ABSTRACT
Dengue viruses (DENV) infect 50 to 100 million people each year. The spread of DENV-associated infections is one of the most serious public health problems worldwide, as there is no widely available vaccine or specific therapeutic for DENV infections. To address this, we developed a novel tetravalent dengue vaccine by utilizing virus-like particles (VLPs). We created recombinant DENV1 to -4 (DENV1-4) VLPs by coexpressing precursor membrane (prM) and envelope (E) proteins, with an F108A mutation in the fusion loop structure of E to increase the production of VLPs in mammalian cells. Immunization with DENV1-4 VLPs as individual, monovalent vaccines elicited strong neutralization activity against each DENV serotype in mice. For use as a tetravalent vaccine, DENV1-4 VLPs elicited high levels of neutralization activity against all four serotypes simultaneously. The neutralization antibody responses induced by the VLPs were significantly higher than those with DNA or recombinant E protein immunization. Moreover, antibody-dependent enhancement (ADE) was not observed against any serotype at a 1:10 serum dilution. We also demonstrated that the Zika virus (ZIKV) VLP production level was enhanced by introducing the same F108A mutation into the ZIKV envelope protein. Taken together, these results suggest that our strategy for DENV VLP production is applicable to other flavivirus VLP vaccine development, due to the similarity in viral structures, and they describe the promising development of an effective tetravalent vaccine against the prevalent flavivirus.
IMPORTANCE Dengue virus poses one of the most serious public health problems worldwide, and the incidence of diseases caused by the virus has increased dramatically. Despite decades of effort, there is no effective treatment against dengue. A safe and potent vaccine against dengue is still needed. We developed a novel tetravalent dengue vaccine by using virus-like particles (VLPs), which are noninfectious because they lack the viral genome. Previous attempts of other groups to use dengue VLPs resulted in generally poor yields. We found that a critical amino acid mutation in the envelope protein enhances the production of VLPs. Our tetravalent vaccine elicited potent neutralizing antibody responses against all four DENV serotypes. Our findings can also be applied to vaccine development against other flaviviruses, such as Zika virus or West Nile virus.
KEYWORDS: DNA vaccine, dengue virus, flavivirus, neutralizing antibodies, vaccine, virus-like particle, VLP, Zika virus
INTRODUCTION
Dengue is a mosquito-borne disease caused by dengue virus (DENV) and has been recognized as a serious public health problem worldwide. DENV is a positive-strand RNA virus that belongs to the Flavivirus genus of the Flaviviridae family. There are four DENV serotypes cocirculating in areas of endemicity, and these share 60 to 75% identity at the amino acid level but are clinically indistinguishable (1). Infection by any of the four serotypes of DENV causes dengue fever, which is a flu-like febrile illness, and occasionally progresses to life-threatening dengue hemorrhagic fever or dengue shock syndrome (2). About 50% of the world's population is currently at risk of DENV infection (3). There remains no effective dengue-specific antiviral treatment or therapy, and vector control efforts to prevent the spread of DENV have been ineffective (4). Therefore, an effective vaccine is viewed as one of the most desired methods for controlling this disease.
A major challenge in dengue vaccine development is the existence of four closely related DENV serotypes. After an initial infection with one DENV serotype, individuals who are subsequently exposed to any of the other serotypes are more likely to develop a more severe case of the disease due to a phenomenon known as antibody-dependent enhancement (ADE); it has been reported that nonneutralizing levels of anti-DENV antibody can enhance viral entry into host cells by forming a DENV-antibody complex (5–7). There is concern that an incomplete immune response upon first immunization may cause ADE-mediated severe dengue disease during the period between the first and the last immunizations. Hence, there is a need for a safe and highly efficacious dengue vaccine that provides long-lasting immunity against all four serotypes simultaneously, with a short immunization schedule.
Currently, CYD-TDV (Dengvaxia) is the only licensed dengue vaccine in the world. CYD-TDV is a live attenuated tetravalent dengue vaccine developed by Sanofi Pasteur, and it requires three injections over one extended year (at 0, 6, and 12 months) to induce a well-balanced antibody response against all four serotypes (6, 8). The overall pooled vaccine efficacy for symptomatic dengue during the first 25 months postdose 1 was 60.3% for all participants (9). However, efficacy in children under 9 years of age was lower (44.6%), with 70.1% efficacy in seropositive children and 14.4% efficacy in seronegative children (9). The vaccine was licensed only for persons of 9 to 45 years of age in countries where dengue is endemic. Furthermore, interim results from long-term safety follow-up studies demonstrated an increased risk for hospitalization of vaccine-sensitized individuals (10), suggesting that the ADE-related concerns are relevant.
A virus-like-particle (VLP) vaccine is a feasible alternative to live attenuated vaccines. VLPs are self-assembled particles consisting of viral structural proteins, which mimic the conformation of the authentic native virus but lack its genomic DNA or RNA (11, 12), and they are the basis for a number of safe, marketed vaccines against hepatitis B virus and human papillomavirus (13). VLPs are highly immunogenic due to the resemblance of their morphology to that of authentic viruses, and they are safe because they are noninfectious and do not pose a risk of reversion to virulence. Another key advantage of using VLPs to develop a dengue vaccine include a short vaccination schedule, which will reduce the risk of ADE-mediated severe dengue cases. Furthermore, a multivalent VLP-based dengue vaccine is expected to elicit balanced antibody responses against all four DENV serotypes, as there is no concern for replication interference, which is a key issue for a live attenuated dengue vaccine (14).
It has been reported that the coexpression of flavivirus precursor membrane (prM) and envelope (E) produces VLPs, with several reports on DENV VLP production in insect, yeast, and mammalian cells published by various groups; however, DENV VLP production levels were relatively low (15–21). For our study, we created novel DENV VLPs for all four serotypes and produced them at high yields by introducing mutations into the E protein. Tetravalent vaccination with DENV VLPs elicited high titers of neutralizing antibody (NAb) against all four serotypes simultaneously in mice. Our tetravalent DENV VLP vaccine thus bears the potential to serve as a next-generation dengue vaccine and as a template for other flavivirus vaccine development strategies.
RESULTS
Amino acid mutation in E produces DENV1 VLPs at high yields.
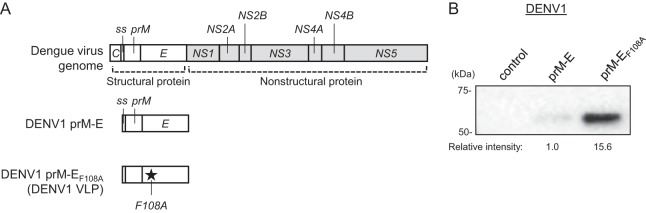
The DENV genome encodes three structural proteins (capsid [C], prM, and E) that form the virus coat and seven nonstructural proteins that take part in virus replication within host cells. The complex of prM and E plays important roles in virus assembly and fusion to the host cell membrane. The E protein lies on the surface of the dengue virion and plays a direct role in cell entry, and it is thus recognized as a target for dengue vaccine development (22). We created a DENV1 prM-E expression plasmid containing the hydrophobic signal sequence (ss; located between the C and prM genes) and the prM and E genes of DENV1 (Fig. 1A). When the plasmid was transfected into 293F cells, DENV1 prM-E VLPs were secreted into the culture supernatant. However, the amount of VLPs in the supernatant was very low as measured by Western blotting using an anti-DENV1 to -4 E (anti-DENV1-4 E) monoclonal antibody (Fig. 1B, middle lane).
FIG 1.
Development of DENV1 VLPs. (A) Schematic illustration of the DENV genome and DENV1 VLP expression vectors. C, capsid; ss, signal sequence; prM, precursor membrane protein; E, envelope. (B) Western blot analysis of DENV1 VLP-containing culture supernatant. 293F cells were transfected with the control vector or an expression plasmid encoding wild-type DENV1 prM-E (prM-E) or prM-E with the F108A mutation (prM-EF108A) and then cultured for 4 days. Secreted DENV1 VLPs were detected using a mouse anti-DENV1-4 E monoclonal antibody. The intensity of each band was measured, and the relative intensity was calculated by setting the intensity of prM-E to 1.0. An image representative of at least 3 independent experiments is shown.
To overcome the low yield, we focused on modifying DENV E because the DENV E protein undergoes structural conformational changes when the dengue virion fuses with the host cell membrane. In a previously reported work on alphavirus VLP vaccine development, it was shown that mutating the E2 protein in the region of the conformational change greatly increased the VLP yield (23). Since flaviviruses and alphaviruses both use mechanisms of class II fusion proteins for viral entry (24), we hypothesized that a modification to the amino acid(s) which effects an E protein conformational change may also increase the DENV VLP yield. We selected 27 amino acids (aa) that could potentially affect the conformational change of the DENV1 E protein, introduced a single amino acid mutation for each residue, and assessed VLP production levels in culture supernatants (Table 1). As a result, we found that mutation of phenylalanine 108 to alanine (F108A) greatly increased the DENV1 VLP production. The production of DENV1 prM-EF108A VLPs (here called DENV1 VLPs) was about 16-fold higher than that of wild-type DENV1 prM-E VLPs (Fig. 1B).
TABLE 1.
VLP secretion into culture supernatants by DENV1 prM-E mutantsa
| DENV1 prM-E construct | VLP secretion |
|---|---|
| Wild type | + |
| K64S | + |
| K64V | − |
| R73A | − |
| R93A | − |
| R99Q | − |
| R99M | + |
| W101F | − |
| L107A | − |
| F108A | +++ |
| K110Q | − |
| K118M | − |
| K120T | − |
| K203N | ++ |
| K204N | − |
| K210N | − |
| H244A | − |
| H244F | − |
| H244Y | − |
| H244R | − |
| K246E | − |
| K246R | − |
| K246N | − |
| K246V | − |
| K246A | + |
| K246T | + |
| K246M | ++ |
| K247N | − |
DENV1 VLPs were detected by use of a mouse anti-DENV1-4 E monoclonal antibody. The intensity of each band was measured, and the relative intensity was calculated by setting the intensity of wild-type DENV1 prM-E to 1.0. −, no DENV1 VLPs were detected in the culture supernatant by Western blotting with anti-DENV E antibody; +, ≤1-fold VLP production compared to that for wild-type DENV1 prM-E; ++, 1.1- to 3-fold VLP production; +++, >3-fold VLP production.
Development of DENV2, DENV3, DENV4, and Zika virus (ZIKV) VLPs.
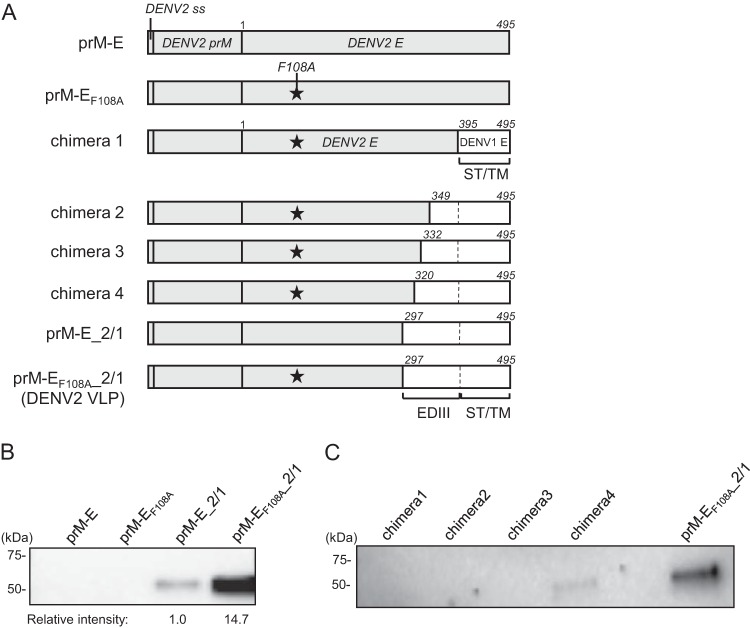
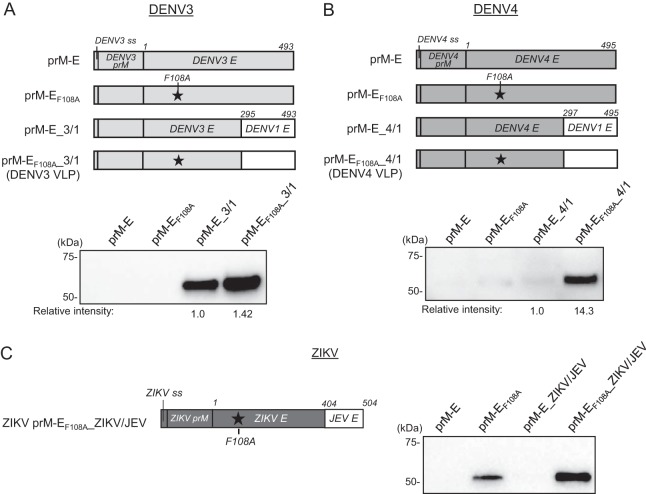
F108 is located in the fusion loop structure of the DENV E protein, which consists of hydrophobic amino acids and is responsible for the insertion of DENV E protein into the host cell membrane (22). As this sequence is highly conserved among flaviviruses, we expected that the F108A mutation would also increase the production of other serotypes of DENV VLPs. Hence, we first constructed expression plasmids encoding DENV2 prM-E with or without the F108A mutation (Fig. 2A, DENV2 prM-EF108A and prM-E). However, when they were transfected into 293F cells, DENV2 prM-E and prM-EF108A did not secrete VLPs into the culture supernatant as measured by Western blotting using an anti-DENV2 E polyclonal antibody (Fig. 2B). The DENV2 E protein is 495 aa long and has structurally distinct domains, namely, envelope domain I (EDI), EDII, EDIII, and the stem and transmembrane anchor (ST/TM) region (22). It has been thought that the ST/TM region contains a membrane retention signal which might contribute to the inefficient secretion of prM-E VLPs (25). To facilitate DENV2 VLP secretion, we next created a chimeric DENV2 construct by replacing the C-terminal region of DENV2 E protein with the corresponding region of DENV1 E protein. We prepared several chimeric DENV2 E proteins with C-terminal regions of different lengths and assessed their VLP production in 293F cells. We found that replacing aa 297 to 495 of the DENV2 E protein, which correspond to the whole EDIII and ST/TM region, with DENV1 E protein aa 297 to 495 enabled efficient DENV2 VLP production (Fig. 2C, prM-EF108A_2/1). The amount of secreted VLPs with the F108A mutation (prM-EF108A_2/1) was about 15-fold higher than that of VLPs without F108 mutation (prM-E_2/1) (Fig. 2B). Next, we used the same approach to produce DENV3 and DENV4 VLPs. Similar to the results for DENV2, the expression plasmids encoding DENV3 and DENV4 prM-E or prM-EF108A did not secrete VLPs into the culture supernatants (Fig. 3A and B). In DENV3, VLP production was greatly increased when the EDIII and ST/TM region of DENV3 E protein was replaced with aa 297 to 495 of DENV1 E protein (prM-E_3/1), and it was further enhanced by introducing the F108A mutation (Fig. 3A, prM-EF108A_3/1). Similar to the DENV2 VLP result, the production of DENV4 VLPs with the F108A mutation and EDIII and ST/TM replacement (prM-EF108A_4/1) was about 14-fold higher than that of VLPs without F108 mutation (prM-E_4/1) (Fig. 3B).
FIG 2.
Development of DENV2 VLPs. (A) Schematic illustration of DENV2 VLP expression vectors. prM-E, wild-type sequence of DENV2 prM-E; prM-EF108A, prM-E with the F108A mutation; chimeras 1 to 4, chimeric DENV2 E constructs with the F108A mutation and different lengths of DENV1 E in the C-terminal region; prM-E_2/1, DENV2 E protein aa 297 to 495 were replaced by the corresponding region of the DENV1 E protein; prM-EF108A_2/1, prM-E_2/1 with the F108A mutation. EDIII, envelope domain III; ST/TM, stem and transmembrane anchor. (B and C) Western blot analysis of DENV2 VLPs in culture supernatants. Expression vectors were transfected into 293F cells, and culture supernatants were tested for VLP production on day 4 by using a goat anti-DENV2 E polyclonal antibody. Images representative of at least 3 independent experiments are shown. (B) Effect of F108A mutation in prM-E or prM-E_2/1. The intensity of each band was measured, and the relative intensity was calculated by setting the intensity of prM-E_2/1 to 1.0. (C) Effects of replacing different lengths of the DENV2 C-terminal region on VLP production.
FIG 3.
Development of DENV3, DENV4, and ZIKV VLPs. (A and B) (Top) Schematic illustrations of VLP expression vectors. (Bottom) Western blot analyses of DENV3 and DENV4 VLPs in culture supernatants. Expression vectors were transfected into 293F cells, and culture supernatants were tested for VLP production on day 4 by using antibodies against DENV1-4 E (A) or DENV4 E (B). The intensity of each band were measured, and the relative intensity was calculated by setting the intensity of the comparator VLPs to 1.0. Images representative of at least 3 replicates are shown. (C) (Left) Schematic illustration of prM-EF108A_ZIKV/JEV. ZIKV E protein aa 404 to 504 were replaced with the corresponding region of JEV, and the F108A mutation was introduced. (Right) Western blot analysis of the ZIKV VLP constructs. Culture supernatants were harvested at 4 days posttransfection, and E proteins were detected by use of a rabbit anti-ZIKV E polyclonal antibody. Images representative of at least 3 independent experiments are shown.
We also tested this strategy to develop ZIKV VLPs. ZIKV is a mosquito-borne flavivirus that is responsible for the recent outbreaks in South and Central America (26), and its structure is similar to that of other flaviviruses, including DENV (27). While the expression of wild-type ZIKV prM-E did not produce VLPs well, prM-E with the F108A mutation in the E protein enabled ZIKV VLP production (Fig. 3C, prM-E and prM-EF108A). Unlike the results for DENV2 to -4, replacement of the ZIKV EDIII and ST/TM region with that of DENV1 did not produce VLPs even with the F108A mutation (data not shown). We found that replacement of the ZIKV ST/TM region (aa 404 to 504) with the Japanese encephalitis virus (JEV) ST/TM region in combination with the F108A mutation further increased ZIKV VLP production levels (Fig. 3C, prM-EF108A_ZIKV/JEV). These data demonstrate that distinct combinations of manipulations to the C-terminal region of the E protein (replacement of ST/TM plus EDIII or ST/TM only) and introduction of the F108A mutation may be applied to increase flavivirus VLP production.
Production and characterization of DENV1-4 VLPs.
The production levels of DENV2 and -4 VLPs were relatively lower than that of DENV3 VLPs. The original DENV2 expression constructs tested for Fig. 2 were derived from the S1 vaccine strain (28). To improve the yield of DENV2 VLPs, we prepared another prM-EF108A_2/1 expression construct, using a different DENV2 strain (D2/TO/UH04/1974) of the American genotype (29), and compared the VLP production level to that of the S1 strain. We ultimately selected the American strain for the following studies based on its higher VLP yield than that from the S1 strain (1.8-fold) (Fig. 4A). Next, to improve the yield of DENV4 VLPs, we introduced an amino acid mutation into the furin recognition site in prM. As DENV3 VLP constructs showed better VLP production than that of the DENV2 VLP constructs, we were prompted to match the DENV4 furin recognition sequence to that of DENV3. Since replacement of glutamic acid 90 by aspartic acid (E90D) slightly improved DENV4 VLP expression (1.2-fold) (Fig. 4B), we selected this construct for the following studies. A summary of the DENV1-4 VLP constructs used for the characterization and immunization studies is shown in Table 2.
FIG 4.
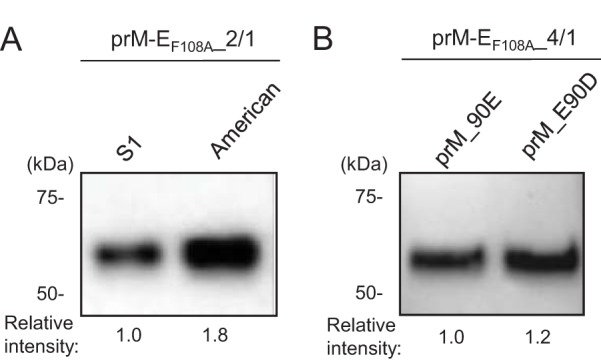
Optimization of DENV2 and DENV4 VLP constructs. (A) VLP production levels of the prM-EF108A_2/1 construct with two different DENV2 strains. Expression vectors were transfected into 293F cells, and culture supernatants were tested for VLP production on day 4 by using a goat anti-DENV2 E polyclonal antibody. (B) Effects of prM E90D mutation on DENV4 VLP production. Culture supernatants were harvested at 4 days posttransfection and assessed by Western blotting using an anti-DENV4 E monoclonal antibody. Images representative of at least 3 independent experiments are shown.
TABLE 2.
Constructs used for DENV VLP vaccine development
| VLP type | Furin recognition site in prM | F108A mutation in E | Chimera |
|---|---|---|---|
| DENV1 VLPs | RDKR (wild type) | + | − |
| DENV2 VLPs | REKR (wild type) | + | + |
| DENV3 VLPs | RDKR (wild type) | + | + |
| DENV4 VLPs | REKR (wild type) → RDKR (prME_90D) | + | + |
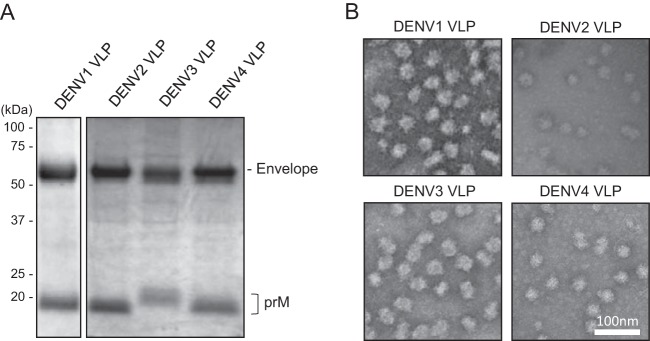
The optimized DENV1-4 VLPs were expressed in 293F cells and purified from culture supernatants by utilizing a combination of anion-exchange column and ceramic hydroxyapatite column chromatography. The purified DENV1-4 VLP samples contained E (53 kDa) and prM (around 20 kDa) as major proteins when they were assessed by SDS-PAGE and Coomassie blue staining (Fig. 5A). The VLP yields after the purification steps, as determined by the Bradford protein assay, were up to 3 mg/liter for DENV3 VLPs and up to 1.5 mg/liter for DENV1, -2, and -4 VLPs. Transmission electron microscopy (EM) images confirmed that DENV1-4 VLPs exhibited electron-dense 35- to 50-nm spherical particles (Fig. 5B), which are similar in size to previously described DENV VLPs (16, 20, 30) but smaller than dengue virions, which have a diameter of around 50 nm (31).
FIG 5.
Characterization of DENV VLPs. (A) SDS-PAGE analysis of purified DENV1 to -4 VLPs. Separated proteins were stained with Coomassie blue dye. Images representative of at least 3 replicates are shown. (B) Transmission electron microscopy images of purified DENV1 to -4 VLPs. Representative images are shown.
Immunogenicity and neutralizing antibody responses in mice induced by monovalent VLP vaccines.
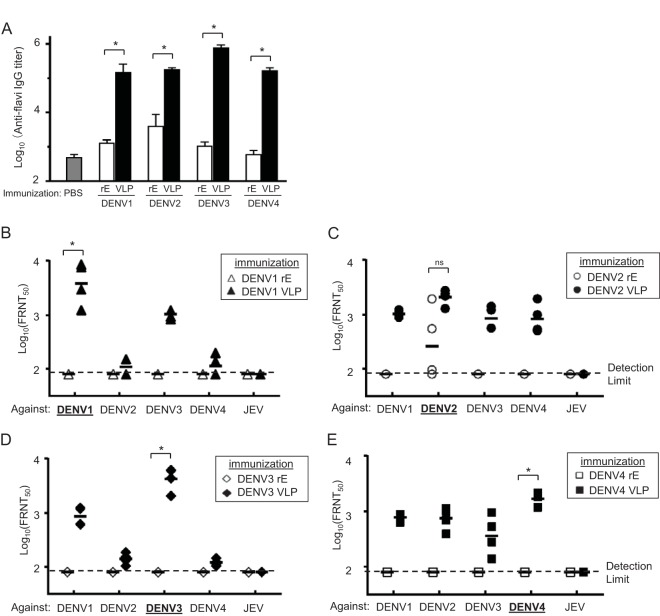
To evaluate the immune responses induced by DENV VLPs, BALB/c mice were immunized with 20 μg of monovalent DENV VLPs of each serotype or 20 μg of recombinant E proteins (rEs; aa 46 to 413) with aluminum hydroxide (alum) adjuvant intramuscularly (i.m.) three times at 3-week intervals. Two weeks after the last immunization, serum anti-flavivirus IgG titers were measured by an enzyme-linked immunosorbent assay (ELISA), using purified JEV antigen (32). In comparison to the phosphate-buffered saline (PBS) control group, mice immunized with rEs elicited anti-flavivirus antibodies. VLPs induced significantly higher anti-flavivirus IgG titers than those elicited by rEs (Fig. 6A).
FIG 6.
Immunogenicity and neutralizing activity of monovalent DENV VLPs. Six-week-old female BALB/c mice (n = 4) were immunized intramuscularly with PBS, 20 μg of DENV VLPs, or recombinant dengue virus E protein (rE) together with alum three times at 3-week intervals. Sera were collected 2 weeks after the last immunization. (A) Serum anti-flavivirus IgG titers were determined by ELISA. Mean log10 ELISA titers (with SD) are shown. *, P < 0.05 (Mann-Whitney U test for homologous serotype of DENV rE versus VLPs). (B to E) Serum neutralization titers of rE- or DENV VLP-immunized mice against DENV1 to -4 and JEV. Each symbol represents the FRNT50 titer from an individual mouse, and the geometric mean titers are represented by horizontal bars. Open and filled symbols indicate DENV rE and VLP immunizations, respectively. *, P < 0.05, ns, not significant (Mann-Whitney U test for homologous serotype of DENV rE versus VLPs).
NAb titers against DENV1 to -4 were determined by the 50% focus reduction neutralization test (FRNT50) (33). NAb titers induced by DENV rEs were below the detection limit except in the case of mice immunized with DENV2 rE. On the other hand, DENV VLPs induced high NAb responses against their homologous DENV serotype (Fig. 6B to E). The NAbs induced by DENV VLPs were serotype cross-reactive, but no cross-reactivity to JEV was detected. Geometric mean (geomean) FRNT50 titers against homologous DENV in DENV VLP-immunized mice were 3,965 (DENV1), 2,071 (DENV2), 4,267 (DENV3), and 1,693 (DENV4).
Immunogenicity and neutralizing antibody responses in mice induced by tetravalent VLP vaccines.
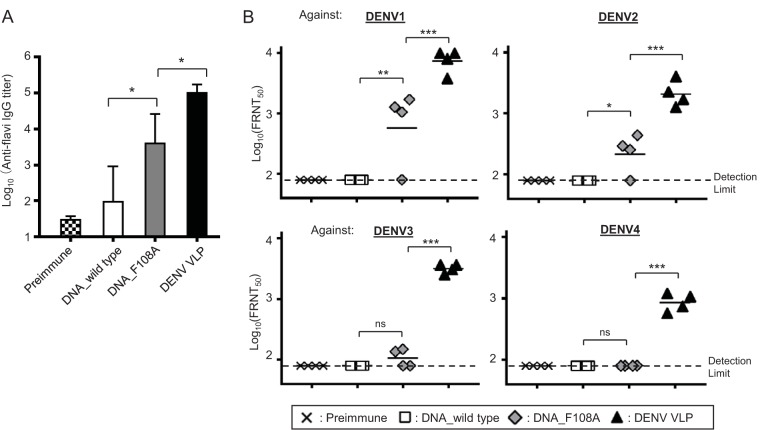
We next evaluated the immunogenicity of DENV VLPs in a tetravalent formulation in mice. BALB/c mice were immunized with PBS or 80 μg of tetravalent DENV1-4 VLPs (20 μg per serotype) with alum adjuvant three times at 3-week intervals. We also compared the immunogenicity of DNA vaccines to that of the VLP vaccine by immunizing mice with plasmid DNA encoding wild-type DENV prM-E (DNA_wild type) or encoding our DENV VLPs containing the F108A mutation and/or chimeric E (DNA_F108A). Eighty micrograms of tetravalent DNA plasmids (20 μg per serotype) was administered by i.m. injection with electroporation as described previously (34). The tetravalent DNA vaccine encoding DENV VLPs (DNA_F108A) induced higher immune responses than those with the DNA vaccine encoding wild-type prM-E (DNA_wild type). The mice immunized with tetravalent VLPs demonstrated the highest anti-flavivirus IgG titers among the three groups (Fig. 7A).
FIG 7.
Immunogenicity and neutralizing activity of tetravalent DNA or VLP vaccines. Six-week-old female BALB/c mice (n = 4) were immunized with 80 μg (20 μg of each serotype) of tetravalent DNA or tetravalent DENV VLPs three times at 3-week intervals. DNAs were administered by intramuscular injection and electroporation, and VLPs were administered by regular intramuscular injection with alum. Sera were collected 2 weeks after the last immunization. DNA_wild type, DENV1-4 prM-E-encoding plasmids; DNA_F108A, DENV1-4 VLP-encoding plasmids; DENV VLP, purified DENV1-4 VLPs. (A) Serum anti-flavivirus IgG titers were determined by ELISA. Mean log10 ELISA titers (with SD) are shown. *, P < 0.05 (one-way analysis of variance [ANOVA] followed by Tukey's multiple-comparison test). (B) Serum neutralization titers against DENV1 to -4. Each symbol represents the FRNT50 titer from an individual mouse, and the geometric mean titers are represented by horizontal bars. ***, P < 0.001; **, P < 0.01; *, P < 0.05; ns, not significant (one-way ANOVA followed by Tukey's multiple-comparison test).
Moreover, tetravalent VLPs elicited a highly potent NAb response (Fig. 7B). Geomean FRNT50 titers against the four DENV serotypes in tetravalent VLP-immunized mice were 7,479 (DENV1), 2,131 (DENV2), 3,202 (DENV3), and 869 (DENV4). The NAb response was not detected in the DNA_wild type group (FRNT50 titers were below the detection limit of 80). The DNA_F108A group showed substantial NAb responses, but the FRNT50 titers were significantly lower, less than 11% of those elicited in the VLP-immunized group. Geomean FRNT50 titers against the four DENV serotypes in DNA_F108A-immunized mice were 656 (DENV1), 227 (DENV2), 107 (DENV3), and below the detection limit of 80 (DENV4). These data suggest that DENV VLP vaccine is a more effective vaccine form than the DNA vaccine format for the induction of potent NAbs.
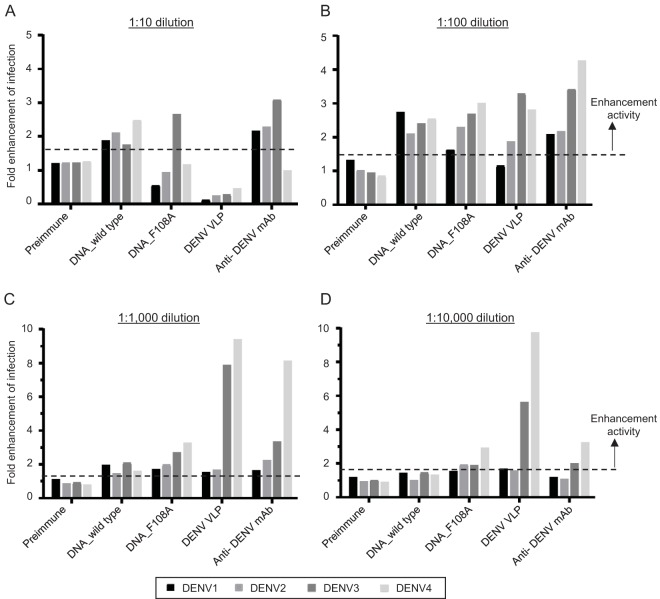
Next, we tested the infection-enhancing capacity of antibodies elicited by DENV VLP immunization. Preexisting, nonneutralizing anti-DENV antibody can enhance viral entry into host cells by forming DENV-antibody complexes (35). This phenomenon is known as ADE. To assess ADE activity, we utilized the Fc gamma receptor (FcγR)-expressing BHK cell system (36).
Sera from mice immunized with DENV DNA vaccines, both DNA_wild type and DNA_F108A, enhanced DENV infection 2- to 3-fold at a 1:10 serum dilution, demonstrating that the induced antibodies possessed ADE activity toward DENV (Fig. 8). In contrast, we did not observe infection enhancement for any of the four serotypes in sera from VLP-immunized mice at a 1:10 serum dilution. When the sera from VLP-immunized mice were diluted more than 1:100, infection enhancement was observed as expected, although no enhancement against DENV1 was observed in the dilutions we tested.
FIG 8.
Antibody-dependent enhancement activity of sera from tetravalent DENV DNA- or VLP-immunized mice. DENV1 to -4 were incubated with serially diluted mouse sera or anti-flavivirus E monoclonal antibody and added to FcγR-expressing BHK cells. After 2 days, infected cell numbers were counted. (A to D) Data were shown as fold infection enhancement values by setting the mean number of infected cells in the absence of serum or antibody to 1.0. The mean value for at least 3 negative-control wells plus 3 SD (indicated as a dotted line) was used as the cutoff value to determine which samples had ADE activity.
NAb responses to DENV3 and DENV4 VLPs in outbred mice.
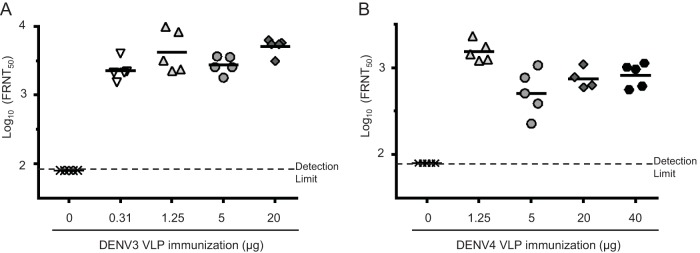
Lastly, to evaluate the immunogenicity of DENV VLPs in a representative, genetically diverse population, we immunized NIH Swiss outbred mice with DENV3 and DENV4 VLPs. We also tested different doses to assess the dose-response relationship between NAb titer and VLP dose. Mice were immunized with monovalent DENV3 or DENV4 VLPs at different doses with alum three times at 3-week intervals (Fig. 9). FRNT50 assays using sera collected 2 weeks after the last immunization demonstrated that both types of VLPs elicited potent NAb responses in the outbred mice (Fig. 9). FRNT50 titers elicited by 20-μg immunizations with DENV3 and DENV4 VLPs in NIH Swiss mice were 5,106 and 751, respectively, similar to the FRNT50 titers in BALB/c mice immunized with 20 μg of VLPs. Potent NAb responses were observed at all doses tested. These results suggest that our VLP vaccine may be effective at doses as low as 1.25 μg per serotype (geomean FRNT50 titers, 4,262 [DENV3] and 1,541 [DENV4]). Taken together, these results demonstrated that our DENV1-4 VLPs were highly immunogenic and that our tetravalent DENV VLP vaccine elicited potent NAb responses against all four DENV serotypes simultaneously.
FIG 9.
Neutralizing activity of monovalent DENV VLPs in outbred mice. Six-week-old female NIH/Swiss mice (n = 5) were immunized intramuscularly with PBS, DENV3 VLPs (0.31, 1.25, 5, or 20 μg), or DENV4 VLPs (1.25, 5, 20, or 40 μg) with alum three times at 3-week intervals. Sera were collected 2 weeks after the last immunization. (A) Serum neutralization titers of DENV3 VLP-immunized mice against DENV3. (B) Serum neutralization titers of DENV4 VLP-immunized mice against DENV4. Each symbol represents the FRNT50 titer from an individual mouse, and the geometric mean titers are represented by horizontal bars.
DISCUSSION
The global spread and persistence of DENV have made dengue vaccine development a pressing matter. In this study, we found an effective strategy to produce DENV VLPs and developed a novel tetravalent dengue VLP vaccine which induces strong neutralizing responses against all four DENV serotypes.
The introduction of an F108A mutation into the E protein increased VLP production for all four serotypes of DENV as well as ZIKV. Amino acid residue F108 is located in the fusion loop of E, which is highly conserved among flaviviruses (37). During viral infection, the E protein undergoes conformational changes that expose the hydrophobic fusion loop on the viral surface. The fusion loop is inserted into the host cell membrane and induces a fusion of the viral and host cell membranes that results in release of the viral genome into the cytosol (22). It has been reported that flavivirus VLPs composed of prM-E also undergo a similar process to fuse with the cell membranes (38). This process may affect the viability of the cells producing VLPs and the yield of VLP production. The F108A mutation may prevent VLPs from fusing to 293F cells during the production process, thereby leading to the high-level VLP production observed.
For DENV2 to -4, in addition to the F108A mutation, replacement of the C-terminal region with the corresponding region of DENV1 was necessary to achieve efficient VLP production (Fig. 2 and 3). The ST/TM region is known to contain a membrane retention sequence, which might prevent VLP budding from the plasma membrane (25). Replacing aa 297 to 495 (EDIII and ST/TM) of DENV2 to -4 with those of DENV1 might affect the membrane retention sequence in a manner that enhances the secretion of these VLPs. Importantly, based on the dengue virus E dimer structure, F108 is in close contact with EDIII (39). The contact of the F108A mutation and DENV1 EDIII in the DENV2 to -4 VLPs might also affect the structural stability in the context of the dengue virus E dimer structure form, which may increase the yield of VLPs. Further studies are needed to support these hypotheses.
Although the EDIII region of our DENV2 to -4 VLPs was replaced with DENV1 EDIII, these VLPs induced serotype-specific NAbs in mice (Fig. 6), suggesting that there are neutralizing epitopes located in EDI, EDII, or the EDI/EDII hinge region. These regions have been recognized as potent targets of NAbs based on several mapping studies using mouse monoclonal antibodies that showed potent neutralizing activities (40). Interestingly, recent studies showed that EDIII-specific antibodies alone are unlikely to account for the strong NAb responses observed in people naturally infected with DENV (41–43). In addition, potent DENV NAbs that bind to epitopes around EDI/EDII were discovered in DENV-immune humans (44, 45). Our results support the observation that EDI/EDII-targeting antibodies contribute to the serotype-specific NAb responses.
When mice were immunized with the plasmid DNAs encoding our DENV VLPs (prM-E with F108A mutation and C-terminal region replacement), we observed higher NAb responses than those of mice immunized with DNAs encoding wild-type prM-E (Fig. 7B). This may be reflective of the different VLP production levels in vivo. Immunization with DNA induces host cells to produce the encoding proteins, resulting in specific immune activation against them (46). Indeed, when mice were immunized with DENV DNA vaccines, there was a significant correlation between the level of in vitro VLP secretion by the DNA vaccine construct and the elicited immune responses (29). Compared to mice immunized with the DNA vaccines or recombinant E proteins, we observed that mice immunized with VLP vaccines induced much higher NAb responses (Fig. 6B to D and 7B). VLP-based vaccines have been successful in protecting humans from various viruses, including hepatitis B virus and human papillomavirus (13). Because of their good safety profiles and strong immunogenicity, many other VLP-based vaccine candidates, such as those against chikungunya virus (CHIKV), influenza virus, or norovirus, are in clinical trials or under preclinical studies (47, 48). Although there is no commercialized VLP-based vaccine against flavivirus, to date, previous studies have demonstrated that coexpression of the prM and E proteins could produce VLPs of West Nile virus, St. Louis encephalitis virus, and JEV (49, 50). Recently, several reports showed that DENV VLPs could induce effective NAb responses in mice immunized with a monovalent vaccine (51, 52). However, tetravalent VLP vaccine development has not been very successful. It has been reported that a tetravalent DENV VLP vaccine candidate produced in Pichia pastoris was immunogenic in mice, but the NAb titers were somewhat low (1:16 to 1:32 by the 50% plaque reduction neutralization test [PRNT50] for immunization with 25 μg per serotype) (53). In our study, tetravalent DENV VLPs induced FRNT50 titers of 1:869 to 1:7,479, which suggests that our tetravalent VLP vaccine can achieve more potent NAb responses than those obtained with existing candidates.
While high levels of neutralizing antibodies were detected in the VLP-immunized group (Fig. 7), ADE activity was absent at the lowest feasible dilution (1:10) (Fig. 8). Strong neutralizing activity in a conventional plaque reduction neutralization assay is associated with an absence of ADE when tested at the lowest feasible dilution (54). It has also been reported that infection enhancement activity is absent at low serum dilutions in convalescent-phase patient samples that possess high levels of neutralizing activity (55). Since immune-enhanced viral replication did not occur at the low dilution (1:10) of sera from the VLP-immunized group but occurred only at higher dilutions, it is unlikely that immune enhancement would occur under in vivo conditions, e.g., with undiluted serum, although further precise preclinical and clinical studies regarding ADE effects are needed.
Viral interference is a phenomenon observed with tetravalent live attenuated dengue vaccines. One or some replication-dominant serotypes interfere with others, resulting in an unbalanced immune response (14). The tetravalent dengue VLP vaccine is highly unlikely to show viral interference because the VLPs do not replicate. While the live attenuated dengue vaccine CYD-TDV requires a 1-year immunization schedule, our DENV VLP vaccine immunizations can be completed within about 2 months, which should greatly reduce the risk of ADE-mediated severe dengue cases. VLPs can be manufactured efficiently under current regulatory standards, and the sequences can be matched easily to those of circulating virus strains or emerging new variants if needed. In addition to these advantages, the high-level NAb response combined with high levels of VLP production make our tetravalent VLP vaccine a promising next-generation dengue vaccine candidate. Furthermore, we demonstrated that the F108A mutation and replacement of the C-terminal region of E similarly enabled ZIKV VLP production in 293F cells. This strategy will facilitate vaccine development not only against DENV but also against other flaviviruses, such as ZIKV or West Nile virus, due to the similarity across flavivirus conserved structures.
MATERIALS AND METHODS
Vector construction.
Plasmids encoding structural proteins of DENV, ZIKV, and JEV were generated by gene synthesis (GeneArt; Thermo Fisher Scientific). The signal sequence, prM, and E genes were cloned into a pUC119-based expression vector. The strains used were as follows: DENV1, Western Pacific strain (GenBank accession no. U88535.1); DENV2, S1 vaccine strain (GenBank accession no. M19197.1) and American strain (GenBank accession no. AY744147); DENV3, Singapore 8120/95 strain (GenBank accession no. AY766104.1); DENV4, ThD4_0476_97 strain (GenBank accession no. AY618988.1); ZIKV (GenBank accession no. KU312312); and JEV, Nakayama strain (GenBank accession no. EF571853). Mutagenesis was performed by use of a QuikChange Lightning site-directed mutagenesis kit (Agilent Technologies) following the manufacturer's instructions. Chimeric constructs of DENV2, DENV3, and DENV4 E with DENV1 E were produced by a standard overlap extension PCR method. Briefly, ss, prM, and aa 1 to 296 of E from DENV2, DENV3, or DENV4 were amplified with universal CMV forward and reverse primers that have connective sequences with DENV1 E at their 3′ ends. The region of aa 297 to 495 of DENV1 E was amplified using a forward primer that has the same connective sequence at the 5′ end and a reverse primer that recognizes a poly(A) region. These PCR products were mixed, amplified, and cloned into NotI and BglII sites of the pUC59 vector. For a chimeric construct of ZIKV and JEV, ZIKV ss, prM, and E aa 1 to 403 and JEV E aa 400 to 500 were connected by the same method.
Production and purification of VLPs.
VLPs were produced in FreeStyle 293F cells (Thermo Fisher Scientific). The cells were cultured in suspension in serum-free FreeStyle 293 expression medium (Thermo Fisher Scientific) and transfected with VLP-expressing plasmids by use of GeneX Plus transfection reagent (ATCC) according to the manufacturer's instructions. Four days after transfection, the cell culture supernatant was harvested and then clarified by centrifugation and filtration through a 0.45-μm polyethersulfone (PES) membrane. The culture supernatant was concentrated and buffer exchanged to 2.5 mM sodium phosphate buffer (Teknova) by use of KrosFlo Research II TFF systems with mPES MidiKros filter modules (Spectrum Laboratories). Further purification was performed by use of NGC Quest 10 chromatography systems (Bio-Rad) with tandemly connected HiTrap Q XL (GE Healthcare Life Sciences) and Foresight CHT type II (Bio-Rad) columns. The VLPs in the flowthrough from the Q XL column were captured by the CHT type II column and eluted with a 2.5 to 400 mM sodium phosphate gradient. The eluates containing VLPs were concentrated by use of Amicon Ultra-15 centrifugal filter units (EMD Millipore) and filtered through a 0.20-μm PES membrane. The total protein concentration of purified DENV VLPs was measured by the Quick Start Bradford protein assay (Bio-Rad). The purity of the DENV VLPs was assessed by SDS-PAGE followed by Coomassie blue dye-based staining, using InstantBlue Stain reagent (Expedeon).
Western blotting.
VLP-containing culture supernatant was separated by electrophoresis on Any kD Mini-Protean TGX precast protein gels (Bio-Rad), and the proteins were transferred onto nitrocellulose membranes by use of a Trans-Blot Turbo transfer system (Bio-Rad). Membranes were blocked with 5% skim milk (Labscientific, Inc.) in Tris-buffered saline containing 0.05% Tween 20 (TBS-T) and then treated with a mouse anti-DENV1-4 E monoclonal antibody (clone 9F10; Santa Cruz Biotechnology) (1:500), a goat anti-DENV2 E polyclonal antibody (Santa Cruz Biotechnology) (1:1,000), a mouse anti-DENV4 E monoclonal antibody (clone 1H10-6; ATCC) (20 μg/ml), or an anti-Zika virus rabbit polyclonal antibody (IBT Bioservices) (0.5 μg/ml). Membranes were washed in TBS-T, incubated with horseradish peroxidase (HRP)-conjugated anti-mouse, -goat, or -rabbit secondary antibody (Santa Cruz Biotechnology) (1:5,000), and developed using Clarity ECL Western blot substrate (Bio-Rad). Imaging and data analysis were done by use of Image Lab software (ver. 5.2.1; Bio-Rad).
Electron microscopy analysis.
The morphologies of DENV VLPs were analyzed at the Johns Hopkins School of Medicine Microscope Facility. Briefly, the purified DENV VLPs were fixed in 4% formaldehyde and placed on glow-discharged carbon-coated 200-mesh copper grids. The grids were then stained with 1% phosphotungstic acid and visualized by use of a Philips CM120 transmission electron microscope at 80 kV and an AMT XR80 8-megapixel camera.
Mouse experiments.
Immunization and serum sample preparation were conducted at Bioqual, Inc. (Rockville, MD). All animal experiments were conducted under Institutional Animal Care and Use Committee-approved and Office of Laboratory Animal Welfare-assured conditions. Female BALB/c mice were purchased from Harlan (Frederick, MD). PBS, rEs (Prospec, den-021, den-022, den-023, and den-024), and VLPs were administered intramuscularly with alum at weeks 0, 3, and 6. DNAs were administered by intramuscular injection followed by electroporation with a BTX 2 needle array and a BTX model 830 electroporation generator (Harvard Instruments), using the following parameters: six pulses of 100 V with 50-ms durations and 200 ms between pulses. Blood samples were taken at weeks 0, 5, and 8. Serum was prepared and heat inactivated by incubation at 60°C for 30 min.
Anti-flavivirus IgG ELISA.
Serum anti-flavivirus IgG titers were measured by enzyme-linked immunosorbent assay (ELISA). A 96-well ELISA plate was coated with purified JEV (strain JaOArS982) at 250 ng/well at 4°C overnight (32). The plate was blocked with Block Ace (DS Pharma Biomedical) for 1 h at room temperature and washed with PBS-0.05% Tween 20 (PBS-T). The test sera and standard control were diluted 1:1,000 and then added to wells, incubated for 1 h at 37°C, and washed. A 1:1,000-diluted HRP-conjugated anti-mouse IgG antibody (American Qualex) was added and incubated for 1 h at 37°C. Wells were washed and developed with o-phenylenediamine dihydrochloride (OPD; Sigma) with 0.05 M citrate phosphate buffer and a 0.03% H2O2 solution by incubation for 30 min in the dark. The reaction was stopped by addition of 1 N HCl, and the optical density at 492 nm (OD492) was read. Sample serum IgG titers were calculated from a standard curve (33). Sample titers of ≥3,000 were interpreted as anti-flavivirus IgG positive.
FRNT50.
Vaccine-immunized mouse sera were serially diluted, mixed with 40 to 50 focus-forming units of virus (DENV1 99st12A strain [genotype IV], DENV2 oost22A strain [Asian 2], DENV3 SLMC50 strain [genotype I], DENV4 SLMC318 strain [genotype I], and JEV S-982 strain [genotype III]), and incubated at 37°C for 1 h. The serum-virus mixtures were inoculated onto Vero cell monolayers in 96-well plates and incubated at 37°C for 1 h, and then 1.25% methylcellulose 4000 in minimum essential medium (MEM) with 2% fetal calf serum (FCS) was added to wells and incubated at 37°C for 3 days for DENV and 36 h for JEV. The plates were washed with PBS, fixed with a 4% paraformaldehyde solution for 30 min, and washed. The cells were then permeabilized with a 1% NP-40 solution for 30 min and washed. The plates were blocked with BlockAce for 30 min and then treated with 1:1,500-diluted pooled human sera with high anti-flavivirus IgG titers (33) for 1 h at 37°C. Subsequently, HRP-conjugated goat anti-human IgG (American Qualex) (1:1,500) was added and incubated at 37°C for 1 h. 3,3′-Diaminobenzidine tetrahydrochloride (0.5 mg/ml; Wako) with 0.03% H2O2 was added and incubated for 10 min for staining. After washing and air drying, the number of foci per well was counted using a biological microscope. The reciprocal of the endpoint serum dilution that provided a 50% or greater reduction in the mean number of foci relative to that in the control wells that contained no serum was considered to be the FRNT50 titer.
Antibody-dependent dengue virus infection enhancement assay.
FcγR-expressing BHK cell lines (36) were seeded into a 96-well plate and cultured in Eagle's MEM (EMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and G418. Vaccine-immunized mouse serum or mouse anti-flavivirus E monoclonal antibody 4G2 was serially diluted from 1:10 to 1:10,000 with 10% FBS-EMEM, mixed with 30 to 50 focus-forming units of virus (same DENV strains as those used in the FRNT50 assay), and incubated at 37°C for 1 h. Virus-immune complexes were added to each well of cells and cultured for 48 h. The cells were washed with PBS once, air dried, and fixed with ice-cold methanol-acetone (1:1). After the plates were air dried, the wells were blocked with 1% horse serum in PBS for 10 min and washed with PBS three times. The plate was then treated with anti-flavivirus antibody (MAb 4G2 at a dilution of 1:1,000) at 37°C for 30 min, biotin-conjugated anti-mouse IgG antibody (Vector Laboratories) (1:500) at room temperature for 30 min, and ABC (Vector Laboratories) solution at room temperature for 30 min. The plate was washed with PBS three times after each incubation step. VIP solution (Vector Laboratories) was added and incubated at room temperature for a few minutes, until the color developed. The plate was washed with PBS once, and infected cells were quantitated (Keyence BZ-X710 microscope). Fold enhancement values were calculated using the following formula: (mean infected cell count using FcγR-expressing BHK cells with the addition of a mouse serum sample)/(mean plaque count using FcγR-expressing BHK cells in the absence of test sample). Infection enhancement (measured as ADE activity) was tested using serum samples diluted from 1:10 to 1:10,000. The fold enhancement values were determined as follows: (mean number of DENV-infected cells in wells treated with serum samples)/(mean number of plaques in wells with monolayers incubated in the absence of test samples). The mean value for at least 3 negative-control wells plus 3 standard deviations (SD) was used as the cutoff value to determine which samples had ADE activity (56).
ACKNOWLEDGMENTS
We thank K. Tolliver, M. Nakata, J. Sastri, E. Cho-Fertikh, and G. Moonsammy (VLP Therapeutics) for facilitation of collaborations, manuscript preparation, and helpful discussions; H. Anderson, S. Kar (Bioqual), and S. Cherukuri (Noble Life Sciences) for managing animal experiments; and B. Smith (Johns Hopkins University) for EM analysis.
A.U., M.I., M.M.N.T., M.L.M., A.S., K.M., and W.A. performed the research; A.U., M.M.N.T., M.L.M., A.S., M.I., K.M., S.K., R.U., K.M., and W.A. analyzed data; A.U., M.M.N.T., M.L.M., A.S., K.M., and W.A. wrote the paper; and all authors participated in manuscript revisions. R.U. and W.A. conceived, directed, and supervised the studies.
This research was funded by VLP Therapeutics and a Global Health Innovative Technology Fund grant (grant G2016-109).
We declare that an intellectual property application has been filed by VLP Therapeutics based on data presented in this paper. A.U., A.S., and M.I. were employees of VLP Therapeutics at the time of the study. S.K., R.U., and W.A. designed the research and are management members and shareholders of VLP Therapeutics. All other authors declare no conflicts of interest.
REFERENCES
- 1.Vannice KS, Durbin A, Hombach J. 2016. Status of vaccine research and development of vaccines for dengue. Vaccine 34:2934–2938. doi: 10.1016/j.vaccine.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 2.Yacoub S, Mongkolsapaya J, Screaton G. 2016. Recent advances in understanding dengue. F1000Res 5:F1000 Faculty Rev-78. doi: 10.12688/f1000research.6233.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. 2012. Global strategy for dengue prevention and control. WHO, Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/75303/1/9789241504034_eng.pdf?ua=1. [Google Scholar]
- 4.Luz PM, Vanni T, Medlock J, Paltiel AD, Galvani AP. 2011. Dengue vector control strategies in an urban setting: an economic modelling assessment. Lancet 377:1673–1680. doi: 10.1016/S0140-6736(11)60246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halstead SB, O'Rourke EJ. 1977. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 6.WHO. 2016. Status of vaccine development. WHO, Geneva, Switzerland: http://www.who.int/immunization/research/development/dengue_vaccines/en/. [Google Scholar]
- 7.Live Dengue Vaccines Technical Consultation Reporting Group, Bentsi-Enchill AD, Schmitz J, Edelman R, Durbin A, Roehrig JT, Smith PG, Hombach J, Farrar J. 2013. Long-term safety assessment of live attenuated tetravalent dengue vaccines: deliberations from a WHO technical consultation. Vaccine 31:2603–2609. doi: 10.1016/j.vaccine.2013.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guy B, Saville M, Lang J. 2010. Development of Sanofi Pasteur tetravalent dengue vaccine. Hum Vaccin 6:12739. doi: 10.4161/hv.6.9.12739. [DOI] [PubMed] [Google Scholar]
- 9.Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, Tran HN, Bouckenooghe A, Chansinghakul D, Cortes M, Fanouillere K, Forrat R, Frago C, Gailhardou S, Jackson N, Noriega F, Plennevaux E, Wartel TA, Zambrano B, Saville M, CYD-TDV Dengue Vaccine Working Group. 2015. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 10.Aguiar M, Stollenwerk N, Halstead SB. 2016. The impact of the newly licensed dengue vaccine in endemic countries. PLoS Negl Trop Dis 10:e0005179. doi: 10.1371/journal.pntd.0005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chackerian B. 2007. Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines 6:381–390. doi: 10.1586/14760584.6.3.381. [DOI] [PubMed] [Google Scholar]
- 12.Noad R, Roy P. 2003. Virus-like particles as immunogens. Trends Microbiol 11:438–444. doi: 10.1016/S0966-842X(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig C, Wagner R. 2007. Virus-like particles—universal molecular toolboxes. Curr Opin Biotechnol 18:537–545. doi: 10.1016/j.copbio.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guy B, Barban V, Mantel N, Aguirre M, Gulia S, Pontvianne J, Jourdier TM, Ramirez L, Gregoire V, Charnay C, Burdin N, Dumas R, Lang J. 2009. Evaluation of interferences between dengue vaccine serotypes in a monkey model. Am J Trop Med Hyg 80:302–311. [PubMed] [Google Scholar]
- 15.Kelly EP, Greene JJ, King AD, Innis BL. 2000. Purified dengue 2 virus envelope glycoprotein aggregates produced by baculovirus are immunogenic in mice. Vaccine 18:2549–2559. doi: 10.1016/S0264-410X(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 16.Sugrue RJ, Fu J, Howe J, Chan YC. 1997. Expression of the dengue virus structural proteins in Pichia pastoris leads to the generation of virus-like particles. J Gen Virol 78:1861–1866. doi: 10.1099/0022-1317-78-8-1861. [DOI] [PubMed] [Google Scholar]
- 17.Konishi E, Fujii A. 2002. Dengue type 2 virus subviral extracellular particles produced by a stably transfected mammalian cell line and their evaluation for a subunit vaccine. Vaccine 20:1058–1067. doi: 10.1016/S0264-410X(01)00446-7. [DOI] [PubMed] [Google Scholar]
- 18.Chang GJ, Hunt AR, Holmes DA, Springfield T, Chiueh TS, Roehrig JT, Gubler DJ. 2003. Enhancing biosynthesis and secretion of premembrane and envelope proteins by the chimeric plasmid of dengue virus type 2 and Japanese encephalitis virus. Virology 306:170–180. doi: 10.1016/S0042-6822(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 19.Kuwahara M, Konishi E. 2010. Evaluation of extracellular subviral particles of dengue virus type 2 and Japanese encephalitis virus produced by Spodoptera frugiperda cells for use as vaccine and diagnostic antigens. Clin Vaccine Immunol 17:1560–1566. doi: 10.1128/CVI.00087-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S, Liang M, Gu W, Li C, Miao F, Wang X, Jin C, Zhang L, Zhang F, Zhang Q, Jiang L, Li M, Li D. 2011. Vaccination with dengue virus-like particles induces humoral and cellular immune responses in mice. Virol J 8:333. doi: 10.1186/1743-422X-8-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coller BA, Clements DE, Bett AJ, Sagar SL, Ter Meulen JH. 2011. The development of recombinant subunit envelope-based vaccines to protect against dengue virus induced disease. Vaccine 29:7267–7275. doi: 10.1016/j.vaccine.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modis Y, Ogata S, Clements D, Harrison SC. 2004. Structure of the dengue virus envelope protein after membrane fusion. Nature 427:313–319. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 23.Akahata W, Nabel GJ. 2012. A specific domain of the Chikungunya virus E2 protein regulates particle formation in human cells: implications for alphavirus vaccine design. J Virol 86:8879–8883. doi: 10.1128/JVI.00370-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmaljohn AL, McClain D. 1996. Chapter 54. Alphaviruses (Togaviridae) and flaviviruses (Flaviviridae). In Baron S. (ed), Medical microbiology, 4th ed University of Texas Medical Branch at Galveston, Galveston, TX. [PubMed] [Google Scholar]
- 25.Hsieh SC, Liu IJ, King CC, Chang GJ, Wang WK. 2008. A strong endoplasmic reticulum retention signal in the stem-anchor region of envelope glycoprotein of dengue virus type 2 affects the production of virus-like particles. Virology 374:338–350. doi: 10.1016/j.virol.2007.12.041. [DOI] [PubMed] [Google Scholar]
- 26.Petersen E, Wilson ME, Touch S, McCloskey B, Mwaba P, Bates M, Dar O, Mattes F, Kidd M, Ippolito G, Azhar EI, Zumla A. 2016. Rapid spread of Zika virus in the Americas—implications for public health preparedness for mass gatherings at the 2016 Brazil Olympic Games. Int J Infect Dis 44:11–15. doi: 10.1016/j.ijid.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, Kuhn RJ. 2016. The 3.8 A resolution cryo-EM structure of Zika virus. Science 352:467–470. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn YS, Galler R, Hunkapiller T, Dalrymple JM, Strauss JH, Strauss EG. 1988. Nucleotide sequence of dengue 2 RNA and comparison of the encoded proteins with those of other flaviviruses. Virology 162:167–180. doi: 10.1016/0042-6822(88)90406-0. [DOI] [PubMed] [Google Scholar]
- 29.Galula JU, Shen WF, Chuang ST, Chang GJ, Chao DY. 2014. Virus-like particle secretion and genotype-dependent immunogenicity of dengue virus serotype 2 DNA vaccine. J Virol 88:10813–10830. doi: 10.1128/JVI.00810-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charoensri N, Suphatrakul A, Sriburi R, Yasanga T, Junjhon J, Keelapang P, Utaipat U, Puttikhunt C, Kasinrerk W, Malasit P, Sittisombut N. 2014. An optimized expression vector for improving the yield of dengue virus-like particles from transfected insect cells. J Virol Methods 205C:116–123. doi: 10.1016/j.jviromet.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717–725. doi: 10.1016/S0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue S, Alonzo MT, Kurosawa Y, Mapua CA, Reyes JD, Dimaano EM, Alera MT, Saito M, Oishi K, Hasebe F, Matias RR, Natividad FF, Morita K. 2010. Evaluation of a dengue IgG indirect enzyme-linked immunosorbent assay and a Japanese encephalitis IgG indirect enzyme-linked immunosorbent assay for diagnosis of secondary dengue virus infection. Vector Borne Zoonotic Dis 10:143–150. doi: 10.1089/vbz.2008.0153. [DOI] [PubMed] [Google Scholar]
- 33.Ngwe Tun MM, Thant KZ, Inoue S, Kurosawa Y, Lwin YY, Lin S, Aye KT, Thet Khin P, Myint T, Htwe K, Mapua CA, Natividad FF, Hirayama K, Morita K. 2013. Serological characterization of dengue virus infections observed among dengue hemorrhagic fever/dengue shock syndrome cases in upper Myanmar. J Med Virol 85:1258–1266. doi: 10.1002/jmv.23577. [DOI] [PubMed] [Google Scholar]
- 34.Bodles-Brakhop AM, Heller R, Draghia-Akli R. 2009. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol Ther 17:585–592. doi: 10.1038/mt.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guzman MG, Vazquez S. 2010. The complexity of antibody-dependent enhancement of dengue virus infection. Viruses 2:2649–2662. doi: 10.3390/v2122649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moi ML, Takasaki T, Saijo M, Kurane I. 2014. Determination of antibody concentration as the main parameter in a dengue virus antibody-dependent enhancement assay using FcgammaR-expressing BHK cells. Arch Virol 159:103–116. doi: 10.1007/s00705-013-1787-3. [DOI] [PubMed] [Google Scholar]
- 37.Rockstroh A, Barzon L, Pacenti M, Palu G, Niedrig M, Ulbert S. 2015. Recombinant envelope-proteins with mutations in the conserved fusion loop allow specific serological diagnosis of dengue-infections. PLoS Negl Trop Dis 9:e0004218. doi: 10.1371/journal.pntd.0004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Esposito DL, Nguyen JB, DeWitt DC, Rhoades E, Modis Y. 2015. Physico-chemical requirements and kinetics of membrane fusion of flavivirus-like particles. J Gen Virol 96:1702–1711. doi: 10.1099/vir.0.000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Modis Y, Ogata S, Clements D, Harrison SC. 2003. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci U S A 100:6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durbin AP, Whitehead SS. 2011. Next-generation dengue vaccines: novel strategies currently under development. Viruses 3:1800–1814. doi: 10.3390/v3101800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliphant T, Nybakken GE, Austin SK, Xu Q, Bramson J, Loeb M, Throsby M, Fremont DH, Pierson TC, Diamond MS. 2007. Induction of epitope-specific neutralizing antibodies against West Nile virus. J Virol 81:11828–11839. doi: 10.1128/JVI.00643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez MD, Pierson TC, Degrace MM, Mattei LM, Hanna SL, Del Piero F, Doms RW. 2007. The neutralizing antibody response against West Nile virus in naturally infected horses. Virology 359:336–348. doi: 10.1016/j.virol.2006.08.047. [DOI] [PubMed] [Google Scholar]
- 43.Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. 2009. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology 392:103–113. doi: 10.1016/j.virol.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE Jr, de Silva AM. 2012. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A 109:7439–7444. doi: 10.1073/pnas.1200566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teoh EP, Kukkaro P, Teo EW, Lim AP, Tan TT, Yip A, Schul W, Aung M, Kostyuchenko VA, Leo YS, Chan SH, Smith KG, Chan AH, Zou G, Ooi EE, Kemeny DM, Tan GK, Ng JK, Ng ML, Alonso S, Fisher D, Shi PY, Hanson BJ, Lok SM, MacAry PA. 2012. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med 4:139ra183. doi: 10.1126/scitranslmed.3003888. [DOI] [PubMed] [Google Scholar]
- 46.Koprowski H, Weiner DB. 1998. DNA vaccination/genetic vaccination. Curr Top Microbiol Immunol 226:V–XIII. [PubMed] [Google Scholar]
- 47.Ramsauer K, Schwameis M, Firbas C, Mullner M, Putnak RJ, Thomas SJ, Despres P, Tauber E, Jilma B, Tangy F. 2015. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect Dis 15:519–527. doi: 10.1016/S1473-3099(15)70043-5. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Limas WA, Sekar K, Tyo KE. 2013. Virus-like particles: the future of microbial factories and cell-free systems as platforms for vaccine development. Curr Opin Biotechnol 24:1089–1093. doi: 10.1016/j.copbio.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Purdy DE, Chang GJ. 2005. Secretion of noninfectious dengue virus-like particles and identification of amino acids in the stem region involved in intracellular retention of envelope protein. Virology 333:239–250. doi: 10.1016/j.virol.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 50.Ohtaki N, Takahashi H, Kaneko K, Gomi Y, Ishikawa T, Higashi Y, Kurata T, Sata T, Kojima A. 2010. Immunogenicity and efficacy of two types of West Nile virus-like particles different in size and maturation as a second-generation vaccine candidate. Vaccine 28:6588–6596. doi: 10.1016/j.vaccine.2010.07.055. [DOI] [PubMed] [Google Scholar]
- 51.Poddar A, Ramasamy V, Shukla R, Rajpoot RK, Arora U, Jain SK, Swaminathan S, Khanna N. 2016. Virus-like particles derived from Pichia pastoris-expressed dengue virus type 1 glycoprotein elicit homotypic virus-neutralizing envelope domain III-directed antibodies. BMC Biotechnol 16:50. doi: 10.1186/s12896-016-0280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tripathi L, Mani S, Raut R, Poddar A, Tyagi P, Arora U, de Silva A, Swaminathan S, Khanna N. 2015. Pichia pastoris-expressed dengue 3 envelope-based virus-like particles elicit predominantly domain III-focused high titer neutralizing antibodies. Front Microbiol 6:1005. doi: 10.3389/fmicb.2015.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Y, Zhou J, Yu Z, Fang D, Fu C, Zhu X, He Z, Yan H, Jiang L. 2014. Tetravalent recombinant dengue virus-like particles as potential vaccine candidates: immunological properties. BMC Microbiol 14:233. doi: 10.1186/s12866-014-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guy B, Chanthavanich P, Gimenez S, Sirivichayakul C, Sabchareon A, Begue S, Yoksan S, Luxemburger C, Lang J. 2004. Evaluation by flow cytometry of antibody-dependent enhancement (ADE) of dengue infection by sera from Thai children immunized with a live-attenuated tetravalent dengue vaccine. Vaccine 22:3563–3574. doi: 10.1016/j.vaccine.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 55.Moi ML, Takasaki T, Saijo M, Kurane I. 2013. Dengue virus infection-enhancing activity of undiluted sera obtained from patients with secondary dengue virus infection. Trans R Soc Trop Med Hyg 107:51–58. doi: 10.1093/trstmh/trs007. [DOI] [PubMed] [Google Scholar]
- 56.Konishi E, Tabuchi Y, Yamanaka A. 2010. A simple assay system for infection-enhancing and -neutralizing antibodies to dengue type 2 virus using layers of semi-adherent K562 cells. J Virol Methods 163:360–367. doi: 10.1016/j.jviromet.2009.10.026. [DOI] [PubMed] [Google Scholar]