Abstract
Insulin autoimmune syndrome (IAS), defined as hyperinsulinemic hypoglycemia with high titers of anti-insulin antibodies, is frequently reported in Japanese patients but rarely observed in whites. We report in this study on a 79-year-old white male without diabetes who developed IAS following exposure to clopidogrel, a drug not previously known to cause hypoglycemia. The patient presented with recurrent symptomatic hypoglycemia. During one episode, serum glucose was 45 mg/dL, whereas insulin and C-peptide levels were 40,000 mIU/mL and 40 ng/mL, respectively. Additional studies revealed no intake of insulin or its secretagogues, whereas anti-insulin antibody titer was high (59.3 nmol/L). Although total insulin levels were consistently high, free insulin concentrations (polyethylene glycol precipitation) were appropriate for ambient glycemia. The patient was found to have HLA-DRB1*0404, a feature often reported in Japanese patients with IAS. Three weeks prior to symptom onset, he was started on clopidogrel, a drug that does not have a sulfhydryl group, but its active metabolite does. Clopidogrel was switched to a nonsulfhydryl antiplatelet agent, and glucocorticoid therapy was initiated. Shortly thereafter, the frequency of hypoglycemic episodes decreased, and glucocorticoids were tapered over the ensuing 3 months. No hypoglycemic episodes were noted during 6 months of observation after discontinuing glucocorticoids, whereas the total insulin and anti-insulin antibody levels normalized. The data indicate that IAS should be considered in the differential diagnosis of hyperinsulinemic hypoglycemia in seemingly well individuals, even when no drugs known to cause IAS were used. Clinical suspicion of IAS can avoid expensive imaging and unnecessary surgery in affected patients.
Keywords: clopidogrel, drug-induced hypoglycemia, hypoglycemia, insulin autoimmune syndrome
We report the development of insulin autoimmune syndrome in a Caucasian male after exposure to clopidogrel, a drug not previously known to cause hypoglycemia, and suggest a potential mechanism.
Clinical hypoglycemia is described as a serum glucose concentration low enough to cause symptoms and/or signs, including impairment of brain function [1]. Common causes of hyperinsulinemic hypoglycemia in patients without diabetes include surreptitious use of drugs, insulinoma, extra pancreatic tumors, and autoimmune hypoglycemia. There are two known forms of autoimmune hypoglycemia [2]. The first form is commonly referred to as insulin autoimmune syndrome (IAS), in which antibodies are directed against endogenous insulin molecule. In contrast, the second form of autoimmune hypoglycemia is associated with antibodies directed against the cell surface insulin receptor (type B insulin resistance). Thus, IAS is defined as hyperinsulinemic hypoglycemia associated with high titers of antibodies to the human insulin molecule in the absence of both pathologic abnormalities of the pancreatic islets and prior exposure to exogenous insulin.
IAS, originally reported by Hirata et al. in 1970 from Japan [3], is the third leading cause of spontaneous hypoglycemia in that country [4]. It is still uncommon in Western countries, with only 58 cases of this disorder reported in white patients until 2009 [2]. In Japanese patients, the onset of IAS has been shown to be strongly associated with HLA-DR4 and the use of medications containing a sulfhydryl group [4]. In contrast, IAS in white patients is more frequently associated with autoimmune diseases or plasma cell dyscrasias, with only 47% triggered by exposure to sulfhydryl containing medications [2]. In this study, we describe a white male who developed IAS following exposure to clopidogrel, a drug not previously known to cause hypoglycemia.
1. Case Report
A 79-year-old white male experienced shakiness, sweating, and presyncope 4 hours after having breakfast. When the Emergency Medical Service evaluated him at home shortly thereafter, his blood glucose level was 29 mg/dL. The symptoms reversed promptly by intravenous dextrose infusion. He was seen subsequently at a nearby emergency room, where his serum glucose level was noted to be 145 mg/dL. No other abnormalities were found on his initial blood work or physical exam, and he was discharged home with follow-up with his primary care physician. He continued to have similar episodes of hypoglycemia, both during fasting and the postabsorptive states, which prompted hospital admission for further evaluation. He did not have diabetes and was not taking any drugs known to cause hypoglycemia. There was no family history of diabetes. General and systemic physical examination did not reveal any abnormalities. Results of routine investigations, including hemogram, renal, liver, and thyroid function tests, were normal. Relevant past medical history included coronary artery disease with coronary artery bypass surgery on two occasions ~20 years earlier and recent stent insertion for new angina. On admission to the hospital, his medications included torsemide, fluticasone nasal spray, ProAir HFA (Teva Respiratory, Horsham, PA) as needed, allopurinol, colchicine, vitamin D, doxazosin, metoprolol, Xarelto (Janssen Pharmaceuticals, Titusville, NJ), clopidogrel, and a multivitamin.
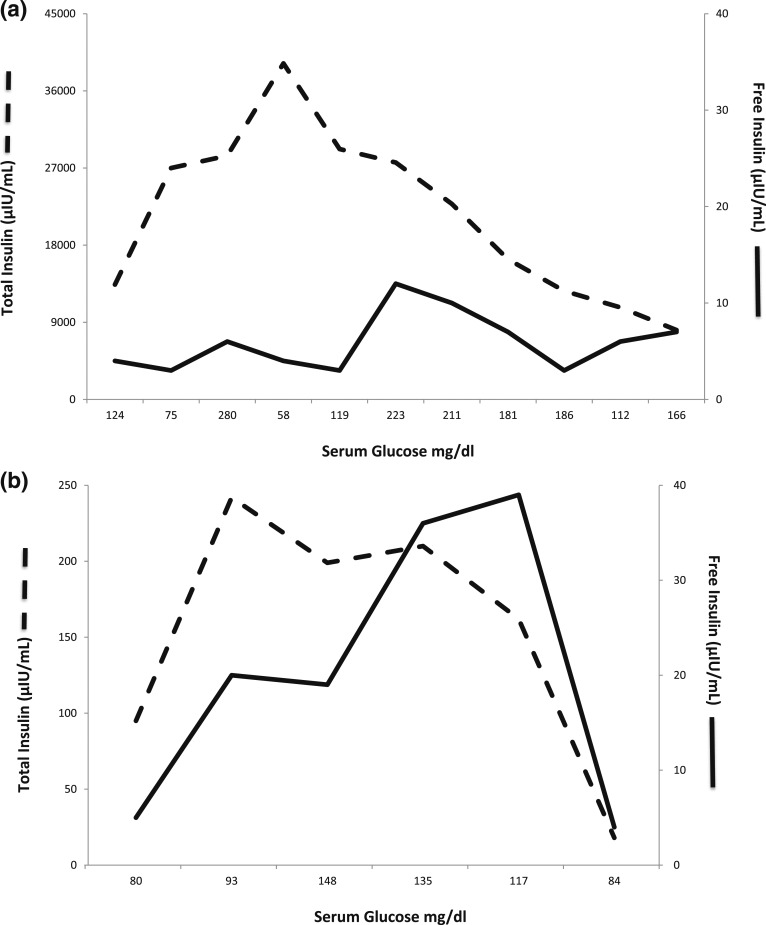
During one of the witnessed symptomatic hypoglycemic episodes during hospitalization, he had serum glucose of 45 mg/dL, whereas the serum insulin and C-peptide levels were quite high at 40,000 mIU/mL and 40 ng/mL, respectively. The serum cortisol response was appropriately elevated at 23.6 µg/dL during the hypoglycemia. Serum proinsulin level was 420 pmol/L (normal fasting value <20 pmol/L). There was no evidence of exogenous insulin administration or its secretagogues, and imaging studies failed to localize an insulinoma. The anti-insulin antibody titer was high at 59.3 nmol/L (0.00–0.02 nmol/L). Although the total serum insulin levels were consistently high, free insulin levels determined by ultrafiltration or polyethylene glycol (PEG) precipitation were appropriate for ambient glycemia (Fig. 1). Thus, the presumptive diagnosis of IAS was made. Samples for HLA studies were collected, which showed HLA-DRB1*0404 (HLA-DR4 subtype).
Figure 1.
(a) Levels of total insulin (dashed line) and free insulin (solid line) plotted against the corresponding serum glucose levels on x-axis prior to treatment. The graph shows that although the total serum insulin levels were consistently high, free insulin levels when determined by PEG precipitation were appropriate for ambient glycemia during the initial treatment phase. (b) Levels of total insulin (dashed line) and free insulin (solid line) plotted against the corresponding serum glucose levels on x-axis during the 3 months of observation after discontinuing glucocorticoids. The graph shows that the total serum insulin levels have greatly decreased (note different scale), although the levels were still higher than free insulin levels. The latest levels are quite comparable with glucose of 84 mg/dL.
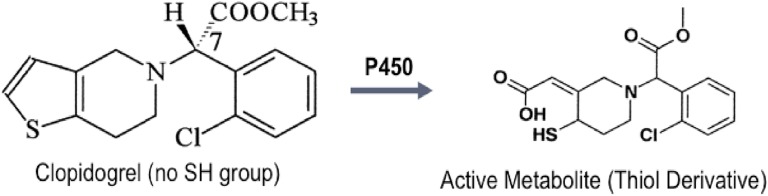
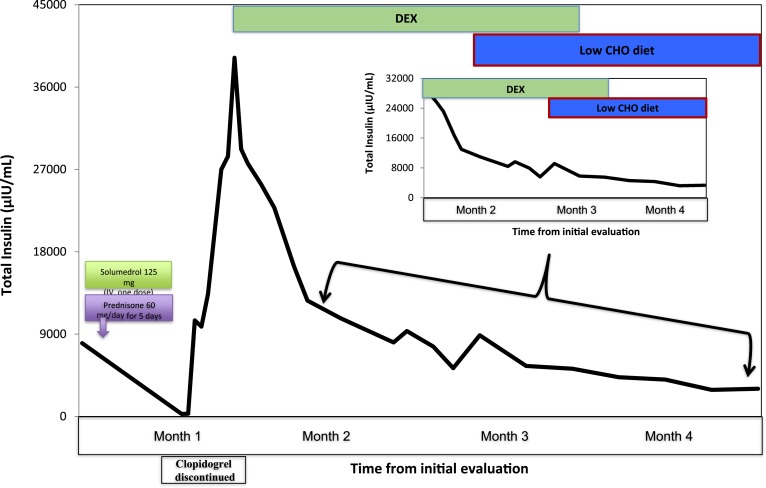
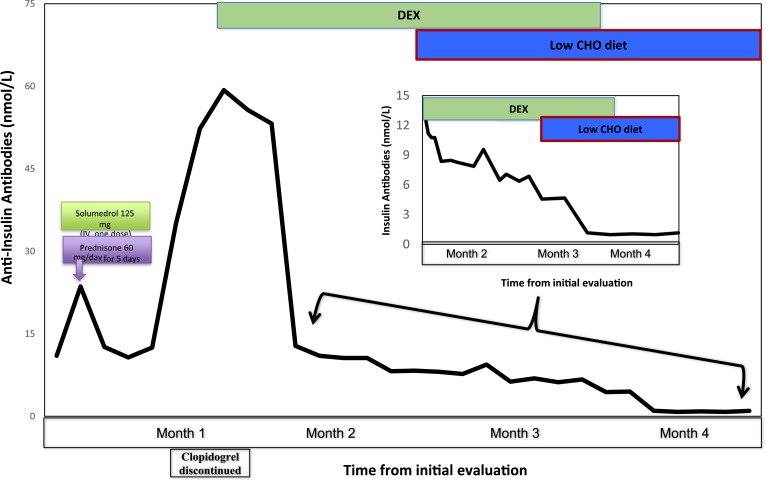
Review of the patient’s drug intake revealed that he was started on clopidogrel 3 weeks prior to onset of his symptoms. Being the latest addition to his medications, clopidogrel was suspected to be related to his hypoglycemia. The chemical structure of clopidogrel does not include a sulfhydryl group, but the active metabolite of the drug does have that chemical feature (Fig. 2). Clopidogrel was discontinued and replaced with another nonsulfhydryl antiplatelet agent. He was initially treated with diazoxide with minimal response to hypoglycemia and subsequently started on glucocorticoid therapy along with small, frequent low-carbohydrate meals. The patient was treated with dexamethasone starting at 2 mg twice daily for 3 days, then 1 mg twice daily for next 2 months, followed by 0.5 mg once daily, which was discontinued over the ensuing 3 weeks. Within 7 days of starting the dexamethasone therapy, the frequency of hypoglycemic episodes decreased. Six months after discontinuing dexamethasone, the patient did not have any further hypoglycemic episodes, whereas his total insulin and insulin antibody levels drastically decreased to 18 μIU/mL and 0.31 nmol/L, respectively, when his serum glucose level was 81 mg/dL (Figs. 3 and 4).
Figure 2.
Chemical structure of clopidogrel is shown not to have a sulfhydryl group. A thiol derivative of the drug is shown after activation by the P450 enzyme system. The active metabolite does contain a sulfhydryl group (arrow).
Figure 3.
Levels of serum total insulin (y-axis) plotted against the time in months (x-axis) during the course of treatment. The initial drop in total insulin levels during the first month was due to methylprednisolone and prednisone given as a treatment of COPD exacerbation. One month after symptom onset, clopidogrel was discontinued, and he was started on dexamethasone (DEX). The graph shows that the total insulin levels decreased to normal levels by the end of 4 months. Inset figure is the magnification of the graph during the last 3 months of treatment and shows a drastic decrease of total insulin levels. CHO, carbohydrate; IV, intravenous.
Figure 4.
Levels of serum anti-insulin antibodies (y-axis) plotted against the time in months (x-axis) during the course of treatment. The initial drop in anti-insulin antibodies levels during the first month was due to methylprednisolone and prednisone given as a treatment of COPD exacerbation. One month after symptom onset, clopidogrel was discontinued, and he was started on dexamethasone (DEX). The graph shows that the anti-insulin antibodies decreased to normal levels by the end of 4 months. Inset figure is the magnification of the graph during the last 3 months of treatment and shows a drastic decrease of the anti-insulin antibodies titers. CHO, carbohydrate; IV, intravenous.
2. Methods
The patient under discussion underwent routine hematologic, immunologic, and biochemical testing during the evaluation. Blood glucose, insulin, C-peptide, proinsulin, and HLA typing were determined at the University Hospitals Cleveland Medical Center laboratories using standard assay systems. Quantitative chemiluminescence immunoassay was used to measure proinsulin, total insulin, and C-peptide. The free insulin was determined in the supernatant after PEG precipitation. Anti-insulin antibodies were determined by radioimmunoassay performed at the Mayo Clinic laboratories (Rochester, MN).
3. Discussion
We present a unique case of IAS in a white patient, with relatively sudden onset of hypoglycemia shortly after exposure to clopidogrel, a drug not previously known to be associated with that symptom. To our knowledge, this is the first case of IAS-induced hypoglycemia reported after exposure to clopidogrel. The case under discussion illustrates that there was more than a temporal association between the onset of symptoms and the introduction of clopidogrel to incriminate that drug as the offending agent. The initial impressive response to glucocorticoids administered to treat chronic obstructive pulmonary disease (COPD) when the patient was still on the drug, followed by the rebound rise in insulin antibody titer after their withdrawal, are supportive of clopidogrel being the offending agent. Similarly, the association of HLA typing and the identification of a sulfhydryl group in the active metabolite of the drug are also supportive of that.
IAS can present with both fasting and postprandial hypoglycemia, and it occurs equally in both sexes. Although the total serum insulin levels are consistently high, the serum-free insulin levels determined by ultrafiltration or PEG precipitation are usually appropriate for the ambient glycemia. C-peptide and proinsulin levels are demonstrated to be high in this setting [2]. Insulin antibodies are typically polyclonal, although monoclonal antibodies have also been described [5]. IAS usually has acute onset, and the total serum insulin levels are high in comparison with those seen in the patients with insulinoma [6]. Thus, the diagnosis of IAS should be seriously considered in a patient with hypoglycemia when the serum levels of insulin, C-peptide, and proinsulin are extremely elevated. In Japanese patients, the development of IAS was shown to be associated with the use of medications containing a sulfhydryl group and HLA-DR4 subtypes [4]. Previously reported whites with IAS were likely to have associated autoimmune diseases or plasma cell dyscrasias, although 47% were triggered by exposure to sulfhydryl-containing medications [2].
IAS usually occurs a few weeks after exposure to medications containing a sulfhydryl group [7]. The proposed mechanism of hypoglycemia in IAS is that sulfhydryl group interacts with the disulfide bond of the insulin molecule, making the latter more immunogenic [8], which, in the appropriate setting, allows the body to make more insulin antibodies. Various explanations have been proposed to account for the hypoglycemia, but the most widely accepted is the buffering effect of insulin antibodies resulting in a binding and release of secreted insulin that is out of synchrony with the prevailing glucose concentration [5]. These insulin antibodies have low affinity and high capacity, which triggers a swift binding of large amounts of insulin during postprandial peaks and then dissociates from the insulin–antibody complex in an unregulated way [8]. This binding reduces the bioavailability of the secreted insulin, resulting in hyperglycemia and further insulin secretion. However, 3 to 5 hours after a meal, due to the low affinity of these insulin antibodies, the insulin–antibody complex shifts toward dissociation, releasing large amounts of biologically active free insulin and triggering episodes of postprandial hypoglycemia [5]. Dozio et al. [9] studied the pattern of insulin biodistribution in a patient with IAS by using the 123I-labeled insulin infusion. They showed that the antibody-bound insulin forms a large and unstable insulin reservoir in patients with IAS, and the insulin is delivered to its receptors not according to blood glucose levels, thus causing hypoglycemia.
Clopidogrel is a prodrug that requires activation by the P450 enzyme system. The chemical structure of clopidogrel does not have a sulfhydryl group. However, the active metabolite of the drug does have a sulfhydryl group (Fig. 2). We postulate that the activated form of clopidogrel may bind with disulfide bond of the insulin molecule, making the latter more immunogenic, and cause hypoglycemia as described previously.
Uchigata et al. [5] suggested that the insulin assay might give erroneous results because of the interference of the antibodies with the assay, but by addition of PEG or ultrafiltration, insulin bound to antibodies, the heavy protein molecule settles down, and free insulin can be measured in the supernatant. Basu et al. [10] reported the clinical, biochemical, and immunologic characteristics of seven white patients with IAS, mostly females, ranging from 46 to 84 years of age. They showed that during hypoglycemia, concentrations of insulin, proinsulin, and C-peptide considerably exceeded those observed in patients with insulinoma, and these concentrations were spuriously elevated as a result of interference by the autoantibodies in the immunoassays. They confirmed the presumption of the high concentrations of insulin due to interference of the autoantibodies in the immunoassay by measuring free insulin during the mixed-meal test [10]. This was clearly evident in our patient (Fig. 1).
Lupsa et al. [2] reported 58 cases of IAS in non-Asians until 2009 (50% in Europe and 41% in the United States), with no sex difference. In non-Asian patients, this syndrome was often associated with other autoimmune diseases or plasma cell dyscrasias, whereas 47% of these patients had a recent exposure to sulfhydryl group–containing medications (captopril, penicillamine, pyritinol, carbimazole, imipenem, propylthiouracil, hydralazine, procainamide, isoniazid, and penicillin G). In that review [2], most patients were treated with a low-carbohydrate diet, whereas only 11 patients (38%) were treated with glucocorticoids. If sulfhydryl-containing medications were implicated, discontinuing the drug led to resolution of the symptoms [2]. There were no data regarding HLA association in non-Asian patients with IAS. Our case is one of the few documented cases of IAS in a non-Asian patient not associated with other autoimmune diseases. However, our patient had a genetic predisposition and was recently exposed to a drug containing a sulfhydryl group, and, given the cumulative clinical and biochemical parameters and spontaneous remission of hypoglycemia, it fits well with this syndrome.
Management of IAS includes discontinuation of the offending agent, a low-carbohydrate diet, and often the use of glucocorticoids. The duration of treatment is ∼3 to 6 months, when a sulfhydryl-containing drug is identified and discontinued. Most of the patients respond to small, frequent low-carbohydrate meals, which serves to lower postprandial hyperglycemia and the subsequent rise in insulin secretion. Although spontaneous improvement of symptoms was reported in the literature [2], most patients were treated differently by various investigators [2]. The therapeutic options used include a low-carbohydrate diet and the use of drugs such as glucocorticoids, diazoxide, acarbose, and somatostatin [2]. In light of our patient’s age, the history of heart disease, and the impressive response observed during the administration of glucocorticoids for the treatment of COPD, we elected to treat him with dexamethasone for several weeks. We chose dexamethasone over other glucocorticoids so that we could maximize the anti-inflammatory benefit and minimize or even eliminate the potential for salt retention. Although it would have been of interest to rechallenge the patient, we did not feel this would be appropriate in light of his medical history.
In summary, this case suggests that IAS should be considered as the differential diagnosis of endogenous hyperinsulinemic hypoglycemia in seemingly well individuals, even when no drugs known to cause IAS were used. Very high total serum insulin levels with relatively low free insulin levels should raise the suspicion for IAS. A clinical suspicion of IAS can avoid expensive imaging and unnecessary surgery in affected patients.
Acknowledgments
Acknowledgments
Financial Support: This study received departmental support.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- COPD
- chronic obstructive pulmonary disease
- IAS
- insulin autoimmune syndrome
- PEG
- polyethylene glycol.
References and Notes
- 1.Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ; Endocrine Society . Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709–728. [DOI] [PubMed] [Google Scholar]
- 2.Lupsa BC, Chong AY, Cochran EK, Soos MA, Semple RK, Gorden P. Autoimmune forms of hypoglycemia. Medicine (Baltimore). 2009;88(3):141–153. [DOI] [PubMed] [Google Scholar]
- 3.Hirata Y, Ishizu H, Ouchi N, Motomura M, Abe M, Hara Y, Wakasugi H, Takahashi L, Sakano H, Tanaka M, Kawano H, Kanesaki T. Insulin autoimmunity in a case of spontaneous hypoglycemia. J Jpn Diabetes Soc. 1970;13:312–320. [Google Scholar]
- 4.Uchigata Y, Hirata Y. Insulin autoimmune syndrome (IAS, Hirata disease). Ann Med Interne (Paris). 1999;150(3):245–253. [PubMed] [Google Scholar]
- 5.Uchigata Y, Tokunaga K, Nepom G, Bannai M, Kuwata S, Dozio N, Benson EA, Ronningen KS, Spinas GA, Tadokoro K, Hirata Y, Juji T, Omori Y. Differential immunogenetic determinants of polyclonal insulin autoimmune syndrome (Hirata’s disease) and monoclonal insulin autoimmune syndrome. Diabetes. 1995;44(10):1227–1232. [DOI] [PubMed] [Google Scholar]
- 6.Redmon JB, Nuttall FQ. Autoimmune hypoglycemia. Endocrinol Metab Clin North Am. 1999;28(3):603–618, vii. [DOI] [PubMed] [Google Scholar]
- 7.Uchigata Y, Eguchi Y, Takayama-Hasumi S, Omori Y. Insulin autoimmune syndrome (Hirata disease): clinical features and epidemiology in Japan. Diabetes Res Clin Pract. 1994;22(2-3):89–94. [DOI] [PubMed] [Google Scholar]
- 8.Taylor SI, Barbetti F, Accili D, Roth J, Gorden P. Syndromes of autoimmunity and hypoglycemia. Autoantibodies directed against insulin and its receptor. Endocrinol Metab Clin North Am. 1989;18(1):123–143. [PubMed] [Google Scholar]
- 9.Dozio N, Scavini M, Beretta A, Sarugeri E, Sartori S, Belloni C, Dosio F, Savi A, Fazio F, Sodoyez JC, Pozza G. Imaging of the buffering effect of insulin antibodies in the autoimmune hypoglycemic syndrome. J Clin Endocrinol Metab. 1998;83(2):643–648. [DOI] [PubMed] [Google Scholar]
- 10.Basu A, Service FJ, Yu L, Heser D, Ferries LM, Eisenbarth G. Insulin autoimmunity and hypoglycemia in seven white patients. Endocr Pract. 2005;11(2):97–103. [DOI] [PubMed] [Google Scholar]