ABSTRACT
Inhibitory receptors have been extensively described for their importance in regulating immune responses in chronic infections and cancers. Blocking the function of inhibitory receptors such as PD-1, CTLA-4, 2B4, Tim-3, and LAG-3 has shown promise for augmenting CD8 T cell activity and boosting pathogen-specific immunity. However, the prevalence of inhibitory receptors on CD4 T cells and their relative influence on CD4 T cell functionality in chronic HIV infection remains poorly described. We therefore determined and compared inhibitory receptor expression patterns of 2B4, CTLA-4, LAG-3, PD-1, and Tim-3 on virus-specific CD4 and CD8 T cells in relation to their functional T cell profile. In chronic HIV infection, inhibitory receptor distribution differed markedly between cytokine-producing T cell subsets with, gamma interferon (IFN-γ)- and tumor necrosis factor alpha (TNF-α)-producing cells displaying the highest and lowest prevalence of inhibitory receptors, respectively. Blockade of inhibitory receptors differentially affected cytokine production by cells in response to staphylococcal enterotoxin B stimulation. CTLA-4 blockade increased IFN-γ and CD40L production, while PD-1 blockade strongly augmented IFN-γ, interleukin-2 (IL-2), and TNF-α production. In a Friend retrovirus infection model, CTLA-4 blockade in particular was able to improve control of viral replication. Together, these results show that inhibitory receptor distribution on HIV-specific CD4 T cells varies markedly with respect to the functional subset of CD4 T cells being analyzed. Furthermore, the differential effects of receptor blockade suggest novel methods of immune response modulation, which could be important in the context of HIV vaccination or therapeutic strategies.
IMPORTANCE Inhibitory receptors are important for limiting damage by the immune system during acute infections. In chronic infections, however, their expression limits immune system responsiveness. Studies have shown that blocking inhibitory receptors augments CD8 T cell functionality in HIV infection, but their influence on CD4 T cells remains unclear. We assessed the expression of inhibitory receptors on HIV-specific CD4 T cells and their relationship with T cell functionality. We uncovered differences in inhibitory receptor expression depending on the CD4 T cell function. We also found differences in functionality of CD4 T cells following blocking of different inhibitory receptors, and we confirmed our results in a Friend virus retroviral model of infection in mice. Our results show that inhibitory receptor expression on CD4 T cells is linked to CD4 T cell functionality and could be sculpted by blockade of specific inhibitory receptors. These data reveal exciting possibilities for the development of novel treatments and immunotherapeutics.
KEYWORDS: CD4 T cells, CTLA-4, HIV, PD-1, inhibitory receptors
INTRODUCTION
Antiviral T cells play a pivotal role in the clearance of acute viral infections. In some cases T cells fail to eliminate infection but then control viral replication to lower, nonpathogenic levels. However, under continuous viral replication and immune activation encountered in certain chronic viral infections, such as human immunodeficiency virus (HIV) and hepatitis C virus, T cells progressively lose the ability to mount antiviral effector activities, such as cytokine secretion, proliferation, and cytotoxicity, necessary to control viral replication (1–3). This progressive loss of function, termed exhaustion, in turn contributes to the lack of viral clearance and may even lead to diminished viral suppression in chronic stages of disease (2, 4, 5). An important feature of exhausted T cells is the upregulation of inhibitory receptors such as CD244 (2B4), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), lymphocyte-activation gene-3 (LAG-3), programmed death-1 (PD-1), and T cell immunoglobulin and mucin domain-containing-3 (Tim-3) (5–8). Inhibitory receptors exert their effect through various mechanisms, resulting in activation or attenuation of signaling cascades, interference with T cell ligation of activating coreceptor, or interference with ligation of major histocompatibility complex (MHC)-peptide complexes (9–15). Notably, these receptors are also commonly found on activated T cells during acute infection in order to limit immunopathology due to an exaggerated immune response or excessive and persistent inflammation (16, 17), yet their continued expression on T cells in chronic infection becomes detrimental by limiting the ability to control viral replication (2, 4, 18–25). Thus, inhibitory receptors and their ligands play crucial roles in shaping the immune response to pathogens, providing the immune system with a mechanism to fine-tune adaptive immune responses and ensuring pathogen control without excessive immune-mediated damage. The identity and prevalence of inhibitory receptor distribution in chronic viral infection is therefore of critical importance to understanding the corresponding potency of virus-specific cellular immunity.
As inhibitory receptors function to limit T cell responses during chronic infections as well as several cancers, their blockade is being actively investigated as a means to restore T cell functionality and achieve therapeutic cures (26–28). Studies in the murine chronic lymphocytic choriomeningitis virus (LCMV), Friend virus (FV), and tumor models have shown a partial or full restoration of cytotoxic CD8 T lymphocyte activity through blockade of CTLA-4, PD-1, LAG-3, or Tim-3 (29). Studies of human chronic infections such as hepatitis B virus (HBV), hepatitis C virus (HCV), and HIV have demonstrated that ex vivo blockade of receptors alone or in combination can rescue cytotoxic CD8 T lymphocyte proliferation, cytokine production, or cytolytic activity (25, 30–42). Importantly, these studies also showed that inhibitory receptor functions are nonredundant, as made apparent by studies showing both rescue of different effector functions dependent on the inhibitory receptor blocked and additional expression of individual inhibitory receptors progressively shutting down effector functions (10, 34, 36, 43–45). The utility of inhibitory receptor blockade has been further demonstrated in clinical trials, where blocking reagents against CTLA-4, PD-1, and LAG-3 improved survival times and reduced tumor burdens for multiple cancers and lowered viral loads in virus infections (46–50).
While most studies have focused on the expression, influence, and blockade of inhibitory receptors on cytotoxic CD8 T lymphocytes, less is known about the influence of inhibitory receptors on CD4 T cell function. Tim-3 has been shown to be important for the generation of gamma interferon (IFN-γ)-secreting CD4 T cells in the setting of acute Mycobacterium tuberculosis and HCV infection. Furthermore, PD-1 and LAG-3 expression on HIV-specific CD4 T cells has been shown to be important for regulating cytokine secretion (37, 51–56). Despite the known role of inhibitory receptors in the restraint of T cell responses in chronic infections, the relative contribution of different inhibitory receptors to CD4 T cell function impairment in chronic HIV infection is poorly understood. As we and others have shown, a robust CD4 T cell response to HIV is influential in controlling infection (57–60). Indeed, factors which modulate CD4 T cell functions in HIV infection, such as the ability of CD4 T cells to produce cytokines supporting CD8 T cell and B cell function and HIV-specific CD4 T cells' ability to directly kill infected cells, are important for disease status (61). The relative prevalence of inhibitory receptors on CD4 T cells and their ability to influence and sculpt HIV-specific CD4 T cell responses therefore would likely have great importance for understanding both the elicitation and control of these crucial antiviral functions.
We therefore assessed the inhibitory receptor profile of functional subsets of HIV-specific CD4 and CD8 T cells from HIV-infected donors able to control viral infection to various degrees. In addition, we studied changes in the functional profiles of T cells after blockade of inhibitory receptors and confirmed these findings in a mouse model of retroviral infection. These results are important for understanding HIV pathology and have important implications for the design of immunotherapeutic interventions.
RESULTS
Marked differences in inhibitory receptor expression between CD4 and CD8 T cells in HIV-infected progressors and controllers.
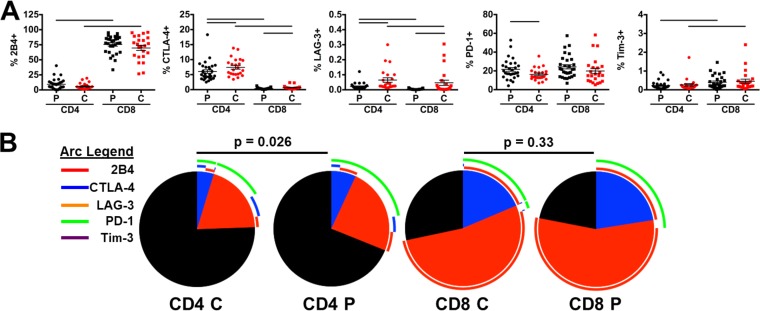
We first determined the expression levels of the inhibitory receptors 2B4, CTLA-4, LAG-3, PD-1, and Tim-3 on CD4 and CD8 T cells from treatment-naive, chronically HIV-infected individuals with detectable viral loads of >2,000 RNA copies/ml (termed HIV progressors; average = 54,248 ± 15,890 HIV RNA copies/ml) and individuals spontaneously able to control HIV replication (<2000 HIV RNA copies/ml) in the absence of antiretroviral medication (termed HIV controllers; average = 566 ± 120 HIV RNA copies/ml). Interestingly, expression levels of inhibitory receptors differed markedly between CD4 and CD8 T cells. The most common inhibitory receptors expressed on CD4 T cells for all individuals were PD-1 and CTLA-4, expressed on ∼20% and 6% of cells, respectively (Fig. 1A). While PD-1 was expressed at similar levels on both CD4 and CD8 T cells, CTLA-4 expression was marginally present on CD8 relative to CD4 T cells. The most commonly expressed receptor on CD8 T cells was 2B4 (∼75% of cells), while less than 10% of CD4 T cells expressed this receptor. The largest difference between HIV controllers and progressors was LAG-3 and CTLA-4 expression, which were both elevated on CD4 and CD8 T cells in controllers (LAG-3, 0.065% versus 0.021% [P = 0.0002] and 0.047% versus 0.004% [P = 0.0003], respectively; CTLA-4, 7.43% versus 6.04% [P = 0.03] and 0.67% versus 0.37% [P = 0.005], respectively). Only PD-1 showed significant upregulation on CD4 T cells in HIV progressors compared to controllers (21.61% versus 16.29%; P = 0.047). CD8 T cells were unique in their larger percentage of 2B4+ cells as well as the predominance of 2B4+ PD-1+ cells, both of which were not observed to the same extent on CD4 T cells.
FIG 1.
Inhibitory receptor distribution on CD4 T cells is diminished in HIV controllers. Levels of 2B4, CTLA-4, LAG-3, PD-1, and Tim-3 were assessed on total CD4 and CD8 T cells in HIV progressors (P; viral load of >2,000 HIV RNA copies/ml; n = 29) and controllers (C; viral load of <2,000 HIV RNA copies/ml; n = 23). (A) Levels were compared for individual receptor prevalence on CD4 (circles) and CD8 (squares) T cells, with progressors in black and controllers in red. Summary data are shown as means ± standard errors of the means (SEM). Bars indicate a P value of <0.05 by Wilcoxon rank-sum tests, with compared groups being at the ends of the bar. (B) Prevalence of multiple and single receptor-expressing subsets on total CD4 and CD8 T cells. Colors of slices indicate the presence of 2 (blue), 1 (red), or 0 (gray) inhibitory receptors. Pies were compared by SPICE permutation tests with 10,000 permutations.
To evaluate levels of redundancy in the inhibitory receptor expression on T cells, we compared coexpression profiles of inhibitory receptors on total CD4 and CD8 T cells using SPICE, version 5.1 (62). While inhibitory receptor distribution on CD8 T cells was similar in progressors and controllers (P = 0.33), we observed significantly lower inhibitory receptor-expressing subsets in CD4 T cells from HIV controllers (P = 0.026) (Fig. 1B). This observation is interesting, as the only significant difference in individual populations between progressors and controllers were higher levels of CTLA-4 single-receptor-expressing cells in controllers relative to progressors (data not shown). This point suggests that an aggregate total of several receptor-negative populations that did not reach monovariate statistical significance contribute to the overall difference in inhibitory receptor distribution between progressors and controllers. Together these results indicate that CD4 T cells in both HIV progressors and controllers express different amounts and combinations of the inhibitory receptors measured relative to CD8 T cells.
Inhibitory receptor distribution differs on CD4 T cell subsets in association with their functional profile but are largely similar between HIV progressors and controllers.
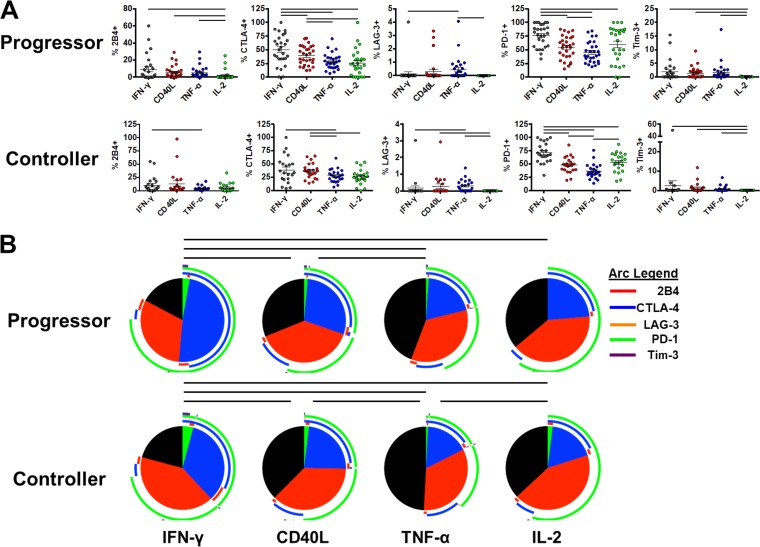
Given the various functions of CD4 T cells in chronic viral infection, we next sought to determine whether inhibitory receptor expression differs on the basis of functionality of HIV-specific CD4 T cells. PBMC from HIV progressors and controllers were stimulated with HIV-1 Gag PTE peptide pools, and production of IFN-γ, CD40L, tumor necrosis factor alpha (TNF-α), and interleukin-2 (IL-2) was determined by intracellular cytokine staining. While Gag-specific CD4 T cell response does not represent the totality of the HIV-specific CD4 T cell responses, we previously determined that it is a good representation of the overall HIV-specific CD4 T cell response (63). Inhibitory receptor expression on these cytokine-producing cells was assessed and compared between groups. Interestingly, we observed marked differences in the inhibitory receptor expression distribution between CD4 T cells based on their functionality (Fig. 2A). A general pattern was observed where HIV-specific IFN-γ-producing CD4 T cells displayed the greatest expression of several inhibitory receptors relative to other subsets, with progressively lower levels observed on CD40L-, TNF-α-, and IL-2-producing CD4 T cells, respectively. This pattern was most evident for CTLA-4 and 2B4 and was observed in HIV progressors as well as HIV controllers. Notably, HIV-specific IL-2-producing CD4 T cells bearing Tim-3 and LAG-3 were negligible in both progressors and controllers. In contrast to other inhibitory receptors, the expression patterns of PD-1 did not fit this general pattern. While IFN-γ-producing cells had the highest levels of PD-1 in progressors and controllers (76.1% and 71.3%, respectively), IL-2-producing cells also displayed elevated levels of PD-1 relative to CD40L- and TNF-α-producing cells in progressors (59.4% versus 54.0% and 45.2%; P values of 0.20 and 0.063) and controllers (58.8% versus 48.3% and 36.5%, respectively; P values of 0.5 and 0.0002). These data show that the inhibitory receptor expression levels on CD4 T cells in both HIV progressors and controllers directly relate to functional capacity.
FIG 2.
Bulk inhibitory receptor distribution varies on different cytokine-producing CD4 T cell subsets but is similar between HIV progressors and controllers. (A) PBMC from HIV progressors (n = 29) and controllers (n = 23) were stimulated with HIV-1 Gag PTE peptide pools and assessed for prevalence of single inhibitory receptors on cytokine-producing subsets: IFN-γ (gray), CD40L (red), TNF-α (blue), and IL-2 (green). Individual receptor prevalence on cytokine-producing subsets in HIV progressors (top) and HIV controllers (bottom) is shown. Summary data are shown as means ± SEM. Bars indicate a P value of <0.05 by Wilcoxon signed-rank tests, with compared groups being at the ends of the bar. (B) Prevalence of single- and multiple-receptor subsets on cytokine-producing CD4 T cells displayed using SPICE. Colors of slices indicate the presence of 3 (green), 2 (blue), 1 (red), or 0 (gray) inhibitory receptors. The prevalence of subsets expressing single and multiple inhibitory receptors in HIV progressors (top; n = 29) and controllers (bottom; n = 23) is shown. Bars indicate a P value of <0.05 by SPICE permutation tests with 10,000 permutations, with compared groups being at the ends of the bar.
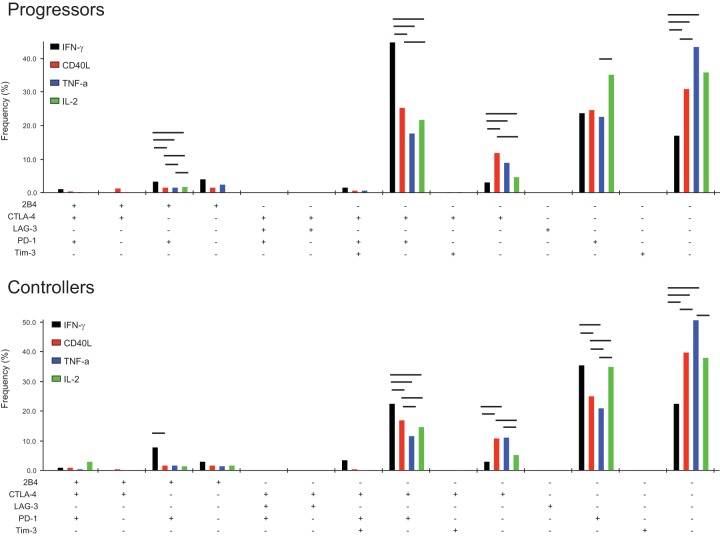
We next analyzed the distribution of single and multiple inhibitory receptor-expressing populations on HIV-specific CD4 T cell subsets in both progressors and controllers using SPICE analysis. Similar to our monovariate analyses, in Gag-stimulated cells, we found that the overall expression of inhibitory receptors was highest on IFN-γ-secreting CD4 T cells followed by CD40L-, TNF-α-, and IL-2-secreting CD4 T cells in both progressors (P = 0.0004, P < 0.0001, and P = 0.0004, respectively; SPICE permutation tests, with 10,000 permutations) and controllers (P = 0.02, P < 0.0001, and P = 0.01, respectively) (Fig. 2B). HIV-specific IFN-γ-secreting CD4 T cells were unique in their high levels of CTLA-4 and PD-1 coexpression, which significantly differed between progressors and controllers (P = 0.023; SPICE permutation tests, with 10,000 permutations) (Fig. 2B and 3). The inhibitory receptor levels were similar between CD40L- and IL-2-producing CD4 T cells, while TNF-α-producing cells displayed the smallest amount of these receptors. Notably, as cytokine production was assessed in a monovariate fashion, it is unclear whether these cytokine-producing subsets represent distinct subpopulations of CD4 T cells or whether they represent cells at different stages of progressive exhaustion.
FIG 3.
Expression of single- and multiple-inhibitory-receptor subsets in cytokine-producing CD4 T cells from HIV progressors (n = 29; top) and controllers (n = 23; bottom) following Gag PTE stimulation. Bars indicate a P value of <0.05 by Wilcoxon signed-rank tests, with compared groups being at the ends of the bar.
Differential impact of inhibitory receptor blockade of HIV-specific CD4 T cell subsets.
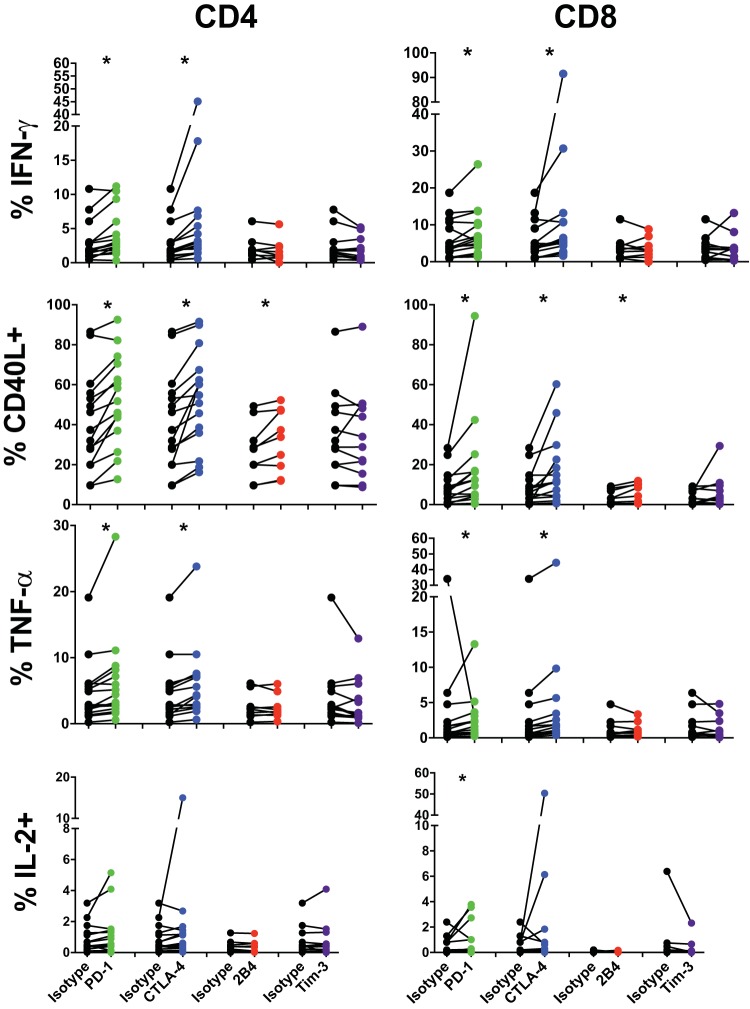
It is widely believed that inhibitory receptors serve to downregulate certain T cell functions, and their actions may synergize to enact successively greater levels of T cell inhibition, with various influences on different T cell functionalities. To investigate the role of inhibitory receptors on the functionality of HIV-specific CD4 and CD8 T cells, we performed in vitro blockade of the most commonly expressed inhibitory receptors, 2B4, CTLA-4, PD-1, and Tim-3, after staphylococcal enterotoxin B (SEB) stimulation in a subset of HIV progressors. Cytokine production by proliferating cells then was measured 5 days following stimulation and compared to an isotype-matched antibody control. LAG-3 was omitted from this analysis due to the exceedingly low level of expression observed on both bulk CD4 and CD8 T cells in our studies. In agreement with previous studies (39, 52), we observed significant differences in the functional responses of CD8 T cells after receptor blockade with PD-L1, which resulted in increased production of IFN-γ (7.4% versus 5.6), CD40L (17.6% versus 8.3%), and IL-2 (0.88% versus 0.45%) (Fig. 4). Blockade of CTLA-4 resulted in increases in IFN-γ (13.2% versus 5.6%), CD40L (16.4% versus 8.3%), and TNF-α (5.1% versus 3.8%) production by CD8 T cells relative to isotype controls.
FIG 4.
Inhibitory receptors differentially control cytokine production by T cells in HIV infection. PBMC from HIV progressors (n = 9 to 15) were stained with CFSE and blocked with an isotype control (black) or for PD-1 (green), CTLA-4 (blue), 2B4 (red), or Tim-3 (purple) and then stimulated with SEB. Five days following stimulation, cells were washed and stimulated with SEB (5 μg/ml), and the prevalence of cytokine-producing CFSE-low CD4 (left) and CD8 (right) cells was assessed. Asterisks indicate a P value of <0.05 by Wilcoxon signed-rank tests.
CD4 T cells showed an overall similar pattern of increased production of IFN-γ (4.2% versus 3.0%), CD40L (52.3% versus 41.4%), and TNF-α (6.3% versus 4.8%) after PD-1 receptor blockade (Fig. 4). Additionally, blockade of CTLA-4 resulted in increased production relative to an isotype control of IFN-γ (7.0% versus 3.0%), CD40L (53.0% versus 41.4%), and TNF-α (6.0% versus 4.8%). Therefore, both PD-1 and CTLA-4 are able to repress cytokine production by CD4 and CD8 T cells in chronic HIV infection, and their blockade is able to partially rescue cytokine production. Interestingly, while blockade of 2B4 had no discernible effect on production of IFN-γ, TNF-α, or IL-2, its blockade resulted in increased levels of CD40L production for both CD4 (31.8% versus 27.0%) and CD8 (4.8% versus 3.7%) T cells. Interestingly, single blockade of Tim-3 had no apparent effect on cytokine elicitation by CD4 T cells.
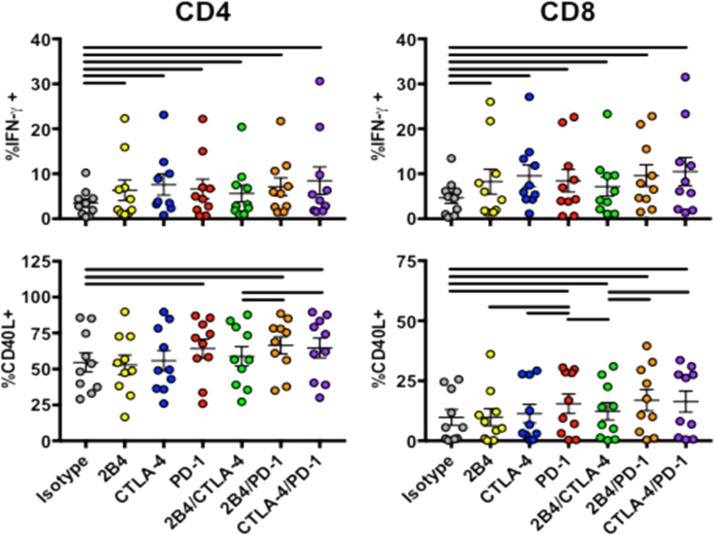
We next sought to explore the hierarchy of inhibitory receptor responses by combining blockade of 2B4, CTLA-4, and PD-1 on T cells in a subset of HIV progressors in response to SEB. Interestingly, combinatorial blockade was unable to augment increases in IFN-γ production by either CD4 or CD8 T cells beyond that achieved by single-receptor blockade (Fig. 5). Together, these data suggest that PD-1 and CTLA-4 share common roles in reducing cytokine production by IFN-γ-, IL-2-, or TNF-α-producing CD4 T cell subsets in chronic HIV, but that CD40L responses are also modulated through 2B4. However, given the subtle degree to which combinations of receptor blockade influenced cytokine production, further exploration of other signatures, such as transcriptional profiles following blockade, would improve our understanding of inhibitory receptor hierarchies.
FIG 5.
PBMC from HIV-positive donors (n = 10) were stained with CFSE and blocked with an isotype control or for PD-1, CTLA-4, 2B4, or a combination of these receptors and then stimulated with SEB. Five days following stimulation, cells were washed and restimulated with SEB (5 μg/ml), and the prevalence of cytokine-producing CFSE-low CD4 and CD8 T cells was assessed. Data are shown as means ± SEM. Bars indicate a P value of <0.05 by Wilcoxon signed-rank tests, with compared groups being at the ends of the bar.
Blockade of CTLA-4 similarly augments T cell responses in murine retroviral infection.
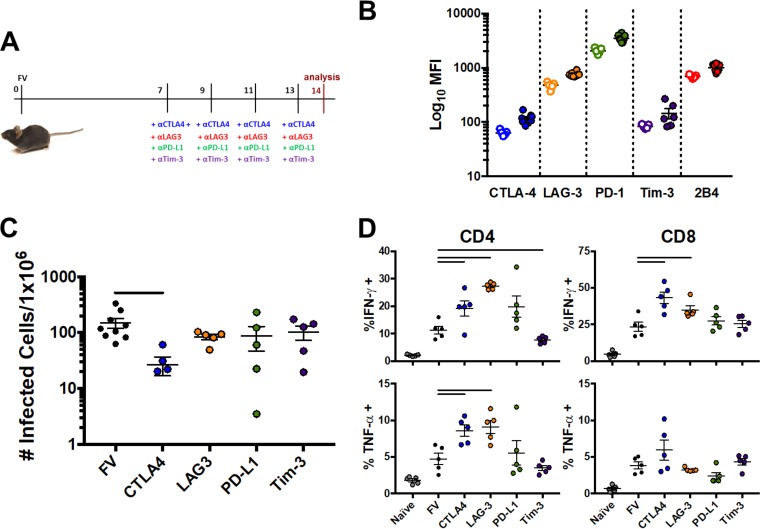
The importance of inhibitory receptor functionality to sculpt antiviral immune responses has been demonstrated in several settings for CD8 T cells. We sought to expand and confirm our observations implicating receptor blockade as a means of supporting CD4 T cell responses in a murine model of retroviral infection. Mice infected with Friend virus (FV) were subjected to inhibitory receptor blockade using antibodies blocking CTLA-4, LAG-3, PD-L1, or Tim-3 or isotype controls. Mice were sacrificed 14 days following infection, and levels of infectious virus as well as cytokine-producing CD4 and CD8 T cells were assessed (Fig. 6A). Relative expression of inhibitory receptors on effector CD4+ CD62L− T cells before and after FV infection was first assessed by mean fluorescence intensity (MFI) in both naive and FV-infected mice. Levels of every inhibitory receptor were found to be increased at day 14 following FV infection (Fig. 6B). In the context of inhibitory receptor-blocking antibodies, only CTLA-4 blockade significantly influenced FV viral loads in the bone marrow, the main site of FV replication, relative to untreated animals (149.7 versus 26.9 infectious cells/1 × 106, respectively; P = 0.01 by Student's t tests) (Fig. 6C). CTLA-4 or LAG-3 inhibition resulted in increased numbers of bone marrow CD4 T cells able to produce IFN-γ (19.3% versus 11.3% and 27.4% versus 11.3%, respectively) or TNF-α (8.6% versus 4.7% and 9.1% versus 4.7%, respectively) relative to untreated animals (Fig. 6D). A similar augmentation of IFN-γ was observed for CD8 T cells in animals blocked with CTLA-4 or LAG-3 antibodies (43.3% versus 32.5% and 35.1% versus 23.5%, respectively). Together, these data highlight the role of CTLA-4 in influencing antiretroviral CD4 and CD8 T cell responses. However, as different viruses and infections covering different time scales were involved in comparing FV to HIV infection, further exploration is warranted.
FIG 6.
CTLA-4 or LAG-3 blockade reduces viral burden and augments CD4 and CD8 T cell responses in murine retroviral infection. Mice (n = 5 to 8/group) were infected with Friend's leukemia virus (FLV) in the presence of control or inhibitory receptor-blocking antibodies. (A) Experimental layout of infection of mice with Friend's leukemia virus (FLV) in the presence of inhibitory receptor-blocking antibodies. (B) Mean fluorescence intensity (MFI) of inhibitory receptors on CD4+ CD62L− effector T cells in spleen in both naive (n = 5) and FV-infected (n = 8; 10 days postinfection) mice. Symbols represent levels in naive (open) and FV-infected (closed) mice. (C) Cells from bone marrow were harvested 14 days following infection, and numbers of cells containing infectious virus were assessed. Data are shown as means ± SEM. Bars represent a P value of <0.05 by Student's t tests, with compared groups being at the ends of the bar. (D) Cells from bone marrow were harvested 14 days following infection, and cells were restimulated with CD3/CD28 antibodies. Levels of cytokine-producing cells were assessed. Levels in naive mice are included for reference. Data are shown as means ± SEM. Bars represent a P value of <0.05 by Student's t tests, with compared groups being at the ends of the bar.
DISCUSSION
Inhibitory receptors play a critical role in regulating immune responses and protecting against immunopathology during acute infections. Despite their crucial role in protecting against hyperactivation of immune cells early in infection, their continued expression in chronic infection is associated with T cell exhaustion and viral persistence. In this study, we found that inhibitory receptor distribution in chronic HIV infection differed significantly between functionally distinct cytokine-producing CD4 T cells and were more elevated on CD4 T cells from progressors than controllers. Treatment with inhibitory receptor blockade yielded generally similar augmentation of cytokine production between CD4 T cells and CD8 T cells, and these results were confirmed in a murine model of retroviral infection. Together these results show that CD4 T cells in chronic HIV infection express inhibitory receptors that can be used to modulate immune responses and that the expression profiles differ across functionally distinct cell subsets. Moreover, blockade of the inhibitory receptors PD-1, CTLA-4, and 2B4 show differential abilities to rescue cytokine production upon stimulation.
The ability to control HIV infection is constrained in part by the cumulative acquisition of inhibitory receptor expression on both CD4 and CD8 T cells (38, 39, 52, 64, 65). In chronic HIV infection, CD8 T cells have been shown to have increased levels of inhibitory receptors, such as PD-1, 2B4, LAG-3, and Tim-3, relative to HIV-uninfected individuals as well as relative to CD8 T cells specific for other chronic infections, such as Epstein-Barr virus or cytomegalovirus (40). Levels of these inhibitory receptors have been suggested to correlate with HIV loads and their accumulation results in an exhausted phenotype, which is at least in part reversible upon receptor blockade (52, 66, 67). Our data show that the CD4 T cell compartment expresses lower overall levels of inhibitory receptors than CD8 T cells, mirroring prior studies (51, 54).
While there indeed appear to be shared mechanisms of CD4 and CD8 T cell exhaustion, there are also unique features of CD4 T cell exhaustion signatures (68). For instance, while CD8 T cell dysfunction in chronic viral diseases is strongly linked to a distinct and predictable exhaustion program, alterations of pathogen-specific CD4 T cell functions result from a broader combination of skewed lineage differentiation and by factors unique to T helper cells. In addition, MHC class II-T cell receptor (TCR) functional avidity is generally lower in HIV infection than MHC class I-TCR interaction, which can further alter apparent CD4 T cell exhaustion (69). In the context of HIV infection, infection and depletion of HIV-specific CD4 T cells also may play a role in observed CD4 T cell dysfunction (70). Therefore, while we have observed differences in exhaustion between CD4 T cell subsets in HIV infection, without assessment of transcriptional programs associated with known exhaustion pathways, such as Blimp-1, it is difficult to specifically assign a mechanism to the observed influence of inhibitory receptors on impaired CD4 T cell functionality we observed. It will therefore be of interest to further explore the genetic signatures of exhaustion between CD4 and CD8 T cells during HIV infection to assess whether CD4 T cells display an exhausted genetic phenotype similar to that of CD8 T cells and whether other inhibitory receptors are involved. In particular, understanding differences in exhaustion signatures using tetramer technology will provide a much cleaner assessment than cytokine secretion.
A hallmark of the exhausted phenotype of CD8 and CD4 T cells in chronic infection is the progressive loss of cellular functions, such as the ability to produce different cytokines as well as the ability to proliferate in response to antigen. Indeed, in chronic simian immunodeficiency virus (SIV) infection, as CD8 T cell exhaustion progresses, SIV-specific CD8 T cells have been shown first to lose the ability to produce IL-2 in response to antigen, then TNF-α, and finally IFN-γ (71–74). Furthermore, the ability to produce several cytokines upon stimulation with antigen has been observed to be sensitive to the amount of PD-1 present on a given cell, with previous studies showing inhibition of IL-2 production requiring little expression of PD-1 while MIP-1β production is only inhibited in the presence of high levels of PD-1 expression (43). Our studies suggest a similar phenomenon occurs with HIV-specific CD4 T cells in chronic HIV infection. IFN-γ-producing CD4 T cells displayed a significantly higher prevalence of inhibitory receptor expression than CD4 T cells that produced CD40L and IL-2. A possible explanation for these differences is that the ability to produce certain cytokines is lost as inhibitory receptors accumulate on a cell type in a manner similar to what has been observed for CD8 T cells. Alternatively, inhibitory receptor expression patterns may delineate distinct functional subsets rather than identify CD4 T cells at various stages of an exhaustion pathway. For example, PD-1 also serves as a marker for T follicular helper (TFH) cells, and its role in TFH function remains uncertain but has been proposed to regulate B cell function (75). It is important to note that differential distribution of memory phenotypes within these subsets could account for some of the observed differences in inhibitory receptor expression. However, in these studies we focus on the ability of the HIV-specific CD4 T cell compartment to execute a given function, regardless of the cells which make up the population providing that function. It would be of interest to further examine the subsets which comprise these functional compartments and their relative influence on observed inhibitory receptor prevalence (63). Lastly, as cytokine production was assessed in a monovariate fashion in these studies, it is unclear whether the larger amounts of inhibitory receptors present on IFN-γ-producing CD4 T cells is the result of progressive loss of functions as inhibitory receptors accumulate, similar to CD8 T cells, or whether the inhibitory receptors delineate different populations of cytokine-producing cells.
The ability of CD4 T cells to maintain help in chronic retroviral infection has numerous benefits that aid in the control of viral infection. Our results indicate that differential blockade of inhibitory receptors can augment CD4 T cell responses to varying degrees, and while the distribution pattern of these receptors remains largely unaltered between HIV progressors and controllers on CD8 T cells, their bulk expression is altered between progressors and controllers on CD4 T cells. Furthermore, in both HIV controllers and progressors, a substantial proportion of cytokine-producing CD4 T cells expresses CTLA-4, PD-1, or both. While it is possible that this phenomenon captures a portion of activated CD4 T cells as well, these receptors likely play a central role in restraining CD4 T cell responses in HIV infection. This central role of CTLA-4 was also observed in a separate retroviral system, Friend virus (FV) infection in mice. This result indicates that inhibitory receptor expression on CD4 T cells in chronic infection follows generalizable rules and that while certain receptors such as LAG-3 or PD-1 are uniquely expressed during FV and HIV infection, respectively, CTLA-4 expression is similarly regulated in both diseases. This possibility is strengthened by previous reports showing the ability of CTLA-4 blockade to augment CD4 T cell responses against other chronic viral infections, such as Epstein-Barr virus, hepatitis B virus, and hepatitis C virus. It is interesting that PD-1 has also been shown to be a prominent marker of CD4 T cells hypothesized to be the main reservoir population in chronic HIV infection (76). Therefore, coexpression of other inhibitory receptors and their relative contribution to the establishment or maintenance of HIV reservoir-harboring cells would be of interest. Further exploration of the hierarchy of inhibitory receptor usage in these systems would build a better understanding of the process by which CD4 T cells become impaired in chronic infection as well as their potential contribution to the HIV reservoir.
Taken together, we demonstrated that CD4 T cells display marked differences in their inhibitory receptor profiles in chronic viral infection dependent on their functionality. Cytokine-producing cellular subsets displayed inhibitory receptor distributions reminiscent of cytokine-production hierarchies previously observed for CD8 T cells, suggesting similarities in T cell exhaustion between CD4 and CD8 T cells, particularly during chronic HIV infection. Notably, we observed an influence of PD-1 and CTLA-4 on HIV Gag-specific CD4 T cell function and confirmed the importance of CTLA-4 in a murine retroviral infection model. These studies suggest that CD4 T cell functionality during chronic viral infection is able to be sculpted by blockade or engagement of different inhibitory receptors, providing exciting avenues for the continued development of HIV immunotherapeutic interventions.
MATERIALS AND METHODS
Ethics statement.
Cryopreserved peripheral blood mononuclear cells (PBMC) from treatment-naive chronically HIV-1-infected individuals were used. Subjects were enrolled either at the United States Military HIV Research Program (MHRP) RV149 cohort or the University of California, San Francisco (UCSF), SCOPE cohort. All study subjects were adults and gave written consent, and IRB approval was obtained by the Walter Reed National Military Medical Center, the Walter Reed Army Institute of Research, the Naval Medical Research Center, and the University of California, San Francisco.
Animal experiments were performed in strict accordance with the German regulations of the Society for Laboratory Animal Science (GV-SOLAS) and the European Health Law of the Federation of Laboratory Animal Science Associations (FELASA). The protocol was approved by the North Rhine-Westphalia State Agency for Nature, Environment, and Consumer Protection (LANUV) (permit number G 1518/15). All efforts were made to minimize suffering.
Mice.
Inbred C57BL/6 (B6) mice were maintained under pathogen-free conditions. C57BL/6 mice (H-2b/b, Fv1b/b, Fv2r/r) are resistant to FV-induced leukemia. All mice were females of 8 to 12 weeks of age at the beginning of the experiments.
Human cell stimulation for cytokine production.
PBMCs were thawed in 37°C RPMI 1640 supplemented with 10% fetal calf serum, l-glutamine, and penicillin-streptomycin (R10) and rested overnight. PBMCs were then diluted to 1 × 106/ml and stimulated with HIV-1 Gag PTE peptide pools (1 μg/ml; NIH AIDS Reagent Bank) in the presence of CD28/49d costimulatory molecules (BD), brefeldin A (Sigma-Aldrich), and monensin (BD) and incubated for 6 h at 37°C, 5% CO2.
Alternatively, PBMC were stained with carboxyfluorescein succinimidyl ester (CFSE) (0.5 μM in phosphate-buffered saline [PBS], 37°C, 5 min) and blocked with 10 μg/ml mouse IgG1 isotype control (MG1-45; BioLegend), anti-human 2B4/CD244 (eBioPP35; eBioscience), anti-human CD152/CTLA-4 (L3D10; BioLegend), anti-human CD274/PD-L1 (29E.2A3; BioLegend) and anti-human CD273/PD-L2 (MIH18; BioLegend), or anti-human CD366/Tim-3 (F38-2E2; BioLegend) 1 h prior to stimulation with staphylococcal enterotoxin B (SEB; 5 μg/ml). Cells were incubated at 37°C, 5% CO2, and then washed with R10 prior to restimulation with SEB and BFA-monensin treatment as described above.
Virus and viral infection.
The FV stock used in these experiments was FV complex containing B-tropic Friend murine leukemia helper virus (F-MuLV) and polycythemia-inducing spleen focus-forming virus (77). Virus stock was prepared as a 10% spleen cell homogenate from BALB/c mice infected 14 days previously with 3,000 spleen focus-forming units of noncloned virus stock. Experimental mice were injected intravenously with 20,000 spleen focus-forming units of lactate dehydrogenase-elevating virus (LDV)-free FV complex.
In vivo receptor blockade.
For blockade of inhibitory pathways in acute FV-infected mice, 200 μg anti-mouse CTLA-4 (9H10), 100 μg anti-mouse LAG-3 (C9B7W), 200 μg rat anti-mouse PD-L1 A (10F.9G2), or 100 μg anti-mouse Tim-3 antibody (RMT3-23) (BioXCell) was administered intraperitoneally every second day for a total of four times. T cell responses and viral loads were analyzed 1 day posttreatment.
Infectious center assays.
Infectious center assays were performed as described previously (78).
Multicolor flow cytometry.
Following peptide stimulation, cells were washed with PBS–2% fetal calf serum (FCS) and stained with an amine-reactive viability dye (Live/Dead aqua; Life Technologies) for 30 min at room temperature (RT). Cells then were washed and blocked with PBS–10% normal mouse serum (Life Technologies) for 15 min at 4°C. Following blocking, cells were stained with anti-human anti-CD14 brilliant violet 510 (BV510) (M5E2; BioLegend), anti-CD19 BV510 (HIB-19; BioLegend), anti-LAG-3/CD223 phycoerythrin-cyanine 7 (PE-Cy7) (3DS223H; eBioscience), anti-2B4/CD244 PE (C1.7; BioLegend), anti-PD-1/CD279 BV605 (EH12.2H7; BioLegend), anti-CTLA-4/CD152 PE-CF594 (BNI3; BD), and anti-Tim-3/CD366 BV421 (F38-2E2; BioLegend) for 30 min at 4°C. Cells then were washed and fixed in Fix/Perm buffer A (Life Technologies) for 15 min at RT. Intracellular staining was done in Fix/Perm Buffer B at RT with anti-human anti-CD3 Alexa Fluor 700 (UCHT1; BD), anti-CD4 fluorescein (RPA-T4; BioLegend), anti-CD8 allophycocyanin (APC)-Cy7 (RPA-T8; BioLegend), and one of the following cytokine-specific antibodies: anti-CD40L/CD154 (24-31; BioLegend), anti-interferon gamma (IFN-γ) (B27; BioLegend), anti-IL-2 (MQ1-17H12; BioLegend), or anti-TNF-α (MAb11; BioLegend), conjugated to APC. Cells were washed twice and resuspended in PBS for flow cytometry. Data were collected on a four-laser LSR II flow cytometer using FACSDiva software (BD) and subsequently analyzed using FlowJo (version 9.410; TreeStar).
Mouse cell surface and intracellular staining by flow cytometry.
Surface and intracellular staining were performed as described previously (79). For surface staining, we used antibodies specific against mouse anti-CD3 APC-Cy7 (17A2; BioLegend), anti-CD4 BV605 (RM4-5; BioLegend), anti-CD8 Alexa Fluor 700 (53-6.7; eBioscience), CD11b BV650 (M17/4; BioLegend), CD43 PE or PerCP (peridinin chlorophyll protein; 1B11; BioLegend), CD62L PE-Cy7 (MEL-14; eBioscience), and CD69 PE (H1.2F3; BioLegend), and for intracellular staining we used a cross-reactive antibody specific against human granzyme B, AF700 (GB11; ThermoFisher) (80). Intracellular staining for IFN-γ fluorescein isothiocyanate (XMG1.2; eBioscience), TNF-α BV510 (MP6-XT22; BioLegend), and IL-2 eF450 (JES6-5H4; eBioscience) was performed as described previously (81). Data were acquired on a four LSR II flow cytometer (Becton Dickinson) from 350,000 to 500,000 lymphocyte-gated events per sample. Analyses were done using FACSDiva software (Becton Dickinson) and FlowJo software v10 (TreeStar).
Statistical analyses.
Monovariate statistical analysis was performed using GraphPad Prism, v6 (GraphPad Software, Inc.). For comparison between flow cytometry groups, nonparametric Mann-Whitney U tests or Wilcoxon signed-rank tests were used for data sets which were nonpaired or paired, respectively. Data are presented as means and standard errors of the means (SEM). Flow cytometry analysis and presentation of distributions was performed using SPICE, version 5-1.2, downloaded from http://exon.niaid.nih.gov/spice (62). Comparison of distributions was performed using a Student's t test and a partial permutation test.
ACKNOWLEDGMENTS
This work was funded by the National Institutes of Health (NIH; R01 AI091450-01 and R01 AI094602-01) and a cooperative agreement (W81XWH-11-2-0174) between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense (DOD). This work was further supported by DFG TRR60 and DFG STR1069/2-1. The SCOPE cohort is supported by P30 AI027763.
The following were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 PTE Gag and Env peptides. Human recombinant IL-2 was obtained from Maurice Gately, Hoffmann-La Roche, Inc.
Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained here are the private views of the author and are not to be construed as official or as reflecting true views of the Department of the Army or the Department of Defense.
REFERENCES
- 1.Antoine P, Olislagers V, Huygens A, Lecomte S, Liesnard C, Donner C, Marchant A. 2012. Functional exhaustion of CD4+ T lymphocytes during primary cytomegalovirus infection. J Immunol 189:2665–2672. doi: 10.4049/jimmunol.1101165. [DOI] [PubMed] [Google Scholar]
- 2.Wherry EJ, Ha S, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. 2007. Resource molecular signature of CD8 + T cell exhaustion during chronic viral infection. Immunity 27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Wherry EJ, Ahmed R. 2004. Memory CD8 T-cell differentiation during viral infection. J Virol 78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DAA, Wherry EJ. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West EE, Youngblood B, Tan WG, Jin H, Araki K, Alexe G, Konieczny BT, Calpe S, Freeman GJ, Terhorst C, Haining WN, Ahmed R. 2011. Tight regulation of memory CD8+ T cells limits their effectiveness during sustained high viral load. Immunity 35:285–298. doi: 10.1016/j.immuni.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Odorizzi PM, Wherry EJ. 2012. Inhibitory receptors on lymphocytes: insights from infections. J Immunol 188:2957–2965. doi: 10.4049/jimmunol.1100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin H-T, Anderson AC, Tan WG, West EE, Ha S-J, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. 2010. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A 107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joller N, Hafler JP, Brynedal B, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. 2011. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol 186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. 2012. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med 209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks DG, Ha S-J, Elsaesser H, Sharpe AH, Freeman GJ, Oldstone MBA. 2008. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A 105:20428–20433. doi: 10.1073/pnas.0811139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eissmann P, Beauchamp L, Wooters J, Tilton JC, Long EO, Watzl C. 2005. Molecular basis for positive and negative signaling by the natural killer cell receptor 2B4 (CD244). Blood 105:4722–4730. doi: 10.1182/blood-2004-09-3796. [DOI] [PubMed] [Google Scholar]
- 12.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. 2014. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity 41:1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider H, Martin M, Agarraberes FA, Yin L, Rapoport I, Kirchhausen T, Rudd CE. 1999. Cytolytic T lymphocyte-associated antigen-4 and the TCRζ/CD3 complex, but not CD28, interact with clathrin adaptor complexes AP-1 and AP-2. J Immunol 163:1868–1879. [PubMed] [Google Scholar]
- 14.Wang CJ, Heuts F, Ovcinnikovs V, Wardzinski L, Bowers C, Schmidt EM, Kogimtzis A, Kenefeck R, Sansom DM, Walker LSK. 2015. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci U S A 112:524–529. doi: 10.1073/pnas.1414576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Way SS, Havenar-Daughton C, Kolumam GA, Orgun NN, Murali-Krishna K. 2007. IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J Immunol 178:4498–4505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelinskyy G, Myers L, Dietze KK, Roggendorf M, Liu J, Lu M, Anke R, Teichgräber V, Hasenkrug KJ. 2011. Virus-specific CD8+ T cells upregulate programmed death-1 expression during acute friend retrovirus infection but are highly cytotoxic and control virus replication. J Immunol 187:3730–3737. doi: 10.4049/jimmunol.1101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akhmetzyanova I, Drabczyk M, Neff CP, Gibbert K, Palmer E, Dittmer U, Zelinskyy G. 2015. PD-L1 expression on retrovirus-infected cells mediates immune escape from CD8 + T cell killing. PLoS Pathog 11:e1005224. doi: 10.1371/journal.ppat.1005224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frebel H, Nindl V, Schuepbach RA, Braunschweiler T, Richter K, Vogel J, Wagner CA, Loffing-cueni D, Kurrer M, Ludewig B, Oxenius A. 2012. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med 209:2485–2499. doi: 10.1084/jem.20121015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, Azuma M, Sharpe AH, Auchincloss H, Sayegh MH, Najafian N. 2005. Analysis of the role of negative T cell costimulatory. J Immunol 174:6648–6656. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 20.Koehn BH, Ford ML, Ferrer IR, Gangappa S, Kirk AD, Larsen CP, Koehn BH, Ford ML, Ferrer IR, Borom K, Gangappa S, Kirk AD, Larsen CP. 2008. PD-1-dependent mechanisms maintain peripheral tolerance of donor-reactive CD8+ T cells to transplanted tissue. J Immunol 181:5313–5322. doi: 10.4049/jimmunol.181.8.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okazaki T, Okazaki I, Wang J, Sugiura D, Nakaki F, Yoshida T, Kato Y, Fagarasan S, Muramatsu M, Eto T, Hioki K, Honjo T. 2011. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J Exp Med 208:395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venner JM, Famulski KS, Badr D. 2014. Molecular landscape of T cell-mediated rejection in human kidney transplants: prominence of CTLA4 and PD ligands. Am J Transplant 14:2565–2576. doi: 10.1111/ajt.12946. [DOI] [PubMed] [Google Scholar]
- 23.Linsley BPS, Greene JL, Tan P, Bradshaw J, Ledbetter JA, Anasetti C, Damle NK. 1992. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J Exp Med 176:1595–1604. doi: 10.1084/jem.176.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. 1996. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 4:535–543. doi: 10.1016/S1074-7613(00)80480-X. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Zhang J-Y, Wherry EJ, Jin B, Xu B, Zou Z-S, Zhang S-Y, Li B-S, Wang H-F, Wu H, Lau GKK, Fu Y-X, Wang F-S. 2008. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology 134:1938–1949. doi: 10.1053/j.gastro.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 26.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. 2007. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuzaki J, Gnjatic S, Mhawech-fauceglia P, Beck A, Miller A, Tsuji T. 2010. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A 107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. 2007. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 13:2151–2158. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 29.Dietze KK, Zelinskyy G, Liu J, Kretzmer F, Schimmer S, Dittmer U. 2013. Combining regulatory t cell depletion and inhibitory receptor blockade improves reactivation of exhausted virus-specific CD8 + T cells and efficiently reduces chronic retroviral loads. PLoS Pathog 9:e1003798. doi: 10.1371/journal.ppat.1003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. 2010. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Investig 120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, Nebbia G, Kennedy PTF, Geretti A-M, Dusheiko G, Maini MK. 2011. Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 53:1494–1503. doi: 10.1002/hep.24249. [DOI] [PubMed] [Google Scholar]
- 32.Kroy DC, Ciuffreda D, Cooperrider JH, Tomlinson M, Hauck GD, Aneja J, Berger C, Wolski D, Carrington M, Wherry EJ, Chung RT, Tanabe KK, Elias N, Freeman GJ, de Kruyff RH, Misdraji J, Kim AY, Lauer GM. 2014. Liver environment and HCV replication affect human T-cell phenotype and expression of inhibitory receptors. Gastroenterology 146:550–561. doi: 10.1053/j.gastro.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, Chinaleong J, Kennedy P, Maini MK. 2012. Upregulation of the Tim-3/Galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One 7:e47648. doi: 10.1371/journal.pone.0047648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bengsch B, Seigel B, Ruhl M, Timm J, Kuntz M, Blum ME, Pircher H, Thimme R. 2010. Exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog 6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raziorrouh B, Schraut W, Gerlach T, Nowack D, Gruner NH, Ulsenheimer A, Zachoval R, Wachtler M, Spannagl M, Haas J, Diepolder HM, Jung M-C. 2010. The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8 1 T-cell function. Hepatology 52:1934–1947. doi: 10.1002/hep.23936. [DOI] [PubMed] [Google Scholar]
- 36.Nakamoto N, Cho H, Shaked A, Olthoff K, Valiga ME, Gostick E, Price DA, Freeman GJ, Wherry EJ, Chang K-M. 2009. Synergistic reversal of intrahepatic HCV-specific CD8 T cell exhaustion by combined PD-1/CTLA-4 blockade. PLoS Pathog 5:e1000313. doi: 10.1371/journal.ppat.1000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. 2009. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues. J Virol 83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun T, Mccune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. 2008. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJR, Klenerman P, Ahmed R, Freeman GJ, Walker BD. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 40.Chew GM, Fujita T, Webb GM, Burwitz BJ, Wu HL, Reed JS, Hammond KB, Clayton KL, Ishii N, Abdel-Mohsen M, Liegler T, Mitchell BI, Hecht FM, Korman AJ, Deeks SG, Sacha JB, Ndhlovu LC. 2016. TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection. PLoS Pathog 12:e1005349. doi: 10.1371/journal.ppat.1005349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raziorrouh B, Heeg M, Kurktschiev P, Schraut W, Zachoval R, Wendtner C, Wächtler M, Spannagl M, Denk G, Ulsenheimer A, Bengsch B, Pircher H, Diepolder HM, Grüner NH, Jung M-C. 2014. Inhibitory phenotype of HBV-specific CD4+ T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS One 9:e105703. doi: 10.1371/journal.pone.0105703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West EE, Jin H, Rasheed A, Penaloza-Macmaster P, Ha S, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R. 2013. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Investig 123:2604–2615. doi: 10.1172/JCI67008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei F, Zhong S, Ma Z, Kong H, Medvec A, Freeman GJ, Krogsgaard M, Riley JL. 2013. Strength of PD-1 signaling differentially affects T-cell effector functions. Proc Natl Acad Sci U S A 110:2–11. doi: 10.1073/pnas.1305394110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Sumire V, Linsley PS, Thompson CB, Riley L, Kobayashi SV, Riley JL. 2005. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol 25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viganò S, Banga R, Bellanger F, Pellaton C, Farina A, Comte D, Harari A, Perreau M. 2014. CD160-associated CD8 T-cell functional impairment is independent of PD-1 expression. PLoS Pathog 10:e1004380. doi: 10.1371/journal.ppat.1004380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gardiner D, Lalezari J, Lawitz E, DiMicco M, Ghalib R, Reddy KR, Chang K-M, Sulkowski M, O'Marro S, Anderson J, He B, Kansra V, McPhee F, Wind-Rotolo M, Grasela D, Selby M, Korman AJ, Lowy I. 2013. Assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One 8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brignone C, Escudier B, Grygar C, Marcu M, Triebel F. 2009. Cancer therapy: clinical A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin Cancer Res 15:6225–6232. doi: 10.1158/1078-0432.CCR-09-0068. [DOI] [PubMed] [Google Scholar]
- 48.Lee PP, Yee C, Savage PA, Fong L, Brockstedt D, Weber JS, Johnson D, Swetter S, Thompson J, Greenberg PD, Roederer M, Davis MM. 1999. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med 5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 49.Callahan MK, Wolchok JD. 2013. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol 94:41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. 2012. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Porichis F, Hart MG, Zupkosky J, Barblu L, Kwon DS, Mcmullen A, Brennan T, Ahmed R, Freeman GJ, Kavanagh DG, Kaufmann DE. 2014. Differential impact of PD-1 and/or interleukin-10 blockade on HIV-1. J Virol 88:2508–2518. doi: 10.1128/JVI.02034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med 203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang B, Wang X, Jiang J, Cheng X. 2013. Involvement of CD244 in regulating CD4+ T cell immunity in patients with active tuberculosis. PLoS One 8:e63261. doi: 10.1371/journal.pone.0063261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Porichis F, Kwon DS, Zupkosky J, Tighe DP, Mcmullen A, Brockman MA, Pavlik DF, Rodriguez-Garcia M, Pereyra F, Freeman GJ, Kavanagh DG, Kaufmann DE. 2011. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood 118:965–974. doi: 10.1182/blood-2010-12-328070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brooks DG, Teyton L, Oldstone MBA, McGavern DB. 2005. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J Virol 79:10514–10527. doi: 10.1128/JVI.79.16.10514-10527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oxenius A, Zinkernagel RM, Hengartner H. 1998. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity 9:449–457. doi: 10.1016/S1074-7613(00)80628-7. [DOI] [PubMed] [Google Scholar]
- 57.Aubert RD, Kamphorst AO, Sarkar S, Vezys V, Ha S-J, Barker DL, Ye L, Sharpe AH, Freeman GJ, Ahmed R. 2011. Antigen-specific CD4 T-cell help rescues exhausted CD8 T cells during chronic viral infection. Proc Natl Acad Sci U S A 108:21182–21187. doi: 10.1073/pnas.1118450109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chevalier MF, Jülg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, Kwon DS, Rychert J, Lian J, Muller MI, Cutler S, McAndrew E, Jessen H, Pereyra F, Rosenberg ES, Altfeld M, Walker BD, Streeck H. 2011. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol 85:733–741. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ranasinghe S, Flanders M, Cutler S, Soghoian DZ, Ghebremichael M, Davis I, Lindqvist M, Pereyra F, Walker BD, Heckerman D, Streeck H. 2012. HIV-specific CD4 T cell responses to different viral proteins have discordant associations with viral load and clinical outcome. J Virol 86:277–283. doi: 10.1128/JVI.05577-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soghoian DZ, Streeck H. 2010. Cytolitic CD4(+) T cells in viral immunity. Expert Rev Vaccines 9:1453–1463. doi: 10.1586/erv.10.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swain SL, McKinstry KK, Strutt TM. 2012. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol 12:136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roederer M, Nozzi J, Nason M. 2012. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytom A 79:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corneau A, Cosma A, Even S, Katlama C, Le Grand R, Frachet V, Blanc C, Autran B. 2017. Comprehensive mass cytometry analysis of cell cycle, activation, and coinhibitory receptors expression in CD4 T cells from healthy and HIV-infected individuals. Cytometry B Clin Cytom 92:21–32. doi: 10.1002/cyto.b.21502. [DOI] [PubMed] [Google Scholar]
- 64.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, Baker B, Zhu B, Le Gall S, Waring MT, Ahern R, Moss K, Kelleher AD, Coffin JM, Freeman GJ, Rosenberg ES, Walker BD. 2007. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol 8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 65.Velu V, Kannanganat S, Ibegbu C, Chennareddi L, Villinger F, Freeman GJ, Ahmed R, Amara RR. 2007. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol 81:5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. 2000. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood 96:3094–3101. [PubMed] [Google Scholar]
- 67.Goepfert PA, Bansal A, Edwards BH, Ritter GD, Tellez I, McPherson SA, Sabbaj S, Mulligan MJ. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J Virol 74:10249–10255. doi: 10.1128/JVI.74.21.10249-10255.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, Wherry EJ. 2014. Resource molecular and transcriptional basis of CD4 + T cell dysfunction during chronic infection. Immunity 40:289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ranasinghe S, Cutler S, Davis I, Lu R, Soghoian DZ, Qi Y, Sidney J, Kranias G, Flanders MD, Lindqvist M, Kuhl B, Alter G, Deeks SG, Walker BD, Gao X, Sette A, Carrington M, Streeck H. 2013. Association of HLA-DRB1-restricted CD4+ T cell responses with HIV immune control. Nat Med 19:930–933. doi: 10.1038/nm.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, Grossman Z, Dybul M, Oxenius A, Price DA, Connors M, Koup RA. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 71.Kuroda MJ, Schmitz JE, Charini WA, Nickerson CE, Lifton MA, Lord CI, Forman MA, Letvin NL. 1999. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J Immunol 162:5127–5133. [PubMed] [Google Scholar]
- 72.Hel Z, Nacsa J, Kelsall B, Tsai WP, Letvin N, Parks RW, Tryniszewska E, Picker L, Lewis MG, Edghill-Smith Y, Moniuszko M, Pal R, Stevceva L, Altman JD, Allen TM, Watkins D, Torres JV, Berzofsky JA, Belyakov IM, Strober W, Franchini G. 2001. Impairment of Gag-specific CD8+ T-cell function in mucosal and systemic compartments of simian immunodeficiency virus mac251- and simian-human immunodeficiency virus KU2-infected macaques. J Virol 75:11483–11495. doi: 10.1128/JVI.75.23.11483-11495.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vogel TU, Allen TM, Altman JD. 2001. Functional impairment of simian immunodeficiency virus-specific CD8+ T cells during the chronic phase of infection. J Virol 75:2458–2461. doi: 10.1128/JVI.75.5.2458-2461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiong Y, Luscher MA, Altman JD, Hulsey M, Robinson HL, Ostrowski M, Barber BH, MacDonald KS. 2001. Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8(+) T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J Virol 75:3028–3033. doi: 10.1128/JVI.75.6.3028-3033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pissani F, Streeck H. 2014. Emerging concepts on T follicular helper cell dynamics in HIV infection. Trends Immunol 35:278–286. doi: 10.1016/j.it.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perreau M, Savoye A-L, De Crignis E, Corpataux J-M, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. 2013. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med 210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lilly F, Steeves R. 1973. B-tropic Friend virus: a host-range pseudotype of spleen focus-forming virus (SFFV). Virology 55:363–370. doi: 10.1016/0042-6822(73)90176-1. [DOI] [PubMed] [Google Scholar]
- 78.Dittmer U, Brooks DM, Hasenkrug KJ. 1998. Characterization of a live-attenuated retroviral vaccine demonstrates protection via immune mechanisms. J Virol 72:6554–6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dietze KK, Zelinskyy G, Gibbert K, Schimmer S, Francois S, Myers L. 2011. Transient depletion of regulatory T cells in transgenic mice reactivates virus-specific CD8+ T cells and reduces chronic retroviral set points. Proc Natl Acad Sci USA 108:2420–2425. doi: 10.1073/pnas.1015148108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zelinskyy G, Kraft ARM, Schimmer S, Arndt T, Dittmer U. 2006. Kinetics of CD8+ effector T cell responses and induced CD4+ regulatory T cell responses during friend retrovirus infection. Eur J Immunol 36:2658–2670. doi: 10.1002/eji.200636059. [DOI] [PubMed] [Google Scholar]
- 81.He H, Messer RJ, Sakaguchi S, Yang G, Robertson SJ, Hasenkrug KJ. 2004. Reduction of retrovirus-induced immunosuppression by in vivo modulation of T cells during acute infection. J Virol 78:11641–11647. doi: 10.1128/JVI.78.21.11641-11647.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]