ABSTRACT
It is well established that interferon gamma (IFN-γ) production by CD4+ T cells is critical for antiviral immunity against herpes simplex virus 2 (HSV-2) genital infection. However, the role of interleukin-17A (IL-17A) production by CD4+ T cells in HSV-2 antiviral immunity is yet to be elucidated. Here we demonstrate that IL-17A plays an important role in enhancing antiviral T helper type 1 (Th1) responses in the female genital tract (FGT) and is essential for effective protection conferred by HSV-2 vaccination. While IL-17A did not play a critical role during primary genital HSV-2 infection, seen by lack of differences in susceptibility between IL-17A-deficient (IL-17A−/−) and wild-type (WT) C57BL/6 mice, it was critical for mediating antiviral responses after challenge/reexposure. Compared to WT mice, IL-17A−/− mice (i) infected intravaginally and reexposed or (ii) vaccinated intranasally and challenged intravaginally demonstrated poor outcomes. Following intravaginal HSV-2 reexposure or challenge, vaccinated IL-17A−/− mice had significantly higher mortality, greater disease severity, higher viral shedding, and higher levels of proinflammatory cytokines and chemokines in vaginal secretions. Furthermore, IL-17A−/− mice had impaired Th1 cell responses after challenge/reexposure, with significantly lower proportions of vaginal IFN-γ+ CD4+ T cells. The impaired Th1 cell responses in IL-17A−/− mice coincided with smaller populations of IFN-γ+ CD4+ tissue resident memory T (TRM) cells in the genital tract postimmunization. Taken together, these findings describe a novel role for IL-17A in regulating antiviral IFN-γ+ Th1 cell immunity in the vaginal tract. This strategy could be exploited to enhance antiviral immunity following HSV-2 vaccination.
IMPORTANCE T helper type 1 (Th1) immunity, specifically interferon gamma (IFN-γ) production by CD4+ T cells, is critical for protection against genital herpesvirus (HSV-2) infection, and enhancing this response can potentially help improve disease outcomes. Our study demonstrated that interleukin-17A (IL-17A) plays an essential role in enhancing antiviral Th1 responses in the female genital tract (FGT). We found that in the absence of IL-17A, preexposed and vaccinated mice showed poor disease outcomes and were unable to overcome HSV-2 reexposure/challenge. IL-17A-deficient mice (IL-17A−/−) had smaller populations of IFN-γ+ CD4+ tissue resident memory T (TRM) cells in the genital tract postimmunization than did wild-type (WT) mice, which coincided with attenuated Th1 responses postchallenge. This has important implications for developing effective vaccines against HSV-2, as we propose that strategies inducing IL-17A in the genital tract may promote more effective Th1 cell immunity and better overall protection.
KEYWORDS: CD4 T cell immunity, IL-17, genital tract immunity, herpes simplex virus, mucosal immunity, sexually transmitted diseases
INTRODUCTION
Genital herpes, caused primarily by herpes simplex virus 2 (HSV-2), is one of the most predominant viral sexually transmitted infections (STIs) in the world (1). Recent estimates show that 417 million people between the ages of 15 and 49 years are infected with HSV-2 globally, and approximately 19.2 million in this age group become newly infected each year (1). Similar to the case with many other STIs, rates of HSV-2 infection are disproportionally higher in women, with approximately 14.8% of women infected, compared to 8.0% of men, globally (1). Efforts to develop effective vaccines against STIs such as HSV-2 have been unsuccessful, as there is little understanding of factors in the local microenvironment of the female genital tract (FGT) that determine the outcome of exposure to HSV-2, making it difficult to develop preventative interventions (2).
To study the immune mechanisms involved in generating protection against HSV-2 infection, a well-established mouse model of genital HSV-2 infection is commonly used, as it closely recapitulates many aspects of human infection. Mouse studies have demonstrated that both humoral and cell-mediated responses are involved in generating protection against genital HSV-2 infection. Vaccine strategies that can induce robust local antibody responses or passively transfer antibodies from immunized mice can protect mice against subsequent intravaginal HSV-2 challenge (3). However, it is well known that CD4+ T helper 1 (Th1) cell immune responses and production of interferon gamma (IFN-γ) are critical for protection both following natural resolution of infection and following immunization (4, 5). Therefore, preventative strategies that aim to induce strong Th1 cell immunity in the genital tract should be able to effectively control HSV-2 infection.
Along with Th1 cell immunity, genital HSV-2 infection also induces a Th17 immune response. Th17 cells are a subset of activated CD4+ T cells, characterized primarily by the secretion of cytokine interleukin-17 (IL-17) as well as IL-21 and IL-22 (6). The IL-17 family of cytokines includes six ligands (IL-17A to IL-17F), and Th17 cells generally produce IL-17A and IL-17F (6). Th17 cell immunity and IL-17A production in particular have been shown to play a fundamental role in resolution of fungal and bacterial infections in the genital tract, including infections with Candida albicans, Neisseria gonorrhoeae, and Chlamydia trachomatis (7–12); however, aberrant Th17 cell responses can lead to autoimmune conditions or chronic inflammatory diseases in other tissues (6, 13, 14). Although IL-17 has been studied in the context of bacterial and fungal infections in the genital tract, the role of IL-17 in genital antiviral responses, particularly during HSV-2 infection, is less understood. Kim et al. reported that here was a significant delay in the deaths of IL-17A-deficient (IL-17A−/−) mice compared to those of C57BL/6 control mice following primary genital HSV-2 infection, implying that IL-17A may have a pathogenic effect (15). Conversely, we have recently shown that Th17 cell immunity and IL-17A production may be involved in mediating better protection following intravaginal HSV-2 challenge (16). Evidently, the role of IL-17A in host defense against genital HSV-2 infection remains unresolved, and therefore, it was the focus of the current study.
In addition to antibacterial and antifungal immunity, IL-17 has also been shown to contribute to the generation of efficient Th1 cell immunity against intracellular pathogens (17–19). In the pulmonary Mycobacterium tuberculosis vaccination model, the absence of IL-17 resulted in reduced and delayed IFN-γ responses and, consequently, delayed bacterial clearance (17). Similarly, in IL-17 receptor A (IL-17RA)-deficient mice, genital tract infection with Chlamydia muridarum also resulted in reduced IFN-γ production (18). Bai et al. further showed that a reduced Chlamydia-specific Th1 cell response was related to impaired dendritic cell (DC) induction of IFN-γ responses upon IL-17A neutralization (19). Parallel to these studies, we recently reported that enhanced Th1 cell immunity against HSV-2 in the genital tract involves Th17 cell responses (16). We showed that estradiol treatment in mice, which is known to protect against HSV-2 (20–23), enhanced antiviral immunity by priming vaginal DCs to induce Th17 cell responses following HSV-2 immunization. This resulted in increased production of IL-17A+ CD4+ (Th17) cells in estradiol-treated mice and coincided with earlier recruitment and increased proportions of IFN-γ+ CD4+ (Th1) cells in the genital tract postchallenge (16). This was the first study to suggest that Th17 responses in the vaginal tract may augment IFN-γ–Th1 cell immunity. However, it was not determined whether this enhanced antiviral immunity was due to IL-17A alone or the result of other cytokines produced by Th17 cells, thus emphasizing the importance of examining directly and in more detail the role of IL-17A in the genital tract model of HSV-2 infection.
In light of these findings, the aim of the present study was to investigate the role and mechanism of IL-17A in regulating IFN-γ–Th1 cell immunity to genital HSV-2 infection. Using IL-17A-deficient (IL-17A−/−) mice, we found no difference in disease severity from that in wild-type (WT) C57BL/6 mice following primary genital HSV-2 infection. However, following HSV-2 reexposure, IL-17A−/− mice had significantly poorer disease outcomes than did WT mice. To further examine the role of IL-17A in postchallenge antiviral responses, IL-17A−/− mice were vaccinated intranasally with an attenuated strain of HSV-2 (thymidine kinase deficient [TK−]); they demonstrated poor disease outcomes following intravaginal challenge with WT HSV-2. In addition, IL-17A−/− mice had significantly higher levels of proinflammatory cytokines and chemokines in vaginal secretions. Most importantly, IL-17A−/− mice showed impaired Th1 responses following both reexposure and challenge. Upon examination, the attenuated recall responses in IL-17A−/− mice were found to correlate with a smaller population of vaginal CD4+ tissue resident memory T (TRM) cells postvaccination. Our data therefore suggest that IL-17A is critical for the induction of optimal Th1 cell responses and overall protection against genital HSV-2 infection.
RESULTS
IL-17A is not critical during primary genital HSV-2 infection.
We recently showed that enhanced protection against HSV-2 involved greater vaginal Th17 cell responses, which coincided with increased Th1 cell responses (16); however, it was not established if the protective effect of Th17 cell immunity was mediated directly by IL-17A. Therefore, we decided to further investigate, in greater detail, the direct role of IL-17A in mediating HSV-2 antiviral immunity in the genital tract.
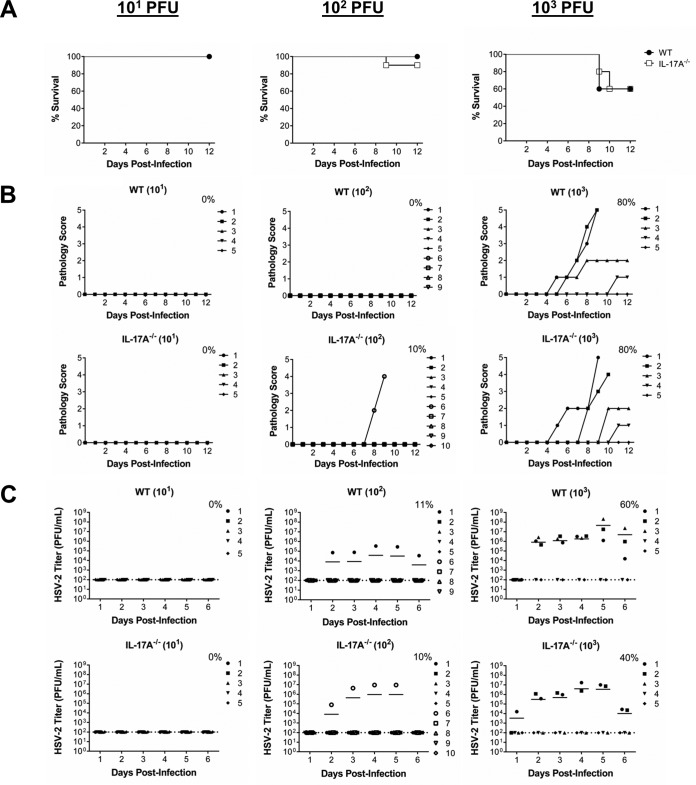
In order to assess if IL-17A−/− mice were more susceptible than WT mice to primary intravaginal infection with HSV-2, ovariectomized (OVX) IL-17A−/− and WT mice were inoculated with sublethal doses of WT HSV-2 virus (101, 102, and 103 PFU/mouse). Survival, genital pathology, and viral shedding were monitored daily to determine disease severity and compare susceptibilities to infection. We found that following primary genital infection, there were no significant differences in survival between IL-17A−/− and WT mice (Fig. 1A). Comparable rates of survival corresponded with similar severities of genital pathology between IL-17A−/− and WT mice (Fig. 1B) and similar numbers of IL-17A−/− and WT mice shedding virus postinfection (Fig. 1C). Overall, regardless of the infectious dose used, there were no differences in mortality, disease pathology, and viral shedding between IL-17A−/− and WT mice, demonstrating that IL-17A does not appear to play a critical role during primary genital HSV-2 infection.
FIG 1.
IL-17A−/− mice demonstrate no significant difference in susceptibility to primary intravaginal HSV-2 infection. OVX WT (C57BL/6) and IL-17A−/− mice (n = 5 to 10/group) were infected intravaginally with sublethal doses of WT HSV-2 (101, 102, or 103 PFU/mouse). Survival was monitored (A) and pathology scores were recorded on a scale of 0 to 5 (B) for 12 days postinfection. Data points superimposed on the x axes of panel B indicate mice without genital pathology, and the percentages represent maximum numbers of mice that demonstrated pathology. (C) Vaginal washes were collected daily for 6 days postinfection and HSV-2 shedding was assessed using a Vero cell-based assay. The bars in panel C indicate mean PFU per milliliter of shed virus. The dotted lines in panel C indicate the lower detection limit of the assay, and data points on this line indicate undetectable viral shedding. The percentages in panel C represent maximum numbers of mice that shed virus on any given day. Each symbol represents a single animal. The results are representative of those from two independent experiments.
IL-17A contributes to antiviral responses following genital HSV-2 reexposure.
We then examined whether IL-17A−/− mice would show compromised antiviral responses following HSV-2 reexposure, as this would determine the role of IL-17A during memory recall responses in the FGT. Previous studies with the intracellular pathogen M. tuberculosis have shown that although IL-17 is not critical during primary infection, it is important for augmenting memory responses (17).
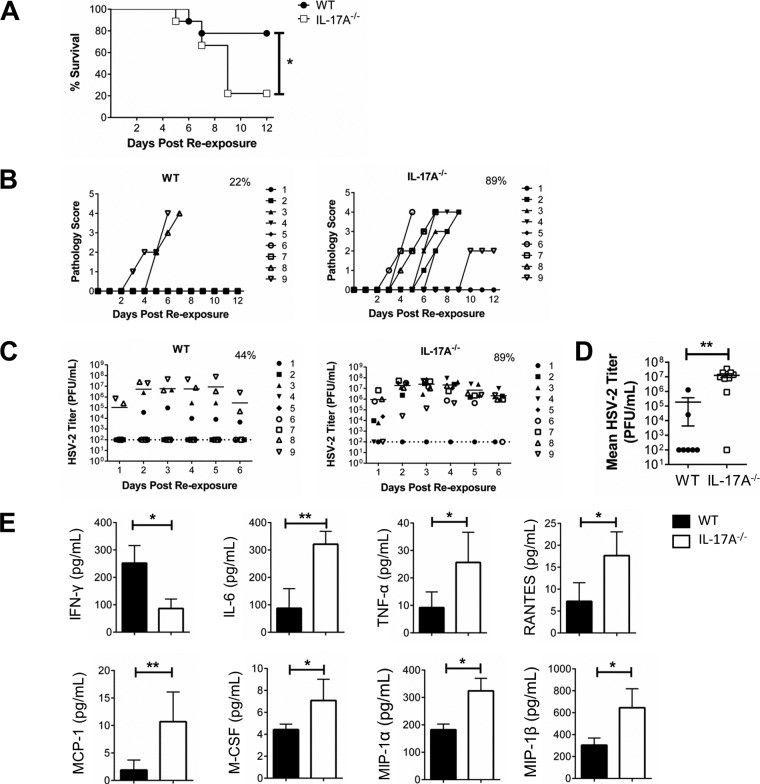
Intravaginally preexposed IL-17A−/− and WT mice (102 PFU/mouse) were reexposed to a higher dose of HSV-2 (5 × 103 PFU/mouse), and survival, genital pathology, and viral shedding were monitored daily. We found that following intravaginal HSV-2 reexposure, IL-17A−/− mice had poor disease outcomes and demonstrated higher rate of mortality (Fig. 2). After reexposure, 78% of WT mice survived, compared to only 22% of IL-17A−/− mice (Fig. 2A), and IL-17A−/− mice developed more than twice as high average cumulative genital pathology (IL-17A−/−, 13.3; WT, 5.8) (Table 1; Fig. 2B). We also collected vaginal washes and found that a greater percentage of IL-17A−/− mice than WT mice were shedding virus (IL-17A−/−, 89%; W, 44%) (Fig. 2C) and had higher viral shedding in the vaginal tract (P = 0.003) (Fig. 2D). We repeated these reexposure experiments with other viral doses (primary exposure, 101 and 103 PFU/mouse; reexposure, 5 × 102 and 5 × 104 PFU/mouse) and consistently found decreased protection in IL-17A−/− mice (Table 1). In addition to quantifying viral shedding, cytokine and chemokine concentrations were also measured in vaginal secretions. Forty-eight hours following reexposure, IL-17A−/− mice had significantly lower levels (means ± standard errors of the means [SEMs]) of IFN-γ (IL-17A−/−, 86.45 ± 15.34 pg/ml; WT, 252.11 ± 31.97 pg/ml; P = 0.016) (Fig. 2E). Interestingly, levels of proinflammatory cytokines and chemokines, including IL-6 (P = 0.006), tumor necrosis factor alpha (TNF-α) (P = 0.038), regulated on activation, normal T-cell expressed and secreted (RANTES) (P = 0.032), monocyte chemoattractant protein 1 (MCP-1) (P = 0.009), macrophage colony-stimulating factor (M-CSF) (P = 0.038), and macrophage inflammatory protein 1 alpha (MIP-1α) (P = 0.017) and beta (MIP-1β) (P = 0.017), were significantly higher in IL-17A−/− mice than in WT mice (Fig. 2E). These findings showcase significant differences between WT and IL-17A−/− mice upon intravaginal HSV-2 reexposure. In the absence of IL-17A, mice demonstrated higher rates of mortality, more severe genital pathology, and greater viral shedding, along with higher concentrations of proinflammatory factors and lower levels of IFN-γ in vaginal secretions. Together, these results indicate that IL-17A plays an important role in the antiviral responses within the genital tract following HSV-2 reexposure.
FIG 2.
Preexposed IL-17A−/− mice are more susceptible to intravaginal HSV-2 reexposure. OVX WT (C57BL/6) and IL-17A−/− mice (n = 9/group) were intravaginally exposed to WT HSV-2 (102 PFU/mouse), and 6 weeks later, they were reexposed intravaginally with a higher dose of WT HSV-2 (5 × 103 PFU/mouse). Survival was monitored (A) and pathology scores were recorded on a scale of 0 to 5 (B) for 12 days after reexposure. Significance in difference in survival (A) was calculated using the log rank (Mantel-Cox) test (*, P < 0.05). Data points superimposed on the x axes of panel B indicate mice without genital pathology, and the percentages represent maximum numbers of mice that demonstrated pathology. Vaginal washes were collected daily for 6 days after reexposure; HSV-2 viral shedding was calculated using a Vero cell-based assay (C and D), and cytokine and chemokine (IFN-γ, IL-6, TNF-α, RANTES, MCP-1, M-CSF, MIP-1α, and MIP-1β) concentrations were measured by multianalyte assays (E). The bars in panel C indicate mean PFU per milliliter of shed virus. The dotted lines in panel C indicate the lower detection limit of the assay, and data points on this line indicate undetectable viral shedding. The percentages in panel C represent maximum numbers of mice that shed virus on any given day. Data shown in panel D represent the viral loads (means ± SEMs) over 6 days. Each symbol represents a single animal. Data shown in panel E represent the means ± SEMs from two independent experiments, done in duplicate (n = 4 to 7/group). Data were analyzed using the unpaired, nonparametric, two-tailed Mann-Whitney test with 95% confidence interval, with the ROUT method used to identify outliers and the Bonferroni correction used to correct for multiple measures.*, P < 0.05; **, P < 0.01.
TABLE 1.
Cumulative pathology scores for HSV-2-reexposed micea
Cumulative pathology is calculated by noting the number of mice with their maximum pathology score and the number of days that score was observed. Mice that did not survive the challenge were given highest pathology score for the duration of the experiment to accurately reflect overall pathology for each group. This takes into consideration that each mouse in a group can reach various degrees of pathology through the experiment. The average pathology score per mouse was calculated by dividing the sum of cumulative pathology by total number of mice.
IL-17A mediates efficient antiviral Th1 responses following genital HSV-2 reexposure.
Next, we sought to better understand why IL-17A−/− mice were more susceptible to HSV-2 reexposure and demonstrated increased mortality (Fig. 2). Since IFN-γ is known to play a key role in clearance of HSV-2 and we saw lower levels of IFN-γ in the vaginal secretions of IL-17A−/− mice (Fig. 2E), we decided to examine the in vivo T cell responses in the vaginal tissue in order to quantify IFN-γ production by CD4+ T cells.
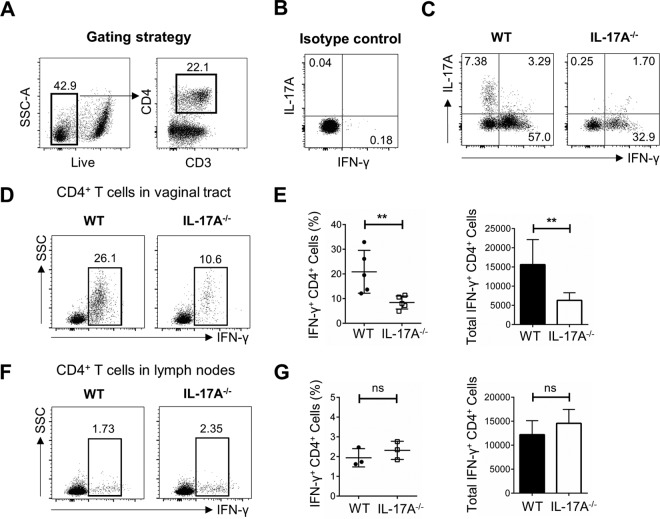
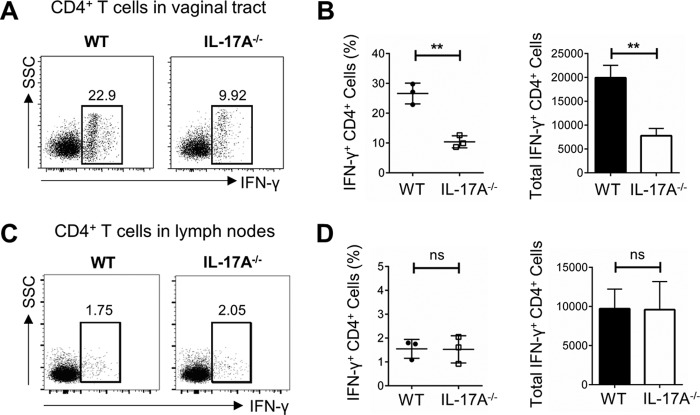
Following intravaginal primary infection and reexposure described above, vaginal tissues and iliac lymph nodes (which drain the genital tract) were collected 3 days later to phenotype and functionally characterize the in vivo T cell responses. CD4+ T cells were gated based on total, live CD3+ cells in the vagina (Fig. 3A), and isotype controls for IFN-γ and IL-17A were included (Fig. 3B). To examine IL-17A production by CD4+ T cells, vaginal cells were stimulated in vitro with phorbol 12-myristate 13-acetate (PMA) and ionomycin, and higher proportions of total Th1 and Th17 cells were seen in WT mice than in IL-17A−/− mice (Fig. 3C), with insignificant IL-17A detected in IL-17A−/− mice. Following in vivo challenge alone, and in the absence of in vitro stimulation, vaginal tissue from IL-17A−/− mice contained lower proportions of IFN-γ+ Th1 cells (10.6% of CD4+ T cells) than that from WT mice (26.1% of CD4+ T cells) (Fig. 3D). Data compiled from multiple independent experiments consistently showed that following reexposure, IL-17A−/− mice had significantly lower IFN-γ production by vaginal CD4+ T cells than did WT mice (IL-17A−/−, 8.42% ± 1.18% of CD4+ T cells; WT, 20.86% ± 3.89% of CD4+ T cells; P = 0.016) (Fig. 3E) and a significantly lower total number of vaginal IFN-γ+ CD4+ T cells (IL-17A−/−, 6,304 ± 883; WT, 15,624 ± 2,912; P = 0.008) (Fig. 3E). Notably, this difference was not present in the lymph nodes, where IL-17A−/− and WT mice had very similar proportions of IFN-γ+ CD4+ cells (IL-17A−/−, 2.32% ± 0.27% of CD4+ T cells; WT, 1.94% ± 0.27% of CD4+ T cells; P = 0.374) and similar total numbers of IFN-γ+ CD4+ cells (IL-17A−/−, 14,562 ± 1,673; WT, 12,194 ± 1,678; P = 0.400) (Fig. 3F and G), suggesting that memory CD4+ T cells, which are readily available to induce IFN-γ production, are compartmentalized in the vaginal tract specifically. Together, these data suggest that the inability of IL-17A−/− mice to effectively resolve HSV-2 reexposure is likely due to impaired local IFN-γ+ Th1 cell responses, indicating that IL-17A plays a critical role in mediating efficient antiviral Th1 cell immunity in the genital tract.
FIG 3.
Preexposed IL-17A−/− mice have lower proportions of IFN-γ+ Th1 cells in the vaginal tract following intravaginal HSV-2 reexposure. OVX WT (C57BL/6) and IL-17A−/− mice (n = 4 to 6/group) were intravaginally exposed to WT HSV-2 (102 PFU/mouse) and 6 weeks later reexposed intravaginally with a higher dose of WT HSV-2 (5 × 103 PFU/mouse). Vaginal tissue and lymph nodes were isolated at day 3 following reexposure, pooled, processed, stained with a panel of antibodies, and examined by flow cytometry. (A) CD4+ T cells were gated among total live CD3+ T cells in the vaginal tissue. (B) Isotype controls for intracellular staining of IL-17A and IFN-γ. (C) Vaginal cells were stimulated in vitro with a cell stimulation cocktail (CSC) containing Golgi inhibitors and PMA plus ionomycin for 16 h to detect intracellular staining of IL-17A and IFN-γ. (D) Intracellular staining for IFN-γ was used to examine the in vivo response to HSV-2 (without in vitro stimulation) and the differentiation of CD4+ T cells into Th1 cells in the vaginal tract. (E) The differences in percentages and total cell numbers of IFN-γ-producing CD4+ T cells after HSV-2 reexposure in the vaginal tract were compared across five independent experiments (n = 4 to 6/group). (F and G) Intracellular staining for IFN-γ (F) and the differences in percentages and total cell numbers of IFN-γ-producing CD4+ T cells after HSV-2 reexposure in the lymph nodes (G) were compared across three independent experiments (n = 5 or 6/group). Data shown in panels E and G represent means ± SEMs. Significant difference in IFN-γ expression was calculated using the unpaired, two-tailed t test with 95% confidence interval. **, P < 0.01. ns, no significance.
IL-17A is essential for establishing efficient Th1 cell responses in the female genital tract following HSV-2 vaccination.
Based on the above-described results, which suggested that IL-17A was critical for augmenting Th1 cell responses (Fig. 3), we wanted to examine whether IL-17A would also play a role in enhancing immune responses following HSV-2 vaccination. To test antiviral immune responses to HSV-2 vaccination, mice were immunized intranasally with an attenuated strain of HSV-2 (TK− HSV-2), followed by intravaginal challenge with WT HSV-2. Intranasal immunization generates immunity against intravaginal HSV-2 challenge, comparable to local immunization, and has been frequently used by us and others to generate potent immune responses in the genital tract (20, 24–26).
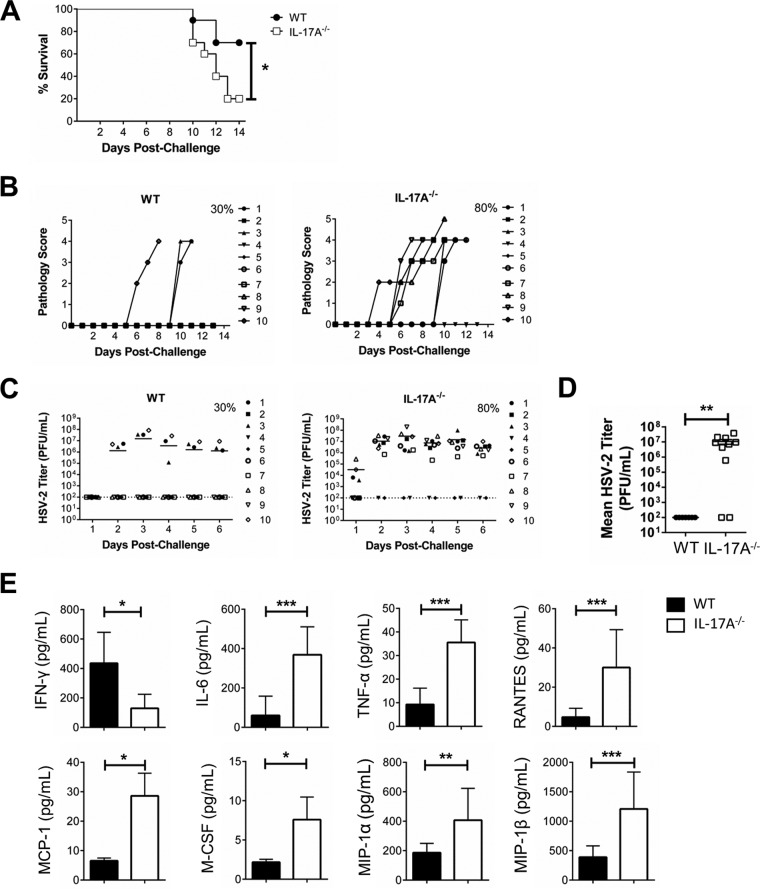
When WT and IL-17A−/− mice were immunized intranasally, we found once again that IL-17A−/− mice were more susceptible to intravaginal HSV-2 challenge (Fig. 4). There was considerably lower survival in IL-17A−/− mice (IL-17A−/−, 20%; WT, 70%) (Fig. 4A), and this coincided with more than twice as high average cumulative genital pathology (IL-17A−/−, 14.4; WT, 5.2) (Table 2; Fig. 4B). There were also more IL-17A−/− mice shedding virus (IL-17A−/−, 80%; WT, 30%) (Fig. 4C), with higher viral shedding in the vaginal tract (P = 0.003) (Fig. 4D). In vaginal secretions collected postchallenge, IL-17A−/− mice had significantly lower levels of IFN-γ (IL-17A−/−, 129.80 ± 38.94 pg/ml; WT, 438.80 ± 93.77 pg/ml; P = 0.017) (Fig. 4E). Levels of other proinflammatory cytokines and chemokines (IL-6, P = 0.0006; TNF-α, P = 0.0003; RANTES, P = 0.0002; MCP-1, P = 0.029; M-CSF, P = 0.029; MIP-1α, P = 0.006; MIP-1β, P = 0.0006) were significantly higher in IL-17A−/− mice than in WT mice (Fig. 4E). These results confirmed that following intranasal vaccination, IL-17A plays an important role in the antiviral immune response after HSV-2 challenge.
FIG 4.
Intranasally immunized IL-17A−/− mice are more susceptible to intravaginal HSV-2 challenge. OVX WT (C57BL/6) and IL-17A−/− mice (n = 10/group) were immunized intranasally with TK− HSV-2 (102 PFU/mouse) and 6 weeks later challenged intravaginally with WT HSV-2 (5 × 103 PFU/mouse). Survival was monitored (A) and pathology scores were recorded on a scale of 0 to 5 (B) for 14 days postchallenge. Significance in difference in survival (A) was calculated using the log rank (Mantel-Cox) test (*, P < 0.05). Data points superimposed on the x axes of panel B indicate mice without genital pathology, and the percentages represent maximum numbers of mice that demonstrated pathology. Vaginal washes were collected daily for 6 days postchallenge; HSV-2 shedding was calculated using a Vero cell-based assay (C and D), and cytokine and chemokine (IFN-γ, IL-6, TNF-α, RANTES, MCP-1, M-CSF, MIP-1α, and MIP-1β) concentrations were measured by multianalyte assays (E). The bars in panel C indicate mean PFU per milliliter of shed virus. The dotted lines in panel C indicate the lower detection limit of the assay, and data points on this line indicate undetectable viral shedding. The percentages in panel C represent maximum numbers of mice that shed virus on any given day. Data shown in panel D represent the viral loads (means ± SEMs) over 6 days. Each symbol represents a single animal. Data shown in panel E represents means ± SEMs from two independent experiments, done in duplicate (n = 4 to 7/group). Data were analyzed using the unpaired, nonparametric, two-tailed Mann-Whitney test with 95% confidence interval, with the ROUT method used to identify outliers and the Bonferroni correction used to correct for multiple measures. *, P < 0.05; **, P < 0.01; ***, P <0.001.
TABLE 2.
Cumulative pathology scores for HSV-2 (5 × 103 PFU)-challenged micea
| Group | Pathology score | No. of mice | No. of days | Cumulative pathology | Avg pathology/mouse |
|---|---|---|---|---|---|
| WT (5 × 103 PFU) | 0 | 7 | 12 | 0 | 5.2 |
| 4 | 1 | 6 | 24 | ||
| 4 | 1 | 4 | 15 | ||
| 4 | 1 | 3 | 12 | ||
| IL-17A−/− (5 × 103 PFU) | 0 | 2 | 13 | 0 | 14.4 |
| 4 | 2 | 3 | 24 | ||
| 4 | 3 | 4 | 48 | ||
| 4 | 1 | 6 | 24 | ||
| 4 | 1 | 7 | 28 | ||
| 5 | 1 | 4 | 20 |
Cumulative pathology is calculated by noting the number of mice with their maximum pathology score and the number of days that score was observed. Mice that did not survive the challenge were given highest pathology score for the duration of the experiment to accurately reflect overall pathology for each group. This takes into consideration that each mouse in a group can reach various degrees of pathology through the experiment. Average pathology score per mouse was calculated by dividing the sum of cumulative pathology by total number of mice.
When we examined T cell responses in the vaginal tract postchallenge, we found a clear difference in the Th1 cell response generated in IL-17A−/− mice from that in WT mice. Consistently, there was significantly lower IFN-γ production by CD4+ T cells in the vaginal tissue of immunized IL-17A−/− mice postchallenge (IL-17A−/−, 10.39% ± 1.16% of CD4+ T cells; WT, 26.60% ± 2.01% of CD4+ T cells; P = 0.002) (Fig. 5A) and a significantly lower total number of vaginal IFN-γ+ CD4+ T cells in IL-17A−/− mice (IL-17A−/−, 7,782 ± 872; WT, 19,923 ± 1,504; P = 0.002) (Fig. 5B). However, there was no difference in the lymph nodes, where the percentage of IFN-γ+ CD4+ T cells (IL-17A−/−, 1.53% ± 0.33% of CD4+ T cells; WT, 1.55% ± 0.23% of CD4+ T cells; P = 0.963) and the total number of IFN-γ+ CD4+ T cells (IL-17A−/−, 9,596 ± 2,067; WT, 9,722 ± 1,438; P = 0.963) were similar between IL-17A−/− and WT mice (Fig. 5C and D). Taken together, these results suggest that IL-17A is important for mounting a proficient Th1 recall response in the FGT that can protect against HSV-2 challenge following vaccination.
FIG 5.
Intranasally immunized IL-17A−/− mice have lower proportions of IFN-γ+ Th1 cells in the vaginal tract following intravaginal HSV-2 challenge. OVX WT (C57BL/6) and IL-17A−/− mice (n = 4 to 6/group) were immunized intranasally with TK− HSV-2 (102 PFU/mouse) and 6 weeks later challenged intravaginally with WT HSV-2 (5 × 103 PFU/mouse). Vaginal tissues and lymph nodes were isolated at day 3 postchallenge, pooled, processed, stained with a panel of antibodies, and examined by flow cytometry. Intracellular staining for IFN-γ was used to examine the in vivo response to HSV-2. (A and B) The differentiation of CD4+ T cells into Th1 cells in the vaginal tract (A) and the differences in percentages and total numbers of IFN-γ-producing CD4+ T cells post-HSV-2 challenge in the vaginal tract (B) were compared across three independent experiments (n = 4 to 6/group). (C and D) The differentiation of CD4+ T cells into Th1 cells (C) and the differences in percentages and total numbers of IFN-γ-producing CD4+ T cells after HSV-2 challenge in the lymph nodes (D) were compared across three independent experiments (n = 4 to 6/group). Data shown in panels B and D represent means ± SEMs. Significant difference in IFN-γ expression was calculated using the unpaired, two-tailed t test with 95% confidence interval. **, P < 0.01.
The absence of IL-17A is associated with decreased vaginal TRM cells following HSV-2 vaccination.
Our results demonstrating more robust CD4+ T cell responses in the vaginal tract than in lymph nodes (Fig. 3 and 5) indicated that the antiviral immune response observed upon HSV-2 reexposure/challenge was compartmentalized in the vaginal tract. These findings suggest that there is a population of CD4+ T cells generated postimmunization which are localized in the vaginal tract and readily available to respond to future HSV-2 exposure. Therefore, we next focused on examining the tissue-resident memory T cell (TRM) population established in the vaginal tract postimmunization. We wanted to see if the IFN-γ-producing CD4+ cells we had observed were consistent with the phenotype of CD4+ TRM cells and if absence of IL-17A would affect the induction of this population, thereby explaining the subsequent inefficient Th1 response generated postchallenge.
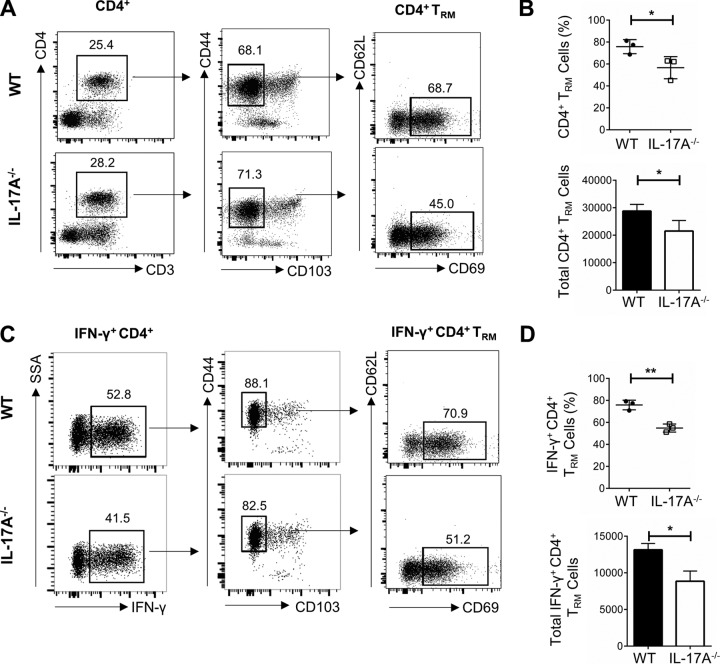
Following vaccination with TK− HSV-2, vaginal cells were isolated, pooled, and stimulated to assess IFN-γ production. CD4+ T cells were identified in the vaginal tract and further classified based on previously reported cell surface markers associated with vaginal TRM populations (27). Following gating strategies previously described in the literature, CD4+ T cells were gated based on live CD3+ cells, followed by gating based on the positive expression of adhesion molecule CD44 and negative expression of the retention marker CD103 (CD44+ CD103−) and, finally, gating based on the positive expression of T cell activation and retention molecule CD69 and negative expression of the adhesion molecule CD62L (CD69+ CD62L−). We found that postimmunization, IL-17A−/− mice had a smaller population of CD4+ TRM cells (CD4+ CD44+ CD103− CD69+ CD62L−) than did WT mice (IL-17A−/−, 45.0%; WT, 68.7%) (Fig. 6A). Data compiled from multiple independent experiments consistently showed that postimmunization, IL-17A−/− mice had significantly smaller populations of CD4+ TRM cells than did WT mice (IL-17A−/−, 56.53% ± 5.82%; WT, 75.77% ± 3.70%; P = 0.05) (Fig. 6B) and a significantly lower total number of vaginal CD4+ TRM cells (IL-17A−/−, 21,521 ± 2,212; WT, 28,791 ± 1,406; P = 0.05) (Fig. 6B). Furthermore, when focusing specifically on the population of CD4+ T cells producing IFN-γ, using the same gating strategy, we found that there was a smaller population of IFN-γ+ CD4+ TRM cells (CD4+ IFN-γ+ CD44+ CD103− CD69+ CD62L−) in the vaginal tract of IL-17A−/− mice (IL-17A−/−, 51.2%; WT, 70.9%) (Fig. 6C). This was true across multiple independent experiments, where IL-17A−/− mice consistently had significantly lower proportions (IL-17A−/−, 54.90% ± 2.11%; WT, 75.83% ± 2.52%; P = 0.003) and total numbers (IL-17A−/−, 8,862 ± 801; WT, 13,149 ± 497; P = 0.01) of IFN-γ+ CD4+ TRM cells (Fig. 6D). Overall, these results suggest that IL-17A helps establish a proficient IFN-γ+ CD4+ TRM cell population postimmunization and, consequently, mediates efficient antiviral Th1 responses postchallenge.
FIG 6.
Phenotypic and functional characteristics of CD4+ TRM cells localized in the vaginal tracts of WT and IL-17A−/− mice following HSV-2 vaccination. OVX WT (C57BL/6) and IL-17A−/− mice (n = 5 to 7/group) were vaccinated intravaginally with TK− HSV-2 (104 PFU/mouse), and 3 weeks later, vaginal tissues were collected, pooled, and processed. Vaginal cells were stimulated in vitro with a cell stimulation cocktail (CSC) containing Golgi inhibitors and PMA plus ionomycin for 16 h, stained with a panel of antibodies, and examined by flow cytometry. (A) CD4+ T cells were gated among total live CD3+ T cells in the vaginal tissue, and CD4+ TRM cells were detected using surface markers CD44, CD103, CD69, and CD62L. CD4+ TRM cells were defined as CD4+ CD44+ CD103− CD69+ CD62L−. (B) The differences in percentages and total numbers of CD4+ TRM cells postimmunization in the vaginal tract were compared across three independent experiments (n = 5 to 7/group). (C) IFN-γ+ CD4+ TRM cells were further gated based on detection of surface markers associated with TRM cells. (D) The differences in percentages and total numbers of IFN-γ+ CD4+ TRM cells postimmunization in the vaginal tract were compared across three independent experiments (n = 5 to 7/group). Data shown in panels B and D represent means ± SEMs. Significant difference in IFN-γ expression was calculated using the unpaired, two-tailed t test with 95% confidence interval. *, P < 0.05; **, P < 0.01.
DISCUSSION
The generation of robust IFN-γ-Th1 cell responses is critical for protective immunity against HSV-2 infection, and recent studies suggest that IL-17 potentiates Th1 immunity, thereby playing an important role in controlling infections with intracellular pathogens (17–19, 28, 29). In the current study, we investigated the role of IL-17A in host defense against genital HSV-2 infection. We found that in the absence of IL-17A, protection against both intravaginal HSV-2 reexposure following primary genital infection and intravaginal HSV-2 challenge following intranasal immunization was significantly decreased. This was evident because IL-17A−/− mice had higher rates of mortality and viral shedding, and more severe disease pathology, than did WT mice. In addition, there were higher levels of inflammatory cytokines and chemokines in the vaginal secretions of IL-17A−/− mice. Furthermore, poor disease outcomes in the absence of IL-17A also coincided with lower Th1 responses than in WT mice, with significantly less IFN-γ production by vaginal CD4+ T cells following reexposure as well as postchallenge in immunized mice. Finally, IL-17A was shown to be important for establishing proficient IFN-γ+ CD4+ TRM cell populations in the FGT postimmunization. To the best of our knowledge, this is the first study demonstrating that IL-17A plays a critical role in mediating efficient antiviral Th1 responses in the female genital tract, thereby improving HSV-2 vaccination efficacy.
The function of IL-17A, a cytokine primarily produced by Th17 cells, in reproductive tract infections initiated by exposure to fungal and bacterial pathogens has been reported by several studies. IL-17A can help control overgrowth of the fungus C. albicans by upregulating proinflammatory cytokines and antimicrobial peptides and recruiting neutrophils through the secretion of neutrophil-recruiting chemokines (10, 30). In addition, Th17 deficiency is associated with chronic candidiasis (31). Similarly, IL-17A also plays a protective role in defense against bacterial STIs. During murine genital tract infection with N. gonorrhoeae, IL-17A is important for the recruitment of neutrophils and resulting clearance of infection (11), while the absence of IL-17A leads to prolonged infection (7). However, the role of IL-17A during viral infections in the genital tract has not been well described. A study by Kim et al. reported that the deaths of IL-17A−/− mice were significantly delayed following intravaginal infection with HSV-2 compared to the deaths of WT mice, suggesting that IL-17A-producing T cell receptor (TCR) γδ+ CD4− CD8− T cells play a pathogenic role during HSV-2 infection (15). However, that study only examined the role of IL-17A in the context of a primary infection with a lethal dose of virus, focusing on IL-17A+ TCRγδ+ T cells, an approach starkly different than the one used in the current study. In this study, we examined the role of IL-17A in closer detail following HSV-2 challenge, as opposed to only after primary infection, as IL-17A−/− mice showed no differences in disease outcome compared to WT mice following primary infection with nonlethal doses of virus (Fig. 1). However, following intravaginal challenge, IL-17A−/− mice consistently demonstrated greater mortality and genital pathology, along with higher viral shedding, than WT mice (Fig. 2 and 4; Tables 1 and 2). Studies examining the role of IL-17 during infection with other intracellular pathogens, such as M. tuberculosis, show similar results, where although IL-17 is dispensable during the primary infection, it appears to play a critical role in inducing immune responses following vaccination (17, 28). Similar to the role of IL-17 in the M. tuberculosis model, our results also show that IL-17 is critical for inducing efficient immune response to HSV-2 postimmunization.
In addition, the study by Kim et al. used a lethal dose of HSV-2 (15), and it is possible that this may mask any effect that IL-17A has in terms of the efficiency in the immune response generated. When administered such a high dose of virus, mice may be able to compensate for the lack IL-17A by producing greater amounts of other protective cytokines and immune factors, making it difficult to assess the efficiency of the immune response generated. Interestingly, we observed significantly increased proinflammatory cytokine and chemokine production in vaginal washes collected from IL-17A−/− mice, even while using lower viral doses (Fig. 2D and 4D). It is possible that the increased production of proinflammatory cytokines in the absence of IL-17A is a compensatory mechanism which is implemented to help protect the mice against HSV-2 infection, and using even higher viral doses further amplifies this compensatory effect. Similarly, a study examining the role of IL-17 during genital C. muridarum infection found that IL-17RA knockout (KO) mice exhibited increased TNF-α production, which acted as a compensatory mechanism to effectively control chlamydial genital infection (18). Furthermore, other studies, including that by Kim et al., utilize medroxyprogesterone acetate (MPA) to make mice susceptible to HSV-2. MPA is an injectable progestin-based formulation that thins the vaginal epithelium and makes mice susceptible to infection. Several studies, including our own, have shown that MPA can significantly dampen mucosal antiviral responses to HSV-2 (32, 33). Specifically, prolonged exposure to MPA has been shown to decrease levels of HSV-2-specific mucosal immune responses following intravaginal immunization. Therefore, the use of MPA may have impacted the results of the study by Kim et al., resulting in an inaccurate assessment of the role of IL-17A during HSV-2 infection. To avoid compromised immune responses as a result of MPA treatment and the presence of endogenous hormones, we use an alternative model in which mice undergo an ovariectomy in order to remove the source of endogenous hormones altogether. This procedure naturally thins the vaginal epithelium, making the mice susceptible to intravaginal HSV-2 infection. This allows us to study antiviral immune responses in the female genital tract under no-hormone or clearly defined hormonal conditions, and it is the technique that was used in the present study.
Several studies show that IL-17 potentiates Th1 cell immune responses and plays a critical role in controlling infections with intracellular pathogens (17–19, 28, 29). The contribution of IL-17 to the development of Th1 cell immunity against intracellular pathogens has been shown during infection with pulmonary M. tuberculosis, Francisella tularensis, and C. muridarum, as well as genital infection with C. muridarum. Khader et al. determined, using the pulmonary M. tuberculosis vaccination model, in which, like anti-HSV-2 immunity, IFN-γ+ CD4+ T cell responses play a key role in generating effective immunity, that Th17 responses induced in the lungs following immunization play a key role in accelerating Th1 cell responses (17). This resulted in the early resolution of bacteria postchallenge, and in the absence of IL-17, IFN-γ responses and clearance of the bacteria were significantly delayed. Similarly, a study examining the role of Th17 cell immunity in host resistance to the intracellular bacterium F. tularensis showed that Th1 cell immunity was compromised in the absence of IL-17A (29). In addition, studies looking at chlamydial infection found that IL-17A is important for the development of Th1 responses in both the lungs and genital tract. Scurlock et al. found that IL-17RA mice demonstrated reduced IFN-γ production within the vaginal tract following genital C. muridarum infection (18). Furthermore, Bai et al. reported that mice treated with anti-IL-17A antibodies showed significantly delayed clearance of C. muridarum and increased disease severity in the lung. This corresponded with significantly reduced Chlamydia-specific Th1 responses and was related to impaired DC induction of IFN-γ responses in the absence of IL-17A (19). Parallel to these studies, we recently showed that IL-17A in the vaginal tract may augment antiviral IFN-γ-Th1 cell responses in the vaginal tract (16). We found that HSV-2 vaccination under the influence of the female sex hormone estradiol resulted in increased production of IL-17A by CD4+ cells, which coincided with earlier recruitment and increased proportions of vaginal IFN-γ+ CD4+ cells postchallenge (16); however, it was not established if IL-17A was directly responsible for the increase in IFN-γ. Interestingly, similar to what was shown by Bai et al. in the C. muridarum model, we also found that in in vitro cocultures conducted with vaginal cells from IL-17A−/− mice and ovalbumin (OVA)-specific OT-II transgenic CD4+ T cells, vaginal antigen-presenting cells (APCs) from IL-17A−/− mice were significantly impaired at inducing IFN-γ production compared to vaginal APCs from WT mice, suggesting that there is an intrinsic impairment in the priming of Th1 cell responses by vaginal DCs in IL-17A−/− mice (16).
The present study advances our understanding regarding the in vivo role of IL-17A, by focusing on the ability of IL-17A−/− mice to initiate an IFN-γ+ CD4+ T cell response in vivo in the context of HSV-2 infection. To assess the differentiation of CD4+ T cells into IFN-γ+ Th1 cells, vaginal cells were isolated following in vivo HSV-2 challenge and left unstimulated to measure spontaneous cytokine production. We have previously shown that levels of cytokine production, including that of IFN-γ, by vaginal CD4+ T cells are comparable between cells which have been stimulated with UV-inactivated HSV-2 in vitro postchallenge and cells grown in culture following in vivo HSV-2 challenge, without further in vitro antigen challenge (34). This is likely because at 3 days after in vivo challenge, T cells in the vaginal tract are already activated. Therefore, we consider T cell responses following in vivo challenge to be good surrogate measures of antigen-specific T cell responses. Similar to the reported in vitro results regarding impaired T cell priming in the absence of IL-17A (16), we show that there is a significant decrease in IFN-γ production by vaginal CD4+ T cells in vivo following HSV-2 challenge (Fig. 3 and 5). Evidently, lower IFN-γ production in the absence of IL-17A is enough to critically impact survival and disease outcomes, as having an approximately 2-fold-lower IFN-γ+ CD4+ T cell response in the vaginal tract after reexposure (Fig. 3) was sufficient to lower survival from 78% in WT mice to 22% in IL-17A−/− mice (Fig. 2). Comparable trends were also seen in vaccinated IL-17A−/− mice, as a loss in IFN-γ+ CD4+ T cell efficiency postchallenge (Fig. 5) resulted in survival decreasing from 70% in WT mice to only 20% in IL-17A−/− mice (Fig. 4). Since IFN-γ plays a critical role in host protection against HSV-2 in the mouse model of infection, decreased IFN-γ production in the vaginal tract can help explain the inability of IL-17A−/− mice to overcome HSV-2 challenge.
Our findings suggest that IL-17A plays an in important role in mediating efficient antiviral immune responses in the vaginal tract following HSV-2 vaccination, and based on previous work, this appears to be linked to a defect in the priming of Th1 cell responses in the absence of IL-17A (16). Interestingly, IL-17A also been shown to play a role in augmenting long-lasting memory responses in the lung (17). In the current study, we found that the protective IFN-γ+ CD4+ T cell response was compartmentalized in the vaginal tract and that in the absence of IL-17A, there was an impaired IFN-γ response in vaccinated mice postchallenge (Fig. 3 and 5). To better understand how IL-17A may be involved in generating efficient recall responses, we examined the repertoire of T cells in the vaginal tracts of IL-17A−/− and WT mice following HSV-2 vaccination, in order to compare the phenotype and functional characteristics of the T cells which localize to the vaginal tract postimmunization. Specifically, we looked at CD4+ TRM cells, a newly described subset of memory T cells, to examine if IL-17A is involved in establishing long-lasting, efficient tissue resident populations. TRM cells are clonally expanded memory T cells which enter peripheral tissues, where they are retained long-term and are able to survive without depending on replenishment from circulating T cells (35). More importantly, TRM cells have the ability to respond rapidly to infection and thereby represent a critical subset of cells involved in immunological protection against invading pathogens. CD8+ and CD4+ TRM cells established in the vaginal tract postimmunization have been shown to be involved in mediating better protection against HSV-2 challenge than that with circulating memory T cells on their own (27, 36); however, this is a new area of research in the field, and very little is known about these populations in the genital mucosa. In particular, it has been shown that vaginal CD4+ TRM cells are established in extremely low numbers, making them especially difficult to detect and characterize. For this reason, postimmunization, we stimulated our cells in vitro with a cell stimulation cocktail including PMA and ionomycin in order to assess maximum IFN-γ production by CD4+ TRM cells. Although this stimulation process may have resulted in T cell responses which are not entirely HSV-2 specific, it allowed us to measure the maximum ability of the vaginal cells to induce cytokine responses, which closely mimic those produced in response to HSV-2. Based on the limited published data, we utilized markers (CD44, CD103, CD69, and CD62L) which have been shown to identify tissue resident memory populations specifically in the vaginal tract (27, 36). CD44 is a cell adhesion molecule and cell activation marker that is highly expressed by TRM cells in most mucosal tissues. CD69 is another activation marker found to be expressed by TRM cells and is thought to play a role in TRM cell retention within periphery tissues, while CD62L is a lymph node homing receptor that is expressed by central memory T cells and not TRM cells. Unlike in other mucosal tissues, vaginal CD4+ TRM cells do not highly express the conventional memory and mucosal homing marker, CD103, as CD103 expression was shown to be detected in only 10% of CD4+ TRM cells in the vagina following infection with TK− HSV-2 (27). We thereby characterized CD4+ TRM cells as follows: CD4+ CD44+ CD103− CD69+ CD62L−. Interestingly, we found that following HSV-2 vaccination, there was a smaller population of CD4+ TRM cells in the vaginal tract of IL-17A−/− mice than in that of WT mice (Fig. 6A and B). Furthermore, the population of vaginal IFN-γ+ CD4+ TRM cells was also smaller in IL-17A−/− mice (Fig. 6C and C). This suggests that in the absence of IL-17A, mice are also impaired in the ability to generate a proficient pool of local, vaginal CD4+ TRM cells following HSV-2 vaccination. Since it has been shown that TRM cells are the primary source of IFN-γ early on during HSV-2 challenge (27), this can help explain why IL-17A−/− mice have a less efficient T-cell response upon subsequent HSV-2 exposure. However, with the limited knowledge available regarding the phenotype and the functional characteristics of vaginal CD4+ TRM cells, further work is ongoing to better understand the importance of this T cell population and how IL-17A may be involved in the establishment of these tissue memory cells. In ongoing studies, we are optimizing a model using transgenic mice expressing CD4+ T cell receptors specific for the HSV-2 glycoprotein D-derived epitope, which will allow us to better study HSV-2-specific CD4+ T cell responses and bypass the use of stimulatory markers, which may influence the expression of our TRM cell markers.
In summary, our study provides insight regarding a novel antiviral role for IL-17A in the genital tract, in which IL-17A improves mucosal vaccination efficacy by mediating efficient Th1 cell immunity. While it is well established that IFN-γ+ CD4+ T cells play a critical role against HSV-2 infection, little is known about the contribution of IL-17A+ CD4+ T cells. We show for the first time that IL-17A contributes significantly to the generation of protective antiviral Th1 cell immunity in the genital tract. In the absence of IL-17A, there is a compromised antiviral Th1 cell response following intravaginal HSV-2 reexposure and challenge, resulting in poor disease outcomes. This has important implications in terms of HSV-2 vaccine development, as a hallmark of an effective vaccination strategy is the ability to generate a rapid, robust local effector response, and we propose that HSV-2 vaccine strategies which induce IL-17A production in the genital tract may promote more effective Th1 cell immunity.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old female C57BL/6 mice were obtained from Charles River Laboratories (Saint-Constant, Quebec, Canada). IL-17A−/− mice (C57BL/6 background) were generated by Yoichiro Iwakura (University of Tokyo, Tokyo, Japan) (37) and bred internally in the Central Animal Facility at McMaster University. All mice were maintained under specific-pathogen-free (SPF) and standard temperature-controlled conditions that followed a 12-h light/dark cycle, and they were given low-fat mouse chow. Routine quality assurance was done by serology and PCR to ensure that mice remained SPF and included testing dirty bedding sentinels, direct resident animals, and exhaust air duct samples of racks. All animal studies performed were approved by, and in compliance with, the Animal Research Ethics Board (AREB) at McMaster University.
OVX.
Endogenous hormones in mice were depleted by ovariectomy (OVX). Prior to OVX, mice were administered analgesic (Temgesic) subcutaneously, and after 30 min, they received an injectable anesthetic preparation of ketamine and xylazine intraperitoneally. Ovaries were removed by making two bilateral incisions, followed by small incisions through the peritoneal wall, and excised through the incisions. Incisions were closed using surgical clips, and mice recovered for 10 to 14 days before the start of experiments.
Viral infection.
For primary HSV-2 infection, OVX mice were anesthetized intraperitoneally and intravaginally infected with 10 μl of WT HSV-2 strain 333 (101, 102, or 103 PFU/mouse). After inoculation, mice were placed on their backs for approximately 30 to 45 min to allow for the inoculum to infect the vaginal tract. For reexposure experiments, mice were reexposed intravaginally 6 weeks later with a higher dose of WT HSV-2 (5 × 102, 5 × 103, or 5 × 104 PFU/mouse). For intranasal immunization experiments, OVX mice were anesthetized using isoflurane. The mice were then immunized intranasally with 5 μl of thymidine kinase-deficient (TK−) HSV-2 strain 333 (102 PFU/mouse) into each nare with a micropipette, for a total of 10 μl. Six weeks following intranasal immunization, mice were challenged intravaginally with WT HSV-2 (5 × 103 PFU/mouse). To examine TRM cell populations following vaccination, mice were immunized intravaginally as described above with TK− HSV-2 strain 333 (104 PFU/mouse).
Collection of vaginal washes.
Vaginal washes were collected daily for up to 6 consecutive days after HSV-2 reexposure or challenge by pipetting 30 μl of phosphate-buffered saline (PBS) into the vagina 5 or 6 times. This was repeated to give a total volume of approximately 60 μl, and washes were stored at −70°C until use.
Genital pathology.
Following infection with HSV-2, genital pathology was monitored daily and scored on a 5-point scale: 0, no infection; 1, slight redness of external vagina; 2, swelling and redness of external vagina; 3, severe swelling and redness of both vagina and surrounding tissue and hair loss in genital area; 4, genital ulceration with severe redness; and 5, severe genital ulceration extending to surrounding tissue. Animals were euthanized by cervical dislocation when they reached stage 4/5. To compare groups, cumulative pathology scores were determined by tabulating the number of mice with the highest score of pathology they achieved and the number of days that score was observed. Mice that did not survive the challenge were given the highest pathology score for the duration of the experiment to accurately reflect overall pathology for each group. The sum of these scores for all the mice was the total level of pathology for each group, and the average pathology score per mouse for each group was then calculated.
Viral titration.
Viral titers in vaginal washes were determined by viral plaque assay on Vero cell (ATCC, Manassas, VA) monolayers. Vero cells were grown in supplemented α-minimum essential medium (α-MEM) (Gibco Laboratories, Burlington, Canada) supplemented with 5% fetal bovine serum (FBS; Gibco Laboratories), 1% penicillin-streptomycin (Invitrogen, Burlington, Canada), l-glutamate (BioShop Canada Inc., Burlington, Canada), and 1% HEPES (Invitrogen). For plaque assays, Vero cells were grown to confluence in 12-well plates. Vaginal lavage samples were removed from −70°C and thawed on ice. Samples were diluted (10−2 to 10−7) in α-MEM and added to monolayers. Infected monolayers were incubated at 37°C for 2 h and were rocked every 15 min to facilitate viral absorption. Infected monolayers were then overlaid with α-MEM to stop viral adsorption. Infection was allowed to occur for 48 h at 37°C. Monolayers were then fixed and stained with crystal violet, and viral plaques were counted under a light microscope. The number of PFU per milliliter was calculated by taking a plaque count for every sample and accounting for the dilution factors.
Multiplex cytokine and chemokine assay.
Vaginal washes collected from mice after reexposure and postchallenge were analyzed for cytokines and chemokines, using the 31-Plex Mouse Cytokine/Chemokine Discovery Luminex assay from Eve Technologies (Calgary, Canada) as per the manufacturer's protocol.
Single-cell preparation and cultures.
Three days postchallenge, iliac lymph nodes (LN), which drain the genital tract, were removed and a single cell suspension was prepared by mechanically disrupting the tissues. Debris was allowed to settle, and the supernatant containing single cells was collected and centrifuged for 5 min (1,500 rpm) at 4°C. Cells then were resuspended in 1 ml of RPMI 1640 medium supplemented with 10% FBS, 100 IU/ml of penicillin, 100 μg/ml of streptomycin, 1% l-glutamine, 0.1% 2-mercaptoethanol, 1× nonessential amino acids, and 1× sodium pyruvate (Gibco Life Technologies, Burlington, Canada). Finally, mononuclear cells were counted and cell preparations were seeded in a 24-well plate at 3 × 106 cells/ml in previously described supplemented RPMI 1640 medium.
Vaginal tracts were removed, pooled, cut lengthwise, washed to remove mucus, and minced into 2- to 4-mm pieces. The vaginal tissue pieces were digested in 15 ml of RPMI 1640 medium containing 0.00157 g/ml of collagenase A (Roche Life Science, USA) at 37°C on a stir plate, stirring with a magnetic bar for 1 h. Following the first digestion, supernatants were collected, and the tissues were digested for a second time in another 15 ml of collagenase A in RPMI 1640 medium for 1 h. At the end of the second digestion, supernatants were collected. The remaining tissue was passed through a 40-μm filter (Small Parts, Miami Lakes, FL), and then all collected supernatants were passed through a 40-μm filter into a 50-ml Falcon tube. Vaginal cell samples were then centrifuged for 10 min (1,200 rpm) at 4°C. Cells were resuspended in 1 ml of previously described supplemented RPMI 1640 medium, mononuclear cells were counted, and cell preparations were seeded in a 24-well plate at a density of 5 × 105 to 1 × 106 cells/well in previously described supplemented RPMI 1640 medium. Cells either were left unstimulated (brefeldin A and monensin) or underwent in vitro stimulation with 2 μl/ml of cell stimulation cocktail (CSC) plus protein transport inhibitors (500×) (cocktail of PMA, ionomycin, brefeldin A, and monensin [eBioscience, San Diego, CA]) for 16 h at 37°C.
Flow cytometry.
Following 16 h of incubation at 37°C with or without CSC, cells were collected and stained with allophycocyanin (APC)-ef780 viability dye (eBioscience) for 30 min. Cells were washed and incubated for 5 to 10 min with 2 μl of Fc block (anti-mouse CD16/32; eBioscience) to reduce nonspecific Fc receptor staining. Cells were then stained for cell surface markers using the following antibodies at concentrations based on manufacturer specification sheets: peridinin chlorophyll protein (PerCP)-labeled anti-mouse CD4, phycoerythrin (PE)-CF594-labeled hamster anti-mouse CD3, brilliant violet 510 (BV510)-labeled rat anti-mouse CD103, brilliant blue 515 (BB515)-labeled rat anti-mouse CD62L, PE-labeled hamster anti-mouse CD69 (BD Biosciences, Mississauga, Canada), BV421-labeled anti-mouse CD4, and Alexa Fluor 700 (AF700)-labeled anti-mouse CD44 (BioLegend, San Diego, CA). Cells were incubated with these antibodies for 30 min and then permeabilized and fixed using a transcription factor buffer set (BD Biosciences) by following the manufacturer's protocol. Cells were then stained for intracellular markers using the following antibodies: BV421- and fluorescein isothiocyanate (FITC)-labeled rat anti-mouse IFN-γ or BV421- and FITC-labeled rat IgG1 isotype control (BD Biosciences), and APC-labeled anti-mouse IL-17A or APC-labeled rat IgG2 isotype control (eBioscience). The validity of intracellular staining was verified by fluorescence-minus-one (FMO) controls and/or appropriate isotype controls. Data were collected by flow cytometric analysis using a BD LSRII flow cytometer system (BD Bioscience Pharmingen), and results were analyzed using FlowJo software.
Statistical analysis.
Statistical analysis and graphical representation were performed using GraphPad Prism 6.0d (GraphPad Software, San Diego, CA). The Mantel-Cox log rank test was used to calculate significant differences in survival. Differences between the groups were identified using the unpaired, nonparametric, two-tailed t test and multiple-comparison Mann-Whitney test with 95% confidence interval, with the ROUT method used to identify outliers. The Bonferroni correction was used to correct for multiple measures in the cytokine analysis. Data are expressed as means ± standard errors of the means.
Ethics statement.
All animals in this study were housed at the McMaster Central Animal facility, and all protocols used were approved by the McMaster University Animal Research Ethics Board (AREB) as per AUP number 14-09-40 in accordance with Canadian Council of Animal Care (CCAC) guidelines.
ACKNOWLEDGMENTS
P.B. and C.K. conceived and designed the experiments. P.B., V.C.A., P.V.N., and D.V. performed the experiments. P.B., V.C.A., and C.K. analyzed the data. M.R.S. provided materials and assisted with data analysis. P.B. and C.K. wrote the manuscript.
We thank Kristen Mueller for providing technical assistance.
This work was supported by operating grants to C.K. from the Canadian Institutes of Health Research (FRN 93615), the Ontario Graduate Scholarship, awarded to P.B., and the Applied HIV Research Chair, awarded to C.K. by the Ontario HIV Treatment Network (AHRC 779).
The funders had no role in the design of the study, data collection and analysis, or the decision to submit the work for publication. We declare no conflict of interest.
REFERENCES
- 1.Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. 2015. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 10:e114989. doi: 10.1371/journal.pone.0114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth K, Ferreira VH, Kaushic C. 2013. HSV-2 vaccine: current state and insights into development of a vaccine that targets genital mucosal protection. Microb Pathog 58:45–54. doi: 10.1016/j.micpath.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Petro C, Gonzalez PA, Cheshenko N, Jandl T, Khajoueinerjad N, Benard A, Sengupta M, Herold BC, Jacobs WR. 2015. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. eLife 4:e06054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milligan GN, Bernstein DI, Bourne N. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol 160:6093–6100. [PubMed] [Google Scholar]
- 5.Harandi AM, Svennerholm B, Holmgren J, Eriksson K. 2001. Differential roles of B cells and IFN-γ-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol 82:845–853. doi: 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- 6.Dubin PJ, Kolls JK. 2008. Th17 cytokines and mucosal immunity. Immunol Rev 226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 7.Feinen B, Jerse AE, Gaffen SL, Russell MW. 2010. Critical role of Th17 responses in a murine model of Neisseria gonorrhoeae genital infection. Mucosal Immunol 3:312–321. doi: 10.1038/mi.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pietrella D, Rachini A, Pines M, Pandey N, Mosci P, Bistoni F, d'Enfert C, Vecchiarelli A. 2011. Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS One 6:e22770. doi: 10.1371/journal.pone.0022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. 2013. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol 190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 10.Conti HR, Gaffen SL. 2015. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J Immunol 195:780–788. doi: 10.4049/jimmunol.1500909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masson L, Salkinder AL, Olivier AJ, McKinnon LR, Gamieldien H, MLisana K, Scriba TJ, Lewis DA, Little F, Jaspan HB. 2015. Relationship between female genital tract infections, mucosal interleukin-17 production and local T helper type 17 cells. Immunology 146:557–567. doi: 10.1111/imm.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vicetti Miguel RD, Calla NEQ, Pavelko SD, Cherpes TL. 2016. Intravaginal Chlamydia trachomatis challenge infection elicits TH 1 and TH 17 immune responses in mice that promote pathogen clearance and genital tract damage. PLoS One 11:e0162445. doi: 10.1371/journal.pone.0162445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guglani L, Khader SA. 2010. Th17 cytokines in mucosal immunity and inflammation. Curr Opin HIV AIDS 5:120. doi: 10.1097/COH.0b013e328335c2f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolls JK, Linden A. 2004. Interleukin-17 family members and inflammation. Immunity 21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Kim J-O, Cha H-R, Kim E-D, Kweon M-N. 2012. Pathological effect of IL-17A-producing TCRγδ+ T cells in mouse genital mucosa against HSV-2 infection. Immunol Lett 147:34–40. doi: 10.1016/j.imlet.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Anipindi VC, Bagri P, Roth K, Dizzell SE, Nguyen PV, Shaler CR, Chu DK, Jiménez-Saiz R, Liang H, Swift S. 2016. Estradiol enhances CD4+ T-cell anti-viral immunity by priming vaginal DCs to induce T h 17 responses via an IL-1-dependent pathway. PLoS Pathog 12:e1005589. doi: 10.1371/journal.ppat.1005589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 18.Scurlock AM, Frazer LC, Andrews CW, O'Connell CM, Foote IP, Bailey SL, Chandra-Kuntal K, Kolls JK, Darville T. 2011. Interleukin-17 contributes to generation of Th1 immunity and neutrophil recruitment during Chlamydia muridarum genital tract infection but is not required for macrophage influx or normal resolution of infection. Infect Immun 79:1349–1362. doi: 10.1128/IAI.00984-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, Jiao L, Yao Z, Yang X. 2009. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J Immunol 183:5886–5895. doi: 10.4049/jimmunol.0901584. [DOI] [PubMed] [Google Scholar]
- 20.Bhavanam S, Snider DP, Kaushic C. 2008. Intranasal and subcutaneous immunization under the effect of estradiol leads to better protection against genital HSV-2 challenge compared to progesterone. Vaccine 26:6165–6172. doi: 10.1016/j.vaccine.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 21.Kaushic C, Roth KL, Anipindi V, Xiu F. 2011. Increased prevalence of sexually transmitted viral infections in women: the role of female sex hormones in regulating susceptibility and immune responses. J Reprod Immunol 88:204–209. doi: 10.1016/j.jri.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Gillgrass AE, Fernandez SA, Rosenthal KL, Kaushic C. 2005. Estradiol regulates susceptibility following primary exposure to genital herpes simplex virus type 2, while progesterone induces inflammation. J Virol 79:3107–3116. doi: 10.1128/JVI.79.5.3107-3116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pennock JW, Stegall R, Bell B, Vargas G, Motamedi M, Milligan G, Bourne N. 2009. Estradiol improves genital herpes vaccine efficacy in mice. Vaccine 27:5830–5836. doi: 10.1016/j.vaccine.2009.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallichan WS, Rosenthal KL. 1998. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J Infect Dis 177:1155–1161. doi: 10.1086/515286. [DOI] [PubMed] [Google Scholar]
- 25.Parr E, Parr M. 1999. Immune responses and protection against vaginal infection after nasal or vaginal immunization with attenuated herpes simplex virus type-2. Immunology 98:639–645. doi: 10.1046/j.1365-2567.1999.00909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milligan GN, Dudley-McClain KL, Chu C-F, Young CG. 2004. Efficacy of genital T cell responses to herpes simplex virus type 2 resulting from immunization of the nasal mucosa. Virology 318:507–515. doi: 10.1016/j.virol.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Iijima N, Iwasaki A. 2014. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science 346:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y. 2007. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol 178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 29.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM. 2009. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Jeffrey JY, Jung JW. 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conti HR, Gaffen SL. 2010. Host responses to Candida albicans: Th17 cells and mucosal candidiasis. Microbes Infect 12:518–527. doi: 10.1016/j.micinf.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C. 2003. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol 77:9845–9851. doi: 10.1128/JVI.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. 2003. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol 77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roth KL, Bhavanam S, Jiang H, Gillgrass AE, Ho K, Ferreira VH, Kaushic C. 2013. Delayed but effective induction of mucsoal memory immune responses against genital HSV-2 in the absence of secondary lymphoid organs. Mucosal Immunol 6:56–68. doi: 10.1038/mi.2012.48. [DOI] [PubMed] [Google Scholar]
- 35.Park CO, Kupper TS. 2015. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat Med 21:688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin H, Iwasaki A. 2012. A vaccine strategy protects against genital herpes by establishing local memory T cells. Nature 491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17:375–387. doi: 10.1016/S1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]