |
| II | Improper protein folding and trafficking | F508del | No | No | Severe |
| III | Defect in ATP-dependent gating of channel | G551D | Yes | No | Severe |
| IV | Defect in channel formation and conductance | R117H | Yes | Reduced | Mild |
| V | Defect in pre-mRNA splicing | A455E | Reduced | Reduced | Mild |
| VI | Decreased protein stability and retention at cell surface | c.120del23 | Reduced | Reduced | Mild |
Abbreviations: ATP, adenosine triphosphate; mRNA, messenger RNA.
1. Risk Factors and Natural History of Diabetes Associated With Cystic Fibrosis
Pancreatic involvement in cystic fibrosis includes exocrine insufficiency with malabsorption, pancreatitis, and insulin sufficiency with abnormal glucose tolerance and cystic fibrosis–related diabetes (CFRD). Diabetes is the most common endocrine complication of cystic fibrosis and affects about 20% of adolescents and 40% to 50% of adults [11]. Certain CFTR genotypes that cause complete lack of protein function, such as ΔF508 (also referred to as F508del, p.Phe508del, or c.1521_1523delCTT), carry a much higher risk of CFRD than do genotypes that partially spare protein function [12]. Nearly all patients with these severe genotypes develop exocrine pancreatic insufficiency by the end of the first year, and about 80% develop CFRD by middle age [13, 14]. Other risk factors include older age, female sex, hepatobiliary disease, and corticosteroid use [12, 13, 15]. The diagnosis of CFRD is associated with worse clinical outcomes in patients with cystic fibrosis, reflected in more frequent pulmonary exacerbations, greater reduction in lung function (as measured by forced expiratory volume in 1 second), poorer nutritional status, and decreased survival, particularly in female patients [11, 13, 16, 17]. Even impaired glucose tolerance occurring well before the diagnosis of overt diabetes has been linked to major clinical deterioration [16, 18–20]. Insulin therapy in CFRD has been shown to improve pulmonary function, increase body weight, and reduce the frequency of lung exacerbations [21–26].
As the average life span of people with cystic fibrosis continues to grow, CFRD is expected to become more common. To enhance early diagnosis and intervention, annual screening of all patients with cystic fibrosis using a 75-g oral glucose tolerance test (OGTT) starting at age 10 years is currently recommended by the Cystic Fibrosis Foundation and the European Cystic Fibrosis Society [22, 27]. Although hemoglobin A1c, fasting plasma glucose, or random plasma glucose level can also establish the diagnosis of diabetes, the OGTT is currently the test of choice for diagnosing CFRD because of its higher sensitivity [22, 28, 29]. The OGTT still has a number of disadvantages, and alternative diagnostic methods that are less time-consuming and more sensitive are being sought [29]. Clinical hyperglycemia may initially manifest only during periods of acute pulmonary infections or corticosteroid therapy. With disease progression, postprandial hyperglycemia develops, followed by CFRD without fasting hyperglycemia, and eventually CFRD with fasting hyperglycemia. The β-cell dysfunction is evident many years before the onset of frank diabetes in the form of impaired first-phase insulin secretion in response to intravenous glucose [30, 31].
A. Comparisons to Type 1 and Type 2 Diabetes
CFRD is classified separately from type 1 diabetes and type 2 diabetes [32]. It is distinguished from type 1 diabetes because of its insidious onset over years to decades, rather than weeks to months, and the persistence of some insulin production long after diagnosis. Accordingly, ketoacidosis is uncommon. Autoantibodies are not detected in most patients with CFRD. CFRD differs from type 2 diabetes because insulin resistance is not the defining feature of the disorder, although substantial insulin resistance may be induced in the context of chronic inflammation and active infections [33]. Similar to both type 1 and type 2 diabetes, CFRD is associated with microvascular complications such as retinopathy and nephropathy, and the risk is dependent on disease duration and glycemic control [34]. Macrovascular disease in CFRD is rare and is not a major source of mortality. However, as an increasing number of CFRD patients reach middle age and beyond, it is conceivable that macrovascular complications may become more recognized in CFRD.
Because of the uniqueness and growing importance of CFRD, a better understanding of how it develops is needed to improve rational therapeutic options. The relative inaccessibility of the pancreatic tissue and the lack of appropriate genetic model systems have hampered mechanistic investigations in the past. With the availability of new animal models and increased focus on human studies examining early phases of CFRD development, there is reason to be optimistic that major conceptual advances are forthcoming. This review addresses new mechanistic insights acquired from cell, animal, and patient studies and the recent and emerging strategies for treatment of CFRD.
2. Updates on CFRD Pathogenesis
A. Collateral Damage or Intrinsic β-Cell Defect?
Decreased insulin secretion from the pancreas is the most prominent defect in CFRD. This has historically been attributed to β-cell destruction that occurs in connection with fibrosis and scarring of the exocrine pancreas [31]. According to this “collateral damage hypothesis” or “bystander hypothesis,” pancreatic duct obstruction from viscous secretions in cystic fibrosis causes tissue autodigestion by the trapped digestive enzymes, and the progressive destruction and fibrosis of the exocrine pancreas eventually damage the adjacent endocrine cells as a result of spillover of inflammation and compromised blood supply. Exocrine pancreatic insufficiency, or the requirement for pancreatic enzyme supplementation, is a readily discernible consequence of pancreatic destruction and is thus often assessed in clinical studies of CFRD.
Only ~3% of patients with cystic fibrosis are born with exocrine pancreatic insufficiency, whereas most are pancreatic sufficient at birth with relatively mild lesions, such as dilation of the duct and acinar lumen [35, 36]. By the end of the first year, about 85% of patients with cystic fibrosis will have developed exocrine pancreatic insufficiency [14]. These individuals typically have two CFTR mutations that result in complete lack of protein function. The other 15% possess at least one copy of the CFTR gene with some residual protein function and will remain pancreatic sufficient or develop exocrine pancreatic insufficiency at an older age.
Patients with exocrine pancreatic insufficiency usually have more severe insulin deficiency, as measured by insulin and glucose response to an OGTT and are much more likely to develop CFRD [14, 30]. Studies have consistently shown that even when patients with pancreatic-insufficient cystic fibrosis exhibit normal glucose tolerance on an OGTT, they have lower β-cell secretory capacity and α-cell function than those with pancreatic-sufficient cystic fibrosis or healthy controls [30, 37]. Individuals with more severe neonatal exocrine pancreatic disease in utero, as reflected by reduced levels of circulating immunoreactive trypsinogen at birth, have a higher CFRD risk [38]. These observations support the idea that exocrine pancreatic insufficiency is intimately tied to CFRD pathogenesis. Alternatively, it could be a marker of more severe CFTR genotypes that cause diabetes via unrelated mechanisms.
Other data suggest that mechanisms in addition to islet destruction and fibrosis may be at play in CFRD development. The incidence of CFRD in patients with pancreatic sufficiency is much lower than in individuals with pancreatic insufficiency, but it is still higher than the rate of type 2 diabetes in the general population of comparable age and body habitus [13]. Impaired insulin secretion has been demonstrated in patients with pancreatic-sufficient cystic fibrosis, albeit not as severe as in those with the pancreatic-insufficient type [39]. The onset of exocrine pancreatic insufficiency in cystic fibrosis does not immediately herald diabetes, similar to diabetes secondary to chronic pancreatitis. CFRD generally lags behind exocrine insufficiency by one or two decades, reflecting a very slow decline in β-cell mass and functional capacity. A β-cell compensatory response may potentially underlie this phenomenon, as discussed later [40, 41]. Autopsy studies have found that the degree of β-cell loss associated with CFRD, which is less than 50%, may not be severe enough to cause overt diabetes on its own [42, 43]. Diabetes secondary to pancreatic disorders such as pancreatitis or pancreatic surgery is said to require destruction of 80% of β-cells [44]. Importantly, the extent of islet destruction and fibrosis is not greater in patients with cystic fibrosis with diabetes than in those without [42], reinforcing the notion that structural damage is most likely not the sole driving factor in the pathogenesis of CFRD.
Autopsy studies have shown that CFRD does correlate with the presence of amyloid deposits in islets; amyloid deposits are absent in islets of patients with cystic fibrosis without diabetes [45]. Amyloid may come from amylin, a polypeptide hormone cosecreted with insulin from β-cells, and whether the islet amyloid contributes to the pathology has not been determined. The islet amyloid accumulation is also frequently seen in type 2 diabetes but generally not in type 1 diabetes. Interestingly, analysis of families with cystic fibrosis has shown that a family history of type 2 diabetes is a risk factor for CFRD, and genome-wide association studies have identified several known susceptibility genes for type 2 diabetes, namely TCF7L2, CDKN2A/B, CDKAL1, and IGF2BP2, as modifier genes for CFRD [46]. These observations suggest that the islet dysfunction in CFRD may share some common mechanisms with that in type 2 diabetes. Indeed, impaired first-phase insulin secretion seen in cystic fibrosis is also found in type 2 diabetes [31]. On the other hand, although insulin resistance is the cardinal hallmark of type 2 diabetes, its role in the development of CFRD is not consistent. Euglycemic hyperinsulinemic clamp studies in nondiabetic subjects with cystic fibrosis have reported conflicting data, finding increased, normal, and decreased insulin sensitivity [33, 47, 48]. It has been suggested that the insulin resistance found in CFRD may be a secondary consequence of sustained hyperglycemia instead of a primary defect in insulin sensitivity [42]. Acute infections tend to be more consistently associated with increased insulin resistance in cystic fibrosis [33], presumably because of elevated levels of inflammatory cytokines and stress hormones. In the setting of reduced insulin secretion, changes in insulin resistance may be a major determinant of glucose tolerance [49].
Aside from β-cell loss from pancreatic destruction and modifier genes shared with type 2 diabetes, some investigators have favored the possibility that a cell-autonomous defect within the β-cell itself directly contributes to the disease process [50]. Intrinsic β-cell defects due to CFTR gene mutations could involve mechanisms such as alterations in cellular membrane potential affecting the insulin secretory apparatus [51], accumulation of misfolded CFTR protein aggregates producing endoplasmic reticulum stress [52], or abnormal reduced glutathione transport with increased oxidative stress [53, 54]. The CFTR protein is primarily expressed in pancreatic ductal cells, whereas its expression in islet cells has been a subject of debate. Recent work using confocal immunolocalization reported the presence of CFTR protein in human and mouse pancreatic β-cells and α-cells [55, 56]. Glucose-stimulated insulin secretion from cultured human and mouse β-cells was significantly inhibited by treatment with CFTR antagonists [55]. In human islets and mouse α-cells, CFTR antagonists increased glucagon secretion in the presence of the cyclic adenosine monophosphate activator forskolin [56]. Depletion of CFTR by short hairpin RNA–mediated gene silencing in a mouse β-cell line reduced glucose-stimulated insulin secretion [57]. However, the expression of CFTR in human endocrine cells and the specificity of the CFTR inhibitor were both questioned in another study [58]. In vivo studies in mice have also produced conflicting results. In one study, the ΔF508 mutant mice displayed attenuated membrane potential and insulin secretion in β-cells isolated from young (12- to 14-week-old) animals, which could be rescued by treatment with the corrector drug lumacaftor [51]. Another study found only a mild β-cell secretory defect in 14-week-old ΔF508 mutant mice, which could be accounted for by a reduction in insulin content, and in older mice found increased insulin resistance and decreased β-cell mass to be the main abnormality, without gross pancreatic pathology [59]. Mice may have limited utility as models of CFRD because they do not spontaneously develop diabetes, although mouse models of cystic fibrosis are more susceptible to streptozotocin-induced diabetes [60], indicating a baseline abnormality.
In addition to β-cells, α-cells may potentially contribute to dysglycemia in cystic fibrosis. Impaired suppressibility of glucagon after oral glucose has been described in patients with cystic fibrosis, possibly predisposing to early impairment in glucose tolerance [61]. In several studies, patients with cystic fibrosis with exocrine pancreatic insufficiency showed reduced glucagon response to arginine and to insulin-induced hypoglycemia [30, 37]. Defective glucagon secretion in cystic fibrosis may increase the risk of hypoglycemia, which is seen even in individuals without CFRD [22]. Other studies have reported normal glucagon response to mixed meals in cystic fibrosis [62, 63]. The possibility of an intrinsic α-cell defect has been raised by an observation that treating mouse islets with CFTR inhibitors increases glucagon secretion, perhaps via alteration of the α-cell membrane potential [56]. Another hormonal system that has been implicated in CFRD pathogenesis is the incretin axis. Active glucagon-like peptide-1 (GLP-1) levels in cystic fibrosis are reportedly diminished in some studies [64]. Both GLP-1 and gastric inhibitory polypeptide (GIP-1) responses to a mixed meal were blunted in the first 30 minutes in patients with pancreatic insufficiency compared with patients with pancreatic sufficiency [37]. Whether CFTR is expressed in intestinal enteroendocrine cells and can affect their secretion of GLP-1 or GIP-1 is not known. Treatment of human patients with cystic fibrosis with the CFTR potentiator ivacaftor improved insulin secretion but not incretin secretion [65], suggesting that CFTR may not directly modulate incretin secretion. Lastly, insulin clearance rate is increased in cystic fibrosis from unclear mechanisms [33, 66], perhaps predisposing to insulin insufficiency.
B. Insights From Ferret and Pig Models
The ferret and pig models mirror the human disease more closely, including age-dependent development of diabetes [67]. Newborn CFTR −/− ferrets have relatively mild disease of the exocrine pancreas and primarily display only acinar duct dilation, but most go on to develop severe inflammation and exocrine pancreatic insufficiency in the first months of life [68]. They serve as a useful model for human infants with cystic fibrosis, most of whom have only mild pancreatic lesions with acinar duct dilation at birth and subsequently undergo pancreatic destruction. The CFTR −/− pigs, on the other hand, develop pancreatic inflammation during late gestation and are all born with exocrine pancreatic insufficiency [69]. The pig is therefore suitable for modeling the later or more severe stages of human pancreatic disease in cystic fibrosis. In terms of the islet pathology, CFTR −/− ferrets exhibit abnormal glucose tolerance and decreased first-phase insulin secretion at birth, before exocrine pancreatic insufficiency [68]. CFTR −/− pigs and ΔF508 pigs also show impaired glucose tolerance and insulin secretion defects at birth and subsequently develop spontaneous hyperglycemia without appreciable loss of islet cell mass [69]. These latter observations imply that structural destruction of the endocrine pancreas may not be required for the development of CFRD, although it is certainly expected to increase the odds of overt diabetes by diminishing the β-cell reserve.
C. How New Human and Animal Data Affect Views on CFRD Pathogenesis
Recent human studies support the presence of early abnormalities in glucose metabolism. Infants and young children aged 3 months to 5 years with cystic fibrosis were found to have abnormal glucose tolerance [70]. Insulin levels were not increased or were only modestly increased relative to controls, suggesting an inability to increase insulin secretion to maintain euglycemia after an oral glucose load. This is reminiscent of the findings in newborn ferret and pig models. In other clinical studies, patients with cystic fibrosis who were given the potentiator drug ivacaftor significantly improved first-phase insulin secretion as well as insulin response to oral glucose load, indicating partial reversibility of the secretion defect [65, 71–73]. Such observations could be consistent with, but do not prove, a primary β-cell defect resulting from CFTR mutations because ivacaftor corrects channel defects in other pancreatic cells as well. To exclude influence from non–β-cells, an inducible β-cell–specific CFTR null mouse was generated that exhibited normal β-cell mass, enhanced sensitivity to glucose-stimulated insulin secretion, and evidence of altered endoplasmic reticulum calcium handling [74]. Although the global CFTR knockout mice do not spontaneously develop diabetes with age, they display a higher predisposition to streptozotocin-induced diabetes, and it would be of interest to see whether the β-cell–specific CFTR null mice can replicate that phenotype. Interestingly, the CFTR transcript was detectable only in a minor subpopulation (10% to 20%) of wild-type mouse β-cells by single-cell RNA sequencing [74]. If the CFTR-expressing β-cells possess special pacemaker properties, quantitatively significant β-cell loss may not be necessary for diabetes to develop.
The ferret model resembles early human disease, as noted previously. No CFTR expression was detected in human or ferret endocrine pancreatic cells by single-molecule fluorescent in situ hybridization [58], conflicting with a recent report of protein detection in human β-cells by confocal immunolocalization [55]. However, islets from newborn CFTR null ferrets still exhibited decreased insulin secretion, as did wild-type islets depleted of CFTR protein by short hairpin RNA, and also showed elevated levels of exocrine ductal markers and markers of stellate cell activation [58]. It was suggested that CFTR may control β-cell function indirectly via a paracrine mechanism involving islet-associated nonendocrine cells, such as duct cells or stellate cells. There may be a role for neuropeptides such as calcitonin gene-related peptide in this context [75]. A paracrine mechanism could account for functional defects in the β-cell without the need to invoke cell autonomous effects or extensive islet destruction. Recent work with the ferrets has also demonstrated the occurrence of a transient glycemic crisis early in life, which is accompanied by loss of β-cell mass and pancreatic inflammation and fibrosis. This is followed by a compensatory response, with a doubling of the residual β-cell mass and enhancement of pancreatic insulin, glucagon, and somatostatin gene expression [70]. The islet protective mechanisms in ferrets are concordant with increased pancreatic expression of the adipogenic transcription factor peroxisome proliferator-activated receptor -γ, which may reflect fatty replacement of pancreatic parenchyma but is hypothesized to have a protective role in conjunction with its anti-inflammatory actions [70]. In the pancreas, adipose tissue stem cells have been reported to promote immunomodulatory and β-cell protective effects [76].
It is conceivable that human patients with cystic fibrosis may undergo a similar glycemic crisis early in life in the setting of exocrine pancreatic insufficiency, but compensatory mechanisms allow sufficient functional recovery to delay the onset of CFRD until decades later. Autopsy studies have documented prominent nesidioblastosis along with classic fibrocystic changes of the pancreas in nondiabetic patients with cystic fibrosis in the first decade of life [41].
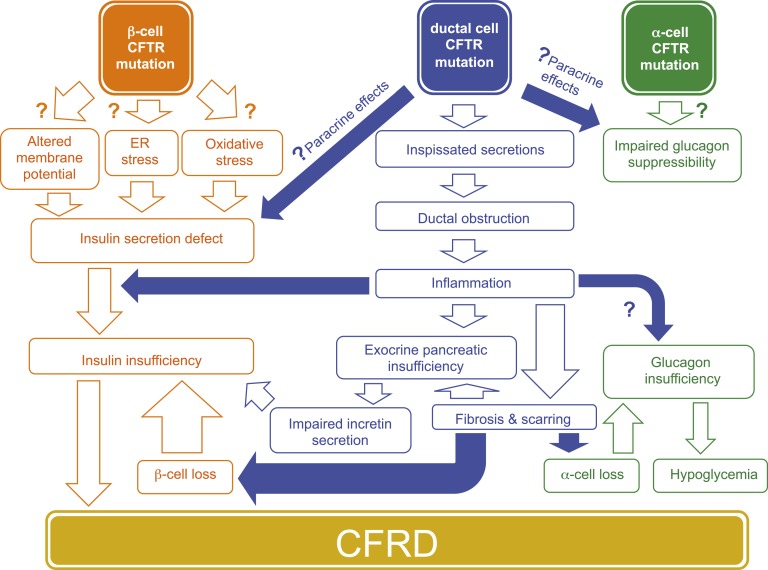
These new findings collectively point to potential contributions from paracrine mechanisms and β-cell compensation (Fig. 1). A role for an intrinsic β-cell defect is supported by experimental studies in human and mouse islets treated with CFTR inhibitors. In ferrets, however, these inhibitors (CFTRinh172) reduced insulin secretion in both wild-type and null islets, suggestive of an off-target effect [58]. It may be informative to perform CFTR expression and inhibition studies using tissues from other CFTR null animal models and patients with CFTR nonsense mutations. The β-cell–specific null mice provide more compelling evidence of an intrinsic abnormality, and a direct connection to CFRD pathogenesis could be more readily established with animal models that spontaneously develop diabetes.
Figure 1.
Possible pathogenetic mechanisms in CFRD. Insulin resistance may also have a role in the setting of infections or glucocorticoid therapy. Modifier genes other than CFTR influence the risk of developing diabetes. ER, endoplasmic reticulum.
3. Advances in CFRD Therapeutic Strategies
A. Insulin Therapy
Currently, insulin remains the only therapy for CFRD officially recommended by the Cystic Fibrosis Foundation and American Diabetes Association [22]. There have been no conclusive data on which insulin regimen is optimal. Insulin glargine, NPH insulin, and fast-acting prandial insulin have all been reported to produce benefits [21, 24, 26]. Recent treatment advances for insulin therapy include the widespread availability of insulin pumps, which lessen variability of administration and increase flexibility with regard to complex meal schedules [77]. With insulin pumps, multiple insulin boluses can be given without separate injections. This can be advantageous to patients with cystic fibrosis who are often encouraged to have frequent meals and snacks throughout the day to maintain high-calorie intake and healthy weight. Insulin pump therapy can be labor-intensive, however, and can add to the treatment burden of patients with cystic fibrosis, which may limit its uptake by patients [78].
The growing use of continuous monitoring with glucose sensors has allowed an unprecedented level of access to patient glucose data for clinical decision-making and can help reduce the occurrence of large glycemic excursions. Continuous glucose monitoring may also have a role in screening for cystic fibrosis–related diabetes [29]. Efforts to integrate the insulin pump and continuous glucose sensor in creating closed-loop systems or an “artificial pancreas” are well under way [79–81], with the promise of ultimately yielding a fully automated glucose sensing and insulin delivery system that requires minimal patient intervention.
B. Oral Antidiabetic Drugs and Incretin Mimetics
The number of therapeutic options for treating diabetes has increased substantially in recent years, but most of them have not been formally evaluated in patients with CFRD. Because the degree of insulin resistance in CFRD is variable, insulin-sensitizing agents such as metformin or thiazolidinediones are not routinely used for treatment of CFRD. α-Glucosidase inhibitors, which prevent digestion of carbohydrates in the intestine, or sodium-glucose co-transporter 2 inhibitors, which block reabsorption of glucose in the kidney, may not be indicated in patients with cystic fibrosis, who are usually trying to increase calorie intake and gain weight. Insulin secretagogues have been used on occasion at some centers, but insufficient evidence exists to establish a clear role in CFRD management. Observational studies have found no difference between insulin and sulfonylureas in clinical outcome [82, 83]. Randomized studies compared prandial insulin with repaglinide [21, 84] and concluded that insulin produced a more favorable response, especially in terms of sustained improvement of body mass index.
There may be some rationale for using drugs that target the incretin axis [85], because impaired postprandial incretin hormone secretion has been reported in patients with cystic fibrosis without CFRD [37, 62]. GLP-1 and GIP-1 secretion was lower in patients with exocrine pancreatic-insufficient cystic fibrosis than in patients with pancreatic sufficiency even when they had a normal OGTT result [37]. The available incretin modulators are GLP-1 receptor agonists, which act as incretin mimetics, and dipeptidyl peptidase-4 inhibitors, which increase GLP-1 levels indirectly by interfering with its degradation. Potential drawbacks of these drugs include GLP-1 receptor agonist–mediated reduction of appetite and weight loss and some concerns over proliferative effects in the pancreas. Of note, fat malabsorption can be a major contributor to postprandial hyperglycemia because digestion of fat serves to slow down gastric emptying and absorption of carbohydrates. Treatment of exocrine pancreatic insufficiency with pancreatic enzyme supplements has the benefit of reducing postprandial hyperglycemia, slowing gastric emptying, and augmenting incretin hormone secretion [62, 86].
C. Islet/Pancreas Transplantation and Stem Cell–Derived β-Cell Replacement Therapy
For patients with CFRD and end-stage lung disease, pancreatic islet transplantation after lung transplantation and combined lung and islet transplantation have been suggested as options [87, 88]. Combined lung-pancreas transplantation is also possible but carries a higher risk of complications than lung-islet transplantation [89]. Total pancreatectomy and islet autotransplantation have been performed in some patients with chronic painful pancreatitis, including those with pancreatic-sufficient cystic fibrosis [90], but a similar strategy has not been attempted in CFRD, largely because of lower numbers of viable islets. An innovative approach to overcome the limited supply of donor cells is to implant islet progenitor cells or β-cells that are differentiated in culture from human embryonic stem cell lines [91]. The implanted cells are encapsulated in semipermeable barrier material, obviating the need for immunosuppression. One such system has entered phase 1/2 clinical trials in patients with type 1 diabetes.
D. CFTR Modulator Therapy
Ivacaftor and lumacaftor are recently approved small-molecule drugs that target the defective CFTR proteins associated with specific genotype classes [10]. Ivacaftor is a potentiator that enhances CFTR channel activity and is effective for mutant proteins with abnormal channel gating (class III mutations) or conductance (class IV mutations). Lumacaftor is a corrector that facilitates CFTR protein folding and maturation and primarily targets patients with class II mutations such as ΔF508. Small pilot studies and case reports have demonstrated that ivacaftor therapy ameliorated impaired insulin secretion in patients with cystic fibrosis who carry common gating mutations, in some cases resulting in resolution of CFRD [65, 71–73]. Longer-term studies involving larger numbers of patients are needed to confirm the proposed benefit of these drugs in treating CFRD. Agents that promote the read-through of premature termination codons have the potential for suppressing certain class I mutations. Structural modification of aminoglycoside antibiotics, which display such read-through activity at sufficiently high concentrations, is being carried out to reduce the cytotoxicity that currently prevents routine use for this purpose [92]. Ataluren, an unrelated small molecule approved in Europe to treat nonsense mutations in the dystrophin gene, has not shown efficacy in cystic fibrosis in recent phase 3 trials [93].
E. Gene Replacement and DNA/RNA Editing Therapy
Because cystic fibrosis is a monogenic, autosomal recessive disorder, direct replacement or repair of the CFTR gene offers a potentially curative strategy [94]. For treatment of pulmonary disease, aerosolized administration of viral or nonviral vectors has been used to deliver the wild-type CFTR gene to the airways; although no CFTR gene therapy has received regulatory approval thus far, some encouraging results have been reported from clinical trials [95]. Systemic administration of pancreas-tropic serotypes of viral vectors, such as adeno-associated virus serotype 8, can in principle target pancreatic disease in cystic fibrosis, and if desired, a tissue-selective promoter can be used to drive transgene expression with viral or nonviral vectors [96]. Genome editing technologies such as CRISPR (clustered regularly interspaced short palindromic repeats), TALENs (transcription activator-like effector nucleases), and ZFNs (zinc finger nucleases) now permit the site-specific correction of CFTR gene mutations at their endogenous chromosomal loci for restoration of function. Culture studies have demonstrated successful repair of a mutant CFTR gene in this fashion [97, 98]. In vivo β-cell–targeted gene editing systems are under development and may someday achieve sufficient efficacy and safety to attempt human trials [99]. RNA editing strategies are another possible alternative. RNA oligonucleotides have been used to rescue deleted segments of CFTR messenger RNA in cultured ΔF508 cells [100]. Delivery of the whole CFTR messenger RNA is also being investigated as an option and has the appeal of being transient, less disruptive to the cell, and easily produced in large quantities [101, 102].
4. Future Perspectives
An interplay of β-cell intrinsic and extrinsic factors is thought to underlie the development of CFRD. At present, it seems reasonable to postulate that a mild β-cell defect, either intrinsic or secondary to altered paracrine communication between β-cells and surrounding islet and nonislet cells, becomes increasingly exacerbated as pancreatic inflammation disrupts the local environment, which may be further compounded by gradual loss of β-cell mass from cell death or dedifferentiation. However, the relative importance of each process is still unknown. Elucidating the early events in CFRD pathogenesis is clearly key, but because it is difficult in practice to recruit large numbers of infants with cystic fibrosis or obtain pancreatic tissues, pig and ferret models may provide critical clues. Although studying the natural course of CFRD in these animals is valuable, a precise dissection of the mechanism may require experimental genetics, such as tissue-selective inactivation of the CFTR gene in β-cells, α-cells, or ductal cells using promoters selective for each cell type [103]. CRISPR-mediated genome editing in nontraditional models such as pigs and ferrets has been demonstrated [104, 105], and both genomes have been sequenced. In principle, cell type–specific CFTR inactivation in these animals can address the question of whether intrinsic β-cell disease or exocrine pancreatic disease alone can produce CFRD or perhaps both are required.
The early occurrence of insulin secretion abnormalities in humans, pigs, and ferrets does not necessarily establish a key role for an intrinsic β-cell defect because concurrent disease processes are ongoing in the exocrine pancreas and in non–β-cells in the islets, which can alter paracrine signals. The sparing of islets in cystic fibrosis pigs suggests that structural destruction of islets is not necessary and perhaps local inflammation is enough to produce functional β-cell insufficiency. In humans, moreover, overt CFRD is not typically diagnosed until years or decades after exocrine pancreatic insufficiency, implying that additional processes, some of which conceivably overlap with type 2 diabetes, must take place. Alternatively, the CFRD diagnosis may be delayed because of a compensatory response and islet remodeling early in life that produce partial recovery of β-cell function and mass. It may be possible to detect such transient glycemic crises with more studies of glycemic patterns in very young children.
The CFTR modulator therapy provides a means to assess the reversibility of insulin-secretion defects in human subjects carrying appropriate CFTR genotypes amenable to such therapy, as well as in certain animal models such as the ΔF508 pig. Because recent clinical data indicate the presence of insulin-secretory defects in young infants, it is desirable to explore the potential benefits of such therapy at the earliest feasible age, with long-term follow-up to determine efficacy in preventing or delaying CFRD [43]. Older patients with established CFRD may also show clinical improvement and even resolution of CFRD, according to case reports [73]. At clinicaltrials.gov, there is an ongoing trial with pediatric patients and a planned trial with adult patients. The pig model may have utility for correlating the islet physiology to the drug response at different stages of pancreatic disease.
5. Search Strategies
A PubMed search was performed for articles published between 2010 and 2017 using the terms “cystic fibrosis” AND “diabetes.” Articles were selected for further evaluation on the basis of whether the abstract conveyed direct relevance to CFRD pathophysiology or therapy. For each article selected, the cited references were screened, and those judged relevant to the subject of the present review were included. Abstracts pertaining to diabetes from the 30th annual North American Cystic Fibrosis Conference in October 2016 [Pediatr Pulmonol. 2016;51(S45)] were also considered for inclusion.
Acknowledgments
Financial Support: J.C.Y. is supported by a grant from the Cystic Fibrosis Foundation.
Acknowledgments
Disclosure Summary: The author has nothing to disclose.
Footnotes
- CFRD
- cystic fibrosis–related diabetes
- CFTR
- cystic fibrosis transmembrane conductance regulator
- GIP-1
- gastric inhibitory polypeptide
- GLP-1
- glucagon-like peptide-1
- OGTT
- oral glucose tolerance test.
References and Notes
- 1.MacNeill SJ. Epidemiology of cystic fibrosis In: Bush A, Bilton D, Hodson M., eds. Hodson and Geddes’ cystic fibrosis. 4th ed.Boca Raton, FL: CRC Press; 2015:18–40. [Google Scholar]
- 2.Cystic Fibrosis Foundation. Available at: https://cftr2.org. Accessed 20 August 2017.
- 3.De Boeck K, Zolin A, Cuppens H, Olesen HV, Viviani L. The relative frequency of CFTR mutation classes in European patients with cystic fibrosis. J Cyst Fibros. 2014;13(4):403–409. [DOI] [PubMed] [Google Scholar]
- 4.Haardt M, Benharouga M, Lechardeur D, Kartner N, Lukacs GL. C-terminal truncations destabilize the cystic fibrosis transmembrane conductance regulator without impairing its biogenesis: a novel class of mutation. J Biol Chem. 1999;274(31):21873–21877. [DOI] [PubMed] [Google Scholar]
- 5.Wilschanski M, Novak I. The cystic fibrosis of exocrine pancreas. Cold Spring Harb Perspect Med. 2013;3(5):a009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishiguro H, Steward MC, Naruse S, Ko SB, Goto H, Case RM, Kondo T, Yamamoto A. CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol. 2009;133(3):315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ooi CY, Durie PR. Cystic fibrosis transmembrane conductance regulator (CFTR) gene mutations in pancreatitis. J Cyst Fibros. 2012;11(5):355–362. [DOI] [PubMed] [Google Scholar]
- 8.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352(19):1992–2001. [DOI] [PubMed] [Google Scholar]
- 9.MacKenzie T, Gifford AH, Sabadosa KA, Quinton HB, Knapp EA, Goss CH, Marshall BC. Longevity of patients with cystic fibrosis in 2000 to 2010 and beyond: survival analysis of the Cystic Fibrosis Foundation patient registry. Ann Intern Med. 2014;161(4):233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowe SM, Verkman AS. Cystic fibrosis transmembrane regulator correctors and potentiators. Cold Spring Harb Perspect Med. 2013;3(7):a009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32(9):1626–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. J Pediatr. 2005;146(5):681–687. [DOI] [PubMed] [Google Scholar]
- 13.Lewis C, Blackman SM, Nelson A, Oberdorfer E, Wells D, Dunitz J, Thomas W, Moran A. Diabetes-related mortality in adults with cystic fibrosis: role of genotype and sex. Am J Respir Crit Care Med. 2015;191(2):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackman SM, Tangpricha V. Endocrine disorders in cystic fibrosis. Pediatr Clin North Am. 2016;63(4):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler AI, Shine BS, Chamnan P, Haworth CS, Bilton D. Genetic determinants and epidemiology of cystic fibrosis-related diabetes: results from a British cohort of children and adults. Diabetes Care. 2008;31(9):1789–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162(3):891–895. [DOI] [PubMed] [Google Scholar]
- 17.Schaedel C, de Monestrol I, Hjelte L, Johannesson M, Kornfält R, Lindblad A, Strandvik B, Wahlgren L, Holmberg L. Predictors of deterioration of lung function in cystic fibrosis. Pediatr Pulmonol. 2002;33(6):483–491. [DOI] [PubMed] [Google Scholar]
- 18.Leclercq A, Gauthier B, Rosner V, Weiss L, Moreau F, Constantinescu AA, Kessler R, Kessler L. Early assessment of glucose abnormalities during continuous glucose monitoring associated with lung function impairment in cystic fibrosis patients. J Cyst Fibros. 2014;13(4):478–484. [DOI] [PubMed] [Google Scholar]
- 19.Hameed S, Morton JR, Jaffé A, Field PI, Belessis Y, Yoong T, Katz T, Verge CF. Early glucose abnormalities in cystic fibrosis are preceded by poor weight gain. Diabetes Care. 2010;33(2):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavie M, Fisher D, Vilozni D, Forschmidt R, Sarouk I, Kanety H, Hemi R, Efrati O, Modan-Moses D. Glucose intolerance in cystic fibrosis as a determinant of pulmonary function and clinical status. Diabetes Res Clin Pract. 2015;110(3):276–284. [DOI] [PubMed] [Google Scholar]
- 21.Moran A, Pekow P, Grover P, Zorn M, Slovis B, Pilewski J, Tullis E, Liou TG, Allen H; Cystic Fibrosis Related Diabetes Therapy Study Group . Insulin therapy to improve BMI in cystic fibrosis-related diabetes without fasting hyperglycemia: results of the cystic fibrosis related diabetes therapy trial. Diabetes Care. 2009;32(10):1783–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, Robinson KA, Sabadosa KA, Stecenko A, Slovis B; CFRD Guidelines Committee . Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nousia-Arvanitakis S, Galli-Tsinopoulou A, Karamouzis M. Insulin improves clinical status of patients with cystic-fibrosis-related diabetes mellitus. Acta Paediatr. 2001;90(5):515–519. [PubMed] [Google Scholar]
- 24.Dobson L, Hattersley AT, Tiley S, Elworthy S, Oades PJ, Sheldon CD. Clinical improvement in cystic fibrosis with early insulin treatment. Arch Dis Child. 2002;87(5):430–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolon MA, Benali K, Munck A, Navarro J, Clement A, Tubiana-Rufi N, Czernichow P, Polak M. Cystic fibrosis-related diabetes mellitus: clinical impact of prediabetes and effects of insulin therapy. Acta Paediatr. 2001;90(8):860–867. [PubMed] [Google Scholar]
- 26.Mozzillo E, Franzese A, Valerio G, Sepe A, De Simone I, Mazzarella G, Ferri P, Raia V. One-year glargine treatment can improve the course of lung disease in children and adolescents with cystic fibrosis and early glucose derangements. Pediatr Diabetes. 2009;10(3):162–167. [DOI] [PubMed] [Google Scholar]
- 27.Smyth AR, Bell SC, Bojcin S, Bryon M, Duff A, Flume P, Kashirskaya N, Munck A, Ratjen F, Schwarzenberg SJ, Sermet-Gaudelus I, Southern KW, Taccetti G, Ullrich G, Wolfe S; European Cystic Fibrosis Society . European Cystic Fibrosis Society standards of care: best practice guidelines. J Cyst Fibros. 2014;13(Suppl 1):S23–S42. [DOI] [PubMed] [Google Scholar]
- 28.Boudreau V, Coriati A, Desjardins K, Rabasa-Lhoret R. Glycated hemoglobin cannot yet be proposed as a screening tool for cystic fibrosis related diabetes. J Cyst Fibros. 2016;15(2):258–260. [DOI] [PubMed] [Google Scholar]
- 29.Boudreau V, Reynaud Q, Dubois CL, Coriati A, Desjardins K, Durieu I, Rabasa-Lhoret R. Screening for cystic fibrosis-related diabetes: matching pathophysiology and addressing current challenges. Can J Diabetes. 2016;40(5):466–470. [DOI] [PubMed] [Google Scholar]
- 30.Moran A, Diem P, Klein DJ, Levitt MD, Robertson RP. Pancreatic endocrine function in cystic fibrosis. J Pediatr. 1991;118(5):715–723. [DOI] [PubMed] [Google Scholar]
- 31.Kelly A, Moran A. Update on cystic fibrosis-related diabetes. J Cyst Fibros. 2013;12(4):318–331. [DOI] [PubMed] [Google Scholar]
- 32.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Suppl 1):S11–S24. [DOI] [PubMed] [Google Scholar]
- 33.Lanng S, Thorsteinsson B, Røder ME, Nerup J, Koch C. Insulin sensitivity and insulin clearance in cystic fibrosis patients with normal and diabetic glucose tolerance. Clin Endocrinol (Oxf). 1994;41(2):217–223. [DOI] [PubMed] [Google Scholar]
- 34.Konrad K, Scheuing N, Badenhoop K, Borkenstein MH, Gohlke B, Schöfl C, Seufert J, Thon A, Holl RW. Cystic fibrosis-related diabetes compared with type 1 and type 2 diabetes in adults. Diabetes Metab Res Rev. 2013;29(7):568–575 [DOI] [PubMed] [Google Scholar]
- 35.Oppenheimer EH, Esterly JR. Cystic fibrosis of the pancreas: morphologic findings in infants with and without diagnostic pancreatic lesions. Arch Pathol. 1973;96(3):149–154. [PubMed] [Google Scholar]
- 36.Sturgess JM. Structural and developmental abnormalities of the exocrine pancreas in cystic fibrosis. J Pediatr Gastroenterol Nutr. 1984;3(Suppl 1):S55–S66. [DOI] [PubMed] [Google Scholar]
- 37.Sheikh S, Gudipaty L, De Leon DD, Hadjiliadis D, Kubrak C, Rosenfeld NK, Nyirjesy SC, Peleckis AJ, Malik S, Stefanovski D, Cuchel M, Rubenstein RC, Kelly A, Rickels MR. Reduced β-cell secretory capacity in pancreatic-insufficient but not pancreatic-sufficient, cystic fibrosis despite normal glucose tolerance. Diabetes. 2017;66(1):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soave D, Miller MR, Keenan K, Li W, Gong J, Ip W, Accurso F, Sun L, Rommens JM, Sontag M, Durie PR, Strug LJ. Evidence for a causal relationship between early exocrine pancreatic disease and cystic fibrosis-related diabetes: a Mendelian randomization study. Diabetes. 2014;63(6):2114–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wooldridge JL, Szczesniak RD, Fenchel MC, Elder DA. Insulin secretion abnormalities in exocrine pancreatic sufficient cystic fibrosis patients. J Cyst Fibros. 2015;14(6):792–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi Y, Sun X, Gibson-Corley K, Xie W, Liang B, He N, Tyler SR, Uc A, Philipson LH, Wang K, Hara M, Ode KL, Norris AW, Engelhardt JF. A transient metabolic recovery from early life glucose intolerance in cystic fibrosis ferrets occurs during pancreatic remodeling. Endocrinology. 2016;157(5):1852–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iannucci A, Mukai K, Johnson D, Burke B. Endocrine pancreas in cystic fibrosis: an immunohistochemical study. Hum Pathol. 1984;15(3):278–284. [DOI] [PubMed] [Google Scholar]
- 42.Moran A, Doherty L, Wang X, Thomas W. Abnormal glucose metabolism in cystic fibrosis. J Pediatr. 1998;133(1):10–17. [DOI] [PubMed] [Google Scholar]
- 43.Ode KL, Moran A. New insights into cystic fibrosis-related diabetes in children. Lancet Diabetes Endocrinol. 2013;1(1):52–58. [DOI] [PubMed] [Google Scholar]
- 44.Czakó L, Hegyi P, Rakonczay Z Jr, Wittmann T, Otsuki M. Interactions between the endocrine and exocrine pancreas and their clinical relevance. Pancreatology. 2009;9(4):351–359. [DOI] [PubMed] [Google Scholar]
- 45.Couce M, O’Brien TD, Moran A, Roche PC, Butler PC. Diabetes mellitus in cystic fibrosis is characterized by islet amyloidosis. J Clin Endocrinol Metab. 1996;81(3):1267–1272. [DOI] [PubMed] [Google Scholar]
- 46.Blackman SM, Commander CW, Watson C, Arcara KM, Strug LJ, Stonebraker JR, Wright FA, Rommens JM, Sun L, Pace RG, Norris SA, Durie PR, Drumm ML, Knowles MR, Cutting GR. Genetic modifiers of cystic fibrosis-related diabetes. Diabetes. 2013;62(10):3627–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moran A, Pyzdrowski KL, Weinreb J, Kahn BB, Smith SA, Adams KS, Seaquist ER. Insulin sensitivity in cystic fibrosis. Diabetes. 1994;43(8):1020–1026. [DOI] [PubMed] [Google Scholar]
- 48.Austin A, Kalhan SC, Orenstein D, Nixon P, Arslanian S. Roles of insulin resistance and beta-cell dysfunction in the pathogenesis of glucose intolerance in cystic fibrosis. J Clin Endocrinol Metab. 1994;79(1):80–85 [DOI] [PubMed] [Google Scholar]
- 49.Boudreau V, Coriati A, Hammana I, Ziai S, Desjardins K, Berthiaume Y, Rabasa-Lhoret R. Variation of glucose tolerance in adult patients with cystic fibrosis: what is the potential contribution of insulin sensitivity? J Cyst Fibros. 2016;15(6):839–845. [DOI] [PubMed] [Google Scholar]
- 50.Manderson Koivula FN, McClenaghan NH, Harper AGS, Kelly C. Islet-intrinsic effects of CFTR mutation. Diabetologia. 2016;59(7):1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo JH, Chen H, Ruan YC, Zhang XL, Zhang XH, Fok KL, Tsang LL, Yu MK, Huang WQ, Sun X, Chung YW, Jiang X, Sohma Y, Chan HC. Glucose-induced electrical activities and insulin secretion in pancreatic islet β-cells are modulated by CFTR. Nat Commun. 2014;5:4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ali BR. Is cystic fibrosis-related diabetes an apoptotic consequence of ER stress in pancreatic cells? Med Hypotheses. 2009;72(1):55–57. [DOI] [PubMed] [Google Scholar]
- 53.Hudson VM. Rethinking cystic fibrosis pathology: the critical role of abnormal reduced glutathione (GSH) transport caused by CFTR mutation. Free Radic Biol Med. 2001;30(12):1440–1461. [DOI] [PubMed] [Google Scholar]
- 54.Ntimbane T, Comte B, Mailhot G, Berthiaume Y, Poitout V, Prentki M, Rabasa-Lhoret R, Levy E. Cystic fibrosis-related diabetes: from CFTR dysfunction to oxidative stress. Clin Biochem Rev. 2009;30(4):153–177. [PMC free article] [PubMed] [Google Scholar]
- 55.Edlund A, Esguerra JL, Wendt A, Flodström-Tullberg M, Eliasson L. CFTR and Anoctamin 1 (ANO1) contribute to cAMP amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med. 2014;12(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edlund A, Pedersen MG, Lindqvist A, Wierup N, Flodström-Tullberg M, Eliasson L. CFTR is involved in the regulation of glucagon secretion in human and rodent alpha cells. Sci Rep. 2017;7(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ntimbane T, Mailhot G, Spahis S, Rabasa-Lhoret R, Kleme ML, Melloul D, Brochiero E, Berthiaume Y, Levy E. CFTR silencing in pancreatic β-cells reveals a functional impact on glucose-stimulated insulin secretion and oxidative stress response. Am J Physiol Endocrinol Metab. 2016;310(3):E200–E212. [DOI] [PubMed] [Google Scholar]
- 58.Sun X, Yi Y, Xie W, Liang B, Winter MC, He N, Yu Y, Uc A, Norris A, Engelhardt JF. CFTR influences beta-cell function and insulin secretion through non-cell autonomous exocrine-derived factors. Pediatr Pulmonol. 2016;51(S45):437 Abstract 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fontés G, Ghislain J, Benterki I, Zarrouki B, Trudel D, Berthiaume Y, Poitout V. The ΔF508 mutation in the cystic fibrosis transmembrane conductance regulator is associated with progressive insulin resistance and decreased functional β-cell mass in mice. Diabetes. 2015;64(12):4112–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stalvey MS, Muller C, Schatz DA, Wasserfall CH, Campbell-Thompson ML, Theriaque DW, Flotte TR, Atkinson MA. Cystic fibrosis transmembrane conductance regulator deficiency exacerbates islet cell dysfunction after β-cell injury. Diabetes. 2006;55(7):1939–1945. [DOI] [PubMed] [Google Scholar]
- 61.Lanng S, Thorsteinsson B, Røder ME, Orskov C, Holst JJ, Nerup J, Koch C. Pancreas and gut hormone responses to oral glucose and intravenous glucagon in cystic fibrosis patients with normal, impaired, and diabetic glucose tolerance. Acta Endocrinol (Copenh). 1993;128(3):207–214. [DOI] [PubMed] [Google Scholar]
- 62.Kuo P, Stevens JE, Russo A, Maddox A, Wishart JM, Jones KL, Greville H, Hetzel D, Chapman I, Horowitz M, Rayner CK. Gastric emptying, incretin hormone secretion, and postprandial glycemia in cystic fibrosis: effects of pancreatic enzyme supplementation. J Clin Endocrinol Metab. 2011;96(5):E851–E855. [DOI] [PubMed] [Google Scholar]
- 63.Goldsweig BK, Sherr JL, Egan ME, Koff J, Weinzimer SA. The importance of alpha: the role of glucagon in cystic fibrosis-related diabetes. Pediatr Pulmonol. 2016;51(S45):445 Abstract 656.26418834 [Google Scholar]
- 64.Hillman M, Eriksson L, Mared L, Helgesson K, Landin-Olsson M. Reduced levels of active GLP-1 in patients with cystic fibrosis with and without diabetes mellitus. J Cyst Fibros. 2012;11(2):144–149. [DOI] [PubMed] [Google Scholar]
- 65.Kelly A, Sheikh S, DeLeon D, Camburn D, Peleckis A, Rickels M, Rubenstein RC. β-cell secretory capacity improves in cystic fibrosis with ivacaftor therapy. Pediatr Pulmonol. 2016;51(S45):438 Abstract 638. [Google Scholar]
- 66.Ahmad T, Nelson R, Taylor R. Insulin sensitivity and metabolic clearance rate of insulin in cystic fibrosis. Metabolism. 1994;43(2):163–167. [DOI] [PubMed] [Google Scholar]
- 67.Gibson-Corley KN, Meyerholz DK, Engelhardt JF. Pancreatic pathophysiology in cystic fibrosis. J Pathol. 2016;238(2):311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Olivier AK, Yi Y, Sun X, Sui H, Liang B, Hu S, Xie W, Fisher JT, Keiser NW, Lei D, Zhou W, Yan Z, Li G, Evans TI, Meyerholz DK, Wang K, Stewart ZA, Norris AW, Engelhardt JF. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest. 2012;122(10):3755–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uc A, Olivier AK, Griffin MA, Meyerholz DK, Yao J, Abu-El-Haija M, Buchanan KM, Vanegas Calderón OG, Abu-El-Haija M, Pezzulo AA, Reznikov LR, Hoegger MJ, Rector MV, Ostedgaard LS, Taft PJ, Gansemer ND, Ludwig PS, Hornick EE, Stoltz DA, Ode KL, Welsh MJ, Engelhardt JF, Norris AW. Glycaemic regulation and insulin secretion are abnormal in cystic fibrosis pigs despite sparing of islet cell mass. Clin Sci (Lond). 2015;128(2):131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yi Y, Norris AW, Wang K, Sun X, Uc A, Moran A, Engelhardt JF, Ode KL. Abnormal glucose tolerance in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 2016;194(8):974–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsabari R, Elyashar HI, Cymberknowh MC, Breuer O, Armoni S, Livnat G, Kerem E, Zangen DH. CFTR potentiator therapy ameliorates impaired insulin secretion in CF patients with a gating mutation. J Cyst Fibros. 2016;15(3):e25–e27. [DOI] [PubMed] [Google Scholar]
- 72.Bellin MD, Laguna T, Leschyshyn J, Regelmann W, Dunitz J, Billings J, Moran A. Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: a small pilot study. Pediatr Diabetes. 2013;14(6):417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hayes D Jr, McCoy KS, Sheikh SI. Resolution of cystic fibrosis-related diabetes with ivacaftor therapy. Am J Respir Crit Care Med. 2014;190(5):590–591. [DOI] [PubMed] [Google Scholar]
- 74.Li C, Chen P, Lam C, Ackermann AM, Rankin MM, Kaestner KH, Stanley CA, Kushner JA, Kelly A, Rubenstein RC. Knockout and overexpression of CFTR in beta-cells reveal its function in regulation of insulin secretion. Pediatr Pulmonol. 2016;51(S45):437 Abstract 637. [Google Scholar]
- 75.Rotti P, Xie W, Sun X, Yi Y, Zhang Y, Winter MC, Liang B, He N, Engelhardt JF. Role of bradykinin and CGRP in pancreatic endocrine dysregulation in cystic fibrosis. Pediatr Pulmonol. 2016;51(S45):441 Abstract 645. [Google Scholar]
- 76.Rahavi H, Hashemi SM, Soleimani M, Mohammadi J, Tajk N. Adipose tissue-derived mesenchymal stem cells exert in vitro immunomodulatory and beta-cell protective functions in streptozotocin-induced diabetic mice model. J Diabetes Res. 2015;2015:878535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hardin DS, Rice J, Rice M, Rosenblatt R. Use of the insulin pump in treat cystic fibrosis related diabetes. J Cyst Fibros. 2009;8(3):174–178. [DOI] [PubMed] [Google Scholar]
- 78.Scheuing N, Badenhoop K, Borkenstein M, Konrad K, Lilienthal E, Laubner K, Naeke A, Rami-Merhar B, Thon A, Wiemann D, Holl RW; German/Austrian Diabetes Prospective Documentation Initiative . Why is insulin pump treatment rarely used in adolescents and young adults with cystic fibrosis-related diabetes? Pediatr Diabetes. 2015;16(1):10–15. [DOI] [PubMed] [Google Scholar]
- 79.Haidar A, Legault L, Messier V, Mitre TM, Leroux C, Rabasa-Lhoret R. Comparison of dual-hormone artificial pancreas, single-hormone artificial pancreas, and conventional insulin pump therapy for glycaemic control in patients with type 1 diabetes: an open-label randomised controlled crossover trial. Lancet Diabetes Endocrinol. 2015;3(1):17–26. [DOI] [PubMed] [Google Scholar]
- 80.Tauschmann M, Allen JM, Wilinska ME, Thabit H, Stewart Z, Cheng P, Kollman C, Acerini CL, Dunger DB, Hovorka R. Day-and-night hybrid closed-loop insulin delivery in adolescents with type 1 diabetes: a free-living, randomized crossover trial. Diabetes Care. 2016;39(7):1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El-Khatib FH, Balliro C, Hillard MA, Magyar KL, Ekhlaspour L, Sinha M, Mondesir D, Esmaeili A, Hartigan C, Thompson MJ, Malkani S, Lock JP, Harlan DM, Clinton P, Frank E, Wilson DM, DeSalvo D, Norlander L, Ly T, Buckingham BA, Diner J, Dezube M, Young LA, Goley A, Kirkman MS, Buse JB, Zheng H, Selagamsetty RR, Damiano ER, Russell SJ. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet. 2017;389(10067):369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosenecker J, Eichler I, Bärmeier H, von der Hardt H. Diabetes mellitus and cystic fibrosis: comparison of clinical parameters in patients treated with insulin versus oral glucose-lowering agents. Pediatr Pulmonol. 2001;32(5):351–355. [DOI] [PubMed] [Google Scholar]
- 83.Onady GM, Langdon LJ. Insulin versus oral agents in the management of cystic fibrosis related diabetes: a case based study. BMC Endocr Disord. 2006;6(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moran A, Phillips J, Milla C. Insulin and glucose excursion following premeal insulin lispro or repaglinide in cystic fibrosis-related diabetes. Diabetes Care. 2001;24(10):1706–1710. [DOI] [PubMed] [Google Scholar]
- 85.Perano S, Rayner CK, Couper J, Martin J, Horowitz M. Cystic fibrosis related diabetes: a new perspective on the optimal management of postprandial glycemia. J Diabetes Complications. 2014;28(6):904–911. [DOI] [PubMed] [Google Scholar]
- 86.Perano SJ, Couper JJ, Horowitz M, Martin AJ, Kritas S, Sullivan T, Rayner CK. Pancreatic enzyme supplementation improves the incretin hormone response and attenuates postprandial glycemia in adolescents with cystic fibrosis: a randomized crossover trial. J Clin Endocrinol Metab. 2014;99(7):2486–2493. [DOI] [PubMed] [Google Scholar]
- 87.Spijker HS, Wolffenbuttel BH, van der Bij W, Engelse MA, Rabelink TJ, de Koning EJ. Islet-after-lung transplantation in a patient with cystic fibrosis-related diabetes. Diabetes Care. 2014;37(7):e159–e160. [DOI] [PubMed] [Google Scholar]
- 88.Kessler L, Bakopoulou S, Kessler R, Massard G, Santelmo N, Greget M, Moreau F, Helms O, Bosco D, Gasche-Soccal P, Morel P, Wolf P, Berney T; GRAGIL group . Combined pancreatic islet-lung transplantation: a novel approach to the treatment of end-stage cystic fibrosis. Am J Transplant. 2010;10(7):1707–1712. [DOI] [PubMed] [Google Scholar]
- 89.Usatin DJ, Perito ER, Posselt AM, Rosenthal P. Under utilization of pancreas transplants in cystic fibrosis recipients in the United Network Organ Sharing (UNOS) data 1987-2014. Am J Transplant. 2016;16(5):1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chinnakotla S, Radosevich DM, Dunn TB, Bellin MD, Freeman ML, Schwarzenberg SJ, Balamurugan AN, Wilhelm J, Bland B, Vickers SM, Beilman GJ, Sutherland DER, Pruett TL. Long-term outcomes of total pancreatectomy and islet auto transplantation for hereditary/genetic pancreatitis. J Am Coll Surg. 2014;218(4):530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, Kelly JR, Haakmeester C, Srijemac R, Wilson AZ, Kerr J, Frazier MA, Kroon EJ, D’Amour KA. Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem Cells Transl Med. 2015;4(10):1214–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xue X, Mutyam V, Tang L, Biswas S, Du M, Jackson LA, Dai Y, Belakhov V, Shalev M, Chen F, Schacht J, Bridges RJ, Baasov T, Hong J, Bedwell DM, Rowe SM. Synthetic aminoglycosides efficiently suppress cystic fibrosis transmembrane conductance regulator nonsense mutations and are enhanced by ivacaftor. Am J Respir Cell Mol Biol. 2014;50(4):805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aslam AA, Higgins C, Sinha IP, Southern KW. Ataluren and similar compounds (specific therapies for premature termination codon class I mutations) for cystic fibrosis. Cochrane Database Syst Rev. 2017;1:CD012040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Villate-Beitia I, Zarate J, Puras G, Pedraz JL. Gene delivery to the lungs: pulmonary gene therapy for cystic fibrosis. Drug Dev Ind Pharm. 2017;43(7):1071–1081. [DOI] [PubMed] [Google Scholar]
- 95.Alton EWFW, Armstrong DK, Ashby D, Bayfield KJ, Bilton D, Bloomfield EV, Boyd AC, Brand J, Buchan R, Calcedo R, Carvelli P, Chan M, Cheng SH, Collie DDS, Cunningham S, Davidson HE, Davies G, Davies JC, Davies LA, Dewar MH, Doherty A, Donovan J, Dwyer NS, Elgmati HI, Featherstone RF, Gavino J, Gea-Sorli S, Geddes DM, Gibson JSR, Gill DR, Greening AP, Griesenbach U, Hansell DM, Harman K, Higgins TE, Hodges SL, Hyde SC, Hyndman L, Innes JA, Jacob J, Jones N, Keogh BF, Limberis MP, Lloyd-Evans P, Maclean AW, Manvell MC, McCormick D, McGovern M, McLachlan G, Meng C, Montero MA, Milligan H, Moyce LJ, Murray GD, Nicholson AG, Osadolor T, Parra-Leiton J, Porteous DJ, Pringle IA, Punch EK, Pytel KM, Quittner AL, Rivellini G, Saunders CJ, Scheule RK, Sheard S, Simmonds NJ, Smith K, Smith SN, Soussi N, Soussi S, Spearing EJ, Stevenson BJ, Sumner-Jones SG, Turkkila M, Ureta RP, Waller MD, Wasowicz MY, Wilson JM, Wolstenholme-Hogg P; UK Cystic Fibrosis Gene Therapy Consortium . Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015;3(9):684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Manzoor F, Johnson MC, Li C, Samulski RJ, Wang B, Tisch R. β-cell-specific IL-35 therapy suppresses ongoing autoimmune diabetes in NOD mice. Eur J Immunol. 2017;47(1):144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, Sasaki N, Boymans S, Cuppen E, van der Ent CK, Nieuwenhuis EE, Beekman JM, Clevers H. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13(6):653–658. [DOI] [PubMed] [Google Scholar]
- 98.Crane AM, Kramer P, Bui JH, Chung WJ, Li XS, Gonzalez-Garay ML, Hawkins F, Liao W, Mora D, Choi S, Wang J, Sun HC, Paschon DE, Guschin DY, Gregory PD, Kotton DN, Holmes MC, Sorscher EJ, Davis BR. Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Reports. 2015;4(4):569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morsy SG, Tonne J, Zhu Y, Belmonte P, Ikeda Y. In vivo beta-cell-targeted gene editing by AAV vectors. Mol Ther. 2016;24(Suppl 1):S217 Abstract 543. [Google Scholar]
- 100.Zamecnik PC, Raychowdhury MK, Tabatadze DR, Cantiello HF. Reversal of cystic fibrosis phenotype in a cultured Δ508 cystic fibrosis transmembrane conductance regulator cell line by oligonucleotide insertion. Proc Natl Acad Sci USA. 2004;101(21):8150–8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bangel-Ruland N, Tomczak K, Fernández Fernández E, Leier G, Leciejewski B, Rudolph C, Rosenecker J, Weber WM. Cystic fibrosis transmembrane conductance regulator-mRNA delivery: a novel alternative for cystic fibrosis gene therapy. J Gene Med. 2013;15(11-12):414–426. [DOI] [PubMed] [Google Scholar]
- 102.Antony JS, Dewerth A, Haque A, Handgretinger R, Kormann MS. Modified mRNA as a new therapeutic option for pediatric respiratory diseases and hemoglobinopathies. Mol Cell Pediatr. 2015;2(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Magnuson MA, Osipovich AB. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab. 2013;18(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Niu D, Wei HJ, Lin L, George H, Wang T, Lee IH, Zhao HY, Wang Y, Kan Y, Shrock E, Lesha E, Wang G, Luo Y, Qing Y, Jiao D, Zhao H, Zhou X, Wang S, Wei H, Güell M, Church GM, Yang L. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science. 2017;357(6357):1303–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kou Z, Wu Q, Kou X, Yin C, Wang H, Zuo Z, Zhuo Y, Chen A, Gao S, Wang X. CRISPR/Cas9-mediated genome engineering of the ferret. Cell Res. 2015;25(12):1372–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]