Abstract
Context:
Specific plasma amino acid (AA) profiles including elevated postabsorptive branched-chain amino acids (BCAAs) have been associated with insulin resistance (IR), mostly estimated by homeostatic model assessment. This study assessed the associations of postabsorptive AAs with IR directly measured by insulin-mediated glucose disposal and determined the quantitative value of AAs and conventional IR predictors.
Design:
Fifty-one healthy, 31 overweight or obese (Ow/Ob), and 52 men and women with type 2 diabetes (T2D) were studied retrospectively. The main outcome measures were the glucose disposal (M/I) index (using 3-[3H]-glucose) during a hyperinsulinemic–euglycemic clamp and whole-body protein turnover (using 1-[13C]-leucine).
Results:
Compared with healthy participants, M/I was lower in Ow/Ob participants and lowest in those with T2D. BCAAs, glutamate, and lysine were higher in the Ow/Ob and T2D groups than in healthy participants; glycine and threonine were lower. Most AAs were higher in men. Principal component analysis identified component 1 (C1: BCAAs, methionine) and C3 (glycine, threonine, serine). Glutamate, C1, ornithine, lysine, methionine, and tyrosine correlated negatively with M/I; C3 and glycine correlated positively. Waist circumference and sex strongly influenced AA–IR relationships; only glutamate correlated after these factors were controlled for. From regression analysis, waist circumference, fasting glucose, insulin, and free fatty acids (FFAs) negatively predicted 64% of the M/I variance; glutamate added 2% more. In nondiabetic participants, IR was predicted by waist circumference, insulin, and FFAs, without contribution from AAs.
Conclusion:
Several postabsorptive AAs correlated with IR but added limited predictive value to conventional markers because levels were determined largely by abdominal adiposity. Data suggest a sex-specific regulation of AA metabolism by excess adiposity, particularly the BCAAs, warranting investigation.
Keywords: amino acids, branched-chain amino acids, insulin resistance, obesity, type 2 diabetes, sex differences
Elevated plasma BCAAs and glutamate correlated with clamp-measured IR but did not add predictive value to conventional markers (i.e., fasting insulin and glucose or abdominal adiposity).
The study of the relationship between plasma amino acids (AAs) and insulin resistance (IR) has recently undergone a renaissance. Elevated AAs in obesity, particularly the branched-chain amino acids (BCAAs) and aromatic AAs phenylalanine and tyrosine, and decreased glycine were reported in 1969. Similar plasma AA suppression occurred despite exaggerated insulin responses to oral glucose in obese compared with healthy participants [1]. This finding pointed to altered insulin regulation of AA metabolism in obesity. Contemporary metabolomic studies consistently found a relationship between elevated BCAAs or aromatic and other AAs and IR, determined mostly by the homeostatic model assessment (HOMA-IR) [2–4], a surrogate, static measure with limitations compared with the gold standard hyperinsulinemic, euglycemic clamp [5]. One clamp study reported glycine having a positive correlation and leucine (Leu)/isoleucine having a negative correlation with insulin sensitivity measured by glucose disposal [6]. Based on the insulin suppression test, elevated AAs were positively associated with steady-state plasma glucose [7]. Isoleucine, Leu, tyrosine, and glutamate showed the strongest correlations; glycine was negatively associated.
It has been proposed that elevated plasma BCAA and AA profiles may be early predictors of type 2 diabetes (T2D) development [8, 9]. However, determination of AAs is more complex and expensive than that of insulin and glucose, and circulating levels are influenced by sex [7, 10], body mass index (BMI) [6], and other potential factors, including protein turnover. We have used the hyperinsulinemic, euglycemic, isoaminoacidemic clamp with isotopic tracers in men and women grouped as healthy, overweight or obese (Ow/Ob), or with T2D. We sought to identify determinants of elevated postabsorptive plasma BCAA concentrations, explore the cross-sectional relationships between postabsorptive AAs and IR by clamp-induced glucose disposal and suppression of production respectively, and determine their independent predictive value on clamp glucose disposal in addition to the conventional predictors (i.e., adiposity, hyperinsulinemia, and hyperglycemia) and assess potential sex differences. Given that both glucose and protein metabolism IR occur concurrently [11], we also examined the relationship of AAs to IR of protein anabolism.
1. Methods
A. Participants
This retrospective analysis includes data from 134 participants (71 men, 63 women, 98% Caucasian) in published studies [11–18]. All studies were approved by the Ethics Review Board of the McGill University Health Centre, and participants provided written informed consent. Participants with hepatic, hematologic, renal, pulmonary, malignant, thyroid, or cardiovascular diseases and smokers were excluded; participants had stable weights (±3 kg/6 mo) and normal dietary habits, and only participants with T2D took antidiabetic, lipid-lowering, or antihypertensive agents. All were admitted 3 to 7 days before the clamp study and consumed an isocaloric, protein-controlled diet [1.7 to 1.9 g protein/kg fat-free mass (FFM)/d and 1.2 g/kg FFM/d in 18 participants with T2D]. Body composition was assessed by bioelectrical impedance analysis (RJL-101A Systems). Premenopausal women were studied during the follicular phase.
B. Hyperinsulinemic, Euglycemic, Isoaminoacidemic Clamp
After an overnight fast, 3-[3H]-glucose and L-[1-13C]-leucine were infused for 6 hours to measure whole-body glucose rate of appearance (Ra) and rate of disposal (Rd) and protein kinetics, postabsorptively and then during the clamp [12]. Human insulin (Humulin R; Eli Lilly) was infused (40 mU/m2/min, representing 0.95 to 1.2 mU/kg FFM/min) [19], yielding physiological postprandial steady-state serum concentrations (560 ± 10 pmol/L). Feedback-controlled 20% glucose was infused to maintain concentrations at 5.5 (euglycemic) or 8.0 mmol/L (isoglycemic, in n = 24 participants with T2D). A solution of AAs (10% TrophAmine®, B. Braun Medical Inc.) was infused to maintain total BCAAs at each participant’s postabsorptive concentrations, measured during the steady state before clamp. The sum of BCAAs was determined by a rapid enzymatic fluorometric assay, every 5 to 10 minutes, as previously described [12], and successfully maintained by feedback control of the infusion rate. Postabsorptive and clamp BCAAs were as follows: in men, healthy, 412 ± 48 and 416 ± 55 μmol/L; Ow/Ob, 444 ± 55 and 441 ± 52 μmol/L; T2D, 446 ± 60 and 456 ± 60 μmol/L; and in women, healthy, 358 ± 45 and 362 ± 53 μmol/L; Ow/Ob: 375 ± 49 and 382 ± 49 μmol/L; T2D, 397 ± 42 and 406 ± 44 μmol/L (all P = nonsignificant, by paired t tests). These and individual AAs have been reported in respective previous articles [12, 13].
Indirect calorimetry was performed to determine resting energy expenditure and respiratory quotient (RQ) (Deltatrac®, Sensor Medics).
C. Calculations
Kinetics were calculated during the last 30 minutes of each steady state. Glucose Ra was calculated from plasma 3-[3H]-glucose specific activity and Rd as the sum of endogenous glucose Ra and infused glucose [20]. The glucose disposal (M/I) index was calculated as ∆glucose Rd divided by clamp serum insulin, ×1000 [19]. Hepatic IR index is the suppression of glucose Ra/serum insulin; less negative values reflect greater hepatic IR. HOMA-IR was calculated as in [21].
L-[1-13C]-leucine kinetics were calculated with plasma [1-13C]-α-ketoisocaproic acid enrichment (reciprocal model) [22]. Fasted Leu turnover = Ra (Leu Ra, protein degradation) = nonoxidative Rd (Leu nonoxidative Rd, protein synthesis) + Leu oxidation. Clamp steady-state Ra + exogenous Leu infusion = nonoxidative Rd + oxidation. Net balance = Leu nonoxidative Rd – Leu Ra, and net anabolism = change in net balance from fasted to clamp state.
D. Analytical Methods
Plasma was kept at −20°C or −80°C in 10% perchloric acid. Twenty individual plasma AAs were measured by fluorometric reverse-phase high-performance liquid chromatography (Beckman Coulter System Gold®) with precolumn derivatization [23]. Calibration and quantitation were by external standard curves. Interassay variability was <5% for all AAs, except ornithine and lysine (<12%).
Plasma glucose–specific activity was determined as described [24]. Isotopic enrichment of plasma (13C)-α-ketoisocaproic acid was determined by gas chromatography mass spectrometry (gas chromatograph model 6890N, mass spectrometer model 5973, Agilent Technologies) and expired air 13CO2 by isotope ratio mass spectrometry (Micromass 903D, Vacuum Generators).
During clamp, glucose was measured by glucose oxidase and total BCAAs by an enzymatic, fluorometric method [12], serum insulin and glucagon by radioimmunoassay (Millipore Corporation), and free fatty acids (FFAs) by colorimetric assay (NEFA C, Wako Chemicals).
E. Statistical Analyses
Results are presented as means ± standard deviations (SDs). Subject characteristics and plasma AAs were analyzed by two-factor analysis of variance (ANOVA), for main effects of sex and group, their interaction, and Bonferroni post hoc test. Skewed data were analyzed by Kruskal–Wallis and Mann–Whitney U tests. Pearson and Spearman coefficients were used to assess bivariate correlations and partial correlations to control for appropriate variables. Principal component analysis was used to identify factor grouping among AAs. Multiple linear regression (hierarchical block entry method) was used to assess independent predictors of BCAA concentrations and identify predictors of IR measured as M/I index. Analyses were performed in SPSS 22.0.
2. Results
A. Participant Characteristics
Nondiabetic participants were categorized as healthy with BMI ≤25 kg/m2 for age <65 years and with BMI ≤29 kg/m2 for ≥65 years (Table 1). Nondiabetic participants with BMIs above these cutoffs were considered overweight or obese (Ow/Ob). Participants with T2D included BMIs 24.6–45.0 kg/m2, with 46 out of 52 Ow/Ob. Age did not differ significantly between groups. Adiposity and FFM were greater in Ow/Ob and T2D than in healthy participants. Men had larger waist circumferences (but not with T2D), greater waist-to-hip ratios, and greater FFMs than women, who had a greater body fat percentages. Ow/Ob and T2D (but not healthy) men showed higher fasting RQ than corresponding groups of women. Fasting insulin was elevated in Ow/Ob and more so in T2D, and glucose was highest in T2D, with corresponding higher HOMA-IR. Serum FFAs were greater in women, without group differences.
Table 1.
Participant Characteristics
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Healthy | Ow/Ob | T2D | Healthy | Ow/Ob | T2D | |
| n | 29 | 11 | 31 | 22 | 20 | 21 |
| Age (y) | 53 ± 20 | 45 ± 18 | 54 ± 6 | 47 ± 24 | 56 ± 19 | 61 ± 10 |
| BMI (kg/m2)a,b,c | 23.3 ± 2.3d | 32.2 ± 3.1e | 31.2 ± 4.0e | 22.3 ± 2.4d | 34.2 ± 4.8e | 34.4 ± 5.4e |
| Waist (cm)a,b,c | 86 ± 8d | 109 ± 7e | 111 ± 12e | 74 ± 9d | 102 ± 12e | 110 ± 11e |
| Waist/hip ratioa,b | 0.91 ± 0.06d | 0.99 ± 0.04e | 1.02 ± 0.06e | 0.78 ± 0.07d | 0.86 ± 0.08e | 0.94 ± 0.09f |
| Body fat (%)a,b,c | 21.3 ± 7.7d | 33.7 ± 4.7e | 30.2 ± 4.8e | 30.9 ± 7.6d | 47.5 ± 4.8e | 46.8 ± 5.4e |
| FFM (kg)a,b | 56.1 ± 6.0d | 66.5 ± 5.5e | 67.0 ± 8.9e | 39.1 ± 3.5d | 46.0 ± 6.6e | 47.2 ± 6.2e |
| FFM index (kg/m2)g,h | 18.3 ± 1.1i | 21.3 ± 1.6j | 21.6 ± 1.9j | 15.3 ± 1.1i | 17.8 ± 1.7j | 18.1 ± 2.0j |
| REE (kcal/d)a,b | 1552 ± 181d | 1916 ± 237e | 1832 ± 194e | 1270 ± 129d | 1506 ± 235e | 1580 ± 265e |
| RQa,b,c | 0.78 ± 0.05 | 0.83 ± 0.06 | 0.78 ± 0.05 | 0.80 ± 0.04d | 0.77 ± 0.04d,e | 0.76 ± 0.03e |
| FFAs (µmol/L)a | 501 ± 186 | 491 ± 67 | 480 ± 154 | 744 ± 194 | 681 ± 174 | 798 ± 228 |
| HOMA-IRh | 2.18 ± 0.64i | 2.78 ± 1.02i | 7.01 ± 2.45j | 1.98 ± 0.48i | 3.70 ± 1.48j | 7.11 ± 3.45k |
| Fasting insulin (pmol/L)h | 58 ± 15i | 72 ± 27i | 120 ± 40j | 53 ± 13i | 95 ± 36j | 126 ± 59j |
| Fasting glucose (mmol/L)h | 5.4 ± 0.4i | 5.3 ± 0.3i | 8.6 ± 2.1j | 5.1 ± 0.3i | 5.4 ± 0.4j | 8.6 ± 2.3k |
| Fasting glucagon (pmol/L)g | 20 ± 7i | 28 ± 11j | 25 ± 10j | 21 ± 7 | 20 ± 5 | 18 ± 5 |
| Clamp IR indexes | ||||||
| Glucose Rd (mg/kg FFM/min)b | 8.7 ± 2.4d | 6.1 ± 1.4e | 4.0 ± 0.9f | 9.1 ± 1.8d | 6.5 ± 1.6e | 4.9 ± 1.0f |
| M/I indexb | 12.5 ± 5.3d | 6.7 ± 3.1e | 1.8 ± 1.9f | 12.6 ± 4.0d | 6.1 ± 2.3e | 2.8 ± 3.0f |
| Hepatic IR indexa,b | −4.6 ± 1.5d | −3.7 ± 1.2d,e | −3.5 ± 1.3e | −5.6 ± 1.4d | −4.3 ± 1.4e | −4.2 ± 1.7e |
Data are means ± SD.
Abbreviation: REE, resting energy expenditure.
M/I index = Δ glucose Rd (mg/kg FFM/min)/clamp plasma insulin (pmol/L) × 1000.
Hepatic IR index = Δ glucose Ra (mg/kg FFM/min)/clamp plasma insulin (pmol/L) × 1000.
Analysis by two-factor ANOVA:
Sex effect.
Group effect.
Sex-by-group interaction, P < 0.05.
Within each sex, groups with different superscripts differ by post hoc Bonferroni test, P < 0.05.
Sex effect by Mann–Whitney U test, P < 0.05.
Group effect by Kruskal–Wallis test in both sexes.
Within each sex, groups with different superscripts differ by post hoc Mann–Whitney U test, P < 0.05.
Glucose Rd and M/I index during the hyperinsulinemic clamp were less in Ow/Ob and even lower in T2D, compared with healthy participants (Table 1). Hepatic IR and protein anabolic indexes were lower in both Ow/Ob and T2D groups, without intergroup differences. Women had less hepatic IR overall.
B. Postabsorptive Plasma Amino Acids
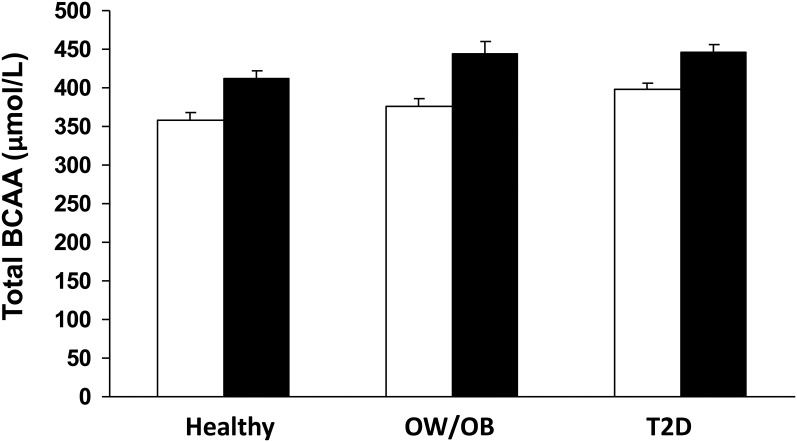
Individual (Table 2) and total BCAAs (Fig. 1) showed an increasing trend from healthy to Ow/Ob to T2D and were higher in men than in women. Lysine and glutamate showed a similar trend. In contrast, threonine, glycine, and taurine were lower in the Ow/Ob and T2D groups. Men had higher total essential AAs, glutamate, and tyrosine.
Table 2.
Postabsorptive Plasma AA Concentrations
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Healthy | Ow/Ob | T2D | Healthy | Ow/Ob | T2D | |
| n | 29 | 11 | 31 | 22 | 20 | 21 |
| AAs | ||||||
| BCAAa,b | 412 ± 48 | 444 ± 55 | 446 ± 60 | 358 ± 45c | 375 ± 49c,d | 398 ± 42d |
| Hisa | 84 ± 14 | 81 ± 12 | 81 ± 14 | 77 ± 12 | 74 ± 13 | 76 ± 14 |
| Ilea,b | 57 ± 8c | 64 ± 12c,d | 68 ± 12d | 52 ± 9 | 53 ± 9 | 56 ± 8 |
| Leua,b | 134 ± 17 | 145 ± 21 | 142 ± 23 | 116 ± 15c | 123 ± 16c,d | 128 ± 15d |
| Lysb | 189 ± 35 | 207 ± 66 | 196 ± 50 | 163 ± 27 | 186 ± 55 | 198 ± 61 |
| Mete | 24 ± 3 | 26 ± 10 | 26 ± 6 | 22 ± 4 | 23 ± 6 | 21 ± 4 |
| Phee | 63 ± 15 | 57 ± 10 | 61 ± 15 | 51 ± 10g | 58 ± 10h | 57 ± 12g,h |
| Thra,b | 124 ± 22c | 106 ± 22c,d | 103 ± 26d | 107 ± 26c | 101 ± 15c,d | 87 ± 23d |
| Trpa | 51 ± 12 | 46 ± 11 | 50 ± 11 | 41 ± 10 | 40 ± 7 | 41 ± 10 |
| Vala,b | 221 ± 25 | 235 ± 24 | 237 ± 30 | 190 ± 24c | 200 ± 28c,d | 214 ± 22d |
| Total EAAsa | 946 ± 96 | 946 ± 141 | 964 ± 145 | 827 ± 91 | 864 ± 110 | 877 ± 126 |
| Ala | 299 ± 74 | 322 ± 49 | 300 ± 45 | 274 ± 52 | 292 ± 58 | 303 ± 63 |
| Arg | 83 ± 22 | 76 ± 16 | 83 ± 17 | 73 ± 18 | 76 ± 16 | 80 ± 18 |
| Asne | 44 ± 8g | 38 ± 8h | 46 ± 8g | 39 ± 10 | 36 ± 6 | 40 ± 7 |
| Cit | 43 ± 11 | 36 ± 9 | 39 ± 10 | 40 ± 11 | 41 ± 11 | 39 ± 11 |
| Gln | 569 ± 94 | 507 ± 60 | 529 ± 62 | 511 ± 103 | 532 ± 61 | 555 ± 114 |
| Glua,b | 66 ± 17c | 92 ± 26d | 104 ± 31d | 61 ± 18c | 73 ± 17c,d | 82 ± 16d |
| Glyb | 211 ± 30c | 186 ± 30c,d | 187 ± 44d | 231 ± 67c | 203 ± 48c,d | 184 ± 48d |
| Orne | 70 ± 20 | 74 ± 32 | 88 ± 39 | 55 ± 18g | 65 ± 24g | 88 ± 35h |
| Ser | 97 ± 20 | 91 ± 20 | 100 ± 18 | 96 ± 18 | 88 ± 20 | 97 ± 29 |
| Tauf | 48 ± 14g | 39 ± 9g,h | 37 ± 10h | 41 ± 7 | 38 ± 8 | 39 ± 13 |
| Tyra | 65 ± 16 | 68 ± 21 | 70 ± 22 | 58 ± 16 | 65 ± 15 | 60 ± 16 |
| Total NEAAs | 1595 ± 143 | 1529 ± 138 | 1578 ± 160 | 1479 ± 176 | 1509 ± 106 | 1566 ± 225 |
| Total AAsa | 2541 ± 221 | 2465 ± 284 | 2540 ± 272 | 2309 ± 261 | 2401 ± 169 | 2443 ± 340 |
Data are means ± SD in μmol/L.
Abbreviations: EAA, essential amino acid; NEAA, nonessential amino acid.
Analysis by two-factor ANOVA:
Sex effect.
Group effect, P < 0.05. No group-by-sex interaction present.
Within each sex, groups with different superscripts differ by post hoc Bonferroni test, P < 0.05.
Sex effect by Mann–Whitney U test, P < 0.05.
Group effect by Kruskal–Wallis test in both sexes.
Within each sex, groups with different superscripts differ by post hoc Mann–Whitney U test, P < 0.05.
Figure 1.
Total postabsorptive BCAAs in healthy, Ow/Ob, and T2D participants, by sex. Means ± standard error of the mean. Two-factor ANOVA: sex effect P < 0.001, group effect P = 0.002, no significant group-by-sex interaction. White bars, women; black bars, men.
Principal component analysis of plasma AAs identified four components, together explaining 71.8% of the cumulative variance. Component 1 (C1) included isoleucine, Leu, valine, and methionine; C2, ornithine and lysine; C3, serine, threonine, and glycine; and C4, alanine, citrulline, and arginine.
C. Determinants of Postabsorptive BCAA Concentrations
In both sexes, all indices of adiposity correlated with BCAAs, as shown in Table 3. In women, all 3 BCAAs correlated, whereas in men only isoleucine did. RQ was negatively related to all 3 BCAAs in women only. Individual BCAAs were positively related to whole-body protein turnover and nonoxidative Rd, in both sexes but more strongly in men. Valine and Leu levels correlated with oxidation rates in men, whereas only valine correlated in women. Fasting insulin correlated with isoleucine in men and valine in women; glucose correlated only with Leu and valine in women. All three BCAAs negatively correlated with the clamp M/I index, reflecting insulin sensitivity.
Table 3.
Correlates of Postabsorptive Plasma BCAA Concentrations
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Ile | Leu | Val | Ile | Leu | Val | |
| BMI | 0.37b | 0.14 | 0.22 | 0.25b | 0.44c | 0.41c |
| FFM | 0.13 | −0.01 | 0.11 | 0.22 | 0.23 | 0.33c |
| Body fat percentage | 0.39c | 0.20 | 0.21 | 0.18 | 0.41c | 0.34c |
| Waist | 0.39c | 0.14 | 0.22 | 0.29b | 0.45c | 0.45c |
| Age | 0.14 | 0.03 | −0.01 | 0.03 | 0.22 | 0.12 |
| RQ | 0.02 | 0.03 | 0.11 | −0.27b | −0.33c | −0.33c |
| Protein turnovera | 0.33c | 0.36c | 0.39c | 0.26b | 0.27b | 0.36c |
| Protein oxidationa | 0.22 | 0.33c | 0.38c | 0.18 | 0.23 | 0.32b |
| Fasting insulin | 0.34c | 0.023 | 0.15 | 0.24 | 0.24 | 0.32b |
| Fasting glucose | 0.13 | 0.034 | 0.11 | 0.18 | 0.30b | 0.37b |
| M/I index | −0.43c | −0.25c | −0.22c | −0.26b | −0.33c | −0.42c |
Pearson r coefficient for total BCAAs.
Whole-body protein turnover and oxidation measured from 13C-leucine flux (described in Methods).
Partial correlation controlled for FFM.
P < 0.05.
cP < 0.01.
The difference in BCAAs between women (377 ± 48 μmol/L) and men (432 ± 56 μmol/L, P < 0.001) (Fig. 1) was examined by analysis of covariance to seek contributing roles for body composition. The sex difference remained highly significant (P = 0.003) when FFM, body fat percentage, and waist circumference were included as covariates, indicating an intrinsic sex difference beyond that conferred by differences in body composition.
Multiple linear regression analysis within each sex identified waist circumference as the single strongest determinant of BCAA levels: in women, F ratio = 15.1, standardized β = 0.45, R2 = 0.20, P < 0.001; in men, F ratio = 4.3, standardized β = 0.24, R2 = 0.09, P = 0.043. Excluded variables from the model were BMI, FFM, body fat percentage, age, and RQ. Hence, sex and waist circumference were considered as confounding variables in subsequent analyses testing the association of AAs with IR.
D. Relationship Between Postabsorptive Plasma AAs and IR
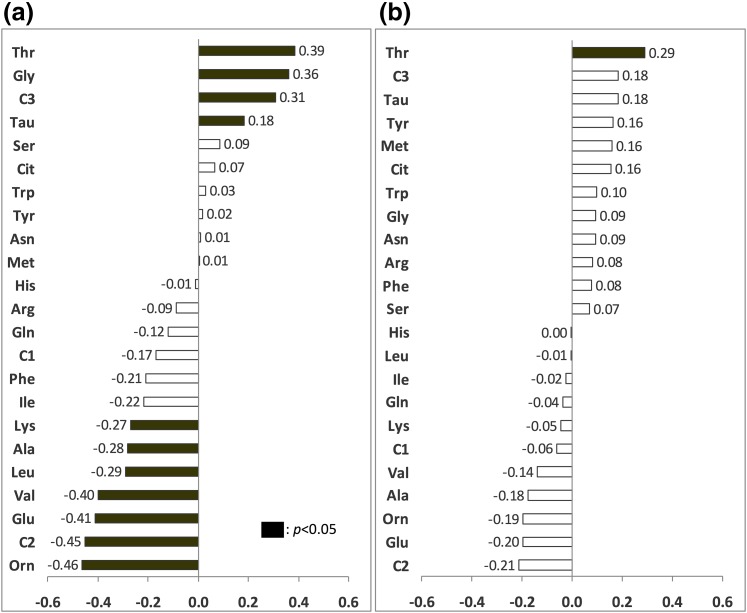
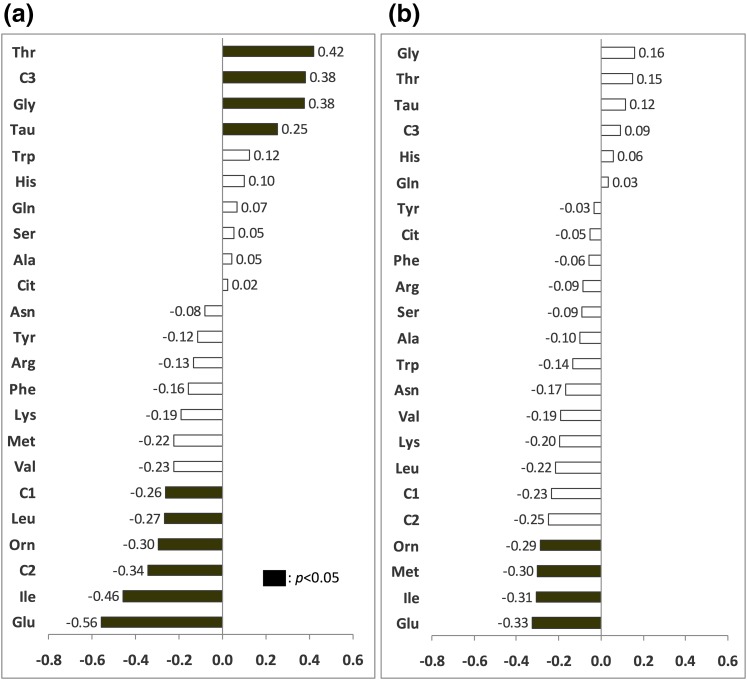
In the whole cohort [Fig. 2(a)], seven individual AAs including BCAAs were negatively associated with M/I index, with glutamate having the strongest relationship. Threonine, C3, glycine, and taurine were positively correlated. After sex and waist circumference were controlled for [Fig. 2(b)], only glutamate, ornithine, isoleucine, C2, and threonine still correlated, reflecting the significant effect of these factors on the AA–IR relationship. In women, only threonine correlated with M/I after waist circumference was controlled for [Fig. 3(a) and 3(b)]. In men, negative correlations (controlled for waist circumference) were between M/I and glutamate, isoleucine, methionine, and ornithine [Fig. 4(a) and 4(b)].
Figure 2.
Correlations between postabsorptive plasma AAs and clamp M/I index. The x-axis indicates Pearson or Spearman correlation coefficients. Black bars are significant correlations, P < 0.05. (a) Zero-order correlations. (b) Partial correlations controlled for sex and waist circumference.
Figure 3.
Correlations between postabsorptive plasma AAs and clamp M/I index, in women. The x-axis indicates Pearson or Spearman correlation coefficients. Black bars are significant correlations, P < 0.05. (a) Zero-order correlations. (b) Partial correlations controlled for waist circumference.
Figure 4.
Correlations between postabsorptive plasma AAs and clamp M/I index, in men. The x-axis indicates Pearson or Spearman correlation coefficients. Black bars are significant correlations, P < 0.05. (a) Zero-order correlations. (b) Partial correlations controlled for waist circumference.
Several AAs correlated with the clamp suppression of glucose Ra (hepatic IR index): glutamate (r = 0.43), phenylalanine (r = 0.33), isoleucine (r = 0.26), tyrosine (r = 0.24), Leu (r = 0.19), and ornithine (r = 0.20), all Ρ s < 0.05. However, none remained correlated after waist circumference was controlled for.
E. Impact of AAs and Conventional Predictors of IR on M/I
Multiple linear regression analysis was performed to determine whether postabsorptive AAs were independent determinants of IR when combined with conventional predictors (Table 4). In the whole cohort, waist circumference alone explained 45% of the variance in M/I index; postabsorptive glucose, insulin, and FFAs together raised the variance to 64%, and glutamate independently contributed an additional 2% to the model. All had a negative predictive value on M/I. In nondiabetic participants, AAs did not enter the regression model, with 48% of the variance explained by waist circumference, insulin, and FFAs. In participants with T2D, glucose and insulin together contributed 40% of the variance in M/I, and isoleucine predicted an additional 8%. Age, BMI, RQ, sex, and C1, C2, and C3 were not independent predictors.
Table 4.
Multiple Linear Regression Analysis Identifying Predictors of IR as Determined by Clamp M/I Index
| Groups | Model | Predictors | R2 | F Ratio | F Change | Std-βa | Sig |
|---|---|---|---|---|---|---|---|
| All subjectsb (n = 129) | 1 | Waist | 0.45 | 100.9 | 100.9c | −0.31 | 0.000 |
| Glucose | −0.33 | 0.000 | |||||
| Insulin | −0.25 | 0.001 | |||||
| 2 | FFA | 0.64 | 53.9 | 21.4c | −0.12 | 0.028 | |
| 3 | Glu | 0.66 | 45.9 | 5.6c | −0.14 | 0.020 | |
| Non-T2D (n = 81) | 1 | Waist | 0.33 | 38.2 | 38.2c | −0.42 | 0.000 |
| Insulin | −0.33 | 0.002 | |||||
| 2 | FFA | 0.48 | 23.7 | 11.4c | −0.20 | 0.031 | |
| T2D (n = 52) | Glucose | −0.59 | 0.000 | ||||
| 1 | Insulin | 0.40 | 13.6 | 13.6c | −0.38 | 0.002 | |
| 2 | Ile | 0.48 | 12.0 | 5.8c | −0.28 | 0.021 |
Dependent variable: clamp M/I index.
Standardized β of each predictive variable in the last inclusive model.
Excluded variables from the last inclusive model were age, BMI, RQ, sex, and C1, C2, and C3.
Significant F change, P < 0.05.
Regression models analyzed separately by sex (not shown) provided similar results to those of combined cohorts, except that the independent value of glutamate (in the whole cohort) and isoleucine (in T2D) on predicting M/I index was not present in either sex. In men with diabetes, C3 independently predicted 13% of the variance in M/I.
F. Impact of Postabsorptive Circulating AAs on Protein Anabolism
Clamp glucose Rd correlated with protein anabolism in response to insulin: r = 0.49, P < 0.001, controlled for FFM. Therefore, waist circumference (r = −0.54), body fat percentage (r = −0.56), fasting insulin (r = −0.45), glucose (r = −0.34), and glutamate (r = −0.26, all Ps < 0.01) were negatively correlated with the protein anabolic response. However, BCAAs and other AAs were not significantly correlated. From multiple regression analysis of the whole cohort, FFM (positively), body fat percentage, fasting insulin, and glucose (negatively) together predicted 49% in the variance of the change in net protein balance (R2 = 0.49, F = 29.4, P < 0.001). In nondiabetic participants, the same variables except fasting glucose predicted 67% of the variance (R2 = 0.67, F = 39.3, P < 0.001). AAs did not have an independent predictive value in any model.
3. Discussion
This study confirmed many components of the AA signature associated with IR reported in the literature. In this cohort of healthy, Ow/Ob nondiabetic, and mostly obese participants with T2D, clamp glucose disposal was negatively related to several AAs, including individual BCAAs, glutamate, lysine, and phenylalanine, and positively correlated with threonine, glycine, and taurine. But only glutamate added a limited independent value (2% of the variance) in predicting low glucose disposal, which was mostly explained (64% of the variance) by excess abdominal adiposity, elevated fasting glucose, insulin, and FFA. In nondiabetic participants, glucose disposal was predicted mainly by waist circumference, insulin, and FFAs, with no added contribution of AAs. Therefore, the potential clinical value of measuring AAs to predict the degree of IR is not supported when simpler measures are available.
The majority of previous studies have used surrogate measures of IR, mostly HOMA [2, 3, 4, 9]. Though practical for large cohort studies, HOMA is a static index, reflecting the homeostasis between fasting glucose production, uptake by all tissues including non–insulin-sensitive tissues, and insulin secretion. Limitations of its use for predicting IR have been raised [5, 21]. The hyperinsulinemic, euglycemic clamp provides a dynamic measure of insulin-induced glucose disposal, mainly into skeletal muscles [19]. Notwithstanding, HOMA-IR correlated well with M/I index (Spearman r = −0.86, P < 0.001) in our whole cohort but less in the nondiabetic subgroup (r = −0.60, P < 0.001).
Our clamp protocol also quantifies glucose production, at physiologically relevant insulin levels. This protocol demonstrated the association of elevated AAs with both peripheral and hepatic IR. Because the magnitude of glucose production suppression is small compared with the stimulation of disposal and is skewed because of complete suppression in insulin-sensitive people, glucose disposal provides a wider range to quantify the degree of IR.
Relationships we identified between elevated AAs and glucose disposal confirm the main findings of the hyperinsulinemic clamp study at supraphysiological insulin in a similar cohort of participants [6] and the insulin suppression test in a nondiabetic cohort [7]. In these and the current study, BCAAs were among the most strongly associated AAs with IR indexes, with isoleucine and Leu more so than valine. Glutamate emerged as the strongest predictor of IR in our study. It was also found highly related in [7] and possibly in [6], though quantified as total with glutamine. Glycine consistently associated positively with insulin sensitivity. We found threonine to have the strongest positive relationship, despite smaller absolute differences due to obesity. Threonine did not correlate with IR in [7] and was not reported in [6]. Apart from this exception, even when we used different analytical methods and dynamic measures of IR, in differing cohorts the same AAs related to IR consistently. These findings also agree with those of cohort studies of mostly young nondiabetic adults, wherein BCAAs and glutamate [4] or glutamate + glutamine [2, 3] most strongly related to HOMA-IR, supporting the robustness of these relationships. Phenylalanine and tyrosine were important AAs in the latter but not highly correlated with glucose disposal in the present or another clamp study [6].
Elevated plasma AAs result from greater rates of appearance into than disposal from the circulation, at least transiently until a new steady state is reached. Both Ra and Rd may be modulated by substrate availability and physiological and hormonal states (reviewed in [25]). Postabsorptive BCAA levels do not appear to be influenced by prior dietary protein intake [3, 9], although large interventional studies are lacking. In the current study, prior protein intakes were controlled, eliminating this possible variable. Because BCAAs are essential, their fasting Ra is mostly determined by protein degradation. Consistently, BCAA levels correlated with Leu Ra but also with nonoxidative Leu Rd and to a lesser extent with Leu oxidation. These observations indicate that elevated BCAAs are related to higher whole-body protein turnover rates, in line with higher protein turnover in obesity and in T2D in most studies [11, 13, 26].
One mechanism common to elevated protein turnover and BCAAs could be the blunted action of insulin in suppressing proteolysis [1]. However, by itself proteolysis does not dictate BCAA levels, given the modest correlations found, and essential AAs were not globally elevated because of increased catabolism in Ow/Ob and T2D. Undoubtedly, other obesity- or T2D-induced alterations in the regulation of BCAA metabolism contribute to their higher concentrations [27, 28]. The first step in BCAA catabolism, transamination, produces branched-chain α-ketoacids (BCKAs) and glutamate from α-ketoglutarate. This probably contributes to the higher glutamate in Ow/Ob and T2D groups, correlating with BCAAs and with IR, as previously reported [7]. Further irreversible oxidative decarboxylation of BCKAs, by the rate-limiting BCKA dehydrogenase complex (BCKDH), results in the formation of acylcarnitines, also shown to relate to IR [2, 29].
The close relationship between excess adiposity and BCAAs points to a role of adipose tissue in BCAA metabolism. Indeed, white adipose tissue enzymatic activity or protein expression of BCKDH components was reduced in obese animals [30, 31] and humans [30–32], and transplantation of adipose tissue into mice defective in peripheral BCAA metabolism reduced BCAA levels [33]. Despite low BCAA uptake by abdominal subcutaneous adipose tissue, decreased messenger RNA transcript abundance for several BCAA catabolic enzymes was found in omental adipose tissue of obese women with the metabolic syndrome compared with those without [30]. Interestingly, we found waist circumference, a surrogate for visceral adiposity, to be the single independent determinant of total BCAAs in both sexes. Alternatively, reduced activity of the hepatic BCKDH was shown to contribute to higher BCAAs, quantitatively more than adipose tissue in rats, and BCKDH protein expression was reduced in obese or diabetic patients [34]. These observations suggest specific roles of visceral fat and liver in BCAA catabolism in addition to those recently reported in muscle [35] and modulation of their circulating levels.
Important sex differences emerged in the current study. Women presented with lower BCAA and glutamate levels than men overall and within each healthy, Ow/Ob, and T2D subgroup. Remarkably, the magnitude of this difference was greater than that conferred by obesity or T2D (Fig. 1). However, women exhibited IR rates similar to those in men, reinforcing the limited and complex role of AAs in predicting IR independently from other factors, especially excess adiposity. Indeed, central adiposity in women was so tightly related to both glucose Rd and AA levels that controlling for waist circumference abolished all correlations between IR and AAs, except for threonine. This result is consistent with findings in >7000 young nondiabetic people, with associations between AAs and HOMA-IR only in women of the highest tertile of waist circumference. In contrast, men showed associations across all tertiles [4].
Although a unifying mechanism underlying sex differences in the regulation of AA levels has yet to be elucidated, sexual dimorphisms in BCAA metabolism have been reported. Hepatic BCKDH protein expression was lower in obese compared with nonobese men only but did not differ in women [34]. In Ow/Ob and T2D women, higher BCAAs correlated with lower RQ, indicating greater fat utilization, perhaps partly contributed by greater oxidation of BCAAs in adipose tissue and conversion of carbon skeletons into newly synthesized FFAs, as supported by markedly higher FFA levels in women. Hormones and adipokines may also be involved. Women display higher adiponectin levels than men [36], which may increase SOGA, a protein that reduces proteolysis and hepatic BCAA release in vitro [37]. Therefore, this impact of sex on AA metabolism, with multiple possible contributing mechanisms, requires further investigation in studies performed concurrently in both sexes.
Limitations of this study include that it is largely correlative and cross-sectional. Thus, any putative causal role in the relationships assessed must be corroborated. Although our total cohort is comparable to that of other published studies, study groups were not equally balanced, limiting statistical power. In particular, the T2D groups had a small sample size and a narrow range of clamp glucose disposal, which limits interpretation of weak correlations with AAs. Though smaller than large cohort studies using surrogate measures of IR, this study directly measured it, and provides simultaneous measurements of both glucose and protein turnover for a more comprehensive understanding of their role in AA levels.
4. Conclusion
We showed correlations between many AAs, including individual BCAAs and glutamate (negative) and threonine and glycine (positive), and IR measured by insulin-stimulated glucose disposal. In men, these associations were stronger and more related to protein turnover, whereas in women associations were almost entirely explained by excess adiposity. These intriguing sex differences warrant further investigation. That the AAs found to correlate most with peripheral and hepatic IR were closely linked with abdominal adiposity suggests that they reflect impaired AA metabolism with excess abdominal adiposity, especially in women. Finally, though associated with IR, AAs contributed limited additional value to conventional markers in predicting the magnitude of IR in both sexes.
Acknowledgments
The authors thank Marie Lamarche, Connie Nardolillo, Ginette Sabourin, Daniel White, Donato Brunetti, Chandra Snarr, Jacqueline MacAdams, Mary Shingler, and Chantal Legaré for their technical assistance. This work was presented in part at the joint International Diabetes Federation/Canadian Diabetes Association, World Diabetes Congress in Vancouver, British Columbia, Canada, 3 December 2015, accessible on the International Diabetes Federation Web site.
Acknowledgments
This work was supported by research grants from the Canadian Institutes of Health Research to R.G. (MOP-77562), to E.B.M. (MOP-62889), to S.C. (MOP-93521), and from the Canadian Diabetes Association to J.A.M. S.C. was a research scholar of the Fonds de Recherche du Québec-Santé.
Author contributions: S.C. designed this study. C.C.L., S.F., and S.C. analyzed data and wrote the manuscript. All authors participated in data collection, interpreting data, and editing the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- amino acid
- ANOVA
- analysis of variance
- BCAA
- branched-chain amino acid
- BCKA
- branched-chain α-ketoacid
- BCKDH
- branched-chain α-ketoacid dehydrogenase complex
- BMI
- body mass index
- C1
- component 1
- FFA
- free fatty acid
- FFM
- fat-free mass
- HOMA-IR
- homeostatic model assessment
- IR
- insulin resistance
- Leu
- leucine
- M/I
- glucose disposal index
- Ow/Ob
- overweight or obese
- Ra
- rate of appearance
- Rd
- rate of disposal
- RQ
- respiratory quotient
- SD
- standard deviation
- T2D
- type 2 diabetes.
References and Notes
- 1.Felig P, Marliss E, Cahill GF Jr. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281(15):811–816. [DOI] [PubMed] [Google Scholar]
- 2.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid–related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, Ilkayeva OR, Wenner BR, Bain JR, Lee JJ, Lim SC, Khoo CM, Shah SH, Newgard CB. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53(4):757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Würtz P, Mäkinen VP, Soininen P, Kangas AJ, Tukiainen T, Kettunen J, Savolainen MJ, Tammelin T, Viikari JS, Rönnemaa T, Kähönen M, Lehtimäki T, Ripatti S, Raitakari OT, Järvelin MR, Ala-Korpela M. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes. 2012;61(6):1372–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisprasert V, Ingram KH, Lopez-Davila MF, Munoz AJ, Garvey WT. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care. 2013;36(4):845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thalacker-Mercer AE, Ingram KH, Guo F, Ilkayeva O, Newgard CB, Garvey WT. BMI, RQ, diabetes, and sex affect the relationships between amino acids and clamp measures of insulin action in humans. Diabetes. 2014;63(2):791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seibert R, Abbasi F, Hantash FM, Caulfield MP, Reaven G, Kim SH. Relationship between insulin resistance and amino acids in women and men. Physiol Rep. 2015;3(5):e12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu FB. Metabolic profiling of diabetes: from black-box epidemiology to systems epidemiology. Clin Chem. 2011;57(9):1224–1226. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel MJ, Batch BC, Svetkey LP, Bain JR, Turer CB, Haynes C, Muehlbauer MJ, Stevens RD, Newgard CB, Shah SH. Race and sex differences in small-molecule metabolites and metabolic hormones in overweight and obese adults. OMICS. 2013;17(12):627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira S, Marliss EB, Morais JA, Chevalier S, Gougeon R. Insulin resistance of protein metabolism in type 2 diabetes. Diabetes. 2008;57(1):56–63. [DOI] [PubMed] [Google Scholar]
- 12.Chevalier S, Gougeon R, Kreisman SH, Cassis C, Morais JA. The hyperinsulinemic amino acid clamp increases whole-body protein synthesis in young subjects. Metabolism. 2004;53(3):388–396. [DOI] [PubMed] [Google Scholar]
- 13.Chevalier S, Marliss EB, Morais JA, Lamarche M, Gougeon R. Whole-body protein anabolic response is resistant to the action of insulin in obese women. Am J Clin Nutr. 2005;82(2):355–365. [DOI] [PubMed] [Google Scholar]
- 14.Chevalier S, Gougeon R, Choong N, Lamarche M, Morais JA. Influence of adiposity in the blunted whole-body protein anabolic response to insulin with aging. J Gerontol A Biol Sci Med Sci. 2006;61(2):156–164. [DOI] [PubMed] [Google Scholar]
- 15.Bassil M, Burgos S, Marliss EB, Morais JA, Chevalier S, Gougeon R. Hyperaminoacidaemia at postprandial levels does not modulate glucose metabolism in type 2 diabetes mellitus. Diabetologia. 2011;54(7):1810–1818. [DOI] [PubMed] [Google Scholar]
- 16.Labonte CC, Chevalier S, Marliss EB, Morais JA, Gougeon R. Effect of 10% dietary protein intake on whole body protein kinetics in type 2 diabetic adults. Clin Nutr. 2015;34(6):1115–1121. [DOI] [PubMed] [Google Scholar]
- 17.Winter A, MacAdams J, Chevalier S. Normal protein anabolic response to hyperaminoacidemia in insulin-resistant patients with lung cancer cachexia. Clin Nutr. 2012;31(5):765–773. [DOI] [PubMed] [Google Scholar]
- 18.Murphy J, Chevalier S, Gougeon R, Goulet ED, Morais JA. Effect of obesity and type 2 diabetes on protein anabolic response to insulin in elderly women. Exp Gerontol. 2015;69:20–26. [DOI] [PubMed] [Google Scholar]
- 19.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. [DOI] [PubMed] [Google Scholar]
- 20.Finegood DT, Bergman RN, Vranic M. Estimation of endogenous glucose production during hyperinsulinemic–euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987;36(8):914–924. [DOI] [PubMed] [Google Scholar]
- 21.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–1495. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-3C]leucine. Am J Physiol. 1980;238(5):E473–E479. [DOI] [PubMed] [Google Scholar]
- 23.Wu G, Knabe DA. Free and protein-bound amino acids in sow’s colostrum and milk. J Nutr. 1994;124(3):415–424. [DOI] [PubMed] [Google Scholar]
- 24.Sigal RJ, Purdon C, Fisher SJ, Halter JB, Vranic M, Marliss EB. Hyperinsulinemia prevents prolonged hyperglycemia after intense exercise in insulin-dependent diabetic subjects. J Clin Endocrinol Metab. 1994;79(4):1049–1057. [DOI] [PubMed] [Google Scholar]
- 25.Tom A, Nair KS. Assessment of branched-chain amino acid status and potential for biomarkers. J Nutr. 2006; 136(1, Suppl)324S–330S. [DOI] [PubMed] [Google Scholar]
- 26.Chevalier S, Burgess SC, Malloy CR, Gougeon R, Marliss EB, Morais JA. The greater contribution of gluconeogenesis to glucose production in obesity is related to increased whole-body protein catabolism. Diabetes. 2006;55(3):675–681. [DOI] [PubMed] [Google Scholar]
- 27.Bassil M, Marliss EB, Morais JA, Pereira S, Chevalier S, Gougeon R. Postprandial hyperaminoacidaemia overcomes insulin resistance of protein anabolism in men with type 2 diabetes. Diabetologia. 2011;54(3):648–656. [DOI] [PubMed] [Google Scholar]
- 28.Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr. 2011;2(6):445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, Tanner CJ, Kuchibhatla M, Houmard JA, Newgard CB, Kraus WE. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32(9):1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH, Karpe F, Humphreys S, Bedinger DH, Dunn TN, Thomas AP, Oort PJ, Kieffer DA, Amin R, Bettaieb A, Haj FG, Permana P, Anthony TG, Adams SH. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab. 2013;304(11):E1175–E1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab. 2007;293(6):E1552–E1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietiläinen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keränen H, Suomalainen A, Götz A, Suortti T, Yki-Järvinen H, Oresic M, Kaprio J, Peltonen L. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med. 2008;5(3):e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem. 2010;285(15):11348–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin AC, Fasshauer M, Filatova N, Grundell LA, Zielinski E, Zhou JY, Scherer T, Lindtner C, White PJ, Lapworth AL, Ilkayeva O, Knippschild U, Wolf AM, Scheja L, Grove KL, Smith RD, Qian WJ, Lynch CJ, Newgard CB, Buettner C. Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Cell Metab. 2014;20(5):898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lerin C, Goldfine AB, Boes T, Liu M, Kasif S, Dreyfuss JM, De Sousa-Coelho AL, Daher G, Manoli I, Sysol JR, Isganaitis E, Jessen N, Goodyear LJ, Beebe K, Gall W, Venditti CP, Patti ME. Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol Metab. 2016;5(10):926–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459–469. [DOI] [PubMed] [Google Scholar]
- 37.Combs TP, Marliss EB. Adiponectin signaling in the liver. Rev Endocr Metab Disord. 2014;15(2):137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]